Abstract
Humans find members of the opposite sex more attractive when their image is spatially associated with the color red. This effect even occurs when the red color is not on the skin or clothing (i.e. is extraneous). We hypothesize that this extraneous color effect could be at least partially explained by a low-level and biologically innate generalization process, and so similar extraneous color effects should be observed in non-humans. To test this possibility, we examined the influence of extraneous color in rhesus macaques (Macaca mulatta). Across two experiments, we determined the influence of extraneous red on viewing preferences (assessed by looking time) in free-ranging rhesus monkeys. We presented male and female monkeys with black and white photographs of the hindquarters of same and opposite sex conspecifics on either a red (experimental condition) or blue (control condition) background. As a secondary control, we also presented neutral stimuli (photographs of seashells) on red and blue backgrounds. We found that female monkeys looked longer at a picture of a male scrotum, but not a seashell, on a red background (Experiment 1), while males showed no bias. Neither male nor female monkeys showed an effect of color on looking time for female hindquarters or seashells (Experiment 2). The finding for females viewing males suggests that extraneous color affects preferences among rhesus macaques. Further, it raises the possibility that evolutionary processes gave rise to extraneous color effects during human evolution.
Keywords: primates, rhesus macaques, sexual signals, attractiveness, body colors, red
1.0 Introduction
Body ornaments and bright colors may be selected through sexual selection for their ability to indicate elements of quality or condition to conspecifics (Darwin, 1872). Species as diverse as mandrills (Mandrillus sphinx: Setchell, 2005), red junglefowl (Gallus gallus: Zuk et al., 1990), and two-spotted goby fish (Gobiusculus flavenscens: Amundsen & Forsgren, 2001) make use of such sexual signals. The form that signals take may originate from sensory biases, which are imperfections in psychological templates for other important environmental elements (e.g. brightly colored foods like fruits and flowers: Arak & Enquiest, 1993; Dawkins & Guildford, 1996; Endler & Basolo, 1998). These imperfections, and the signal recipient’s tendency to respond, can be exploited by conspecific traits that advertise quality or condition, and over evolutionary time, further preferences and elaborations of those traits can result in a sexual signal. Thus, unorthodox stimuli that replicate some aspects of the original sexual signal can reveal sensory biases.
Consistent with this idea, some animals exhibit greater attraction to others in the presence of a color even when it is not a natural part of the body (Arak & Enquiest, 1993). For example, several bird species exhibit preferences for opposite sex conspecifics that have artificial leg bands of the appropriate color (e.g. zebra finches Taeniopygia guttata: Burley et al., 1982; Hunt et al., 1997; double-bar finches Poephila bichenovii: Burley, 1986; American goldfinches Carduelis tristis: Johnson et al., 1993; Bluethroats Luscinia svecica: Johnsen et al., 1997). Similarly, uncrested zebra finches show preferences for individuals to whom white crests (but not those of any other color) have been experimentally added (Burley & Symanski, 1998). These examples demonstrate that a receiver can have a generalized template for the signal they seek, which can be exploited under unusual or unnatural circumstances that mimic some aspects of the original signal.
The sensory bias concept may apply to a known red-attraction association in humans. Research indicates that humans are particularly attracted to individuals displaying or in close proximity to the color red. Increased facial redness is perceived as more attractive (Re et al., 2011; Stephen et al., 2012) and individuals wearing red are considered more attractive and sexually desirable (Elliot & Niesta, 2008; Gueguen, 2012; Roberts et al., 2010). Even extraneous red (i.e., red not on skin or clothing) has an effect, as men and women rate black and white photographs of opposite sex individuals framed in red as more attractive and sexually desirable (Elliot et al., 2010; Schwarz & Singer, 2012). In humans, the red effect could reflect cultural influences. Indeed, in many cultures red is linked to sex and romance, and red may enhance attraction entirely due to learned associations (Hutchings, 2004; Elliot & Maier, 2012). However, as the human phenomenon resembles sensory biases for colors in non-humans, such effects could also have biological roots.
Whether human and animal color-attraction effects are derived from similar evolutionary processes is unclear. Non-natural cues in birds do not produce effects in some instances (zebra finches: Ratcliffe & Boag, 1987; bluethroats: Johnsen et al., 2000); one possible reason is that these color biases do not extend beyond manipulations of the original mate or require natural contexts (Hunt et al., 1997). The aim of the present research was to test for the extraneous color effect observed in humans in a non-human primate, using a method matched as closely as possible to that used in human studies. Documenting such an effect would provide evidence that responses to extraneous colors in mating contexts may have similar biological roots across species, including humans.
Here, we measured rhesus macaques’ looking time as a proxy for interest in or preference for black and white photographs of the scrota/hindquarters of opposite sex conspecifics overlaid on a red or blue background (Figure 1a). Both male and female rhesus macaques use red body signals in sexual displays. Mating season brings a redder scrotum to males, and redder hindquarters and faces to both sexes, but little or no sexual swelling (Baulu, 1976). Female facial coloration darkens around ovulation (Higham et al., 2010) and attracts the attention of males (Waitt et al., 2006; Higham et al., 2011). While the correlation of male coloration with male condition remains unclear (Higham et al., 2013), experimental manipulations have also suggested that females are attracted to artificially reddened male faces (Waitt et al., 2003). If these sexual signals of male and female rhesus macaques have evolved from, or have led to the evolution of, a more generalized sensory bias, then unorthodox stimuli that contain aspects of the signal – in this case black and white photographs of conspecifics on a red background – should still elicit greater interest in that image, as measured in increased looking-time, in opposite sex macaques. The absence of such a result would be indicated by similar looking-times across conditions, regardless of stimulus content.
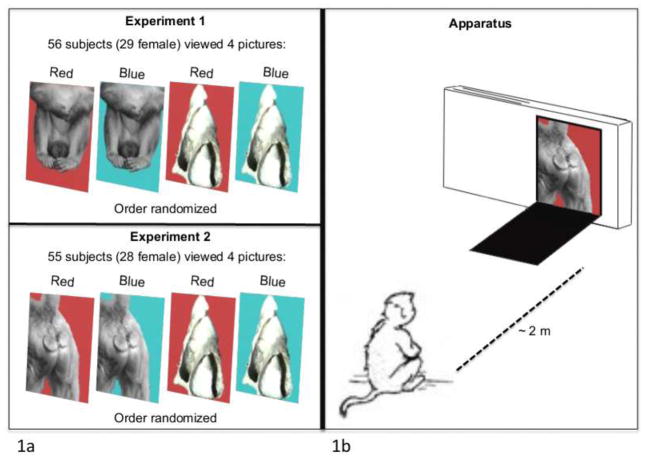
Figure 1.
1a: In Experiment 1, male and female rhesus macaques viewed four images in random order: a black and white male scrotum on a red background, a black and white male scrotum on a blue background, a black and white seashell on a red background, and a black and white seashell on a blue background. In Experiment 2, male and female macaques viewed four images in a random order: a black and white female hindquarter image on a red background, a black and white female hindquarter image on a blue background, a black and white seashell image on a red background, and a black and white seashell image on a blue background. 1b: These images were presented to the subject in a white foamcore box (50 cm long x 5 cm deep x 25 cm tall), with a port through which the image could be displayed. The apparatus was placed ~2 m from the subject.
2.0 Experiment 1: Males and females viewing male scrota
2.1 Methods
2.1.1 Subjects
We tested rhesus macaques (Macaca mulatta) from the semi-provisioned free-ranging population of approximately 1000 monkeys at the Cayo Santiago field site in Puerto Rico. These monkeys are well studied and habituated to human observation (Rawlins & Kessler, 1986). Their conditions are semi-natural, living in naturally formed social groups, but about 50% of their diet is provisioned monkey chow (Rawlins & Kessler, 1986). Individuals are identifiable by tattoos and ear notches and complete records on individual ages are maintained. This research with this population was approved by the Institutional Animal Care and Use Committee at the University of Puerto Rico and was therefore in compliance with ethical animal use rules and laws.
We completed a full session – four trials – with 56 subjects (29 female, 27 male) for this experiment. We approached 78 additional subjects but they did not complete a session due to subject inattention, movement toward or away from the apparatus, or interference from other monkeys. We did not include individuals that did not complete a full session in the data analysis. This failure rate (55%) is typical for field studies with this population using very similar methods (e.g. Cheries et al., 2006). Note that, because failures were independent from the task parameters, they are unlikely to bias our results. During data collection, we discarded and replaced an additional 6 sessions because the monkey was found to have served as a subject for a different experiment that month that used somewhat similar stimuli, and we did not want previous exposure to those stimuli to affect the results of the present experiment.
2.1.2 Stimuli
Our stimuli consisted of a black and white image of an adult male from the mating season that focused on the scrotum, or a large seashell (neutral control) cut from their original backgrounds and superimposed on a background of solid color (red or blue, Fig. 1a). Previous studies suggest that monkeys (Pokorny & de Waal, 2009; Schell et al., 2011), including rhesus macaques (Sliwa et al., 2011), match photographs of conspecifics to their real-life counterparts. Studies at Cayo Santiago (Higham et al., 2011) and elsewhere (Waitt et al., 2003, 2006) have already used photographs to investigate sexual signaling preferences in rhesus macaques. The photo of the male was of the lower half of the male body. Although this image contains other body information, it does not include additional areas that have been shown to or are hypothesized to be used by female rhesus macaques in mate selection. Furthermore, this image of the male contained no other areas besides the genitals that are known to carry color-based regions (i.e. the face and hindquarters). A large seashell was selected as a control because it is: 1) organically shaped, like body parts; and 2) familiar to the monkeys of Cayo Santiago, as there are many seashells on the beaches of the island. Note that the image of a scrotum on a red background [Scrotum Red] is our critical test image, for which we predict that females (opposite sex) will have longer looking-times if the extraneous red effect is present. Two additional images, a scrotum on a blue background [Scrotum Blue] and a seashell on a red background [Seashell Red], serve as tests for independent photo and color effects. By comparing the results of experiments these different stimuli, we can identify whether there is an effect only for the combination of photo and color (critical test image), as predicted, rather than one or two independent effects for photo (scrotum vs. seashell) or color (red vs. blue).
To create the specific shade of red used for our study, we collected 457 standardized images of 27 male and 17 female rhesus macaques on Cayo Santiago using a calibrated camera (Stevens et al., 2009). These were taken over a period of several weeks in order to capture temporal variations in face color. From these photographs, we measured the average color of the cheek region in all images using previously published methods and transformed this color directly from camera space into rhesus visual space, based on the retinal receptor catches of rhesus macaques to the colors shown (Stevens et al., 2007, 2009). We used data and models of rhesus spectral sensitivities as reported elsewhere (Higham et al., 2010), with the exception that instead of utilizing data on human short-wave sensitivity, we estimated a rhesus macaque short-wave sensitivity curve from its peak sensitivity value (Harosi, 1987) using a rhodopsin template (Govardovskii et al., 2000). We calculated a geometric mean of this cheek color to serve as our red color. We calculated a blue color as being perfectly opposed in rhesus color space to the red mean and of the same luminance (Kelber et al., 2003). This method of choosing an alternative color to red has been used in human studies of the extraneous red effect (Elliot & Niesta, 2008; Elliot et al., 2010). Based on this red and blue, we printed red and blue colored sheets, which we compared to this mean using a visual threshold discrimination model (Vorobyev & Osorio, 1998). The printed red in sRGB colorspace was R = 0.77, G = 0.45, B = 0.44. This color was 3.3 just noticeable differences (JND) from the hue of the calculated mean, and 4.2 JND in luminance from the calculated mean. The printed color was within the range of natural variation in our rhesus sample. The printed blue in sRGB colorspace was R = 0.46, G = 0.77, B = 0.77, which was 3.5 JND from the calculated mean hue, and 5.9 JND in the calculated mean luminance.
2.1.3 Apparatus and Procedure
We displayed our stimuli pictures using a simple box-like apparatus made of white foamcore and open on the top (50 cm long x 5 cm deep x 25 cm tall, Fig. 1b). The right side of the apparatus facing the subject (the experimenter’s left side) contained a port with a door (20 x 22.5 cm) that could be opened and closed to reveal a 20 x 22.5 cm picture. We attached the pictures to a solid foamcore backing and then placed them into the right side of the apparatus, with a tag sticking above the box top. This tag was labeled with the condition code and was used to move the pictures within the box. The slim size of the box prevented the experimenters from seeing the picture stimuli, blinding them to condition.
We ran each session with two experimenters: a presenter who handled the stimuli and a cameraperson who recorded the subject’s behavior using a video camera. The cameraperson initiated the session by choosing a subject. Subjects were selected opportunistically when a macaque was found seated, alone, and attentive. Then the presenter set the apparatus ~ 2 m from the subject, while the cameraperson stood ~ 1m behind the presenter and began to film the session, capturing a tight portrait shot of the subject. Recording only stopped when the session was aborted or completed; the decision to terminate was made solely by the cameraperson. Both the cameraperson and the presenter were blind to condition; the cameraperson was never informed of the conditions and the presenter knew the conditions only in code. Condition orders were taken from a pre-generated, randomized list of Latin squares design.
A session consisted of the sequential presentation of 4 picture stimuli (4 trials); the order of these stimuli were randomized across sessions. Male and female subjects saw male scrota and seashells against red and blue backgrounds. A trial started when the presenter identified that the monkey was looking in the direction of the apparatus, and then simultaneously opened the port, revealing the appropriate picture and said “Now”. This verbally marked the beginning of the trial; the cameraperson then monitored time until 10 seconds passed, at which time she said “Stop”. Hearing this, the presenter closed the port, ending the trial. Sessions were aborted when the cameraperson identified that the subject was not visually orienting toward the apparatus, which was required for the initiation of a new trial. Note, the decision to end a session was never made during a trial when subjects were exposed to experimental stimuli, only between trials.
2.1.4 Data Analysis
We used MPEG streamclip software (Cinque, 2008) to code the 10-second video clips for looking duration for each picture. Total look duration (looking-time) was measured in number of frames (videos were recorded at 30 frames/second) and could include multiple looks during the 10-second video. Looking direction was defined as the direction the subject was looking when the presenter said “Now”. The total time a subject spent looking in this direction was the total looking-time. Coding was undertaken later by assistants who were blind to condition. A second blind coder recoded the looking-time of 20% of sessions to establish reliability (correlation, r = 0.925). Three trials were identified in which the subject looked away the instant the presenter said “Now” giving a looking-time of zero; these trials (but not the entire session) were excluded from analysis. Statistics were computed using the Generalized Estimating Equations (GEE) module in PASW 20 (guided by Pan & Connett, 2002; details reported in Table 1). This statistical procedure allows mixed experimental designs, and accommodates missing values and complex data structures, specifically our four different conditions that fall randomly in order during a session. Into this GEE, we entered the dependent variable looking duration, and we entered 3 factor variables: place order of stimuli in a session (4 places in order, recoded into 3 dummy variables), condition (4 conditions, recoded into 3 dummy variables), and sex (recoded as 1 dummy variable). We found a significant effect of stimulus order within a session on looking-time, indicating that with additional trials within the same subject, looking-times decreased. An image first in order in the presentation (p < 0.001), second (p < 0.001) or third (p < 0.001) was statistically significant from other ordered positions and from the final and fourth position. We had anticipated this effect, and so balanced presentation orders across conditions while including a presentation order variable in all analyses. Finally, we included interaction factor variables for sex*condition (recoded as 3 dummy variables) to test our hypothesis that a male scrotum on a red background (condition 1) seen by a female viewer would generate the longest looking-times.
Table 1. Summary of experimental parameters and results from Experiments 1 and 2.
Statistical Test: Generalized Estimating Equation (GEE) using a normal probability function, identity link, and independent working correlation matrix
| Variable | Experiment 1: Viewing Male Scrota N = 56; 29 female, 27 male |
Experiment 2: Viewing Female Hindquarters N = 55, 28 female, 27 male |
|---|---|---|
| (1) Sex: 2 sexes recoded into 1 dummy variable, Exp 1 reference variable = male, Exp 2 reference variable = female | ||
| Sex | B = −5.898, X2 = 0.238, p = 0.626 | B = 2.764, X2 = 0.035, p = 0.852 |
| (2) Condition: 4 stimuli recoded into 3 dummy variables, seashell blue = reference variable; Exp 1 Macaque = scrotum; Exp 2 Macaque = hindquarters | ||
| Macaque Red | B = −1.672, X2 = 0.026, p = 0.871 | B = 10.628, X2 = 0.469, p = 0.493 |
| Macaque Blue | B = −5.410, X2 = 0.357, p = 0.550 | B = −2.567, X2 = 0.048, p = 0.827 |
| Seashell Red | B = 2.998, X2 = 0.073, p = 0.787 | B = 9.586, X2 = 0.707, p = 0.401 |
| (3) Interaction Terms: Exp 1 Sex = female, Macaque = scrotum; Exp 2 Sex = male, Macaque = hindquarters | ||
| [Sex]*[Macaque Red] | B = 34.454, X2 = 3.860, p = 0.049 | B = −11.036, X2 = 0.381, p = 0.537 |
| [Sex]*[Macaque Blue] | B = 19.537, X2 = 1.910, p = 0.167 | B = 33.874, X2 = 3.125, p = 0.077 |
| [Sex]*[Seashell Red] | B = 2.904, X2 = 0.037, p = 0.846 | B = −4.168, X2 = 0.065, p = 0.799 |
2.2 Results
We found no significant main effect of stimulus or sex, indicating that no looking duration differences could be attributed to a particular image or sex alone. However, the interaction term [Female]*[Scrotum Red] was significantly associated with longer looking durations, indicating the females looked longer at male scrotum on red background (p = 0.049, Fig. 2, Table 1). We considered the possibility that this interaction effect was actually driven by both an interest in photos of scrota and the color red in a cumulative effect rather than a true interaction. To address this possibility, we did simple effects analyses within females comparing the reference condition [Seashell Blue] to the different color condition [Seashell Red], the different photo condition [Scrotum Blue], and the critical test condition [Scrotum Red]. These contrasts revealed no statistical difference for the color test (p = 0.55) or for the photo test (p = 0.198) indicating that neither the color red nor the image of a scrotum alone was enough to drive a statistical change in looking duration. However, there was a statistical difference between the reference condition and the critical test condition (p = 0.020), indicating that an effect was only observed when females viewed both a scrotum and the color red together.
Figure 2.
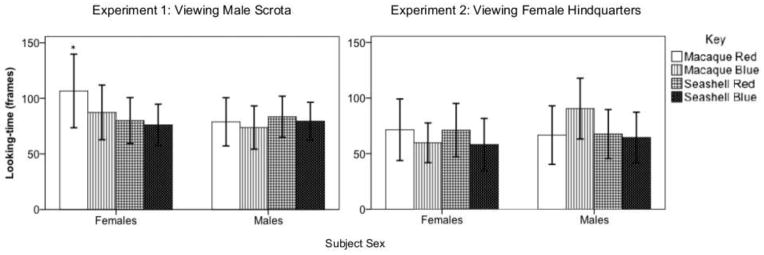
In Experiment 1, female but not male macaques looked longer at a male scrotum on a red background compared to a male scrotum on a blue background or a seashell on either color background, or compared to the looking behavior of males. * = the interaction term [Female]*[Scrotum Red], labeled as Macaque Red on the chart, was significant. In Experiment 2, there were no significant differences in looking-time across conditions. Bars represent mean looking duration, and error bars represent 95% C.I.
3.0 Experiment 2: Males and females viewing female hindquarters
3.1 Methods
3.1.1 Subjects
Subjects were rhesus macaques from the same population at Cayo Santiago as those in Experiment 1. We tested 55 rhesus macaques (28 female, 27 male) in a full session in Experiment 2. An additional 11 sessions were completed, but were not included in analysis because the monkey had already served as a subject in a different experiment that month. There was no overlap in use of subjects between Experiment 1 and Experiment 2, and the timing of data collection for these two experiments also did not overlap. We approached an additional 85 subjects but they did not complete testing due to subject inattention, movement toward or away from the apparatus, or interference from other monkeys (failure rate 56%). This failure rate is typical for these kinds of studies, and these failures were independent of any experimental parameters and so did not affect the study.
3.1.2 Stimuli, apparatus, procedure, and data analysis
The stimuli for Experiment 2 were very similar to those in Experiment 1 except for one critical change. We used a black and white image of an adult female’s hindquarters from the mating season instead of a black and white image of a male scrotum as our test image. Otherwise, we continued to use a seashell as a neutral control, and, as before, these black and white images were cut from their original backgrounds and superimposed on a solid red or blue background, the same red and blue from Experiment 1 (Fig. 1a).
All aspects of the procedure were the same as in Experiment 1. A research assistant blind to condition coded the 10-second video clips for looking duration toward the stimuli; no anomalous trials were found. A second blind coder recoded the looking-time of 20% of sessions to establish reliability (correlation, r = 0.917). We analyzed the data using approaches that were similar to that in Experiment 1 except for one change. For Experiment 2, when entering the factor variable sex into the regression, female was the reference variable instead of male. As in Experiment 1, we again found a significant effect of order of presentation; later order elicited shorter looking times (1st position in order p < 0.001, 2nd order in presentation p = 0.031, 3rd order in presentation n.s.).
3.2 Results
The main effects of stimulus and sex, and their interactions, were all non-significant, indicating that none of the stimuli presented in Experiment 2 altered the looking behavior of male or female subjects. Altogether, we did not find any evidence that male rhesus macaques responded differentially to female hindquarters on a red background, suggesting that they do not exhibit an extraneous color effect, at least concerning female hindquarters (Table 1).
4.0 General Discussion
We found that female macaques looked longer at a picture of a male scrotum on a red background compared to all other conditions. This effect was not solely due to the red background, as this did not affect looking times for seashell photographs, nor was it specific to male scrota, as the scrotum on a blue background did not generate statistically significant longer looking-times. In addition, the longer looking-times for the scrotum on a red background where specific to females, and were not found in males. The greater looking-time by females for the male scrotum on a red background suggests that females saw this stimulus as more interesting compared to other stimuli (Waitt et al., 2003; Higham et al., 2011), and the absence of an effect for looking duration in males suggests they did not find this stimulus more interesting.
To our knowledge, this is the first demonstration of an extraneous color effect in non-human primates. Extraneous red increased females’ interest in viewing a male scrotum while extraneous blue did not. Although we did not explore a full range of colors in this set of experiments, we can still conclude that some colors, such as red, increase interest in images in rhesus macaques, while other colors, like blue, do not. It is notable that red, the color of sexual signaling in this species, was the color that elicited the extraneous color effect, and that the effect was only found for opposite sex stimuli (females looking at a male scrotum). In Experiment 2, female macaques did not look longer at female hindquarters on a red background. As such, our data are consistent with the idea that extraneous red mimics and extends the sexual signal of this species and suggests that female macaque preferences for red in a mating context may stem from a generalized sensory bias (Arak & Enquiest, 1993; Dawkins & Guildford, 1996; Endler & Basolo, 1998). The finding is in accord with human observations that a red frame increases the attractiveness of black and white portraits of members of the opposite sex for women viewing men (Elliot et al., 2010; Roberts et al., 2010), and raises the possibility that the effects of extraneous colors in a mating context, in this case red, in human and non-human females may have similar biological roots.
Our main finding - greater looking time by females looking at a male scrotum on a red background – strongly suggests that female macaques attend to male anogenital sex skins as a sexual signal. This was not directly tested, as we did not show females a red male scrotum, but if our result is correctly interpreted as a sensory bias, our results are at least consistent with a female preference for reddened male scrota. Previous experimental work had shown that female macaques respond to male faces with more red color (Waitt et al., 2003), but it was previously unknown if reddened male anogenital sex skin also elicited increased interest from female rhesus macaques. In one suggestive experimental study, Watson & colleagues (2012) found that female rhesus macaques have a greater preference for photographs of the face or genitals of conspecifics, including male perineal areas, compared to socially neutral stimuli such as a colored square. Using post hoc tests, this same study did not find that redness in images influenced preference for that image. In contrast, the present experiment does show that red in conjunction with male genitalia has increased salience to female macaques, suggesting that females do attend to color cues in this region of male anatomy. Our data also suggest a second novel insight into rhesus macaque sexual signaling: that female viewers have a generalized template for the male sexual signal as our result was obtained even though the potential mate was represented as a black and white photograph and the relevant color – red – was extraneous to the potential mate.
Future investigation can illuminate if and how females make use of color signals in males. One possibility is that females use body color information in mate selection. Recent research shows that rhesus macaque females are indeed attracted to males with darker red faces, as measured by the number of solicitations females direct towards males (Dubuc et al., 2014), even though current behavioral evidence does not clearly indicate what information on male quality might be potentially related to body color (Higham et al., 2013). Another possibility is that females attend to red color signals in males in order to be vigilant for dominance and aggression. Research in primate species that are subject to more direct contest competition has shown that brightness of color cues is related to dominance rank (e.g. mandrills: Setchell & Dixson, 2001; Setchell, 2005; drills, Mandrillus leucophaeus: Marty et al., 2009; gelada, Theropithecus gelada: Bergman et al., 2009) and in such species color may be more related to intra-sexual selection and male-male competition than to inter-sexual selection and mate choice. One experimental study suggests that this may also apply to rhesus macaques; working with the Cayo macaque population, researchers found that macaques were more likely to steal food from a researcher wearing green or blue rather than red shirt (Khan et al., 2011). The authors suggested that macaques act submissively, and so did not steal, from individuals exhibiting a red dominant signal. However, data from rhesus macaques suggests that dominance does not correlate with red coloration in this species (Higham et al., 2013, Dubuc et al. 2014). This should be expected; male rhesus macaques do not achieve dominant social positions based on male-male contests and physical aggression (Manson, 1995) and so signals of competitive dominance status seem unlikely (Higham et al., 2013). Instead, our data are in agreement with other evidence (Waitt et al., 2006; Dubuc et al., 2014) suggesting that male red coloration in rhesus macaques has evolved through inter-sexual selection processes related to female attraction.
We did not find that male macaques looked longer at a picture of female hindquarters on a red background compared to other object/color combinations although previous experimental work found that males look longer at artificially reddened hindquarters in female macaques (Waitt et al., 2006). We can think of two possibilities to explain this result. One possibility for the lack of an extraneous color effect in male rhesus macaques in the present work is that we used a suboptimal stimulus. In using an image of female hindquarters separate from an entire female, a critical aspect of the image may have been missing. Other work has also shown that red hindquarter coloration does not reflect reproductive state, while facial color changes do (Dubuc et al., 2009, Higham et al., 2010), and that males in the field do not pay attention to the hindquarters of ovulating females more than those of non-ovulating females (Higham et al., 2011). These data could suggest that facial coloration is the more critical signal of female fertile status in rhesus macaques, and that using images of female faces would elicit an extraneous color effect. The second possibility is that males do indeed have a preference for reddened female hindquarters, but that this preference has not resulted from, or resulted in, such a “fuzzy” template for recognizing this sexual signal that it will be triggered by photographs in frames. In short, males may prefer the reddened signal, but have a less extreme sensory bias for the signal components, and therefore do not show an extraneous color effect.
That female rhesus macaques’ interest in opposite sex individuals can be influenced by extraneous color shows that the extraneous color effect in humans is not unique. As such, it may not result solely from human processes like enculturation. Instead, the red-attraction association could be (at least partially) supported by an evolved biological mechanism. However, before firm conclusions about human sensory mate choice biases are reached, further work is needed to evaluate the consistency with which such effects are observed in diverse human populations and cultures.
Supplementary Material
Acknowledgments
We would like to thank Drue Sokol, Lauren Wolfe, and Matias Piva for research assistance at Cayo Santiago in data collection. We also thank Mark Bell for research assistance in data coding at the University of Rochester. We thank two anonymous reviewers for their comments on previous versions of this manuscript. We thank the CPRC and the University of Puerto Rico for permission to work at Cayo Santiago. This work was supported by the Sloan Foundation, by NIDA, and by two Reach fellowships provided by the University of Rochester to undergraduate assistants. The population of Cayo Santiago is currently supported by the National Center for Research Resources (NCRR grant number: 8 P40 OD012217) and the Office of Research Infrastructure Programs (ORIP) of the National Institute of Health and the Medical Science Campus of the University of Puerto Rico.
Footnotes
The content of the publication is solely the responsibility of the authors and does not necessarily represent the official views of NCRR or ORIP.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
6.0 References
- Amundsen T, Forsgren E. Male mate choice selects for female coloration in a fish. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:13155–13160. doi: 10.1073/pnas.211439298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arak A, Enquiest M. Hidden preferences and the evolutionary of signals. Philosophical Transactions of the Royal Society Series B. 1993;340:270–213. [Google Scholar]
- Baulu J. Seasonal sex skin coloration and hormonal fluctuations in free-ranging and captive monkeys. Hormones and Behaviour. 1976;7:481–494. doi: 10.1016/0018-506x(76)90019-2. [DOI] [PubMed] [Google Scholar]
- Bergman TJ, Ho L, Beehner JC. Chest color and social status in male geladas (Theropithecus gelada) International Journal of Primatology. 2009;30:791–806. [Google Scholar]
- Burley N. Comparison of the band-color preferences of two species of estrildid finches. Animal Behaviour. 1986;34:1732–1741. [Google Scholar]
- Burley BYN, Krantzberg G, Radman P. Influence of colour-banding on the conspecific preference of zebra finches. Animal Behaviour. 1982;30:444–455. [Google Scholar]
- Burley NT, Symanski R. “A taste for the beautiful”: Latent aesthetic mate preferences for white crests in two species of Australian grassfinches. The American Naturalist. 1998;152:792–802. doi: 10.1086/286209. [DOI] [PubMed] [Google Scholar]
- Cheries EW, Newman GE, Santos LR, Scholl BJ. Units of visual individuation in rhesus macaques: objects or unbound features? Perception. 2006;35:1057–1071. doi: 10.1068/p5551. [DOI] [PubMed] [Google Scholar]
- Cinque S. Squared 5 MPEG Stremclip [Internet] 2008 Available from: http://www.squared5.com/svideo/mpeg-streamclip-win.html.
- Darwin C. The expression of the emotions in man and animals. Oxford University Press; 1872. 1998. [Google Scholar]
- Dawkins MS, Guilford T. Sensory bias and the adaptiveness of female choice. The American Naturalist. 1996;148:937–942. [Google Scholar]
- Dubuc C, Brent LJN, Accamando AK, Gerald MS, MacLarnon A, Semple S, et al. Sexual skin color contains information about the timing of the fertile phase in free-ranging Macaca mulatta. International Journal of Primatology. 2009;30:777–789. [Google Scholar]
- Dubuc C, Allen WL, Maestripieri D, Higham JP. Is male rhesus macaque red color ornamentation attractive to females? Behavioral Ecology and Sociobiology. 2014 doi: 10.1007/s00265-014-1732-9. [Online] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliot AJ, Kayser DN, Greitemeyer T, Lichtenfeld S, Gramzow RH, Maier MA, et al. Red, rank, and romance in women viewing men. Journal of Experimental Psychology, General. 2010;139:399–417. doi: 10.1037/a0019689. [DOI] [PubMed] [Google Scholar]
- Elliot AJ, Maier MA. Color-in-context theory. Advances in Experimental Social Psychology. 2012;45:61–125. [Google Scholar]
- Elliot AJ, Niesta D. Romantic red: Red enhances men’s attraction to women. Journal of Personality and Social Psychology. 2008;95:1150–1164. doi: 10.1037/0022-3514.95.5.1150. [DOI] [PubMed] [Google Scholar]
- Endler JA, Basolo A. Sensory ecology, receiver biases and sexual selection. Trends in Ecology and Evolution. 1998;13:415–420. doi: 10.1016/s0169-5347(98)01471-2. [DOI] [PubMed] [Google Scholar]
- Govardovskii VI, Fuhrqist N, Reuter T, Kuzman DG, Donner K. In search of a visual pigment template. Visual Neuroscience. 2000;4:509–528. doi: 10.1017/s0952523800174036. [DOI] [PubMed] [Google Scholar]
- Gueguen N. Color and women attractiveness: When red clothed women are perceived to have more intense sexual intent. Journal of Social Psychology. 2012;152:261–265. doi: 10.1080/00224545.2011.605398. [DOI] [PubMed] [Google Scholar]
- Hárosi FI. Cynomolgus and rhesus monkey visual pigments. Application of Fourier transform smoothing and statistical techniques to the determination of spectral parameters. The Journal of General Physiology. 1987;89:717–743. doi: 10.1085/jgp.89.5.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higham JP, Brent LJ, Dubuc C, Accamando AK, Engelhardt A, Gerald MS, et al. Colour signal information content and the eye of the beholder: A case study in the rhesus macaque. Behavioral Ecology. 2010;21:739–746. doi: 10.1093/beheco/arq047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higham JP, Hughes KD, Brent LJN, Dubuc C, Engelhardt A, Heistermann M, et al. Familiarity affects the assessment of female facial signals of fertility by free-ranging male rhesus macaques. Proceedings of the Royal Society B: Biological Sciences. 2011;278:3452–3458. doi: 10.1098/rspb.2011.0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higham JP, Pfefferle D, Heisterman M, Maestripieri D, Stevens M. Signaling in multiple modalities in male rhesus macaques: Sex skin coloration and barks in relation to androgen levels, social status, and mating behavior. Behavioral Ecology and Sociobiology. 2013 doi: 10.1007/s00265-013-1521-x. [Online] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt S, Cuthill I, Swaddle J, Bennett A. Ultraviolet vision and band-colour preferences in female zebra finches, Taeniopygia guttata. Animal Behaviour. 1997;54:1383–1392. doi: 10.1006/anbe.1997.0540. [DOI] [PubMed] [Google Scholar]
- Hutchings J. Color in folklore and tradition – The principles. Color Research and Application. 2004;29:57–66. [Google Scholar]
- Johnsen A, Fiske P, Amundsen T, Lifjeld J, Rohde P. Color bands, mate choice and paternity in the bluethroat. Animal Behaviour. 2000;59:111–119. doi: 10.1006/anbe.1999.1274. [DOI] [PubMed] [Google Scholar]
- Johnsen A, Lifjeld J, Rohde P. Colored leg bands affect male mate-guarding behaviour in the bluethroat. Animal Behaviour. 1997;54:121–130. doi: 10.1006/anbe.1996.0437. [DOI] [PubMed] [Google Scholar]
- Johnson K, Dalton R, Burley N. Preferences of female American goldfinches (Carduelis tristis) for natural and artificial male traits. Behavioural Ecology. 1993;4:138–43. [Google Scholar]
- Kelber A, Vorobyev M, Osorio D. Animal colour vision - behavioural tests and physiological concepts. Biological Reviews. 2003;78:81–118. doi: 10.1017/s1464793102005985. [DOI] [PubMed] [Google Scholar]
- Khan SA, Levine WJ, Dobson SD, Kralik JD. Red signals dominance in male rhesus macaques. Psychological Science. 2011;22:1001–1003. doi: 10.1177/0956797611415543. [DOI] [PubMed] [Google Scholar]
- Manson JH. Do female rhesus macaques choose novel males? American Journal of Primatology. 1995;37:285–296. doi: 10.1002/ajp.1350370403. [DOI] [PubMed] [Google Scholar]
- Marty JS, Higham JP, Gadsby EL, Ross C. Dominance, coloration, and social and sexual behavior in male drills Mandrillus leucophaeus. International Journal of Primatology. 2009;30:807–823. [Google Scholar]
- Pan W, Connett JE. Selecting the working correlation structure in generalized estimating equations with application to the lung health study. Statistica Sinica. 2002;12:475–490. [Google Scholar]
- Pokorny JJ, de Waal FB. Monkeys recognize the faces of group mates in photographs. Proceedings of the National Academy of Sciences. 2009;106:21539–21543. doi: 10.1073/pnas.0912174106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlins RG, Kessler MJ. The Cayo Santiago macaques: History, behaviour, and biology. State University of New York Press; 1986. [Google Scholar]
- Ratcliffe L, Boag P. Effects of color bands on male competition and sexual attractiveness in zebra finches (Poephila quttata) Canadian Journal of Zoology. 1987;65:333–338. [Google Scholar]
- Re DE, Whitehead RD, Xiao D, Perrett DI. Oxygenated blood colour change thresholds for perceived facial redness, health, and attractiveness. PloS One. 2011;6:e17859. doi: 10.1371/journal.pone.0017859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephen ID, Oldham FH, Perrett DI, Barton RA. Redness enhances perceived aggression, dominance and attractiveness in men’s faces. Evolutionary Psychology. 2012;10:562–72. [PubMed] [Google Scholar]
- Roberts SC, Owens RC, Havilcek J. Distinguishing between perceiver and wearer effects in clothing color-associated attributions. Evolutionary Psychology. 2010;8:350–364. [PubMed] [Google Scholar]
- Schell A, Rieck K, Schell K, Hammerschmidt K, Fischer J. Adult but not juvenile Barbary macaques spontaneously recognize group members from pictures. Animal Cognition. 2011;14:503–509. doi: 10.1007/s10071-011-0383-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz S, Singer M. Romantic red revisited: Red enhances men’s attraction to young, but not menopausal women. Journal of Experimental Social Psychology. 2012;49:161–164. [Google Scholar]
- Setchell JM. Do female mandrills prefer brightly colored males? International Journal of Primatology. 2005;26:715–35. [Google Scholar]
- Setchell JM, Dixson AF. Changes in the secondary sexual adornments of male mandrills (Mandrillus sphinx) are associated with gain and loss of alpha status. Hormones and Behavior. 2001;39:177–184. doi: 10.1006/hbeh.2000.1628. [DOI] [PubMed] [Google Scholar]
- Sliwa J, Duhamel JR, Pascalis O, Wirth S. Spontaneous voice face identity matching by rhesus monkeys for familiar conspecifics and humans. Proceedings of the National Academy of Sciences. 2011;108:1735–1740. doi: 10.1073/pnas.1008169108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephen ID, Oldham FH, Perrett DI, Barton RA. Redness enhances perceived aggression, dominance and attractiveness in men’s faces. Evolutionary Psychology. 2012;10:562–72. [PubMed] [Google Scholar]
- Stevens M, Párraga CA, Cuthill IC, Partridge JC, Troscianko T. Using digital photography to study animal coloration. Biological Journal of the Linnean Society. 2007;90:211–237. [Google Scholar]
- Stevens M, Stoddard MC, Higham JP. Studying primate color: towards visual system-dependent methods. International Journal of Primatology. 2009;30:893–917. [Google Scholar]
- Vorobyev M, Osorio D. Receptor noise as a determinant of color thresholds. Proceedings of the Royal Society B: Biological Sciences. 1998;265:351–358. doi: 10.1098/rspb.1998.0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waitt C, Gerald MS, Little AC. Selective attention toward female secondary sexual colour in male rhesus macaques. American Journal of Primatology. 2006;744:738–744. doi: 10.1002/ajp.20264. [DOI] [PubMed] [Google Scholar]
- Waitt C, Little AC, Wolfensohn S, Honess P, Brown AP, Buchanan-Smith HM, et al. Evidence from rhesus macaques suggests that male coloration plays a role in female primate mate choice. Proceedings of the Royal Society B: Biological Sciences. 2003;270(Suppl):S144–S146. doi: 10.1098/rsbl.2003.0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson KK, Ghodasra JH, Furlong MA, Platt ML. Visual preferences for sex and status in female rhesus macaques. Animal Cognition. 2012;15:401–407. doi: 10.1007/s10071-011-0467-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuk M, Thornhill R, Ligon JD, Johnson K, Austad S, Ligon SH, et al. The role of male ornaments and courtship behavior in female mate choice of red jungle fowl. The American Naturalist. 1990;136:459–473. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.