Abstract
Delivery of vaccine antigens from controlled-release poly(lactic/glycolic acid) (PLGA) microspheres is a novel approach to reduce the number of antigen doses required for protection against infection. A major impediment to developing single-shot vaccines is encapsulated antigen instability during months of exposure to physiological conditions. For example, efforts to control neonatal tetanus in developing countries with a single-dose TT vaccine have been plagued by poor stability of the 150 kDa formaldehyde-detoxified protein antigen, tetanus toxoid (TT) in PLGA microspheres. We examined the denatured states of PLGA-encapsulated TT, revealing two primary TT instability mechanisms: 1) protein aggregation mediated by formaldehyde and 2) acid-induced protein unfolding and epitope damage. Further, we systemically identified excipients which can efficiently inhibit TT aggregation and retain TT antigenicity under simulated deleterious conditions, i.e., elevated temperature and humidity. By employing these novel additives in the PLGA system, we report the slow and continuous release of high doses of TT for one month with retained antigen stability during bioerosion of PLGA.
Keywords: vaccine delivery, protein aggregation, stabilization, PLGA, formaldehyde
INTRODUCTION
Since vaccine delivery from polymers was first discovered,1 research has focused on developing polymer formulations that would be capable of reducing the required number of antigen doses for protection to as few as a single shot.2 Efforts to this end have intensified after recognition that effective controlled-release vaccines are among the best hope to improve vaccine coverage in developing countries.3 To reduce injection frequency, antigens are encapsulated in biodegradable polymers such as poly(lactic/glycolic acid) (PLGA) microspheres (1–100 μm), which are easily administered through a syringe needle. PLGAs can be formulated to slowly release soluble antigens from days to several months. This release duration, for which the antigen must remain encapsulated in the polymer at physiological temperature and humidity, poses significant challenges to retain both the structural integrity and native epitopes of the antigen. Encapsulated antigen instability is considered the primary obstacle impeding the development of effective single-dose vaccines.4–9
Tetanus remains a major killer in developing countries, e.g., > 400,000 deaths/yr10 from neonatal tetanus alone, largely due to the logistical difficulty of delivering 2 to 3 doses of vaccine required for protection to pregnant women whose immunity can be passively transferred to the fetus.11 Spear-headed by the World Health Organization, tetanus toxoid (TT), the formaldehyde-treated protein antigen against tetanus, was selected as one of the first antigens for single-dose vaccine development for human use based on PLGA microsphere system.12 Despite several promising reports of neutralizing antibody responses in test animals following a single dose of TT encapsulated in PLGA microspheres,13–19 the instability of the antigen emerged as a ubiquitous problem.20–26 In limited cases, anti-TT antibody titers in vivo were reported to be in accordance with the in vitro release characteristics of immunoreactive TT from the particles. For example, Raghuvanshi et al. reported that anti-TT antibody titers from the stabilized particles were much better than that observed from particles made without stabilizers.26 In addition, other formulation parameters such as polymer hydrophobicity, particle size and use of additional adjuvants may also impact on the generation of immune responses in experimental animals.16,18, 19, 25
Previous attempts to stabilize encapsulated TT include: employing basic inorganic salts and BSA as a proton scavenger to inhibit acid-induced instability,23, 24, 27 using rat serum albumin to enhance TT stability during primary emulsification step of particle formation, 24, 26 optimizing encapsulation methods,28, 29 reducing the interaction of TT with the polymer or polymer degradation products,28, 30 adjusting water uptake of the polymer,23, 31, 32 and co-encapsulating some commonly used protein stabilizers, e.g., sucrose, trehalose, cyclodextrin, dextran, heparin in PLGA microspheres to enhance the stability of TT.24, 29 Although some stability improvements have been noted,23, 24, 26, 29, 31 few study has clearly characterized the instability mechanisms of TT inside PLGA microspheres and systemically analyzed the effect of stabilizers on the biophysical and biochemical properties of TT. In addition, although co-encapsulation of serum albumin in the polymer formulations significantly increased the continuous release of antigenically active TT,23 potential autoimmune reactions of serum albumin may hinder its further use in humans.33
The purpose of this study is to investigate the instability mechanisms of TT in PLGA microspheres. By examining the denatured state of encapsulated TT and co-encapsulating novel stabilizers into the polymer, we confirmed that acid- and formaldehyde-based mechanisms as the two primary deleterious pathways of the encapsulated TT and more fully describe formulations that we originally reported to exhibit exceptional stability of encapsulated TT.34, 35
Experimental Section
Chemicals
Poly(DL-lactide-co-glycolide) 85/15 (inherent viscosity of 0.69 and 0.86 dl/g in CHCl3) was from Birmingham Polymers (Birmingham, AL). TT (specific activity of 3300 Lf mg–1), was a kind gift from Chiron Corp. (San Francisco, CA). TT010 monoclonal antibody, which detects a neutralizing TT epitope, and anti-TT guinea pig IgG were generously provided by National Institute of Biological Standard Control (UK). Goat anti-guinea pig IgG peroxidase conjugate and 2,2′-azino-di-3-ethylbenzthiazoline-6-sulphonate (ABTS) tablets were obtained from Sigma (Milwaukee, WI). Fine MgCO3 (<5 μm) powder was from Aldrich (Milwaukee, WI). All other substances used were of pharmaceutical or analytical grade and purchased from commercial suppliers.
Microsphere preparation
TT was encapsulated by an oil-in-oil emulsion and solvent extraction method. To avoid TT aggregates during grinding, TT with or without stabilizers was micronized by adding 150 μl aqueous antigen solution into 1.2 ml of 300 mg/ml PLGA (0.86 dl/g) in acetonitrile. The suspension was homogenized at 15,000 rpm (Model IQ2, Virtis Co., Gardiner, NY) for 3 min at 4°C and dropwise added to 100 ml cottonseed oil containing 1.6 g Span 85 under stirring at 700 rpm. After 5 h, petroleum ether (b.p. 50–110 °C) was poured into the cottonseed oil bath to extract the acetonitrile from the polymer. After an additional 15 min of stirring, the microspheres were sieved (120 μm screen), washed with 250 ml of petroleum ether, collected and lyophilized. The loading of antigen was determined by both destructive and nondestructive methods (see below). MgCO3 was incorporated in microspheres by suspension in the polymer solution before addition of antigens. All microspheres were well formed with a mean diameter of 80–90 μm for TT/PLGA microspheres.
Determination of antigen loading
The amount of TT encapsulated in microspheres was determined by two methods. The first was to extract the protein antigen from the microspheres by removing the polymer and determine the protein content by modified Bradford assay (Coomassie brilliant blue plus protein assay, Pierce, Rockford, IL) and ELISA. The second was to determine encapsulated protein content by amino acid analysis after direct acid hydrolysis of microspheres (see Amino Acid Analysis below). In the former, acetone was added in microspheres to dissolve the polymer. The mixture was vortexed, centrifuged and then the supernatant was removed. After removal of polymer was repeated 3 times, the remaining protein pellet was reconstituted in phosphate buffer saline containing 0.02% Tween 80 (PBST) at 37ºC for 2 hrs and protein content was determined by modified Bradford assay and ELISA.
Evaluation of antigen release from microspheres and aggregation within microspheres
Microspheres (15–20 mg) were incubated under mild agitation at 37 °C in 0.5 ml PBST, or in PBST containing 0.2% BSA (PBSTB) to inhibit potential TT adsorption to the polypropylene eppendorf tube.36 Release medium was collected periodically by centrifugation and replaced with new medium. The soluble and antigenic protein content collected from PBST was determined by using a modified Bradford assay and ELISA, respectively. For the release medium collected from PBSTB, only the amount of antigenic TT was determined by ELISA. Soluble and antigenic protein content during release were normalized by the antigen content from the loading measurement (modified Bradford and ELISA) to determine total and antigenic release kinetics, respectively.
At the end of release, incubated microspheres were collected, dried and dissolved in acetone. After centrifugation and removal of the polymer solution, the remaining protein pellet was reconstituted in phosphate buffer saline containing 0.02% Tween 80 (PBST) at 37ºC for 2 hrs and soluble protein content was determined by modified Bradford assay and ELISA. If any insoluble aggregates were observed in the reconstituted solution, aggregates were collected, washed with distilled water and then freeze dried. These insoluble aggregates were reconstituted in denaturing agent (6 M Guanidine-HCl (GnCl)). Determination of any aggregate soluble in denaturing agent gave the amount of non-covalently bonded aggregates. With the further addition of reducing agent (10 mM dithiothreitol, 1 mM EDTA), any disulfide-bonded aggregates were dissolved. The total dissolved portion in denaturing and reducing agents gave the total amount of non-covalent and disulfide-bonded aggregates. For any insoluble aggregates which are not soluble in both denaturing and reducing agents, they will be further subjected to amino acid analysis or counted as formaldehyde-mediated aggregates.
Amino acid analysis
Dry microspheres or insoluble protein aggregates were weighed and dissolved in 6 N HCl/TFA (1:1) and hydrolyzed under 6 N HCl vapor at 125 °C for 24 h. An Applied Biosystems (ABI, Foster city, CA) 420 H was used to derivatize hydrolyzed amnio acids with phenylisothiocyanate (PITC) at alkaline pH to form PTC-amino acid derivatives, which were injected onto an ABI model 130A HPLC and detected at 269 nm.
Solid state stability of antigens
For solid-state stability, TT was diluted to ~1 mg/ml and dialyzed (10 kDa cut-off) against 1 mM sodium phosphate buffer pH 7.3 at 4 °C. Stabilizers were added to TT solutions, flash frozen in liquid N2 and lyophilized for 48 h on a Freezezone 6 freeze-drying system (Labconco, Kansas City, MO) at 133 × 10−3 mbar or less with a condenser temperature of −46 °C. Lyophilized antigen samples were incubated at 37 °C and 80% relative humidity (R.H.). Incubated samples were reconstituted in 1 mM phosphate buffer and assessed for soluble protein. Any protein aggregates were further analyzed with the addition of denaturing and reducing agent described in the section Evaluation of antigen release from microspheres and aggregation within microspheres.
Structural Analysis of TT
Far-UV circular dichroic (CD) spectra were taken with a J-810 Jasco spectropolarimeter (Hachioji, Japan) at room temperature to assess secondary structure of TT. Tertiary structural changes were monitored with a fluorimeter (Fluoromax-2, ISA Instrument Inc., NJ). TT solutions were excited (λex) at 280 nm. The ratio of emission intensity at 350 nm over that at 329 nm (I350/I329) was monitored. Both changes in emission wavelength maximum (λmax) and I350/I329 are sensitive indices of conformational changes.27, 37
ELISA for TT
Microtitre plates were coated with 100 μl of 1 μg ml−1 of the monoclonal anti-TT in coating buffer overnight at 4 °C. After blocking, and washing with PBST, serial dilutions of reference tetanus toxoid and samples in PBSTB were added and incubated for 2 h at 37 °C. After PBST washing, plates were incubated with purified guinea pig polyclonal anti-TT IgG 5 μg/ml for 2 hrs at 37 °C, followed by incubation with goat anti-guinea pig IgG-horseradish peroxidase (HRP) conjugate in PBSTB (1 in 2000) for 1 hr at 37 °C. After adding the substrate solution containing 0.5 mg ml−1 of ABTS and 0.04% H2O2 in 0.05 M citric acid, pH 4.0 plates were read after 25–30 min at room temperature at 405 nm.
RESULTS AND DISCUSSION
Encapsulation of TT in PLGA
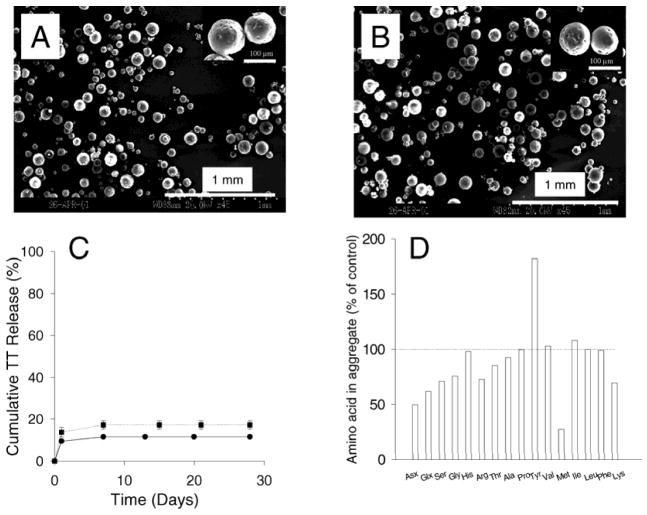
To investigate TT stability in the polymer, we encapsulated TT in PLGA 85/15 microspheres (1.5% w/w TT) (Fig 1A, Table 1) by an oil-in-oil emulsion and solvent extraction method. Fig. 1A shows the size and morphology of TT microspheres. Microsphere particles were spherical but not smooth. Small pores were observed on micropshere surfaces, possibly formed after water and acetonitrile evaporation during freeze drying process.
Fig. 1.
Scanning electron micrographs of TT/PLGA micropsheres (A) and TT/PLGA micropsheres containing lysine, sorbitol and MgCO3 (B); In vitro release of TT from TT/PLGA microspheres without stabilizers: protein content (mean ± s.d., n=2) (●), and antigenic TT (mean ± s.d., n=2) (■) (C); and changes in the amino acid composition of TT aggregates formed in PLGA microspheres after 28 days of release (D). The amino acid composition of the acid hydrolysate of the aggregated TT is given relative to the unencapsulated TT standard control. All amino acid concentrations were normalized for the concentration of leucine.
Table 1.
Encapsulation of TT
| Formulations | TT Loading (%) | ||
|---|---|---|---|
| Solublea,e | Antigenicb,e | Total (AAA)c,d | |
| No stabilizers | 1.5 ± 0.1 | 0.82 ±0.02 | 1.6 |
| Lysine + MgCO3 f | 1.7 ± 0.4 | 1.4 ± 0.1 | 1.7 |
| Lysine + Sorbitol + MgCO3g | 2.1 ± 0.2 | 1.1 ± 0.2 | 2.3 |
| Lysine + Trehalose + MgCO3g | 2.5 ± 0.1 | 1.5 ± 0.4 | 2.8 |
Extracted TT detected by modified Bradford
Extracted TT determined by ELISA
TT loading % determined by amino acid analysis (AAA).
Encapsulation efficiency was 54–93% as determined by AAA loading/theoretical loading.
mean ± s.d. (n = 2).
The weight ratio of TT:Lysine was 1:3 and MgCO3 was loaded at 3%.
The weight ratio of TT : Lysine : sorbitol (or trehalose) was 1:1.5:1.5 and MgCO3 was loaded at 3%.
Theoretical TT loading in PLGA microspheres was 3%. We determined the actual TT loading value by quantification of total protein and antigenically active protein. Total protein content encapsulated in PLGA microspheres was determined by two methods: 1) Modified Bradford assay following TT extracted from PLGA micropsheres, and 2) amino acid analysis after complete acid digestion of the TT microsphere.
As seen in Table 1, TT loading determined by modified Bradford assay was 1.5%, indicative of 50% encapsulation efficiency obtained by the O/O encapsulation method. For the extracted soluble protein, the antigenicity was further determined as 0.8%. This result suggested that ~ 50% of encapsulated TT lost antigenicity, likely due to rapid precipitation and micronization of the protein in the polymer solution.
TT loading estimated by amino acid analysis is 1.6% after complete acid hydrolysis. The amino acid analysis method can accurately determine the levels of total protein entrapped in PLGA microspheres, however, it leads to the inactivation of toxoid. For TT microspheres, similar loading values were obtained when determined by modified Bradford assay and amino acid analysis, indicating that modified Bradford assay after extraction process can have an accurate estimation of the encapsulated TT content.
Identifying instability mechanisms of TT in PLGA
We monitored TT release kinetics in a phosphate buffer and recovery of the antigen from the polymer after the release interval. TT release was brief (< 7 days) and incomplete (only 12% and 17% of total and antigenic TT released, Fig. 1C). The remaining unreleasable TT residue was removed from the microspheres and found mostly insoluble in phosphate buffer. In Table 2, it was shown that 2% of encapsulated TT remained soluble, whereas 10% and 9% of encapsulated TT formed non-covalent and disulfide-bonded aggregates, respectively. This low TT recovery indicates that ~ 60% of encapsulated TT formed aggregates which were held together by covalent, non-disulfide bonds.38, 39 To further assess the aggregation mechanism, the aggregate residue was subjected to amino acid analysis. As seen in Fig. 1D, much higher levels of the strongly formaldehyde-interacting tyrosine40, 41 were recorded and a noticeable loss of lysine and other amino acids were observed, consistent with previously reported solid-state aggregation of TT via formaldehyde-mediated aggregation pathway.39 Hence, the analysis revealed two salient features of the instability of TT in the PLGA system: the formation of insoluble, non-disulfide bonded covalent aggregates and alterations in strongly formaldehyde-interacting amino acid content.
Table 2.
Stability of controlled-released TT
| Stabilizers | Released Soluble (%) a | Released Antigenic (%) b | Soluble Residue (%) c | Non-covalent aggregate (%) d | Non covalent + disulfide-bonded aggregate (%) e | Recovery (%) f |
|---|---|---|---|---|---|---|
| No stabilizers | 12 ± 2 g | 17 ± 2 | 2 ± 1 | 10 ± 3 | 19 ± 6 | 33 ± 6 |
| Lysine + MgCO3 h | 60 ± 1 | 52 ± 1 | 1 ± 1 | 25 ± 3 | 40 ± 1 | 100 ± 3 |
| Lysine + Sorbitol + MgCO3 i | 66 ± 1 | 64 ± 2 | 1 ± 1 | 10 ± 2 | 20 ± 1 | 87 ± 2 |
| Lysine + Trehalose + MgCO3 i | 64 ± 2 | 78 ± 1 | 2 ± 1 | 11 ± 1 | 18 ± 3 | 84 ± 3 |
Cumulative released TT detected by modified Bradford assay and ELISA after 28 days, respectively
TT extracted from microspheres after 28 days soluble in PBST, in GnCl but not in PBST, and in GnCl/DTT but not in PBST, respectively
Recovery accounted for the total of soluble residue, non-covalent and disulfide bonded aggregates
mean ± s.d. (n = 2)
The weight ratio of TT:Lysine was 1:3 and MgCO3 was loaded at 3%.
The weight ratio of TT : Lysine : sorbitol (or trehalose) was 1:1.5:1.5 and MgCO3 was loaded at 3%.
The denatured state of the unreleased TT is characteristic of insoluble aggregation mediated by formaldehyde reversibly bound to the antigen following formaldehyde detoxification of the dangerous tetanus toxin.39 According to the formaldehyde-mediated aggregation pathway (FMAP), during exposure of the solid antigen to intermediate moisture levels, reactive formaldehyde-bound species (e.g., Schiff base of lysine residues) become exposed and react with another strongly formaldehyde-interacting side-chain on a second TT molecule (e.g., another lysine or tyrosine) to form inter-molecular cross-linking, further leading to the formation of insoluble aggregates.39
Besides formaldehyde-mediated aggregation, like most proteins, TT is susceptible to irreversible acid denaturation, and loses higher order structure and antigenicity rapidly below pH ~ 4.27, 37 PLGA microspheres are known to commonly develop an acidic microclimate (e.g., pH < 342, 43) during incubation at physiological conditions, which has been hypothesized to cause damage to TT.37 Therefore we hypothesized that formaldehyde- and acid-induced instability were the primary instability mechanisms during release from PLGA microspheres. Other potential stresses of instability such as reactions initiated by the surface-active water-soluble oligomers of PLGA or by adsorption to the polymer surface are expected to be insignificant. For example, losses of antigenic TT were found to depend solely on pH (3–7.4) and be independent of the presence of either PLGA (MW 2000 Da, 100% L-lactide) oligomers or microspheres,44 also shown in our studies described below.
Identifying inhibitors of FMAP and the acidic microclimate in PLGA
TT was found to form aggregates by FMAP during release from microspheres. Previously, to investigate FMAP, we studied a model formalized antigen, f-BSA, by treating bovine serum albumin (BSA) with formaldehyde under routine detoxification conditions. We discovered that strongly formaldehyde-interacting amino acids such as histidine and lysine could efficiently inhibit FMAP in the solid state by scavenging the reactive species in FMAP.38 Co-encapsulating the f-BSA with histidine and a sugar/polyol (trehalose) provided complete inhibition of FMAP in PLGA microspheres for one month.45 We note that both trehalose23 and sorbitol46 have also been identified to improve TT stability in the solid state when exposed to moisture and in PLGA microspheres.
To determine if FMAP inhibitors identified in studies with model formalinized antigen f-BSA would be effective for TT, we compared the aggregation behavior of TT, BSA, and f-BSA in both solid state and PLGA microspheres. When exposed to intermediate moisture levels, solid state f-BSA exhibits nearly identical aggregation kinetics as TT and such aggregates are insoluble in 10 mM DTT/1 mM EDTA/6 M urea.38 The pH-aggregation profile of TT (Table 3) and f-BSA38 followed the same trend—aggregation is accelerated with increasing pH. Similarly, both f-BSA aggregates and TT aggregates formed in PLGA microspheres were characterized as covalent and non-disulfide bonded.38, 45 By contrast, the BSA control under either neutral or acidic conditions in the solid state, or in acidic PLGA microspheres, forms exclusively non-covalent and/or disulfide-bonded aggregates that are completely soluble in combined denaturing and reducing solvents.38, 45, 47, 48 The distinct difference in aggregation behavior of f-BSA and BSA in both the solid state and PLGA microspheres proved the existence of a FMAP in the polymer. Furthermore, close similarity of TT and f-BSA aggregation behavior indicates that those covalent, non-disulfide bonded aggregates are not TT-specific, but formaldehyde-specific, which suggests the stabilization approaches developed with f-BSA may be also efficient to inhibit FMAP in TT.
Table 3.
TT aggregation as a function of pH before lyophilization
| pH | Soluble TT* (%) |
|---|---|
| 2 | 48 ± 1 |
| 5 | 48 ± 8 |
| 7 | 15 ± 1 |
| 10 | 7 ± 1 |
after 6-day incubation at 37 °C and 80% RH, soluble TT content was determined by modified Bradford assay.
As for the second critical deleterious instability pathway for TT, namely, acid denaturation in PLGA, earlier studies in our lab have demonstrated that inorganic basic additives (MgCO3 or Mg(OH)2)48, 49 or pore-forming agent, poly(ethylene-glycol) (PEG)50 can prohibit the large microclimate pH drop in the PLGA induced by the water-soluble acidic polymer degradation products51. These excipients directly neutralized acids and/or increased the acid diffusion out of the polymer during bioerosion of PLGA, resulting in successful inhibition or prevention of acid-induced hydrolysis and aggregation of proteins such as BSA, basic fibroblast growth factor and several other proteins.52
Therefore, we selected three classes of stabilizers to inhibit acid- and formaldehyde-induced damage of TT encapsulated in PLGA: 1) strongly formaldehyde-interacting amino acids such as free histidine and lysine;38, 45 2) polyols and sugars such as sorbitol and trehalose;23, 45, 46 3) and poorly soluble bases such as MgCO3.48, 52 Any highly soluble base, such as sodium bicarbonate used in TT microsphere formulation by Katare et al., was not selected considering its fast diffusion and short duration in the polymer system.24
Effect of stabilizers on heat and moisture-induced TT stability
Before co-encapsulation in PLGA system, putative stabilizing excipients including MgCO3, histidine, lysine, trehalose and sorbitol, were examined for their effects on the physicochemical properties of TT when exposed to heat or moisture. MgCO3 was selected in this study relative to Mg(OH)2 because the carbonate salt is a better neutralizer of microclimate pH throughout PLGA microspheres.53
The excipients were first added to TT solution at a weight ratio of 10:1 (excipients:protein) and incubated at 45 °C for 22 days. At the end of incubation, TT was taken out for CD, fluorescence and antigenicity analysis. Table 4 shows the effect of excipients on fluorescent properties and antigenicity of TT. As seen in Table 4, when excited at 280 nm, the maximum emission wavelength (λmax) of TT standard was 329 nm and A350/329 was 0.75. For TT at pH 7.3 (1 mM phosphate buffer), with no excipients added, after incubation at 45 °C for 22 days, the λmax of TT was shifted to a longer wavelength, 339 nm, and the A350/329 was increased to 0.99. Also, the antigenicity of TT was decreased to 28%. With the addition of most excipients, TT antigenicity was significantly improved. For example, TT co-incubated with histidine, lysine and sorbitol retained above 80% of antigenicity after 22 days of incubation. Also, these samples showed the least alterations in λmax and A350/329. Their CD spectra were almost identical to that of TT standard (Fig 2). In contrast, fluoresecent properties of TT incubated with trehalose showed a significant change. λmax and A350/329 exceeded 330 nm and 0.80, respectively. Furthermore, CD spectra of the sample deviated largely from that of TT standard. The antigenicity of TT with trehalose retained 44%.
Table 4.
Effect of excipients on fluorescent properties and antigenicity after exposure to heat (45 °C and 22 days)
| Excipients | Fluorescent properties | Antigenicity (%) | |
|---|---|---|---|
| λmax | I350/I329 | ||
| TT standard | 329 | 0.75 | 100 |
| None | 339 | 0.99 | 28 ± 1 |
| Histidine | 329 | 0.76 | 96 ± 22 |
| Lysine | 329 | 0.78 | 82 ± 12 |
| Trehalose | 331 | 0.82 | 44 ± 10 |
| Sorbitol | 330 | 0.78 | 83 ± 6 |
| Monomers (pH 2.9) | 335 | 0.88 | 23 ± 1 |
| MgCO3/monomers pH 5.7 | 329 | 0.78 | 88 ± 5 |
| MgCO3/monomers pH 8 | 331 | 0.82 | 56 ± 6 |
Fig. 2.
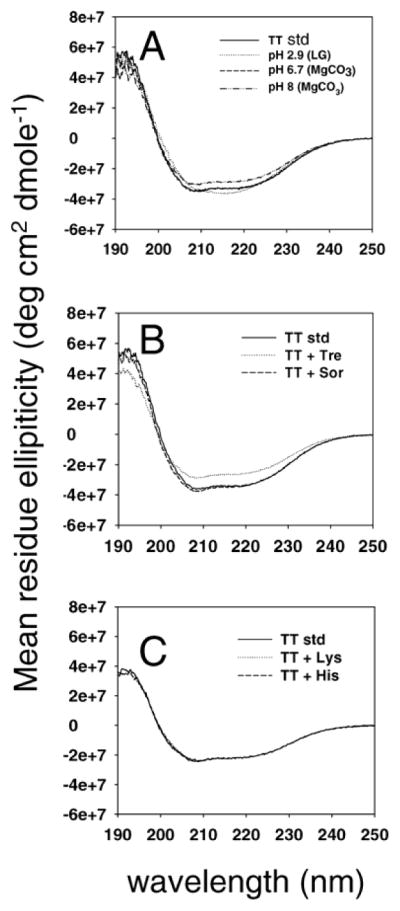
(A) Far UV -CD spectra of TT standard (—), TT incubated with lactic and glycolic acid monomer at pH 2.9 (···), monomers adjusted by MgCO3 to pH 6.7 (-□-□-) and pH 8 (–··) at 45 °C for 22 days. (B) TT standard (—), TT incubated with trehalose (···) and sorbitol (-□-□-) at 45 °C for 22 days. (C) TT standard (—), TT incubated with lysine (···) and histidine (-□-□-) at 45 °C for 22 days.
We also investigated the effect of PLGA degradation products (lactic acid and glycolic acid monomers) and MgCO3 on the structure of TT. As seen in Fig. 2, when TT was incubated with 22 mM lactic acid and 24 mM glycolic acid (pH 2.9) at 45 °C for 22 days, both 208 and 222 nm, characteristic peaks of TT α helix were diminished in its CD spectra. For fluorescent properties, the λmax and A350/329 of this sample, was changed to 335 nm and 0.88, respectively (Table 4), corresponding to an antigenicity decrease to 23%. When pH of the TT solution was adjusted to 6.7 with MgCO3, an identical CD spectrum with that of standard TT was exhibited. The λmax and A350/329 of TT approached that of TT standard, and we also observed that 88% of TT antigenicity was retained. When pH of the TT solution was further increased to 8 by the addition of MgCO3, the whole CD spectrum curve was shifted up. The λmax and A350/329 of TT also shifted away from that of TT standard. Only 56% of TT antigenicity was retained after 22 days of incubation. The above results suggested a good correlation of high-order structural loss of TT with antigenicity loss. These changes are easily monitored by CD and fluorescence spectra. Excipients, which improved the structural integrity of TT, such as lysine, sorbitol, histidine, significantly improved TT antigenicity upon exposure to heat. As expected, low solution pH with the addition of lactic acid and glycloic acid monomers largely deteriorated the secondary, tertiary structure and antigenicity of TT. With pH neutralization by the addition of MgCO3, secondary and tertiary structure of TT was recovered, corresponding to the antigenicity recovery of TT.
The effect of excipients on moisture-induced instability of TT is shown in Table 5 (excipient to protein ratio is 10:1). TT samples after incubation were first analyzed for soluble protein by visual observation, modified bradford assay and A280 absorbance determinations. For TT samples which retained significant solubility after incubation, their higher order structure and antigenicity were further evaluated. As seen in Table 5, TT lyophilized with lactic acid and glycolic acid (pH 2.9) formed aggregates before incubation. When pH was adjusted to 6.7 and 8 by MgCO3, still significant amount of TT aggregates were formed. TT co-lyophilized with histidine and lysine remained 100% soluble after incubation, whereas TT incubated with trehalose and sorbitol formed a small amount of aggregates.
Table 5.
Effect of excipients on fluorescent properties and antigenicity after exposure to Moisture (37 °C and 80% RH for 30 days)
| Fluorescence properties | ||||
|---|---|---|---|---|
| Excipients | Soluble protein (%) | λmax | I350/I329 | Antigenicity (%) |
| TT standard | 329 | 0.75 | 100 | |
| Lyophilized TT standard | 327 | 0.73 | 100 | |
| None | 15 ± 1 (15 d) | * | * | * |
| Histidine | 101 ± 5 | 329 | 0.72 | 24 |
| Lysine | 103 ± 1 | 329 | 0.76 | 81 ± 12 |
| Trehalose | 92 ± 4 | 328 | 0.73 | 47 |
| Sorbitol | 87 ± 1 | 329 | 0.75 | 90 ± 8 |
| Monomers (pH 2.9) | Aggregates formed before incubation | * | * | * |
| MgCO3/monomers pH 5.7 | 9 ± 1 | * | * | * |
| MgCO3/monomers pH 8 | 4 ± 2 | * | * | * |
Not determined
We further analyzed the higher order structure and antigenicity properties of the soluble TT fraction. Similar CD spectra were observed for TT samples incubated with excipients and TT standard, indicating similar secondary structure among these samples (data not shown). As for fluorescent properties (see Table 5), for TT just after lyophilization, the λem and A350/A329 were shifted to 327 nm and 0.73 respectively. However, TT antigenicity remained the same as standard. Fluorescent properties of TT lyophilized with sorbitol and lysine were closest to the TT standard, and similarly the antigenicity in these samples was highly retained after incubation. Although TT lyophilized with histidine and trehalose also had similar fluorescent properties with untreated TT, their antigenicities differed from untreated TT substantially. We speculate that incubation of these excipients with TT may not alter the higher order structure, but chemical compositions of their epitopes (i.e., incorporation of excipients in the TT molecule), therefore causing large differences in antigenicity.
Overall, Lysine and sorbitol were excellent inhibitors of aggregation and antigenicity losses during exposure to moisture in the solid state and heat in solution. The stabilization effect of lysine on TT was consistent with the finding that the presence of lysine, of all tested amino acids, imparted superior antigenicity during formaldehyde detoxification of tetanus toxin.54 It was proposed that the high antigenicity and relative stability of TT with lysine may be associated with the presence of side chains derived from the incorporation of lysine into protein molecules. 54 TT antigenicity was also largely improved after monomers were neutralized by magnesium salts. Although trehalose and histidine can significantly inhibit TT aggregation but TT antigenicity was compromised in the presence of these species. CD and fluorescence spectroscopies appear to be useful to predict TT antigenicity losses in most instances.
Stabilization of TT in PLGA
Based on the above studies, we co-encapsulated with TT in PLGA microspheres putative stabilizers against formaldehyde- and acid-induced inactivation uncovered by our mechanistic analysis (Table 4 and 5), lysine, lysine/sorbitol, or lysine/trehalose and MgCO3. Lysine was chosen over histidine as the inhibitor of FMAP because of large histidine-induced losses of TT antigenicity during solid-state stability assessment of FMAP.
Fig 1B shows the morphology of microspheres co-encapsulated with lysine, sorbitol and MgCO3. Similar SEM image of this formulation to TT microspheres without any stabilizer indicated that co-encapsulation of these excipients did not change microsphere morphology.
As shown in Table 1, the antigenic TT loading for each formulation with stabilizers (MgCO3/lysine, MgCO3/lysine/sorbitol, and MgCO3/lysine/trehalose) was > 1% w/w with an encapsulation efficiency of 54–93%. Whereas negligible TT solubility was lost during encapsulation, some noticeable antigenicity losses of the antigen were noted. These losses appeared not to affect the protein during release, as indicated by the similarity between antigenic and soluble TT release profiles (e.g., Fig 3B). We also note that no antigenicity loss was observed if solid protein powder was homogenized in acetonitrile at 15,000 rpm for 1 min (data not shown). In future formulation, to minimize antigencity loss during encapsulation, micronized solid TT powder can be used or precipitation/micronization of TT from aqueous concentrate should be performed in organic solvent alone.
Fig. 3.
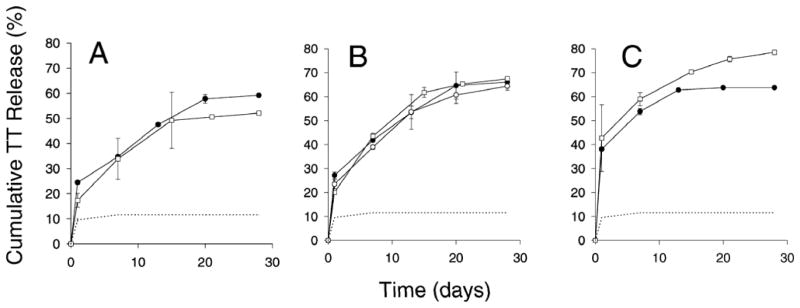
Release kinetics of TT from PLGA 85/15 (i.v. = 0.86 dl/g) microspheres co-encapsulated with lysine + MgCO3 (A), lysine + sorbitol + MgCO3 (B), lysine + trehalose + MgCO3 (C). Protein content determined by modified Bradford assay (mean ± s.d., n=2) (●) and antigenic TT (mean ± s.d., n=2) (○) when release medium contained no BSA; Antigenic TT (mean ± s.d., n=2) (□) released from microspheres when release medium contained 0.2% BSA. Dashed line represents release kinetics of TT (determined by modified Bradford assay) from PLGA microspheres containing no stabilizers.
TT loading data in Table 1 indicated that co-encapsulation of the stabilizers influenced the TT encapsulation efficiency favorably. When MgCO3/lysine was co-encapsulated, TT loading based on soluble protein content was 1.7% and 80% of encapsulated TT retained antigenicity. When sorbitol/trehalose is further added, a substantial increase of TT loading was noted, especially with the co-encapsulation of trehalose. Johansen et al. also reported that co-encapsulation of trehalose increased the encapsulation efficiency and exerted a dominant increase in the initial burst.23 The underlying mechanism by which trehalose increase encapsulation efficiency and burst release is unclear and warrants further investigation.
In Fig 3, release kinetics of formulations with and without stabilizers was displayed. As predicted, with stabilizers added, slow and continuous antigenic TT release was observed for the 1-month incubation. For lysine/sorbitol/MgCO3 and lysine/trehalose/MgCO3 PLGA microspheres (Fig. 3B and 3C), 64–78% antigenic release was observed after a ~25–40% initial burst without any signs of antigenic losses in the released protein. Equally compelling was the recovery data, as shown in Table 2. Without stabilizers, TT aggregation via FMAP was extensive resulting in only 33% recovery. With lysine and MgCO3 added, 60% of TT was released and the remaining TT (40%), although aggregated, was now soluble in DTT + GnCl. The 100% recovery confirms the complete inhibition of FMAP by the addition of lysine, allowing secondary aggregation mechanisms involving disulfide scrambling and hydrophobic interactions to become dominant.38, 39 Further addition of either sorbitol or trehalose to the MgCO3/lysine formulation slightly decreased FMAP inhibition (i.e., < 100% recovery) but improved the antigenic release (i.e., 64–78% with sugar/polyol vs. 52% without), indicating that the sugars/polyols, while not as effective as lysine to inhibit FMAP, are necessary to retain TT antigenicity during release. The absence of antigenicity losses in the released TT from the sugar/polyol-containing samples also indicates that pH was effectively neutralized in the polymer.
Previously Johanson et al. reported that experiment set-up exerts a great effect on the release of encapsulated TT from PLGS microspheres due to strong TT adsorption to hydrophobic surfaces such as glass.36 In our study, when quantified by ELISA, similar TT release kinetics was observed in the release medium with and without 0.2% BSA (Fig 3B), indicating that TT adsorption was not substantial during release evaluation. This discrepancy with literature report is possibly due to higher TT loading in our microsphere formulation, causing greater protein concentration to mask the adsorption artifacts. Moreover, a large burst release may potentially block the adsorption sites in the release vessel and make TT adsorption less significant. In addition, some TT samples during later release were poorly quantitated by the modified Bradford assay, which has a higher quantitation limit than the ELISA, causing an artificial flattening in some of the release curves.
In closing, there are two principal mechanisms of TT instability during release from PLGA microspheres, acid-induced and formaldehyde-induced inactivation. We used stabilizers to neutralize acidic microclimate and inhibit FMAP and systemically evaluate their effect on TT stability under elevated high temperature and humidity. This strategy uncovered potent additives for stabilizing encapsulated TT, i.e., lysine, sorbitol, and trehalose to inhibit FMAP and MgCO3 to bypass acid-induced damage. Co-encapsulation of these additives resulted in an unparalleled stability of TT encapsulated in PLGA microspheres, and slow and continuous release of the antigen.
Acknowledgments
We thank Dr. Derek T. O’ Hagan at Chiron Corp. for providing the tetanus toxoid. We are very grateful to Dr. Dorothea Sesardic and Mr. Robert Tierney at the NIBSC for providing the TT antibodies and for their advice concerning the TT ELISA. This work was supported in part by NIH HL 68345.
References
- 1.Preis I, Langer R. A single-step immunization by sustained antigen release. J Immunol Methods. 1979;28:193–197. doi: 10.1016/0022-1759(79)90341-7. [DOI] [PubMed] [Google Scholar]
- 2.Gibbons A. A booster shots for children’s vaccines. Nature. 1992;255:1351. doi: 10.1126/science.1542784. [DOI] [PubMed] [Google Scholar]
- 3.Aguado MT. Future approaches to vaccine development: single-dose vaccines using controlled-release delivery systems. Vaccine. 1993;11:596–597. doi: 10.1016/0264-410x(93)90241-o. [DOI] [PubMed] [Google Scholar]
- 4.O’Hagan DT, Singh M, Gupta RK. Poly(lactide-co-glycolide) microparticles for the development of single-dose controlled-release vaccines. Adv Drug Del Rev. 1998;32:225–246. [PubMed] [Google Scholar]
- 5.Hanes J, Cleland JL, Langer R. New advances in microsphere-based single-dose vaccines. Adv Drug Del Rev. 1997;28:97–119. doi: 10.1016/s0169-409x(97)00053-7. [DOI] [PubMed] [Google Scholar]
- 6.Schwendeman SP, Costantino HR, Gupta RK, Langer R. Peptide Protein, and Vaccine Delivery from Implantable Polymeric Systems. In: Park K, editor. Controlled Drug Delivery, Challenges and Strategies. The American Chemical Society; Washington, DC: 1997. pp. 229–267. [Google Scholar]
- 7.Jiang W, Gupta RK, Deshpande MC, Schwendeman SP. Biodegradable poly(lactic-co-glycolic acid) microparticles for injectable delivery of vaccine antigens. Adv Drug Deliv Rev. 2005;57:391–410. doi: 10.1016/j.addr.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 8.Tamber H, Johansen P, Merkle HP, Gander B. Formulation aspects of biodegradable polymeric microspheres for antigen delivery. Adv Drug Deliv Rev. 2005;57:357–376. doi: 10.1016/j.addr.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 9.Singh M, Ugozzoli M, Kazzaz J, Chesko J, Soenawan E, Mannucci D, Titta F, Contorni M, Volpini G, Guidice G, O’Hagan DT. A preliminary evaluation of alternative adjuvants to alum using a range of established and new generation vaccine antigens. Vaccine. 2006;24:1680–1686. doi: 10.1016/j.vaccine.2005.09.046. [DOI] [PubMed] [Google Scholar]
- 10.Farrar JJ, Yen LM, Cook T, Fairweather N, Binh N, Parry J, Parry CM. Tetanus. J Neurol Neurosurg Psychiatry. 2000;69:292–301. doi: 10.1136/jnnp.69.3.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aguado MT, Lambert PH. Controlled-release vaccines - biodegradable polylactide-polyglycolide (PL/PG) microspheres as antigen vehicles. Immunobiol. 1992;184:113–125. doi: 10.1016/S0171-2985(11)80470-5. [DOI] [PubMed] [Google Scholar]
- 12.Bloom BR. Vaccines for the third world. Nature. 1989;342:115–120. doi: 10.1038/342115a0. [DOI] [PubMed] [Google Scholar]
- 13.Alonso MJ, Gupta RK, Min C, Siber GR, Langer R. Biodegradable microspheres as controlled-release tetanus toxoid delivery systems. Vaccine. 1994;12:299–306. doi: 10.1016/0264-410x(94)90092-2. [DOI] [PubMed] [Google Scholar]
- 14.Men Y, Thomasin C, Merkle HP, Gander B, Corradin G. A single administration of tetanus toxoid in biodegradable microspheres elicits T cell and antibody responses similar or superior to those obtained with aluminum hydroxide. Vaccine. 1995;113:683–689. doi: 10.1016/0264-410x(94)00046-p. [DOI] [PubMed] [Google Scholar]
- 15.Gupta RK, Alroy J, Alonso MJ, Langer R, Siber GR. Chronic local tissue reactions, long-term immunogenicity and immunologic priming of mice and guinea pigs to tetanus toxoid encapsulated in biodegradable polymer microspheres composed of poly lactide-co-glycolide polymers. Vaccine. 1997;15:716–1723. doi: 10.1016/s0264-410x(97)00116-3. [DOI] [PubMed] [Google Scholar]
- 16.Raghuvanshi RS, Katare YK, Lalwani K, Ali MM, Singh O, Panda AK. Improved immune response from biodegradable polymer particles entrapping tetanus toxoid by use of different immunization protocol and adjuvants. Int J Pharm. 2002;245:109–121. doi: 10.1016/s0378-5173(02)00342-3. [DOI] [PubMed] [Google Scholar]
- 17.Jung T, Koneberg R, Hungerer, Klaus D, Kissel T. Tetanus toxoid microspheres consisting of biodegradable poly(lactide-co-glycolide)-ABA-triblock-copolymers: immune response in mice. Int J Pharm. 2002;234:75–90. doi: 10.1016/s0378-5173(01)00957-7. [DOI] [PubMed] [Google Scholar]
- 18.Raghuvanshi RS, Mistra A, Talwar GP, Levy RJ, Labhasetwar V. Enhanced immune response with a combination of alum and biodegradable nanoparticles containing tetanus toxoid. J microencapsulation. 2001;18:723–732. doi: 10.1080/02652040110055261. [DOI] [PubMed] [Google Scholar]
- 19.Katare YK, Panda AK, Lalwani K, Haque IU, Ali MM. Potentiation of Immune Response from Polymer-Entrapped Antigen: Toward Development of Single Dose Tetanus Toxoid Vaccine. Drug Delivery. 2003;10:231–238. doi: 10.1080/drd_10_4_231. [DOI] [PubMed] [Google Scholar]
- 20.Gupta RK, Chang AC, Siber GR. Biodegradable polymer microspheres as vaccine adjuvants and delivery systems. Dev Biol Stand. 1998;92:63–78. [PubMed] [Google Scholar]
- 21.Kissel T, Koneberg R, Hilbert AK, Hungerer KD. Microencapsulation of antigens using biodegradable polyesters: facts and phantasies. Behring Inst Mitt. 1997;98:172–183. [PubMed] [Google Scholar]
- 22.Kersten GFA, Donders D, Akkermans A, Beuvery EC. Single shot with tetanus toxoid in biodegradable microspheres protects mice despite acid-induced denaturation of the antigen. Vaccine. 1996;14:1627–1632. doi: 10.1016/s0264-410x(96)00145-4. [DOI] [PubMed] [Google Scholar]
- 23.Johansen P, Men Y, Audran R, Corradin G, Merkle HP, Gander B. Improving stability and release kinetics of microencapsulated tetanus toxoid by co-encapsulation of additives. Pharm Res. 1998;15:1103–1110. doi: 10.1023/a:1011998615267. [DOI] [PubMed] [Google Scholar]
- 24.Katare YK, Panda AK. Influences of excipients on in vitro release and in vivo performance of tetanus toxoid loaded polymer particles. Euro J Pharm Sci. 2006;28:179–188. doi: 10.1016/j.ejps.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 25.Katare YK, Muthukumaran T, Panda AK. Influence of particle size, antigen load, dose and additional adjuvant on the immune response from antigen loaded PLA microparticles. Inter J Pharm. 2005;301:149–160. doi: 10.1016/j.ijpharm.2005.05.028. [DOI] [PubMed] [Google Scholar]
- 26.Raghuvanshi RS, Singh O, Panda AK. Correlation Between In Vitro Release and In Vivo Immune Response from Biodegradable Polymer Particles Entrapping Tetanus Toxoid. Drug Delivery. 2002;9:113–120. doi: 10.1080/10426500290095557. [DOI] [PubMed] [Google Scholar]
- 27.Johansen P, Merkle HP, Gander G. Physico-chemical and antigenic properties of tetanus and diphtheria toxoids and steps towards improved stability. Biochim Biophys Acta. 1998;1425:425–436. doi: 10.1016/s0304-4165(98)00097-x. [DOI] [PubMed] [Google Scholar]
- 28.Schwendeman SP, Tobio M, Joworowicz M, Alonso MJ, Langer R. New strategies for the microencapsulation of tetanus vaccine. J Microencapsul. 1998;15:299–318. doi: 10.3109/02652049809006859. [DOI] [PubMed] [Google Scholar]
- 29.Sanchez A, Villamayor B, Guo Y, McIver J, Alonso MJ. Formulation strategies for the stabilization of tetanus toxoid in poly(lactide-co-glycolide) microspheres. Int J Pharm. 1999;185:255–266. doi: 10.1016/s0378-5173(99)00178-7. [DOI] [PubMed] [Google Scholar]
- 30.Tobio M, Nolley J, Guo Y, McIver J, Alonso MJ. A novel system based on a poloxamer/PLGA blend as a tetanus toxoid delivery vehicle. Pharm Res. 1999;16:682–688. doi: 10.1023/a:1018820507379. [DOI] [PubMed] [Google Scholar]
- 31.Schwendeman SP, Costantino HR, Gupta RK, Tobio M, Chang AC, Alonso MJ, Siber GR, Langer R. Strategies for stabilising tetanus toxoid towards the development of a single-dose tetanus vaccine. Dev Biol Stand. 1996;87:293–306. [PubMed] [Google Scholar]
- 32.Sanchez A, Gupta RK, Alonso MJ, Siber GR, Langer R. Pulsed controlled-released system for potential use in vaccine delivery. J Pharm Sci. 1996;85:547–552. doi: 10.1021/js960069y. [DOI] [PubMed] [Google Scholar]
- 33.Chang AC, Gupta RK. Stabilization of tetanus toxoid in poly(DL-lactic-co-glycolic acid) microspheres for the controlled release of antigen. J Pharm Sci. 1996;85:129–132. doi: 10.1021/js950365v. [DOI] [PubMed] [Google Scholar]
- 34.Jiang W, Schwendeman SP. Stabilization of Tetanus Toxoid encapsulated in PLGA microspheres. Proceed Int’l Symp Control Rel Bioact Mater Podium presentation. 2002 doi: 10.1021/mp800027f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schwendeman SP, Jiang W. Polymer compositions that stabilize and control the release of formaldehyde-treated vaccine antigens. 20040071715 US Pub App No.
- 36.Johansen P, Corradin G, Merkle HP, Gander B. Release of tetanus toxoid from adjuvants and PLGA microspheres: how experimental set-up and surface adsorption fool the pattern. J Control Release. 1998;56:209–217. doi: 10.1016/s0168-3659(98)00084-4. [DOI] [PubMed] [Google Scholar]
- 37.Xing DKI, Crane DKDT, Bolgiano B, Corbel MJ, Jones C, Sesardic D. Physicochemical and immunological studies on the stability of free and microsphere-encapsulated tetanus toxoid in vitro. Vaccine. 1996;14:1205–1213. doi: 10.1016/s0264-410x(96)00032-1. [DOI] [PubMed] [Google Scholar]
- 38.Jiang W, Schwendeman SP. Formaldehyde-mediated aggregation of protein antigens: comparison of untreated and formalinized model antigens. Biotechnol Bioeng. 2000;70:507–517. [PubMed] [Google Scholar]
- 39.Schwendeman SP, Costantino HR, Gupta RK, Siber GR, Klibanov AM, Langer R. Stabilization of tetanus and diphtheria toxoids against moisture-induced aggregation. Proc Natl Acad Sci USA. 1995;92:11234–11238. doi: 10.1073/pnas.92.24.11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fraenkel-Conrat H, Cooper M, Olcott HS. The Reaction of formaldehyde with Proteins. J Am Chem Soc. 1945;67:950–954. [Google Scholar]
- 41.French D, Edsall JT. Formaldehyde with Amino Acids and Proteins. Adv Protein Chem. 1945;2:277–335. [Google Scholar]
- 42.Shenderova A, Burke TG, Schwendeman SP. The acidic microclimate in poly(lactide-co-glycolide) microspheres stabilizes camptothecins. Pharm Res. 1999;16:241–248. doi: 10.1023/a:1018876308346. [DOI] [PubMed] [Google Scholar]
- 43.Fu K, Pack DW, Klibanov AM, Langer R. Visual evidence of acidic environment within degrading poly(lactic-co-glycolic acid) (PLGA) microspheres. Pharm Res. 2000;17:100–106. doi: 10.1023/a:1007582911958. [DOI] [PubMed] [Google Scholar]
- 44.Schwendeman SP, Gupta RK, Siber GR, Langer R. Pathways of inactivation of tetanus toxoid in the presence of PLA 2000. Pharm Res. 1995;12:S–80. [Google Scholar]
- 45.Jiang W, Schwendeman SP. Stabilization of a model formalinized protein antigen encapsulated in poly(lactide-co-glycolide)-based microspheres. J Pharm Sci. 2001;90:1558–1569. doi: 10.1002/jps.1106. [DOI] [PubMed] [Google Scholar]
- 46.Costantino HR, Schwendeman SP, Griebenow K, Klibanov AM, Langer R. The secondary structure and aggregation of lyophilized tetanus toxoid. J Pharm Sci. 1996;85:1290–1293. doi: 10.1021/js960148+. [DOI] [PubMed] [Google Scholar]
- 47.Liu WR, Langer R, Klibanov AM. Moisture-induced aggregation of lyophilized proteins in the solid state. Biotechnol Bioeng. 1991;37:177–184. doi: 10.1002/bit.260370210. [DOI] [PubMed] [Google Scholar]
- 48.Zhu G, Mallery SR, Schwendeman SP. Stabilization of proteins encapsulated in injectable poly(lactide-co-glycolide) Nat Biotechnol. 2000;18:52–57. doi: 10.1038/71916. [DOI] [PubMed] [Google Scholar]
- 49.Zhu G. PhD Thesis. The Ohio State University; 1999. Stabilization and controlled release of proteins encapsulated in poly(lactide-co-glycolide) delivery systems. [Google Scholar]
- 50.Jiang W, Schwendeman SP. Stabilization and controlled release of bovine serum albumin encapsulated in poly(D, L-lactide) and poly(ethylene-glycol) microsphere blends. Pharm Res. 2001;18:878–885. doi: 10.1023/a:1011009117586. [DOI] [PubMed] [Google Scholar]
- 51.Ding AG, Schwendeman SP, Shenderova A. Prediction of Microclimate pH in Poly(lactic-co-glycolic Acid) JACS. 2006;128:5384–5390. doi: 10.1021/ja055287k. [DOI] [PubMed] [Google Scholar]
- 52.Schwendeman SP. Recent advances in the stabilization of proteins encapsulated in injectable PLGA delivery systems. Crit Rev Ther Drug Carrier syst. 2002;19:73–98. doi: 10.1615/critrevtherdrugcarriersyst.v19.i1.20. [DOI] [PubMed] [Google Scholar]
- 53.Zhu G, Schwendeman SP. Stabilization of proteins encapsulated in cylindrical poly(lactide-co-glycolide) implants: mechanism of stabilization by basic additives. Pharm Res. 2000;17:351–357. doi: 10.1023/a:1007513425337. [DOI] [PubMed] [Google Scholar]
- 54.Linggood FV, Stevens MV, Fulthorpe AJ, Woiwod AJ, Pope CG. The toxoiding of purified diphtheria toxin. Br J exp Path. 1963;44:117–188. [Google Scholar]