Summary
Oral testosterone undecanoate (TU) is used to treat testosterone deficiency; however, oral TU treatment elevates dihydrotestosterone (DHT), which may be associated with an increased risk of acne, male pattern baldness and prostate hyperplasia. Co-administration of 5α-reductase inhibitors with other formulations of oral testosterone suppresses DHT production and increases serum testosterone. We hypothesized that finasteride would increase serum testosterone and lower DHT during treatment with oral TU. Therefore, we studied the steady-state pharmacokinetics of oral TU, 200 mg equivalents of testosterone twice daily for 7 days, alone and with finasteride 0.5 and 1.0 mg po twice daily in an open-label, three-way crossover study in 11 young men with experimentally induced hypogonadism. On the seventh day of each dosing period, serum testosterone, DHT and oestradiol were measured at baseline and 1, 2, 4, 8, 12, 13, 14, 16, 20 and 24 h after the morning dose. Serum testosterone and DHT were significantly increased into and above their normal ranges similarly by all three treatments. Co-administration of finasteride at 0.5 and 1.0 mg po twice daily had no significant effect on either serum testosterone or DHT. Oral TU differs from other formulations of oral testosterone in its response to concomitant inhibition of 5α-reductase, perhaps because of its unique lymphatic route of absorption.
Keywords: 5α-reductase, androgen, dihydrotestosterone (DHT), drug delivery, oestradiol
Introduction
Testosterone is the most important male sex hormone and is crucial for male health and development. Approximately 6–10% of men, depending on age, have low testosterone concentrations and symptoms of testosterone deficiency, including low libido, erectile dysfunction, osteoporosis, sleep disturbance, depression, lethargy and diminished physical performance (Araujo et al., 2007). These men benefit from testosterone replacement, which improves mood, energy and sense of well-being, increases bone and muscle mass and maintains sexual function (Katznelson et al., 1996; Wang et al., 1996; Snyder et al., 2000).
Testosterone undecanoate (TU) is a highly lipophilic oral formulation of testosterone that has been used for the treatment of hypogonadism (Maisey et al., 1981; Skakkebaek et al., 1981; Gooren, 1994). TU is absorbed almost exclusively via the intestinal lymphatics (Coert et al., 1975; Nieschlag et al., 1975; Horst et al., 1976), thereby bypassing hepatic metabolism. For reasons of the dependence on lymphatic absorption, oral TU must be ingested with a meal containing some fat to allow for its optimal absorption and the attainment of serum testosterone concentrations within the normal range of adult men (Houwing et al., 2003; Schnabel et al., 2007). Once TU is absorbed into the intestinal lymphatics, a portion of TU is acted upon by 5α-reductase to form dihydrotestosterone undecanoate (DHTU) (Horst et al., 1976). After the TU and DHTU are released into circulation, non-specific plasma esterases enzymatically cleave off the undecanoate ester resulting in the liberation of testosterone and DHT in the serum.
In contrast to oral TU, less lipophilic testosterone esters and testosterone itself are absorbed into the portal circulation and undergo significant hepatic metabolism (Nieschlag et al., 1975; Daggett et al., 1978), making them poorly suited for the treatment of testosterone deficiency. Therefore, alkylated formulations of testosterone such as methyltestosterone were created which were resistant to hepatic metabolism and allowed for oral testosterone dosing. Unfortunately, these formulations of alkylated oral testosterone have been associated with liver toxicity, including cholestatic jaundice and peliosis hepatis in some long-term users (Westaby et al., 1977; Turani et al., 1983) and greater increases in low-density lipoprotein and decrease in high-density lipoprotein cholesterol levels than aromatizible testosterone esters (Friedl et al., 1990). Unlike alkylated forms of testosterone, oral TU has not been associated with liver toxicity even after long-term use (Gooren, 1994). The risk of hepatotoxicity from oral TU is probably alleviated both by the absence of alkylation, aromatization and by the avoidance of hepatic metabolism (Hong & Ahn, 2007).
Another potential issue regarding the use of TU for the treatment of testosterone deficiency is the observation that serum DHT concentrations are elevated above the normal range during treatment (Nieschlag et al., 1975; Franchimont et al., 1978). These elevations in DHT may be disadvantageous as DHT probably plays a role in the pathophysiology of androgenic alopecia, acne and benign prostatic hyperplasia. Therefore, methods to attenuate the increase in serum DHT seen after oral TU administration may have clinical application to reduce side effects such as acne and baldness, and to minimize the potential for adverse effects on the prostate.
We have previously demonstrated that when other non-lymphatically absorbed formulations of oral testosterone are co-administered with a 5α-reductase inhibitor, the resulting serum testosterone concentrations are roughly doubled and serum DHT concentrations are significantly reduced (Amory & Bremner, 2005; Amory et al., 2006). If the co-administration of a 5α-reductase inhibitor with oral TU were to similarly increase the resulting concentrations of serum testosterone and decrease serum DHT, clinicians could consider co-prescription of a 5α-reductase inhibitor to patients receiving oral TU to improve testosterone pharmacokinetics and potentially decrease the risk of DHT-mediated adverse effects.
Therefore, in this study, we sought to determine if the combination of the 5α-reductase inhibitor finasteride and oral TU would be superior to oral TU alone in terms of testosterone delivery and attenuation of the supraphysiological elevations in serum DHT seen previously with administration of oral TU alone. We conducted a randomized, open-label, three-arm crossover trial of the steady-state pharmacokinetics of a fixed oral TU dose combined with two different oral doses of finasteride and placebo on serum testosterone and DHT concentrations in 11 normal men with experimentally induced hypogonadism.
Materials and methods
Subjects
Eleven men, 18–52 years of age, in good health, were recruited through local newspapers and campus flyers. After informed consent was obtained, subjects underwent screening procedures consisting of a medical history, a physical examination, measurements of serum hormone levels and routine laboratory tests, including complete blood counts, serum chemistries, liver function tests and prostate-specific antigen. Specific exclusion criteria included current use of testosterone, infertility, poor general health, clinically significant abnormal laboratory results, history of testicular disease or severe testicular trauma, major psychiatric disorders, use of illicit drugs or the use of more than three alcoholic beverages daily, participation in a drug study within the last month, a history of bleeding disorders or the use of anti-coagulants or the use of medications known to interfere with testosterone metabolism including finasteride and dutasteride.
Study design
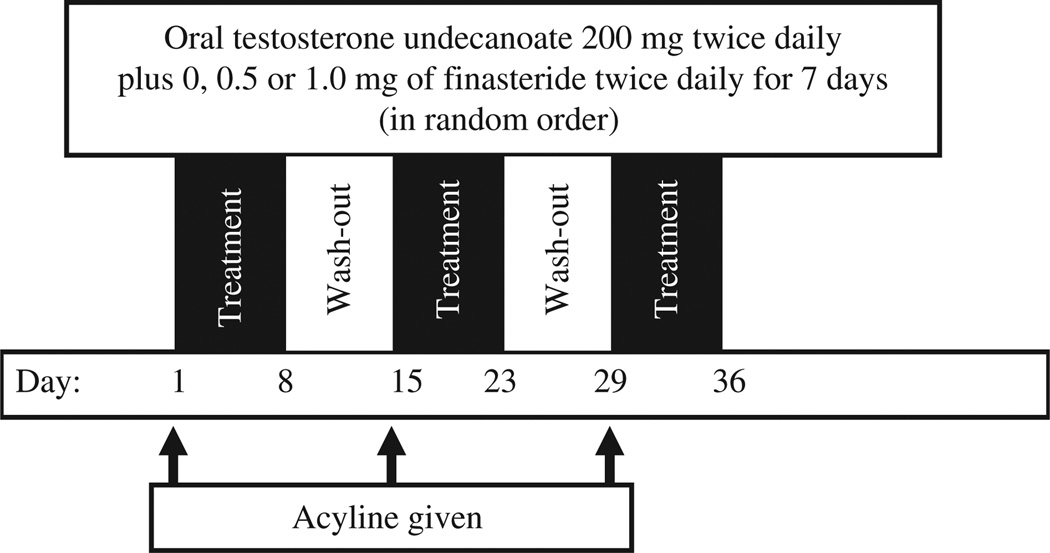
We conducted an open-label, randomized, crossover three-arm pharmacokinetic study of a fixed dose of a novel formulation of oral TU (200 mg twice daily) alone or with one of two doses (0.5 or 1.0 mg twice daily) of the 5α-reductase inhibitor finasteride. The study was conducted in normal men whose endogenous testosterone production was suppressed by the subcutaneous administration of 300 µg/kg of the potent gonadotropin-releasing hormone (GnRH) antagonist, acyline, shown previously to suppress testosterone to castrate levels within 24 h, an effect lasting 2 weeks in normal healthy men (Herbst et al., 2004). Twelve subjects were screened and 11 were enrolled, and they completed all study procedures. One subject was excluded for abnormal liver function tests at baseline and excessive alcohol use. The oral TU was administered 30 min after ingestion of a 500–700 kcal meal containing 30% fat (i.e. a regular meal). A dose of 158 mg of TU (equivalent to 100 mg of testosterone) in a proprietary self-emulsifying drug delivery system was encapsulated in capsules by Clarus Therapeutics (North-brook, IL, USA). Therefore, subjects took two of these capsules twice daily during each of the three 7-day study periods. Finasteride tablets (1 mg, Propecia; Merck & Co., Inc.) were purchased commercially and, for the 0.5 mg dose, finasteride tablets were cut into two by the University Investigational Pharmacy. Acyline was obtained from NeoMPS (San Diego, CA, USA). Each subject was administered each of the three treatments over a 6-week period (see Fig. 1), with 1 week of no treatment between each of the 3-week long treatment periods. The order of treatments was randomly assigned by a preordained sequence to minimize any carryover effects. On the seventh and last days of each of the three treatments, subjects were admitted to the General Clinical Research Center at the University of Washington overnight and had blood sample drawn prior to dosing and 1, 2, 4, 8, 12, 13, 14, 16, 20 and 24 h after dosing for measurement of serum testosterone, DHT and oestradiol. Study drugs were self-administered by subjects for the first 6 days of each of the three treatments, but were administered by study personnel on the last day of dosing. Acyline was administered 1 day prior to dosing for the first treatment to ensure suppression of testosterone before the first dose of TU, and on the first day of dosing during the second and third treatment periods. Finasteride and oral testosterone were taken at the same time for the first 6 days. For safety monitoring, subjects had blood counts, serum chemistries and liver function tests measured before and after each week of dosing. The Western Investigational Review Board approved all aspects of this study before study initiation. This study was registered in advance at www.clinicaltrials.gov as study # NCT00842751.
Figure 1.
Study design. Treatment with oral testosterone undecanoate 200 mg twice daily for three separate 1-week periods, with 1-week wash-out periods in between dosing, co-administered with 0, 0.5 or 1.0 mg finasteride twice daily. Acyline (300 mcg/kg) was administered on days 1, 15 and 29 to induce hypogonadism. The sequence of finasteride doses was randomized to minimize carryover effect.
Measurements
After clotting, blood samples were centrifuged; the serum was decanted and stored at −20 °C prior to analysis. Serum total testosterone, DHT and oestradiol were measured by liquid chromatography with tandem mass spectrometry as described previously (Shiraishi et al., 2008). Intra- and inter-assay coefficients of variation for this assay are both less than 5%. The normal range for testosterone was 265–973 ng/dL; the normal range for DHT was 13.7–77 ng/dL and the same for oestradiol was 7.5–30.6 pg/mL. The lower limits of quantification for testosterone, DHT and oestradiol are 3 ng/dL, 2 ng/dL and 2 pg/mL, respectively. Blood counts, serum chemistries and liver function tests were measured by the University of Washington Hospital clinical laboratory.
Statistical analysis
The predetermined primary outcomes for each of testosterone, DHT and oestradiol were the pharmacokinetic measures: maximum concentration after initial dosing (Cmax), time to maximum concentration (Tmax) and time-weighted mean concentration calculated as area under the concentration curve (AUC) divided by time from initiation of dosing for the morning dose and corrected for differences in baseline hormone concentration. The two dosing regimens that included finasteride were compared with the TU regimen using repeated measures analysis of variance that included the effects of regimen, the crossover sequence of the regimens and the treatment week. Compound symmetry covariance structure was used as unstructured covariance did not significantly improve model fit. All measures were log-transformed and summary statistics are reported as antilog least squares means. A significance level of 0.05 was used without correction for multiple statistical comparisons. Analyses were performed using stata (College Park, TX, USA) and sas 9.2 (Cary, NC, USA).
Results
Subjects
Eleven men who were enrolled completed all aspects of the study and were included in the analysis. The baseline characteristics of the study subjects are displayed in Table 1. One subject each experienced headache, testicular pain, depression and hot flashes. All symptoms were transient and resolved without specific medical intervention. In addition, one subject had dyspepsia and mild reflux with each dose of oral testosterone, probably because of the oily nature of the TU formulation. These symptoms remitted after completion of the drug exposure phase of the study. There were no clinically significant laboratory abnormalities in any subject during or after treatment.
Table 1.
Baseline characteristics of the 11 study subjects (mean ± SD)
| Age (years) | 32 ± 9 |
| Weight (kg) | 80 ± 13 |
| Height (cm) | 181 ± 7 |
| BMI (kg/m2) | 24 ± 3 |
| Serum testosterone (ng/dL) | 405 ± 14 |
| Serum DHT (ng/dL) | 32 ± 12 |
| Serum oestradiol (pg/mL) | 15 ± 5 |
BMI, body mass index; DHT, dihydrotestosterone.
Serum hormone pharmacokinetics
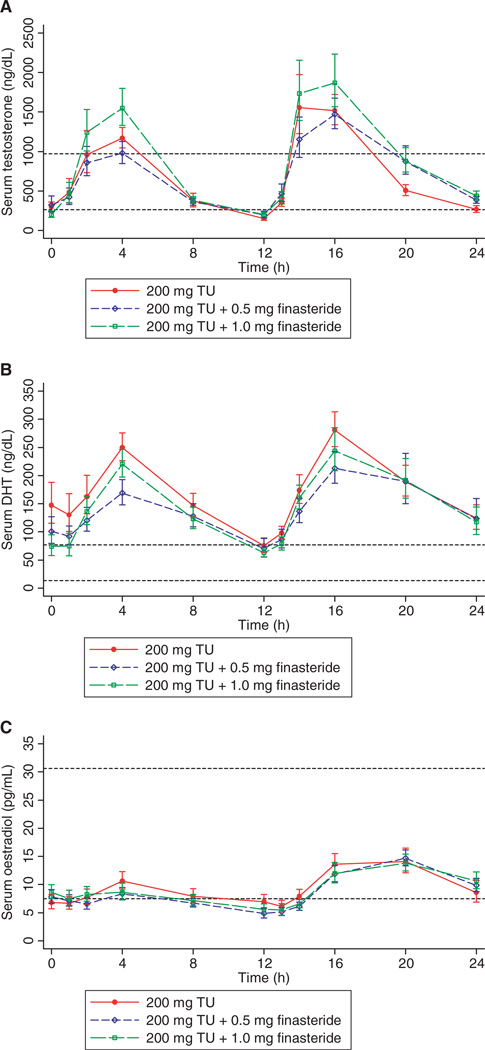
Twenty-four hours after acyline administration, serum testosterone concentrations in all subjects were markedly suppressed from 405 ± 14 ng/dL to 46 ± 10 ng/dL (p < 0.0001). Similarly, serum DHT was suppressed from 32 ± 12 ng/dL to 9.9 ± 5.4 ng/dL and serum oestradiol from 15 ± 5.0 pg/mL to 6.6 ± 3.3 pg/mL (p < 0.01 for both comparisons with baseline). On the seventh day of dosing with all treatments, serum testosterone increased significantly with oral TU administration, with mean testosterone concentrations peaking 4 h after dosing (Fig. 2A). Serum testosterone then fell below the lower limit of the normal range 12 h after the morning dose. Serum testosterone concentrations after the evening dose were similarly increased, but did not fall below the lower limit of the normal range by 12 h after dosing. There were no significant differences between the treatments in any of the measures of testosterone pharmacokinetics (Table 2).
Figure 2.
Serum testosterone (A), dihydrotestosterone (DHT) (B) and oestradiol (C) after 7 days of dosing with oral testosterone undecanoate (200 mg) by mouth twice a day alone or with 0.5 or 1.0 mg of finasteride twice a day in 11 normal men with experimentally induced hypogonadism. Each subject underwent all three treatments in random order. The short dashed lines represent the upper and lower limits of the normal range. All values are geometric means ± SE. TU, testosterone undecanoate.
Table 2.
Daytime hormone pharmacokinetics after 7 days of dosing with oral testosterone undecanoate (TU) 200 mg twice a day alone, or with 0.5 mg finasteride twice a day or 1.0 mg finasteride twice a day in 11 normal men with pharmacologically induced hypogonadism. All subjects received all three treatments
| Cmax (ng/dL) | Tmax (h) | AUC0–12 h/12 (ng/dL) | |
| Testosterone | |||
| TU + placebo | 1707 (1225–2378) | 2.4 (1.9–2.9) | 471 (381–581) |
| TU + 0.5 mg finasteride | 1380 (985–1933) | 2.3 (1.9–2.8) | 444 (358–551) |
| Ratio: TU + 0.5 mg finasteride/TU + placebo | 0.81 (0.51–1.28) | 0.96 (0.72–1.27) | 0.94 (0.70–1.27) |
| p = 0.35 | p = 0.76 | p = 0.69 | |
| TU + 1 mg finasteride | 1976 (1416–2758) | 2.5 (2.1–3.1) | 543 (439–671) |
| Ratio: TU + 1.0 mg finasteride/TU + placebo | 1.16 (0.73–1.83) | 1.07 (0.81–1.41) | 1.15 (0.86–1.54) |
| p = 0.51 | p = 0.61 | p = 0.31 | |
| DHT | |||
| TU + placebo | 287 (245–336) | 2.4 (1.9–3.1) | 154 (125–190) |
| TU + 0.5 mg finasteride | 221 (188–260) | 2.3 (1.7–2.9) | 118 (96–146) |
| Ratio: TU + 0.5 mg finasteride/TU + placebo | 0.77 (0.62–0.96) | 0.94 (0.66–1.34) | 0.77 (0.57–1.02) |
| p = 0.02 | p = 0.72 | p = 0.06 | |
| TU + 1 mg finasteride | 250 (213–293) | 2.8 (2.2–3.6) | 125 (102–154) |
| Ratio: TU + 1.0 mg finasteride/TU + placebo | 0.87 (0.70–1.08) | 1.17 (0.83–1.66) | 0.81 (0.61–1.08) |
| p = 0.20 | p = 0.36 | p = 0.14 | |
| Cmax (pg/mL) | Tmax (h) | AUC0–12 h/12 (pg/mL) | |
| Oestradiol | |||
| TU + placebo | 12.4 (9.9–15.5) | 2.4 (1.6–3.4) | 7.9 (6.4–9.7) |
| TU + 0.5 mg finasteride | 10.2 (8.1–12.8) | 1.8 (1.2–2.6) | 6.5 (5.2–8.0) |
| Ratio: TU + 0.5 mg finasteride/TU + placebo | 0.82 (0.60–1.13) | 0.75 (0.44–1.28) | 0.82 (0.61–1.11) |
| p = 0.21 | p = 0.28 | p = 0.18 | |
| TU + 1 mg finasteride | 10.1 (8.0–12.6) | 1.9 (1.3–2.8) | 7.2 (5.8–8.9) |
| Ratio: TU + 1.0 mg finasteride/TU + placebo | 0.81 (0.60–1.11) | 0.81 (0.48–1.36) | 0.91 (0.68–1.22) |
| p = 0.18 | p = 0.40 | p = 0.53 | |
Cmax, maximum concentration; Tmax, time of maximum concentration; AUC0–12 h/12, weighted mean concentration for serum testosterone, dihydrotestosterone (DHT) and oestradiol are shown. All values are through 12 h post-dosing and are summarized as geometric means and 95% confidence intervals.
Serum DHT was significantly increased from baseline during all three treatment periods, staying above the upper limit of the normal range throughout the 24-h sampling period on the seventh day of dosing (Fig. 2B). Surprisingly, there were almost no significant differences among the three treatments, with the two treatments containing finasteride exhibiting almost identical serum DHT concentrations and pharmacokinetics (Table 2). The only difference between the treatments that attained significance was a slight decrease in the ratio of Cmax DHT between the 0.5 mg of finasteride twice daily treatment and placebo. However, there was no difference in this ratio with the larger dose of finasteride. There was a trend towards a higher serum DHT concentration prior to the morning dose in the group receiving no finasteride, but this difference was not statistically significant (Fig. 2B). Serum oestradiol after 7 days of dosing remained within the normal range in all treatments (Fig. 2C). There was a slight increase in the average serum oestradiol concentrations after the evening dose of testosterone; however, this difference did not attain statistical significance.
Discussion
In this study, we have demonstrated that the pharmacokinetics of orally dosed TU is not improved by the concomitant administration of the 5α-reductase inhibitor finasteride. This finding is in sharp contrast to our earlier work demonstrating that the concomitant administration of either finasteride or dutasteride significantly increased serum testosterone concentrations and significantly suppressed serum DHT concentrations when used in combination with oral testosterone in oil (Amory & Bremner, 2005; Amory et al., 2006). What could explain the inability of finasteride to suppress serum DHT and increase serum testosterone after the oral administration of TU as compared with the other tested formulations of oral testosterone? One possible explanation involves the variable methods of absorption between these two oral formulations. Oral TU is absorbed via intestinal lymphatics (Coert et al., 1975; Nieschlag et al., 1975), whereas the oral formulations of non-esterified testosterone are via the portal circulation (Amory & Bremner, 2005). Finasteride is also absorbed via the portal circulation (Carlin et al., 1992), and its absorption is not thought to be affected by food (Steiner et al., 1996). Therefore, finasteride may not be able to prevent the 5α-reduction of the oral TU because of the variable routes of absorption and appearance in the systemic circulation. Consistent with this hypothesis is the work of Horst et al. (1976) that demonstrated the presence of significant amounts of DHTU in the thoracic ducts of men dosed orally with TU while their thoracic ducts were cannulated during neck surgery. If this hypothesis is correct, 5α-reductase inhibitors with greater lipophilicity, such as dutasteride (Bramson et al., 1997), may be more successful in suppressing the elevations in serum DHT observed with dosing of oral TU. Alternatively, we may have chosen too low a dose of finasteride to inhibit the production of DHT from testosterone; however, previous studies with finasteride have demonstrated that 2 mg daily can reduce circulating levels of DHT by 50% (Steiner, 1996) and therefore it seems unlikely that the failure of finasteride to suppress DHT after oral dosing of TU was because of an insufficient dose of finasteride. Indeed, the finasteride probably reduced DHT, as the pre-dose serum DHT in the testosterone-only group appeared to be higher than that in the groups receiving finasteride, although this difference was not statistically significant. Therefore, it seems more likely that the lack of effects was because of the differing routes of absorption of these two medications.
Our study clearly demonstrates that oral dosing of TU is associated with supraphysiological elevations in serum DHT. Other forms of testosterone therapy such as patches and gels are also associated with slightly increased concentrations of DHT, presumably because of the presence of 5α-reductase in the skin (Swerdloff et al., 2000). In terms of testosterone therapy, such increases may increase the risk of DHT-related conditions such as acne, benign prostatic hypertrophy and androgenic alopecia. As 5α-reductase inhibitors are useful clinically to treat acne, androgenic alopecia and prostate hypertrophy, these conditions must be more sensitive to DHT than testosterone. A case in point is the poorly understood and controversial relationship between serum DHT and the risk of prostate cancer. Increased serum DHT associated with oral TU administration does not seem to be associated with an increased risk of prostate cancer long-term (Gooren et al., 1994). This may be because intraprostatic DHT does not appear to change in response to even high levels of circulating DHT – an observation recently made by Page et al. (2010) in men treated with a topical DHT gel. In addition, most epidemiological studies do not find an association between serum DHT and the risk of prostate cancer (Gann et al., 1996; Gill et al., 2010). However, two large, prospective, placebo-controlled trials have demonstrated that the administration of either finasteride or dutasteride reduces the risk of prostate cancer by approximately 25% (Thompson et al., 2003; Andriole et al., 2010). However, both these studies raised the concern that the reduction in serum DHT might actually increase the risk of prostate cancers with higher Gleason scores that are potentially less sensitive to androgen deprivation therapies. Clearly, a greater understanding of the association between DHT and prostate cancer is required.
In contrast to the elevations in serum DHT with oral TU administration, serum oestradiol concentrations were in the low-normal range with all three treatments, demonstrating that 5α-reductase inhibition with finasteride or dutasteride has no effect on the aromatization of oestradiol from testosterone, consistent with other studies of these agents (Amory et al., 2007). Interestingly, the evening serum oestradiol concentrations appeared to be elevated compared with those observed after the morning dose of oral TU. The reason for this remains unclear; however, it has been observed previously with orally dosed testosterone (Amory et al., 2008).
The oral TU was well-tolerated with only one subject complaining of gastrointestinal discomfort during treatment. It is important to note that there were no significant alterations in the liver or kidney function consistent with previous reports of the safety of oral TU (Gooren et al., 1994) and in contrast to the reports of liver inflammation observed with alkylated formulations of oral testosterone (Westaby et al., 1977; Turani et al., 1983).
In conclusion, we have shown that administration of oral TU results in significantly elevated serum DHT concentrations, and that the co-administration of finasteride has no apparent effect on the elevated serum DHT concentrations. Additional studies of oral testosterone with and without the co-administration of finasteride and dutasteride will be necessary to find a combination that leads to normal concentrations of serum testosterone and DHT. Such a formulation may allow for treatment of testosterone deficiency without increasing the risk of any DHT-mediated side effects.
Acknowledgements
This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, a division of the National Institute of Health through cooperative agreement U54 HD42454 as part of the Cooperative Contraceptive Research Centers Program and Clarus Therapeutics, Inc. Portions of this work were conducted through the Clinical Research Center facility at the University of Washington, and supported by the NIH grant UL1-RR-025014, the GCRC Core Laboratory at Harbor-UCLA Medical Center and NIH grant (M01 RR-00425).
References
- Amory JK, Bremner WJ. Oral testosterone in oil plus dutasteride: a pharmacokinetic study in men. J Clin Endocrinol Metab. 2005;90:2610–2617. doi: 10.1210/jc.2004-1221. [DOI] [PubMed] [Google Scholar]
- Amory JK, Page ST, Bremner WJ. Oral testosterone in oil: pharmacokinetic effects of 5α-reduction with finasteride or dutasteride and food intake in men. J Androl. 2006;27:72–78. doi: 10.2164/jandrol.05058. [DOI] [PubMed] [Google Scholar]
- Amory JK, Wang C, Swerdloff RS, Anawalt BD, Matsumoto AM, Bremner WJ, Walker SE, Haberer LJ, Clark RV. The effect of 5α-reductase inhibition with dutasteride and finasteride on semen parameters and serum hormones in healthy men. J Clin Endocrinol Metab. 2007;92:1659–1665. doi: 10.1210/jc.2006-2203. [DOI] [PubMed] [Google Scholar]
- Amory JK, Kalhorn TF, Page ST. Pharmacokinetics and pharmacodynamics of oral testosterone enanthate in oil plus dutasteride for four weeks in normal men: implications for male hormonal contraception. J Androl. 2008;29:260–271. doi: 10.2164/jandrol.107.004226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andriole GL, Bostwick DG, Brawley OW, Gomella LG, Marberger M, Montorsi F, et al. Effect of dutasteride on the risk of prostate cancer. N Engl J Med. 2010;362:1192–1202. doi: 10.1056/NEJMoa0908127. [DOI] [PubMed] [Google Scholar]
- Araujo AB, Esche GR, Kupelian V, O’Donnell AB, Travison TG, Williams RE, Clark RV, McKinlay JB. Prevalence of symptomatic androgen deficiency in men. J Clin Endocrinol Metab. 2007;92:4241–4247. doi: 10.1210/jc.2007-1245. [DOI] [PubMed] [Google Scholar]
- Bramson HN, Hermann D, Batchelor KW, Lee FW, James MK, Frye SV. Unique preclinical characteristics of GG745, a potent dual inhibitor of 5AR. J Pharmacol Exp Ther. 1997;282:1496–1502. [PubMed] [Google Scholar]
- Carlin JR, Höglund P, Eriksson LO, Christofalo P, Gregoire SL, Taylor AM, Andersson KE. Disposition and pharmacokinetics of [14C] finasteride after oral administration in humans. Drug Metab Dispos. 1992;20:148–155. [PubMed] [Google Scholar]
- Coert A, Geelen J, de Visser J, van der Vies J. The pharmacology and metabolism of testosterone undecanoate (TU), a new orally active androgen. Acta Endocrinol (Copenh) 1975;79:789–800. doi: 10.1530/acta.0.0790789. [DOI] [PubMed] [Google Scholar]
- Daggett PR, Wheeler MJ, Nabarro JDN. Oral testosterone, a reappraisal. Hormone Res. 1978;9:121–129. doi: 10.1159/000178904. [DOI] [PubMed] [Google Scholar]
- Franchimont P, Kicovic PM, Mattei A, Roulier R. Effects of oral testosterone undecanoate in hypogonadal male patients. Clin Endocrinol (Oxf) 1978;9:313–320. doi: 10.1111/j.1365-2265.1978.tb02216.x. [DOI] [PubMed] [Google Scholar]
- Friedl KE, Hannan CJ, Jr, Jones RE, Plymate SR. High-density lipoprotein cholesterol is not decreased if an aromatizable androgen is administered. Metabolism. 1990;39:69–74. doi: 10.1016/0026-0495(90)90150-b. [DOI] [PubMed] [Google Scholar]
- Gann PH, Hennekens CH, Ma J, Longcope C, Stampfer MJ. Prospective study of sex hormone levels and risk of prostate cancer. J Natl Cancer Inst. 1996;88:1118–1126. doi: 10.1093/jnci/88.16.1118. [DOI] [PubMed] [Google Scholar]
- Gill JK, Wilkens LR, Pollak MN, Stanczyk FZ, Kolonel LN. Androgens, growth factors, and risk of prostate cancer: the multi-ethnic cohort. Prostate. 2010;70:906–915. doi: 10.1002/pros.21125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooren LJ. A ten-year safety study of the oral androgen testosterone undecanoate. J Androl. 1994;15:212–215. [PubMed] [Google Scholar]
- Herbst KL, Coviello AD, Page ST, Amory JK, Anawalt BD, Bremner WJ. A single dose of the potent gonadotropin-releasing hormone antagonist acyline suppresses gonadotropins and testosterone for 2 weeks in healthy young men. J Clin Endocrinol Metab. 2004;89:5959–5965. doi: 10.1210/jc.2003-032123. [DOI] [PubMed] [Google Scholar]
- Hong BS, Ahn TY. Recent trends in the treatment of testosterone deficiency syndrome. Int J Urol. 2007;14:981–985. doi: 10.1111/j.1442-2042.2007.01882.x. [DOI] [PubMed] [Google Scholar]
- Horst HJ, Holtje WJ, Dennis M, Coert A, Geelen J, Voigt KD. Lymphatic absorption and metabolism of orally administered testosterone undecanoate in man. Klin Wochenschr. 1976;54:875–879. doi: 10.1007/BF01483589. [DOI] [PubMed] [Google Scholar]
- Houwing NS, Maris F, Schnabel PG, Bagchus WM. Pharmacokinetic study in women of three different doses of a new formulation of oral testosterone undecanoate, Andriol Testocaps. Pharmacotherapy. 2003;23:1257–1265. doi: 10.1592/phco.23.12.1257.32707. [DOI] [PubMed] [Google Scholar]
- Katznelson L, Finkelstein JS, Schoenfeld DA, Rosenthal DI, Anderson EJ, Klibanski A. Increase in bone density and lean body mass during testosterone administration in men with acquired hypogonadism. J Clin Endocrinol Metab. 1996;81:4358–4365. doi: 10.1210/jcem.81.12.8954042. [DOI] [PubMed] [Google Scholar]
- Maisey NM, Bingham J, Marks V, English J, Chakraborty J. Clinical efficacy of testosterone undecanoate in male hypogonadism. Clin Endocrinol (Oxf) 1981;14:625–629. doi: 10.1111/j.1365-2265.1981.tb02974.x. [DOI] [PubMed] [Google Scholar]
- Nieschlag E, Mauss J, Coert A, Kicovic P. Plasma androgen levels in men after oral administration of testosterone and testosterone undecanoate. Acta Endocrinol (Copenh) 1975;79:366–374. doi: 10.1530/acta.0.0790366. [DOI] [PubMed] [Google Scholar]
- Page ST, Lin DW, Mostaghel E, Marck B, Wright J, Wu J, Amory JK, Nelson PS, Matsumoto AM. Exogenous dihydrotestosterone does not increase intraprostatic dihydrotestosterone concentrations or androgen action in healthy men: a randomized-controlled trial. Poster P1-388 presented at the 90th Annual Meeting of the Endocrine Society; San Diego, CA. 2010. [Google Scholar]
- Schnabel PG, Bagchus W, Lass H, Thomsen T, Geurts TB. The effect of food composition on serum testosterone levels after oral administration of Andriol Testocaps. Clin Endocrinol (Oxf) 2007;66:579–585. doi: 10.1111/j.1365-2265.2007.02781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiraishi S, Lee PW, Leung A, Goh VH, Swerdloff RS, Wang C. Simultaneous measurement of serum testosterone and dihydrotestosterone by liquid chromatography-tandem mass spectrometry. Clin Chem. 2008;54:1855–1863. doi: 10.1373/clinchem.2008.103846. [DOI] [PubMed] [Google Scholar]
- Skakkebaek NE, Bancroft J, Davidson DW, Warner P. Androgen replacement with oral testosterone undecanoate in hypogonadal men: a double blind controlled study. Clin Endocrinol (Oxf) 1981;14:49–61. doi: 10.1111/j.1365-2265.1981.tb00364.x. [DOI] [PubMed] [Google Scholar]
- Snyder PJ, Peachey H, Berlin JA, Hannoush P, Haddad G, Dlewati A, et al. Effects of testosterone replacement in hypogonadal men. J Clin Endocrinol Metab. 2000;85:2670–2677. doi: 10.1210/jcem.85.8.6731. [DOI] [PubMed] [Google Scholar]
- Steiner JF. Clinical pharmacokinetics and pharmacodynamics of finasteride. Clin Pharmacokinet. 1996;30:16–27. doi: 10.2165/00003088-199630010-00002. [DOI] [PubMed] [Google Scholar]
- Swerdloff RS, Wang C, Cunningham G, Dobs A, Iranmanesh A, Matsumoto AM, Snyder PJ, Weber T, Longstreth J, Berman N. Long-term pharmacokinetics of transdermal testosterone gel in hypogonadal men. J Clin Endocrinol Metab. 2000;85:4500–4510. doi: 10.1210/jcem.85.12.7045. [DOI] [PubMed] [Google Scholar]
- Thompson IM, Goodman PJ, Tangen CM, Lucia MS, Miller GJ, Ford LG, et al. The influence of finasteride on the development of prostate cancer. New Engl J Med. 2003;349:215–224. doi: 10.1056/NEJMoa030660. [DOI] [PubMed] [Google Scholar]
- Turani H, Levi J, Zevin D, Kessler E. Hepatic lesions in patients on anabolic androgenic therapy. Isr J Med Sci. 1983;19:332–337. [PubMed] [Google Scholar]
- Wang C, Alexander G, Berman N, Salehian B, Davidson T, McDonald V, Steiner B, Hull L, Callegari C, Swerdloff RS. Testosterone replacement therapy improves mood in hypogonadal men – a clinical research center study. J Clin Endocrinol Metab. 1996;81:3578–3583. doi: 10.1210/jcem.81.10.8855804. [DOI] [PubMed] [Google Scholar]
- Westaby D, Ogle SJ, Paradinas FJ, Randell JB, Murray-Lyon IM. Liver damage from long-term methyltestosterone. Lancet. 1977;2:262–263. [PubMed] [Google Scholar]