Abstract
Background
Atrial fibrillation (AF) is associated with increased morbidity. P-wave indices (PWI) measure atrial electrical function and are associated with AF. Study of PWI has been limited to single-cohort investigations, and their contributions to risk enhancement are unknown.
Methods
We examined PWI from the Framingham Heart Study (FHS) and Atherosclerosis Risk in Communities (ARIC) Study. We calculated 10-year AF risk using adjusted Cox models. We conducted cross-cohort meta-analyses for the PWI estimates and assessed their contributions to risk discrimination (C-statistic), net reclassification index, and integrated discrimination improvement.
Results
After exclusions the analysis included 3,110 FHS (62.6±9.8 years, 56.9% women) and 8,254 ARIC participants (62.3±5.6 years, 57.3% women, 20.3% black race). Over 10-years, 217 FHS and 458 ARIC participants developed AF. In meta-analysis, P-wave duration >120 ms was significantly associated with AF (hazard ratio [HR] 1.55, 95% CI [confidence interval] 1.29 to 1.85) compared to ≤120 ms. P-wave area was marginally but not significantly related to AF (HR 1.31, 95% CI 0.95 to 1.80). P-wave terminal force was strongly associated with AF in ARIC but not FHS. PWI had a limited contribution towards predictive risk beyond traditional risk factors and markers.
Conclusions
PWI are intermediate phenotypes for AF. They are associated with AF in cross-cohort meta-analyses but contribute minimally toward enhancing risk prediction.
Keywords: Atrial fibrillation, atrial, epidemiology, electrocardiography, risk prediction
Atrial fibrillation (AF) is the most commonly encountered clinical arrhythmia and is associated with profound medical costs and clinical morbidity. P-wave indices (PWI) quantify atrial electrical function derived from the electrocardiogram (ECG) and are comprised of summary measures of duration, area, and amplitude. PWI have been associated with AF and related to established AF risk factors including age, hypertension and obesity.1-3 Consequently, PWI reflect subclinical atrial remodeling, which occurs secondary to the cumulative exposure to heterogeneous insults.4,5 PWI have consequently been employed as quantifiable, intermediate phenotypes for characterizing AF risk.6,7
We developed and validated a multicohort AF prediction algorithm for 5-year AF risk (Cohorts for Heart and Aging Research in Genomic Epidemiology atrial fibrillation [CHARGE-AF]).8 The ECG contributions to the CHARGE-AF risk score included PR interval and assessments of left ventricular hypertrophy (LVH), which did not contribute substantively towards model discrimination. However, PWI, which include P-wave duration, area and terminal force (specific to left atrial function) measure atrial conduction. Hence, they may perform better in AF risk prediction than other ECG markers.
To date, prior assessments of PWI and their relations to AF remain limited. Studies have been done in single cohorts, many of which have been limited by sample size, covariate ascertainment and brevity of follow-up.9 The cross-cohort heterogeneity in the contribution of PWI towards AF risk assessment has not been examined. Similarly, studies have not examined how PWI contribute towards metrics of risk prediction, specifically model discrimination (C-statistic) and reclassification.10 Digitized tracings facilitate automated PWI quantification, further obviating prior challenges to measurement techniques in some studies.
Our objectives were two-fold in investigating PWI and AF in the Framingham Heart Study (FHS) and Atherosclerosis Risk in Communities (ARIC) Study. First, we investigated the cross-cohort contributions of PWI towards risk of AF in meta-analysis following multivariable adjustment with the covariates included in the CHARGE-AF risk score. Second, we examined the predictive contributions of PWI towards reclassifying risk of AF in the two cohorts using statistical metrics such as the net reclassification index (NRI) and integrated discrimination improvement (IDI).
Methods
Study Populations
FHS
The FHS is a prospective, longitudinal study investigating cardiovascular diseases with multigenerational design.11,12 We included participants attending Original Cohort examination 20 (1986-1990), Offspring Cohort examination 6 (1995-1998), and the Third Generation initial examination (2002-2005) who were between ages 50 and 90 (n=4742). We excluded participants with prevalent AF or atrial flutter (n=27), used medications that may alter atrial or atrioventricular nodal electrophysiology (beta-blockers, calcium channel blockers, cardiac glycosides, and anti-arrhythmics, n=1457), or missing essential covariates (n=148).
The ARIC Study
The ARIC Study consists of a predominantly biracial (blacks and whites) sample recruited from 4 communities (Washington County, MD; suburbs of Minneapolis, MN; Jackson, MS; and Forsyth County, NC) enrolled from 1987 to 1989 (n=15,792). ARIC Study participants subsequently underwent 4 examinations at approximately 3-year intervals with annual telephone updates to capture hospital admission and vital status.13,14 The present study included participants attending ARIC Study visit 4 (1996-1998, n=11,656). Exclusions included participants with race or ethnicity other than white or black, or non-white in the Minneapolis and Washington Country field centers (n=69), prevalent AF or atrial flutter (n=297), atrial or atrioventricular nodal medications as listed above (n=2665), lacking or unreadable ECG data (n=252), or missing covariates (n=119).
FHS and ARIC were approved by the institutional review each participating institution. Study participants provided informed consent.
PWI acquisition and measurement
In FHS, digitized ECGs were performed using the Marquette (now General Electric, Fairfield, CT) MAC 5000. In ARIC, participants had ECGs recorded on MAC PC Personal Cardiographs (Marquette Electronics, Milwaukee, WI) and submitted for interpretation to the Epidemiological Cardiology Research Center, Wake Forest University, Winston-Salem, NC.
Both studies employed the Marquette 12SL ECG (General Electric) analysis program for PWI quantification. PWIs were defined uniformly in both studies and included the median PR interval, maximum P-wave duration, maximum P-wave area, and P-wave terminal force. The PR interval (ms) was defined by measurement from the onset of the P-wave to the initiation of the QRS segment. The median value across all 12-leads was used in the present analysis. P-wave duration (ms) was measured from the P-wave onset (conclusion of the T-P segment) to its offset (return to baseline for the remaining PR interval). P-wave area (μV·ms) was measured as the area underneath the positive deflection demarcating the P-wave. The maximum area from among the 12 leads was selected for analysis. P-wave terminal force (μV·ms), specific to right precordial lead V1, was determined as the product of the negative P-wave deflection in lead V1 (μV) and the duration (ms) from onset of the negative deflection to its nadir. P-wave dispersion was not included in our analysis, primarily because of its uncertain electrophysiologic significance.15
Ascertainment of AF
AF was ascertained in FHS from electrocardiograms, Holter monitors, and tracings at or external to FHS following adjudication by 2 physicians. Ascertainment of AF in the ARIC Study entailed review of hospital discharge records for International Classification of Diseases, Ninth Revision (ICD-9) codes 427.31 or 427.32. AF events associated with cardiac surgery were not included. The ARIC Study has reported this method for AF identification as 80-85% sensitive and 97-99% specific in a validation study of participants with stroke.16 AF also was identified from death certificates (ICD-9 427.3x or ICD-10 I48 listed as any cause of death).
Covariate definitions
Participant covariates were obtained at the same examination as PWI quantification in both cohorts. In FHS, smoking was defined by self-report. Blood pressures were recorded as the mean of two measurements obtained 5 minutes apart by a physician. LVH was defined by voltage criteria.17 Diabetes was defined as a fasting glucose ≥126 mg/dL at an examination, use of hypoglycemic agents (oral or insulin), or diagnosis of diabetes by a physician. Myocardial infarction (MI) and heart failure were determined by adjudication. 18,19 In the ARIC Study, race and smoking status were defined by self-report. Anthropometric assessments followed a standardized protocol.16 LVH was determined by Cornell criteria.20 Criteria for diabetes were reporting a diagnosis of diabetes by a physician, a fasting blood sugar ≥126 mg/dL, a nonfasting glucose ≥200 mg/dL, or use of hypoglycemic medications. MI was determined by committee adjudication and heart failure as described elsewhere.21,22
Statistical analysis
The mean and standard deviations (SD) of continuous variables and distributions of categorical variables were summarized by cohort. The Kaplan-Meier 10-year percentages for AF were calculated and then by stratifying PWI at <95th and ≥95th percentile in both cohorts. We constructed cohort-specific Cox proportional hazards models to examine the 10-year risk of AF for each of the PWI using cut-points established by prior investigations: PR interval >200, consistent with first-degree atrioventricular block;23 P-wave duration >120 ms;15 P-wave area ≥95th percentile;6 and P-wave terminal force >4000 μV·ms.24 A baseline model adjusted for age, sex, and race (in ARIC). The model adjusted for the variables comprising the CHARGE-AF risk score developed in 18,556 individuals and validated in 7,762 individuals.8 These included current smoking, height, weight, systolic and diastolic blood pressures, diabetes, history of myocardial infarction, and prevalent heart failure. We also adjusted for heart rate, total/high density lipoprotein cholesterol, and ECG-based LVH. The assumption of proportional hazards was tested. We constructed restricted cubic splines to display the relations of PWI and the 10-year hazard ratio for AF with knots at 5, 27.5, 50, 72.5, and 95 quantiles as per Harrell.25 We meta-analyzed the baseline and multivariable-adjusted hazard ratio for the 10-year risk of AF for each of the PWI using a fixed-effects model. We tested for heterogeneity of the hazard ratio estimates using the I2 index of heterogeneity.26 We evaluated the contribution of individual PWI to the model by determining the C-statistic, categorical NRI with risk categories of <5%, 5% to 10%, and >10%, and relative IDI.10 All analyses were conducted using SAS 9.2 (Cary, North Carolina). Spline graphics were constructed with STATA/MP (College Station, Texas). The authors are solely responsible for the design and conduct of this study, all study analyses, and the drafting and editing of the paper and its final contents.
Funding
Funding
This research was funded by American Heart Association Award 09FTF2190028 (JWM) and 09SDG2280087 (AA), NHLBI grant 5R21 HL106092, and a Boston University School of Medicine Department of Medicine Career Investment Award (JWM). The Framingham Heart Study is supported by National Institute on Aging (NIA) Contracts N01-AG-6-2101; N01-AG-6-2103; N01-AG-6-2106; NIA grant R01-AG028050; and NINR grant R01-NR012459. The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). Additional support was provided by NHLBI grant RC1 HL099452.
Results
The analysis included 3,110 FHS participants (62.6±9.8 years, 56.9% women) and 8,254 ARIC participants (62.3±5.6 years, 57.3% women, 20.3% black race; see Table 1). In both cohorts prevalent MI and heart failure were fairly low. The distributions of PWI were similar in both cohorts.
Table 1.
Descriptive characteristics of the Framingham Heart Study and Atherosclerosis Risk in Communities Cohorts.
| Framingham | ARIC | |
|---|---|---|
| N, total | 3110 | 8254 |
| Age, years | 62.6 (9.8) | 62.3 (5.6) |
| Women | 1771 (56.9%) | 4733 (57.3%) |
| Black race | ---- | 1673 (20.3%) |
| Current smoking | 423 (13.6%) | 1292 (15.7%) |
| Height, cm | 166 (10) | 168 (9) |
| Weight, kg | 75 (17) | 80 (17) |
| Systolic BP, mm Hg | 132 (20) | 126 (18) |
| Diastolic BP, mm Hg | 76 (10) | 71 (10) |
| Heart rate, bpm | 66 (11) | 63 (10) |
| Total/HDL cholesterol ratio | 4.4 (1.5) | 4.3 (1.5) |
| ECG-based LVH | 258 (8.3%) | 153 (1.9%) |
| Diabetes | 251 (8.1%) | 1143 (13.9%) |
| History of MI | 56 (1.8%) | 72 (0.9%) |
| Prevalent heart failure | 9 (0.3%) | 53 (0.6%) |
| P-wave indices | ||
| PR interval, ms | 163 (24) | 165 (25) |
| Maximum P-wave duration, ms | 109 (12) | 109 (12) |
| Maximum P-wave area, μV·ms | 330 (98) | 334 (104) |
| P-wave terminal force, μV·ms | 2162 (1808) | 1989 (1790) |
Continuous variables described as mean (SD) and categorical as N (%).
Framingham indicates Framingham Heart Study; ARIC, Atherosclerosis Risk in Communities Study; BP, blood pressure; HDL, high-density lipoprotein; LVH, left ventricular hypertrophy; MI, myocardial infarction.
During 10-year follow-up, 217 FHS and 458 ARIC participants developed incident AF. Table 2 describes the Kaplan-Meier 10-year percentage risk for AF in both cohorts, overall and stratified by PWI at specified cut-points. In both cohorts, the 10-year event Kaplan-Meier percentages were greater for the higher range PWI compared to those in the lower range. The largest magnitude differences were seen for P-wave duration in FHS and P-wave terminal force in ARIC.
Table 2.
Kaplan-Meier 10-year percentage risk (% [95% CI]) of atrial fibrillation by cohort, overall and stratified by P-wave indices using the specified cut-points.
| Framingham | ARIC | |
|---|---|---|
| 10-year % risk of event, overall | 8.0 (7.0, 9.0) | 5.9 (5.4, 6.4) |
| PR interval | ||
| <120 | --* | 5.6 (5.1, 6.2) |
| 120 to 200 ms | 7.8 (6.8, 8.9) | 5.6 (5.1, 6.2) |
| >200 ms | 13.9 (8.5, 22.4) | 9.0 (7.0, 11.5) |
| P-wave duration | ||
| ≤120 ms | 6.8 (5.8, 7.9) | 5.1 (4.6, 5.7) |
| >120 ms | 14.6 (11.5, 18.3) | 9.9 (8.4, 11.7) |
| Maximum P-wave area | ||
| <95th percentile | 7.9 (6.9, 9.0) | 5.8 (5.3, 6.3) |
| ≥95th percentile | 9.3 (5.5, 15.6) | 8.0 (5.6, 11.2) |
| P-wave terminal force | ||
| ≤4000 μV·ms | 7.4 (6.4, 8.6) | 5.3 (4.8, 5.8) |
| >4000 μV·ms | 11.4 (8.6, 15.0) | 10.4 (8.6, 12.6) |
CI, indicates confidence interval; Framingham, Framingham Heart Study; ARIC, Atherosclerosis Risk in Communities Study. Atrial fibrillation and P-wave indices determined as described by text.
No Framingham Heart Study participants with PR<120 ms developed atrial fibrillation during the 10-year follow-up.
The results of the 10-year Cox proportional hazards models are presented in Table 3. P-wave duration was the only measure significantly associated with 10-year risk of AF in both cohorts. In multivariable-adjusted analysis, the estimates were similar in FHS (HR 1.54, 95% CI 1.13 to 2.11) and ARIC (HR 1.55, 95% CI 1.25 to 1.93). PR interval, P-wave area, and P-wave terminal force were not associated with AF in FHS. In contrast, all 3 PWI were significantly associated with incident AF in the ARIC Study in the age- and sex-adjusted models; only P-wave area did not continue to have a significant association following multivariable adjustment (HR 1.35; 95% CI 0.92-1.98). The largest cross-cohort difference was in the estimates for P-wave terminal force. In the ARIC Study, P-wave terminal force >4000 μV·ms was associated with a 1.6-fold increased AF risk. The associations of the covariates included in the multivariable model and the 10-year risk of AF are presented in the Supplementary Table.
Table 3.
Hazard ratios (95% confidence intervals) for the 10-year risk of atrial fibrillation for cohort participants according to P-wave indices, categorized using specified clinical cut-points.
| Framingham | ARIC | |
|---|---|---|
| PR interval, >200 ms | ||
| Age- and sex-adjusted* | 1.24 (0.72-2.15) | 1.37 (1.04-1.82) |
| Multivariable-adjusted† | 1.21 (0.69-2.11) | 1.36 (1.02-1.81) |
| P-wave duration, >120 ms | ||
| Age- and sex-adjusted* | 1.70 (1.26-2.30) | 1.71 (1.39-2.11) |
| Multivariable-adjusted† | 1.54 (1.13-2.11) | 1.55 (1.25-1.93) |
| Maximum P-wave area, ≥95th percentile | ||
| Age- and sex-adjusted* | 1.21 (0.69-2.12) | 1.52 (1.05-2.21) |
| Multivariable-adjusted† | 1.21 (0.68-2.15) | 1.35 (0.92-1.98) |
| P-wave terminal force, >4000 μV·ms | ||
| Age- and sex-adjusted* | 1.09 (0.78-1.52) | 1.75 (1.39-2.20) |
| Multivariable-adjusted† | 1.00 (0.71-1.40) | 1.56 (1.24-2.00) |
Hazard ratios are for 10-year follow-up. Framingham indicates Framingham Heart Study; ARIC, Atherosclerosis Risk in Communities Study. Events and P-wave indices referent as described by text.
Includes adjustment for race in ARIC.
Multivariable-adjusted for age, sex, race (in ARIC), current smoking, height, weight, systolic and diastolic blood pressures, heart rate, total/HDL cholesterol, ECG-based LVH, diabetes, history of myocardial infarction, and prevalent heart failure.
Figure 1 shows the meta-analyses of the hazard ratio estimates across cohorts for each of the PWI. The figure further shows the heterogeneity (I2) estimates with corresponding significance levels. In the pooled multivariable-adjusted meta-analysis, P-wave duration (HR 1.60, 95% CI 1.24 to 2.06) was significantly associated with AF. P-wave area had a weak, non-significant association with AF (HR 1.31, 95% CI 0.95 to 1.80). Whereas the cohort-specific estimates demonstrated heterogeneity (I2 =91.0%, P=0.001), the association of P-wave terminal force with AF was highly significant in ARIC (HR 1.56, 95% CI 1.24 to 2.00) but not FHS (HR 1.00, 95% CI 0.71 to 1.40). Given the significance of the heterogeneity estimate and a concern for racial differences in PWI, we repeated the meta-analysis selecting whites only from the ARIC cohort (n=6,581, 79.7%). The association of P-wave terminal force with AF in ARIC was similar in whites (HR, 1.56, 95% CI 1.20 to 2.03) and blacks (HR 1.65, 95% CI 0.96 to 2.83) following multivariable adjustment. The meta-analysis including FHS and only ARIC whites also indicated persistence of significant heterogeneity (I 2=75.7%, P=0.042).
Figure 1.
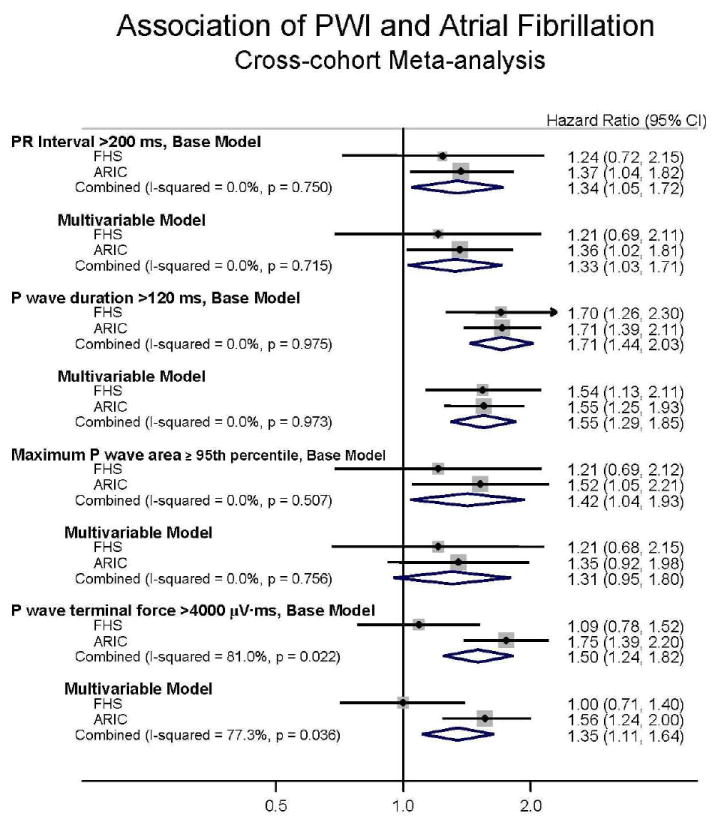
Cross-cohort meta-analysis for the 10-year risk of incident atrial fibrillation in the FHS (Framingham Heart Study) and the Atherosclerosis Risk in Communities (ARIC) Study cohorts. Hazard ratios (95% Confidence Intervals) are shown for each cohort. Results are adjusted for age, sex and race (in ARIC) and multivariable-adjusted as per text. The I-squared presents the heterogeneity index for meta-analysis across the two cohorts.
The assessments of reclassification and discrimination are summarized in Table 4. The multivariable model in FHS had a C-statistic of 0.78 (95% CI 0.75 to 0.80) and in ARIC 0.71 (95% CI 0.69 to 0.73). In neither cohort did the C-statistic improve with the addition of PWI. The largest NRI was that of P-wave duration >120 ms in FHS (2.9%) and P-wave terminal force >4000 μV·ms in ARIC (2.0%). P-wave terminal force showed the largest improvement in IDI, reaching 5.0% (95% CI 1.5-8.4) in the ARIC Study.
Table 4.
C-statistics, categorical NRI, and IDI for different PWI models in the prediction of atrial fibrillation.
| C-statistic (95% CI) | NRI | Relative IDI, % (95% CI) | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Framingham | ARIC | Framingham | ARIC | Framingham | ARIC | |
|
|
||||||
| Multivariable-adjusted base model* | 0.78 (0.75-0.80) | 0.71 (0.69-0.73) | ---- | ---- | ---- | ---- |
| + PR interval >200 ms | 0.78 (0.75-0.80) | 0.71 (0.69-0.74) | -0.2% | 0.3% | 0.8 (-0.5-8.2) | 2.3 (0.3-4.4) |
| + P duration >120 ms | 0.78 (0.75-0.80) | 0.72 (0.69-0.74) | 2.9% | -0.1% | 2.4 (-0.2-10.8) | 6.1 (2.5-9.8) |
| + P area ≥95th percentile | 0.78 (0.75-0.80) | 0.71 (0.69-0.74) | -0.1% | 1.5% | 0.1 (-0.4-1.7) | 0.8 (-0.4-2.0) |
| + P terminal force >4000 μV·ms | 0.78 (0.75-0.81) | 0.72 (0.70-0.74) | 0.04% | 2.0% | 0.003 (-0. 6-0.6) | 5.0 (1.5-8.4) |
Multivariable-adjusted for age, sex, race (in ARIC), current smoking, height, weight, systolic and diastolic blood pressures, heart rate, total/HDL cholesterol, ECG-based LVH, diabetes, history of myocardial infarction, and prevalent heart failure. P-wave indices added as dichotomous variable (<95th vs ≥95th percentile).
CI indicates confidence interval; Framingham, Framingham Heart Study; ARIC, the Atherosclerosis Risk in Communities Study; NRI, net reclassification index; IDI, integrated discrimination improvement.
Figures 2A and 2B shows the association of individual PWI with AF incidence by cohort modeled as restricted cubic splines. In the ARIC cohort, P-wave duration >110 ms had a proportionately increasing association with AF, but was more modest in FHS. The risk of AF increased up to 5-fold at the highest values of P-wave terminal force in the ARIC cohort. However, in FHS P-wave terminal force had <2-fold risk of AF; the wide CI underscore the uncertainty in the estimates of the association.
Figure 2.
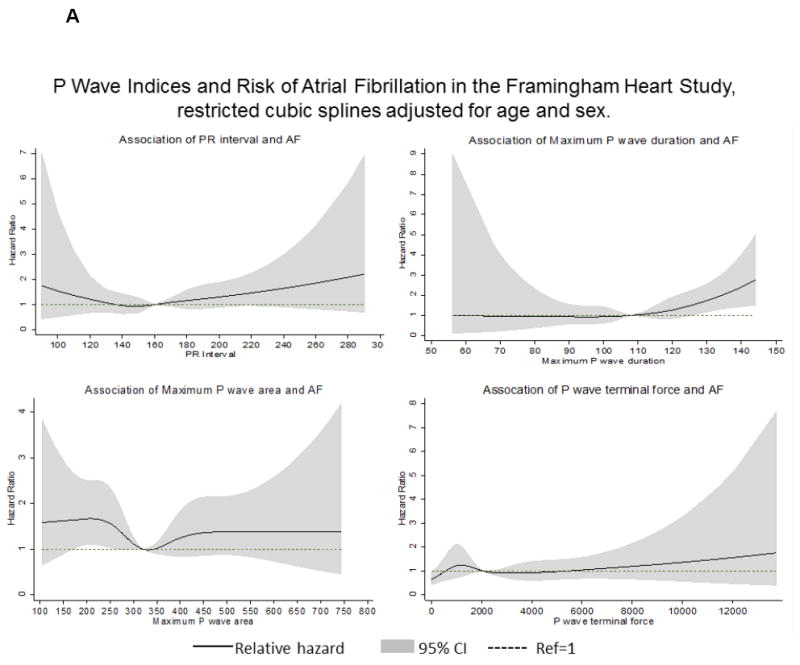
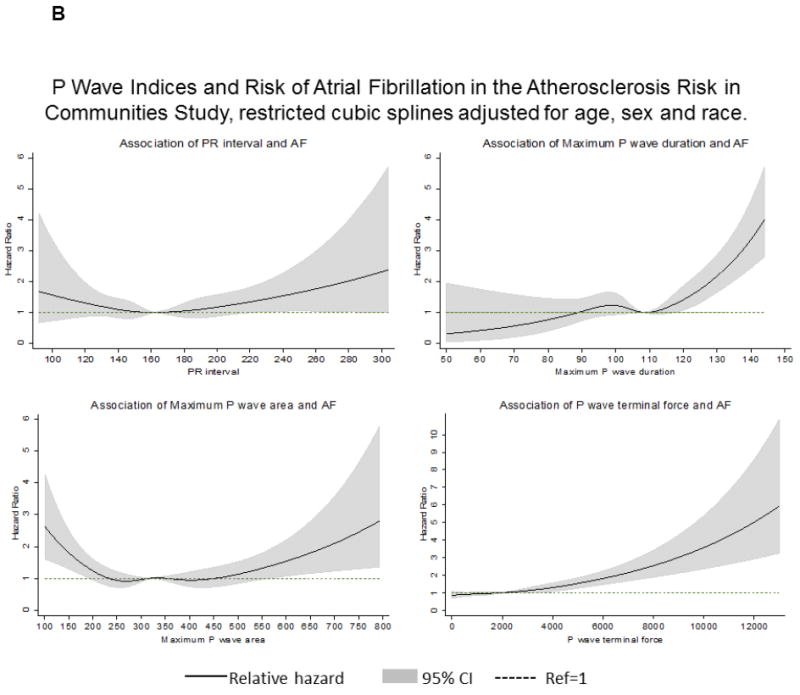
Restricted cubic splines illustrating the associations between the P-wave indices and the relative hazard for atrial fibrillation (AF) during 10-year follow-up (2A, Framingham Heart Study; 2B, Atherosclerosis Risk in Communities Study). Models adjusted for age, sex, and race (in ARIC).
Discussion
We conducted a novel assessment of the contribution of PWI towards AF risk by studying two large-sized, community-based cohorts. FHS and the ARIC Study have robust covariate and AF ascertainment, and we determined that PWI have strong associations with increased AF risk in cross-cohort meta-analyses. We selected covariates for multivariable adjustment that were included in the well-validated CHARGE-AF risk model and are readily available in the primary care setting. We determined that PWI did not enhance risk metrics beyond such readily available covariates.
Our findings are consistent with previous work done in FHS, ARIC, and other studies examining the relation of PWI to AF. In ARIC, PWI ≥95th percentile were previously associated with AF.6 However, the prior ARIC analysis had fewer AF events (n=117) and did not examine risk discrimination and reclassification. A prior FHS analysis examined selected PWI using a manual measurement technique.27 The FHS analysis was conducted in a smaller and older cohort, included fewer covariates, and had the significant limitation of using manually derived measurement of P-wave duration only. Neither the prior FHS nor ARIC analysis examined the additive contributions of PWI towards AF risk. In a large, retrospective Veterans Affairs analysis, P-wave duration was associated with a 2.7-fold increased risk of AF during approximately 5-year follow-up, albeit with limited covariate adjustment.7 PWI have been examined in a range of clinical conditions28 and particularly been related to AF recurrence following pulmonary vein isolation and cardioversion.29,30
The electrophysiologic significance of PWI has been demonstrated by intracardiac, catheter-based studies. PWI are markers of adverse atrial electrical remodeling related to electroanatomic mapping and electrophysiologic functional assessments. PWI and catheter-based atrial studies are altered by aging, increased atrial pressure, and cardiomyopathy in the absence of AF.5,31,32 An evident strength of using PWIs is that they are obtained from the 12-lead surface ECG. Contemporary software facilitates automated analysis and quantification.
Our meta-analyses enhanced our evaluation of the relations of PWI to 10-year risk of AF. In both FHS and ARIC, the associations of the PR interval, maximum P-wave duration and maximum P-wave area with AF were consistent. Meta-analysis of the two increased statistical power and improved generalizability. In contrast, we did observe significant heterogeneity in the assessment of P-wave terminal force (I2=91.0% and P=0.001, for the multivariable-adjusted model). P-wave terminal force had a striking relation to AF in the ARIC cohort, yet was all but absent in FHS.
There are multiple potential reasons for the cross-cohort heterogeneity of the relations of P-wave terminal force and AF that we observed. Differences in cohort design, AF ascertainment, and covariate measurement may contribute towards heterogeneity. ECGs were performed in a standardized manner in both cohorts with identical software for PWI quantification. Hence, methodological differences in PWI acquisition and measurement are unlikely to explain the P-wave terminal force heterogeneity. The meta-analysis results were unchanged in a subgroup analysis of whites only, suggesting as well that racial differences in P-wave terminal force do not account for the heterogeneity. We are interested in further study incorporating larger cohorts in order to continue to investigate the associations of non-invasive ECG markers of atrial function and adverse outcomes.
Our analysis satisfied a number of essential criteria for appraisal of novel biomarkers.33 First, PWI are easily measured with contemporary software algorithms. Second, PWI add novel information about atrial electrical function, describing an intermediate phenotype. Importantly, our assessment showed that PWI have highly limited contribution towards enhancing AF risk modeling metrics. We used a robust multivariable model that remained essentially unchanged after adding PWI. Similarly, reclassification metrics did not improve by adding PWI to the model.
Strengths and limitations
Our work has important limitations. Blacks were not well represented in our study and the generalizability of our findings to other races or ethnicities is unknown. Racial differences in PWI have been identified, but the limited enrollment of blacks here precludes generalizing our findings to other black cohorts or other races or ethnicities. Second, the P-wave is a low amplitude signal; it is possible that P-waves would not be detected by software algorithms, yielding systematic measurement error. We did not perform a sensitivity analysis to exonerate this possibility. However, FHS and ARIC ECG data underwent extensive data cleaning; we consider such measurement error to be non-differential with respect to AF. In addition, our results may not be generalizable to studies using manual measurement techniques. However, we would assert that an automated approach incorporating digitized tracings and software algorithms is superior to manual measurements. Third, we conducted a multivariable adjustment that included the covariates identified by CHARGE-AF but are unable to exclude residual confounding. We were further unable to incorporate imaging assessments, such as echocardiographic left atrial diameter, that have been associated with AF. However, inclusion of atrial diameter would not alter the assessments of reclassification and discrimination. Fourth, it is possible that systematic bias in either or both FHS and ARIC affected the identification of AF cases. Whether such bias would be differential with respect to the PWI exposure is not clear. Fifth, our analysis excluded individuals using atrioventricular nodal medications at baseline because of our concern for their potential effects on PWI measurement. This exclusion markedly diminished the sample size and limits generalizability of the study to those not taking atrioventricular nodal medications. Further analyses are essential to examine the effect of such medications on PWI.
Strengths of our analysis include studying PWI in a cross-cohort meta-analysis in two cohorts with complementary designs yet similar baseline covariates. Our statistical approach allowed us to comment on the cross-sectional consistency and heterogeneity of estimates of PWI and AF. The use of contemporary statistical assessments of risk reclassification and discrimination is essential and informative for further studies of PWI and AF risk assessment.
In conclusion, we demonstrated that PWI are significantly associated with AF but have a limited contribution to incremental risk prediction when added to a model with well-established clinical covariates related to AF.
Supplementary Material
Acknowledgments
The authors thank the staff and participants of the ARIC study for their important contributions.
Footnotes
Disclosures
The authors have no disclosures or conflicts of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Magnani JW, Lopez FL, Soliman EZ, Maclehose RF, Crow RS, Alonso A. P wave indices, obesity, and the metabolic syndrome: the atherosclerosis risk in communities study. Obesity (Silver Spring) 2012 Mar;20(3):666–672. doi: 10.1038/oby.2011.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Magnani JW, Gorodeski EZ, Johnson VM, et al. P wave duration is associated with cardiovascular and all-cause mortality outcomes: the National Health and Nutrition Examination Survey. Heart Rhythm. 2011 Jan;8(1):93–100. doi: 10.1016/j.hrthm.2010.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alonso A, Soliman EZ, Chen LY, Bluemke DA, Heckbert SR. Association of blood pressure and aortic distensibility with P wave indices and PR interval: the multi-ethnic study of atherosclerosis (MESA) J Electrocardiol. 2013 Jul-Aug;46(4):359, e351–356. doi: 10.1016/j.jelectrocard.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Platonov PG. P-wave morphology: underlying mechanisms and clinical implications. Ann Noninvasive Electrocardiol. 2012 Jul;17(3):161–169. doi: 10.1111/j.1542-474X.2012.00534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sanders P, Morton JB, Davidson NC, et al. Electrical remodeling of the atria in congestive heart failure: electrophysiological and electroanatomic mapping in humans. Circulation. 2003 Sep 23;108(12):1461–1468. doi: 10.1161/01.CIR.0000090688.49283.67. [DOI] [PubMed] [Google Scholar]
- 6.Soliman EZ, Prineas RJ, Case LD, Zhang ZM, Goff DC., Jr Ethnic distribution of ECG predictors of atrial fibrillation and its impact on understanding the ethnic distribution of ischemic stroke in the Atherosclerosis Risk in Communities (ARIC) study. Stroke; a journal of cerebral circulation. 2009 Apr;40(4):1204–1211. doi: 10.1161/STROKEAHA.108.534735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perez MV, Dewey FE, Marcus R, et al. Electrocardiographic predictors of atrial fibrillation. Am Heart J. 2009 Oct;158(4):622–628. doi: 10.1016/j.ahj.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 8.Alonso A, Krijthe BP, Aspelund T, et al. Simple Risk Model Predicts Incidence of Atrial Fibrillation in a Racially and Geographically Diverse Population: the CHARGE-AF Consortium. J Am Heart Assoc. 2013;2(2):e000102. doi: 10.1161/JAHA.112.000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Magnani JW, Williamson MA, Ellinor PT, Monahan KM, Benjamin EJ. P wave indices: current status and future directions in epidemiology, clinical, and research applications. Circ Arrhythm Electrophysiol. 2009 Feb;2(1):72–79. doi: 10.1161/CIRCEP.108.806828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pencina MJ, D’Agostino RB, Sr, D’Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008 Jan 30;27(2):157–172. doi: 10.1002/sim.2929. discussion 207-112. [DOI] [PubMed] [Google Scholar]
- 11.Dawber TR, Meadors GF, Moore FE., Jr Epidemiological approaches to heart disease: the Framingham Study. American journal of public health and the nation’s health. 1951 Mar;41(3):279–281. doi: 10.2105/ajph.41.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Splansky GL, Corey D, Yang Q, et al. The Third Generation Cohort of the National Heart, Lung, and Blood Institute’s Framingham Heart Study: design, recruitment, and initial examination. American Journal of Epidemiology. 2007 Jun 1;165(11):1328–1335. doi: 10.1093/aje/kwm021. [DOI] [PubMed] [Google Scholar]
- 13.White AD, Folsom AR, Chambless LE, et al. Community surveillance of coronary heart disease in the Atherosclerosis Risk in Communities (ARIC) Study: methods and initial two years’ experience. J Clin Epidemiol. 1996 Feb;49(2):223–233. doi: 10.1016/0895-4356(95)00041-0. [DOI] [PubMed] [Google Scholar]
- 14.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol. 1989 Apr;129(4):687–702. [PubMed] [Google Scholar]
- 15.Soliman EZ, Alonso A, Misialek JR, et al. Reference ranges of PR duration and P-wave indices in individuals free of cardiovascular disease: The Multi-Ethnic Study of Atherosclerosis (MESA) J Electrocardiol. 2013 Jun 24; doi: 10.1016/j.jelectrocard.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alonso A, Agarwal SK, Soliman EZ, et al. Incidence of atrial fibrillation in whites and African-Americans: the Atherosclerosis Risk in Communities (ARIC) study. American Heart Journal. 2009 Jul;158(1):111–117. doi: 10.1016/j.ahj.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mosterd A, D’Agostino RB, Silbershatz H, et al. Trends in the prevalence of hypertension, antihypertensive therapy, and left ventricular hypertrophy from 1950 to 1989. The New England journal of medicine. 1999 Apr 22;340(16):1221–1227. doi: 10.1056/NEJM199904223401601. [DOI] [PubMed] [Google Scholar]
- 18.Parikh NI, Gona P, Larson MG, et al. Long-term trends in myocardial infarction incidence and case fatality in the National Heart, Lung, and Blood Institute’s Framingham Heart study. Circulation. 2009 Mar 10;119(9):1203–1210. doi: 10.1161/CIRCULATIONAHA.108.825364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ho KK, Pinsky JL, Kannel WB, Levy D. The epidemiology of heart failure: the Framingham Study. J Am Coll Cardiol Oct. 1993;22(4 Suppl A):6A–13A. doi: 10.1016/0735-1097(93)90455-a. [DOI] [PubMed] [Google Scholar]
- 20.Casale PN, Devereux RB, Alonso DR, Campo E, Kligfield P. Improved sex-specific criteria of left ventricular hypertrophy for clinical and computer interpretation of electrocardiograms: validation with autopsy findings. Circulation. 1987 Mar;75(3):565–572. doi: 10.1161/01.cir.75.3.565. [DOI] [PubMed] [Google Scholar]
- 21.Myerson M, Coady S, Taylor H, Rosamond WD, Goff DC., Jr Declining severity of myocardial infarction from 1987 to 2002: the Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 2009 Feb 3;119(4):503–514. doi: 10.1161/CIRCULATIONAHA.107.693879. [DOI] [PubMed] [Google Scholar]
- 22.Loehr LR, Rosamond WD, Chang PP, Folsom AR, Chambless LE. Heart failure incidence and survival (from the Atherosclerosis Risk in Communities study) The American Journal of Cardiology. 2008 Apr 1;101(7):1016–1022. doi: 10.1016/j.amjcard.2007.11.061. [DOI] [PubMed] [Google Scholar]
- 23.Cheng S, Keyes MJ, Larson MG, et al. Long-term outcomes in individuals with prolonged PR interval or first-degree atrioventricular block. JAMA : the journal of the American Medical Association. 2009 Jun 24;301(24):2571–2577. doi: 10.1001/jama.2009.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Munuswamy K, Alpert MA, Martin RH, Whiting RB, Mechlin NJ. Sensitivity and specificity of commonly used electrocardiographic criteria for left atrial enlargement determined by M-mode echocardiography. The American Journal of Cardiology. 1984 Mar 1;53(6):829–832. doi: 10.1016/0002-9149(84)90413-2. [DOI] [PubMed] [Google Scholar]
- 25.Harrell FE., Jr . Regression Modeling Strategies with Applications to Linear Models, Logistic Regression, and Survival Analysis. New York: Springer-Verlag; 2001. [Google Scholar]
- 26.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002 Jun 15;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 27.Magnani JW, Johnson VM, Sullivan LM, et al. P wave duration and risk of longitudinal atrial fibrillation in persons >/= 60 years old (from the Framingham Heart Study) Am J Cardiol. 2011 Mar 15;107(6):917–921. e911. doi: 10.1016/j.amjcard.2010.10.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Magnani JW, Williamson MA, Ellinor PT, Monahan KM, Benjamin EJ. P wave indices: current status and future directions in epidemiology, clinical, and research applications. Circulation Arrhythmia and electrophysiology. 2009 Feb;2(1):72–79. doi: 10.1161/CIRCEP.108.806828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chao TF, Sung SH, Wang KL, et al. Associations between the atrial electromechanical interval, atrial remodelling and outcome of catheter ablation in paroxysmal atrial fibrillation. Heart. 2011;97(3):225–230. doi: 10.1136/hrt.2010.212373. [DOI] [PubMed] [Google Scholar]
- 30.Raitt MH, Volgman AS, Zoble RG, et al. Prediction of the recurrence of atrial fibrillation after cardioversion in the Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) study. Am Heart J. 2006 Feb;151(2):390–396. doi: 10.1016/j.ahj.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 31.John B, Stiles MK, Kuklik P, et al. Reverse remodeling of the atria after treatment of chronic stretch in humans: implications for the atrial fibrillation substrate. J Am Coll Cardiol. 2010 Mar 23;55(12):1217–1226. doi: 10.1016/j.jacc.2009.10.046. [DOI] [PubMed] [Google Scholar]
- 32.Kistler PM, Sanders P, Fynn SP, et al. Electrophysiologic and electroanatomic changes in the human atrium associated with age. J Am Coll Cardiol. 2004 Jul 7;44(1):109–116. doi: 10.1016/j.jacc.2004.03.044. [DOI] [PubMed] [Google Scholar]
- 33.Hlatky MA, Greenland P, Arnett DK, et al. Criteria for evaluation of novel markers of cardiovascular risk: a scientific statement from the American Heart Association. Circulation. 2009 May 5;119(17):2408–2416. doi: 10.1161/CIRCULATIONAHA.109.192278. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


