Abstract
Following reports of elevated antiviral antibodies in MS patient sera and viral DNA detection in MS plaques nearly two decades ago, the neurovirology community has actively explored how herpesviruses such as HHV-6 might be involved in MS disease pathogenesis. Though findings across the field are nonuniform, an emerging consensus of viral correlates with disease course and evidence of HHV-6-specific immune responses in the CNS provide compelling evidence for a role, direct or indirect, of this virus in MS. Ultimately, the only way to demonstrate the involvement, or lack thereof, of HHV-6 or other herpesviruses in this disease is through a controlled clinical trial of an efficacious antiviral drug.
Introduction: Pathogens in Multiple Sclerosis
Multiple Sclerosis (MS), a neurodegenerative, inflammatory demyelinating disease of the central nervous system (CNS), is idiopathic, despite its description over 150 years ago [1]. For the past two decades, following reports of elevated anti-human herpesvirus 6 (HHV-6) antibodies in MS patient sera [2, 3] and HHV-6 viral DNA detection in MS plaques [4], the neurovirology community has actively explored if and how this virus is involved in MS disease pathogenesis.
The discussion of any pathogen implicated in MS should be contextualized by the long history of infectious agents in this disease. Proponents of an infectious etiology of MS can be traced back to the mid 19th century, when descriptions of the disease were beginning to coalesce [1]. The idea of an infectious etiology resurged in the 1930s with the observation that, by histopathology, the perivenous demyelination of MS and post-infectious encephalomyelitis were indistinguishable. From this time forward, there were many reports of agents detected in MS patient spinal fluid including spirochetes and Toxoplasma gondii [1]. There were also reports of agents recovered from laboratory animals following immunization with tissue from MS patients. These agents have been largely dismissed due to confirmed contamination or irreproducibility, but the list once included rabies, a Scrapie agent, measles and chimpanzee cytomegalovirus, to name a few. Interestingly, viruses have dominated the list of suspected agents; there have been few bacteria or parasites by comparison [5]. However, despite the subsequent isolation of the specific viruses responsible for the demyelinating diseases subacute sclerosing panencephalitis (SSPE: measles virus) and progressive multifocal leukoencephalopathy (PML: JC virus), the focus of the MS field has largely transitioned away from a single, unidentified agent (though some hold this view [6]) towards ubiquitous agents, particularly herpesviruses [5]. While there are numerous reports for other herpesviruses in MS, notably the sero-epidemiological data for human herpesvirus 4 (Epstein-Barr virus (EBV)) reviewed in [7, 8], this current review will focus solely on HHV-6.
Traces of HHV-6 in the CNS: virus detection and virus-specific immune responses
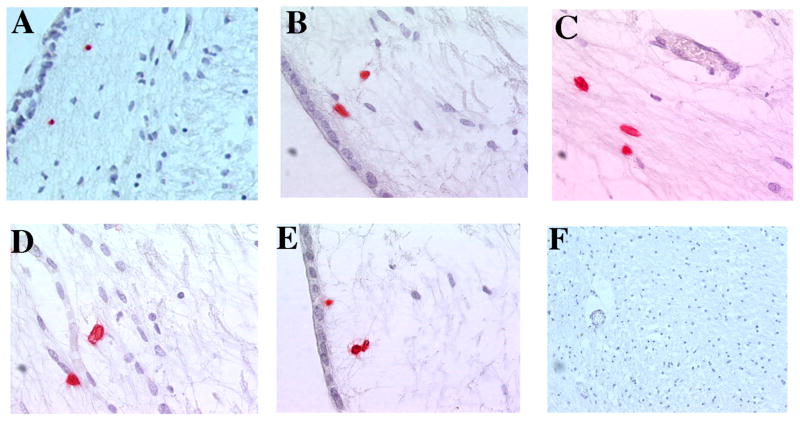
Early studies reporting HHV-6 viral DNA in the brains [9, 10] and CSF [11] of MS patients and controls supported that HHV-6 possessed strong neurotropism that was associated with a CNS reservoir [9]. This was supported by concomitant studies reporting higher levels of HHV-6 expression in MS brains compared to control brains [12], and greater levels of viral DNA [13, 14] and viral mRNA [12] specifically in the demyelinated plaques. An example of HHV-6 expression, as detected by immunohistochemistry (IHC), in a periventricular MS lesion is shown in Figure 1. HHV-6 positivity (red) is evident in the lesion (A–E), but notably absent in non-lesional areas and normal appearing white matter (F). The observations of viral mRNA [12] and protein expression [4] specifically in oligodendrocytes proved central to the hypothesis that HHV-6 may be a driver of MS pathogenesis. Collectively, these studies demonstrated that while HHV-6 may be a commensal of normal brain, its replication and activity is enriched in the context of MS pathology. This is highlighted in Table 1, which summarizes the pathologic, inflammatory and virologic findings of 20 lesions from a subset of MS lesions previously reported [14]. HHV-6 expression was greater in the acute relative to chronic lesions, associating viral expression with earlier stages of MS lesion formation. This appears specific for HHV-6 since IHC for three other herpesviruses were uniformly negative (Table 1).
Figure 1.
HHV-6 expression is detectable by immunohistochemistry in a periventricular MS lesion (A–E), but not in the normal appearing white matter (F). Red: HHV-6 gp116. MS lesions were obtained from a subset of patient material previously reported [14].
Table 1.
MS lesion activity and viral infection
| Lesion | Classification | Lesion pathology | Inflammation | Herpesviral expression | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Axonal damage (NFTP) |
Astrocytosis (GFAP) |
Myelin loss (LFB) |
Oligo loss (S100) |
CD4+ T cells |
CD8+ T cells |
CD20+ B cells |
CD68+ MF |
HSV-1 | CMV | EBV | HHV-6 | ||
| 1 | Acute | Intact | Reactive | Minor | Normal | ++ | ++ | − | ++ | − | − | − | + |
| 2 | Acute | Intact | Reactive | Major | Normal | + | ++ | − | +++ | − | − | − | +++ |
| 3 | Chronic active | Major | Reactive | Major | Minor | ++ | + | − | + | − | − | − | + |
| 4 | Chronic | Major | Normal | Major | Major | − | + | − | + | − | − | − | + |
| 5 | Acute | Minor | Reactive | Minor | Normal | ++ | ++ | − | +++ | − | − | − | ++ |
| 6 | Acute | Intact | Reactive | Minor | Normal | ++ | ++ | − | ++ | − | − | − | ++ |
| 7 | Chronic active | Intact | Reactive | Major | Normal | − | + | − | +++ | − | − | − | + |
| 8 | Acute | Intact | Reactive | Minor | Normal | − | + | − | +++ | − | − | − | ++ |
| 9 | Acute | Minor | Reactive | Minor | Normal | + | + | − | ++ | − | − | − | + |
| 10 | Chronic | Major | Normal | Major | Major | − | − | − | − | − | − | − | − |
| 11 | Acute | Intact | Reactive | Minor | Normal | + | + | − | ++ | − | − | − | ++ |
| 12 | Acute | Minor | Reactive | Minor | Normal | + | + | − | ++ | − | − | − | + |
| 13 | Chronic active | Major | Normal | Major | Minor | + | + | − | + | − | − | − | + |
| 14 | Chronic active | Intact | Reactive | Major | Normal | + | + | + | ++ | − | − | − | + |
| 15 | Chronic | Minor | Reactive | Major | Minor | + | + | − | ++ | − | − | − | − |
| 16 | Acute | Minor | Reactive | Major | Normal | − | + | + | +++ | − | − | − | + |
| 17 | Chronic active | Minor | Reactive | Major | Normal | + | + | − | ++ | − | − | − | − |
| 18 | Chronic active | Minor | Reactive | Major | Minor | + | ++ | + | ++ | − | − | − | + |
| 19 | Chronic active | Major | Reactive | Major | Major | ++ | ++ | ++ | + | − | − | − | − |
| 20 | Shadow | Minor | Normal | Minor | Normal | − | − | − | + | − | − | − | ++ |
Compelling evidence that HHV-6 may be a key component in MS pathology stems from the observation that in approximately 20% of patients, a subset of oligoclonal bands (OCB) demonstrates HHV-6 specificity [15, 16]. A 2014 publication by Pietläinen-Nicklén and colleagues analyzed patients with demyelinating disease (mostly MS) and HHV-6-reactive CSF OCB, and determined that patients with HHV-6 OCB appear to form a separate group, which was significantly younger, with greater IgG OCB relative to patients without HHV-6 OCB [17]. OCB, representing intrathecally-produced immunoglobulins, are a hallmark of MS but are not specific for the disease. In fact, OCB are common among CNS disorders with an infectious component, and when the inciting agent is known, OCB are often specific to that agent (for example measles virus in SSPE). For this reason, the identification of HHV-6-specific bands in a subset of MS patients has strengthened the idea that HHV-6 from within the CNS is involved in the disease (Figure 2) [18]. Furthermore, the hypothesis of an antigen-driven immune response in MS is supported by data of clonally expanded B cells in MS brains, similar to SSPE brains [19]. A recent study observed interesting correlates between the presence of herpesvirus-specific OCB (HHV-6 and EBV) and several clinical parameters [20]. Virtanen and colleagues reported that herpesvirus-specific CSF OCB inversely correlated with the detection of CSF viral DNA, and that MS patients with CSF viral DNA had significantly more contrast enhancing lesions compared to those without detectable CSF viral DNA. These data suggest that anti-viral antibodies may be necessary for the maintenance of viral latency, as the reduction in such antibodies corresponded to both detectable CSF virus and MRI activity indicative of an active inflammatory process [20].
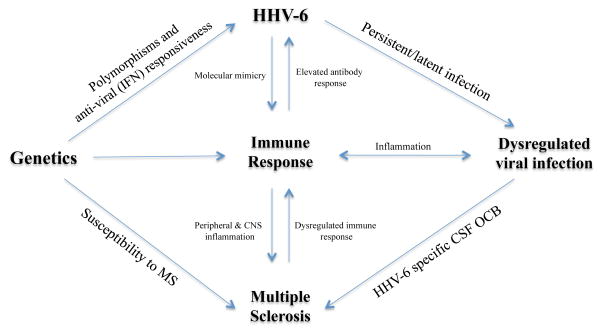
Figure 2.
A complex interplay between genetics, immune response and viral infections (such as HHV-6) influences the development of MS. Genetics have been implicated in the susceptibility to the disease, as well as in the response to antiviral therapy. Under certain inflammatory conditions, potentially in genetically susceptible individuals, the latency and persistence of herpesviruses may result in a dysregulated infection. Anti-viral immune responses in the periphery and CNS of MS patients suggest that a dysregulated viral infection is a key component of the disease.
Adapted from Owens, Bennet. 2012 Mult Scler.
While OCB reflect CNS B cell reactivity toward HHV-6, less is known about CNS T cell reactivity toward HHV-6. A recent study by Wuest and colleagues reported significant enrichment of HHV-6 specific CD4 T cell responses in CSF compared to peripheral blood of MS patients (progressive and relapsing-remitting subtypes), suggesting that HHV-6-expanded T cells in the CNS may contribute to disease activity [21].
Traces of HHV-6 in the periphery: virus detection and virus-specific immune responses
It is not solely studies of the CNS that have established an association between HHV-6 and MS; early observations of HHV-6 in the periphery of MS patients linked the detection of, or an immune response to, the virus with clinically active disease [22, 23]. Recent studies with MS cohorts in different geographical areas have largely confirmed these previously reported observations. Two recent studies found greater levels of HHV-6 IgM and IgG in MS cohorts compared to controls, one in an Iranian population [24] and one in a Tunisian population [25]. A separate study of another Iranian MS cohort detected a higher frequency of viral DNA in the serum of patients, along with a relative increase in viral load during disease exacerbation [26]. Such observations of increased antibody responses and elevated viral loads in the serum, especially during disease exacerbation, confirm earlier observations of HHV-6 in MS and appear to be valid across geographically varied populations.
Many serologic and DNA studies published in the past several years have stratified MS patients into the clinical phases of relapse or remission, and provide mounting evidence for a role—direct or indirect—of HHV-6 in the switch from remission to relapse. A 2012 study of a Tasmanian cohort found HHV-6 IgG titer to be a significant predictor of relapse risk [27]. This was echoed in a 2014 study of a Spanish MS cohort, which reported that a decrease in HHV-6 antibody titers correlated with fewer relapses and less disease progression [28]. Interestingly, the authors noted that IgG titers reached their highest value two weeks, and IgM titers one month, before relapse [28]. A 2011 study of a Latvian MS cohort reported HHV-6 DNA in the plasma of a majority of RRMS and SPMS patients during relapse, which was confirmed by enhancing MRI lesions, and correlated with higher serum concentrations of the inflammatory cytokines IL-12 and TNF-alpha relative to periods of remission [29]. These data agree with earlier studies of serum HHV-6 detection during relapse and add the observation of cytokine correlates, complementing a recent study suggesting that TNF-alpha may be predictive of HHV-6 reactivation [30]. However, if HHV-6 is involved in relapses, the nature of its involvement remains unknown. Does the virus have an active role in initiating or potentiating the inflammation associated with relapse, or is it a marker of disease activity, activated from latency as a result of the surrounding inflammation?
Other serological studies have focused on the immune response to a specific portion of the virus, an approach that may provide functional insights into the role of HHV-6 in disease. A 2013 study examined antibodies to a latency-promoting protein, U94/REP, and found elevated IgG levels in Tunisian MS patients compared to controls; for eight patients with samples collected during relapsing and remitting phases, significantly higher titers were detected during the relapsing phases [31]. The finding of an elevated U94 IgG response in MS patients versus controls agrees with previous findings in an Italian cohort [32], and adds the observation of higher titers during relapse versus remission. Elevated antibodies against a latency-promoting protein may be one mechanism leading to the increased viral levels observed across many MS cohorts. Another approach to investigating the immune response against a specific viral protein is identification of the antigenic target of anti-viral antibodies. In a recently published study, Alenda and colleagues purified IgG from the CSF of RRMS and PPMS patients, then incubated the IgG with HHV-6 and characterized peptides of the bound antigens. They reported that the peptides matched the major capsid protein of HHV-6A, a structural protein needed to assemble the viral capsid [33]. This approach provides a framework for exploring the antigenic targets of HHV-6 antibodies, and whether there are differences between the periphery and CSF, MS patients and controls or MS patients in different stages of the disease.
HHV-6 status post-interferon treatment: examining the influence of polymorphisms
A long-standing argument in support of a viral etiology of MS is the effectiveness of interferon beta, a potent antiviral [34]. Several studies published in the past few years have formally examined the relationship between interferon treatment and HHV-6 status in MS patients. In a 2011 publication, Garcia-Montojo and colleagues observed that patients with HHV-6 viral DNA in whole blood and serum exhibited a higher risk of MS relapse and comprised a lower proportion of IFN-beta-1b responders [35]. These data agree with the many studies that detect an increase in serum viral DNA during relapse compared to remission, and add the observation of an inverse correlation with IFN-beta responsiveness.
Several studies have adopted a gene-environment interactions approach to the study of HHV-6 and interferon therapy, correlating polymorphisms with HHV-6 status and therapy responsiveness. For instance, Vandenbroeck and colleagues reported elevated odds ratios for specific polymorphisms of the transcription factor IRF5 (interferon regulatory factor 5) and HHV-6 infection and interferon responsiveness [36]. In a separate study, Garcia-Montojo and colleagues studied polymorphisms in MHC2TA, which encodes a transcriptional coactivator of MHC class II genes, and reported significant differences in genotype frequency between MS patients with and without detectable serum HHV-6 [37]. In a follow up study, they observed that a significantly higher proportion of MS patients with higher MHC2TA mRNA levels and without detectable serum HHV-6 were clinical responders to interferon beta therapies compared to patients with decreased MHC2TA mRNA levels and detectable serum HHV-6. The authors concluded that MHC2TA mRNA levels might be decreased by the active replication of HHV-6 [38]. Interestingly, human cytomegalovirus (HCMV), a beta herpesvirus like HHV-6, has been reported to decrease MHC2TA mRNA levels, resulting in the suppression of MHC class II expression [39]. While this study found no correlation between polymorphisms and the development of interferon-neutralizing antibodies [38], future studies should examine polymorphisms that correlate with interferon-neutralizing antibodies and HHV-6 viral DNA.
Potential mechanism of HHV-6 involvement in MS: molecular mimicry with myelin
Associations of viruses with human demyelinating disease and virally-induced animal models of demyelination provide compelling, though indirect, evidence of a viral etiology of MS [19]. Additionally, studies of mechanisms of demyelination and oligodendrocyte injury have reinforced the idea that viruses can lead to MS or MS-like pathology [5]. One such mechanism is molecular mimicry, whereby sequence homology between a pathogen and a self-molecule leads to the generation of an immune response that is cross-reactive between both the pathogen and self. There is a stretch of identical amino acids between HHV-6 U24 (an integral membrane protein [40]) and human myelin basic protein (MBP), which has bolstered the idea that molecular mimicry may be at play in the relationship between HHV-6 and MS. In 2003, Tejada-Simon and colleagues reported that MS patients, compared to healthy controls, exhibited a much higher frequency of T cells that were reactive to both (HHV-6 U24)1–13 and (MBP)93–105 [41]. These observations were recently confirmed in a cohort of Chinese MS patients, in a 2012 study by Cheng and colleagues [42].
While positive findings continue to provide an impetus to study the role of HHV-6 in MS, much about the mechanisms remain unknown. Are elevated levels of HHV-6 in MS a hallmark of an aberrant immune response or a reflection of the failure of the immune response to contain infection (Figure 2)? As inflammation can induce reactivation in T cells trafficking through the CNS [19], to what extent is the virus causal or simply a reactivated byproduct of vast peripheral and CNS inflammation?
Controversy: findings and suggestions
Despite a publication bias toward positive results, not all published reports of HHV-6 in MS are positive; several recent studies have found a non-significant or low incidence of HHV-6 in their respective MS populations. A 2014 study of South African MS patients and controls reported no difference between HHV-6 viral DNA detection in whole blood between MS patients and controls [43]. Another study of Swedish patients reported a low incidence of HHV-6 in the plasma and CSF of possible MS patients compared to controls. These investigators also detected a low incidence of HHV-6 in the serum samples of IFN-beta treated MS patients, without any difference between patients with or without neutralizing antibodies [44]. Another study of a Tasmanian MS cohort prospectively analyzed levels of HHV-6 IgM as a marker of viral reactivation; the authors detected HHV-6 IgM in only 1/198 patients, and concluded that HHV-6 reactivation does not drive relapse or disability in this MS population [45].
Many factors can account for discordant results, including differences in patient and control populations, technical differences and the timing of sample acquisition, to name a few. In a multitude of positive studies, HHV-6 appears in only a subset of MS patients and yet, the findings are often interpreted broadly. Investigators of both positive and negative studies should carefully parse out characteristics of the patient and control populations in question, in an effort to foster hypothesis generation and present more nuanced conclusions than HHV-6 is or is not involved in MS.
Future directions
Ultimately, a controlled clinical trial of an efficacious [CNS penetrable] anti-HHV-6 drug in MS may be the only way to ascertain the involvement of this agent in MS (it is important to consider that a positive outcome demonstrating robust clinical efficacy would be persuasive, while a negative outcome would only add controversy to the field). However, additional basic research on the biology of HHV-6—especially differences between the two viruses that comprise this group [46]—is required for the discovery or development of such an anti-viral. For example, several studies have reported more HHV-6A relative to HHV-6B in MS patient material [38, 47, 48]. Understanding the properties of each virus and knowing to what extent one or both are involved in MS is crucial to furthering this field, and all publications should diligently distinguish HHV-6A from HHV-6B viral DNA sequences. Serological differentiation between these two viruses is an active area of research [49] and once validated, will provide great insight into the relative antibody reactivity to each virus, and importantly, the time of acquisition of HHV-6A. The acquisition time of one or both viruses may be a factor in MS development, as has been proposed for EBV [50].
Sequencing additional HHV-6 genomes may also lead to a more thorough understanding of each virus. A 2013 study examined the oral shedding of EBV from pediatric MS patients and controls, and reported that changes in the predominant EBV variants were higher in MS patients, suggesting a lack of immunologic control of this virus [51]. Perhaps there are different frequencies of HHV-6 variants between MS patients and controls? Or perhaps there are HHV-6 variants that differ between sites, for example the periphery and CNS? Studies of JC virus have identified sequences that lend to its classification as non-virulent (found in non-PML patients) or virulent (found in the brain and CSF of PML patients) [52]. As HHV-6 is at once ubiquitous and implicated in a non-ubiquitous pathology, perhaps there are genetic variants that are analogously associated with MS.
In conclusion, there is sufficient and compelling evidence that HHV-6 may be involved, albeit to an unknown extent, in the disease pathogenesis of a subset of MS cases. To elucidate the possible mechanisms of HHV-6A and/or HHV-6B involvement in this disease, or the involvement of other herpesviruses, future studies are encouraged to ask focused questions, using material from well-characterized patient populations and well-matched control populations.
Highlights.
Additional observations of HHV6-specific B and T cell reactivity in MS CSF
Evidence of correlations between HHV6 status, polymorphisms and response to therapy
Controlled trial of anti-HHV6 therapeutic needed to demonstrate MS involvement
Acknowledgments
Funding was provided by the intramural program of the National Institutes of Health, National Institute of Neurologic Disease and Stroke (NINDS). We thank Bridgette Jeanne Billioux and Breanna Caruso for critical reading of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading recent references are noted as
• of special interest
•• of outstanding interest
- 1.Kesselring J. History of Multiple Sclerosis. In: Raine CS, McFarland HF, Hohlfeld R, editors. Multiple Sclerosis: A comprehensive text. Saunders/Elsevier; 2008. p. 474. [Google Scholar]
- 2.Sola P, et al. Human herpesvirus 6 and multiple sclerosis: survey of anti-HHV-6 antibodies by immunofluorescence analysis and of viral sequences by polymerase chain reaction. J Neurol Neurosurg Psychiatry. 1993;56(8):917–9. doi: 10.1136/jnnp.56.8.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilborn F, et al. A potential role for human herpesvirus type 6 in nervous system disease. J Neuroimmunol. 1994;49(1–2):213–4. doi: 10.1016/0165-5728(94)90198-8. [DOI] [PubMed] [Google Scholar]
- 4.Challoner PB, et al. Plaque-associated expression of human herpesvirus 6 in multiple sclerosis. Proc Natl Acad Sci U S A. 1995;92(16):7440–4. doi: 10.1073/pnas.92.16.7440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••5.Venkatesan A, Johnson RT. Infections and Multiple Sclerosis. In: Goodin DS, editor. Handbook of Clinical Neurology. Elsevier B.V; 2014. This chapter provides an exhaustive overview of infections in multiple sclerosis, highlighting animal models of viral demyelination, mechanisms of virally-induced demyelination and the ways by which infectious agents, of both historic and current interest, were implicated in multiple sclerosis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lipton HL, et al. A specific viral cause of multiple sclerosis: one virus, one disease. Ann Neurol. 2007;61(6):514–23. doi: 10.1002/ana.21116. [DOI] [PubMed] [Google Scholar]
- 7.Lucas RM, et al. Epstein-Barr virus and multiple sclerosis. J Neurol Neurosurg Psychiatry. 2011;82(10):1142–8. doi: 10.1136/jnnp-2011-300174. [DOI] [PubMed] [Google Scholar]
- 8.Pakpoor J, Giovannoni G, Ramagopalan SV. Epstein-Barr virus and multiple sclerosis: association or causation? Expert Rev Neurother. 2013;13(3):287–97. doi: 10.1586/ern.13.6. [DOI] [PubMed] [Google Scholar]
- 9.Merelli E, et al. Human herpes virus 6 and human herpes virus 8 DNA sequences in brains of multiple sclerosis patients, normal adults and children. J Neurol. 1997;244(7):450–4. doi: 10.1007/s004150050121. [DOI] [PubMed] [Google Scholar]
- 10.Gordon L, McQuaid S, Cosby SL. Detection of herpes simplex virus (types 1 and 2) and human herpesvirus 6 DNA in human brain tissue by polymerase chain reaction. Clin Diagn Virol. 1996;6(1):33–40. doi: 10.1016/0928-0197(95)00203-0. [DOI] [PubMed] [Google Scholar]
- 11.Liedtke W, et al. Human herpesvirus 6 polymerase chain reaction findings in human immunodeficiency virus associated neurological disease and multiple sclerosis. J Neurovirol. 1995;1(3–4):253–8. doi: 10.3109/13550289509114021. [DOI] [PubMed] [Google Scholar]
- 12.Opsahl ML, Kennedy PG. Early and late HHV-6 gene transcripts in multiple sclerosis lesions and normal appearing white matter. Brain. 2005;128(Pt 3):516–27. doi: 10.1093/brain/awh390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sanders VJ, et al. Detection of herpesviridae in postmortem multiple sclerosis brain tissue and controls by polymerase chain reaction. J Neurovirol. 1996;2(4):249–58. doi: 10.3109/13550289609146888. [DOI] [PubMed] [Google Scholar]
- 14.Cermelli C, et al. High frequency of human herpesvirus 6 DNA in multiple sclerosis plaques isolated by laser microdissection. J Infect Dis. 2003;187(9):1377–87. doi: 10.1086/368166. [DOI] [PubMed] [Google Scholar]
- 15.Derfuss T, Hohlfeld R, Meinl E. Intrathecal antibody (IgG) production against human herpesvirus type 6 occurs in about 20% of multiple sclerosis patients and might be linked to a polyspecific B-cell response. J Neurol. 2005;252(8):968–71. doi: 10.1007/s00415-005-0794-z. [DOI] [PubMed] [Google Scholar]
- 16.Virtanen JO, et al. Intrathecal human herpesvirus 6 antibodies in multiple sclerosis and other demyelinating diseases presenting as oligoclonal bands in cerebrospinal fluid. J Neuroimmunol. 2011;237(1–2):93–7. doi: 10.1016/j.jneuroim.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 17.Pietilainen-Nicklen J, et al. HHV-6-positivity in diseases with demyelination. J Clin Virol. 2014 doi: 10.1016/j.jcv.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 18.Gilden DH. Infectious causes of multiple sclerosis. Lancet Neurol. 2005;4(3):195–202. doi: 10.1016/S1474-4422(05)01017-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bennet JL, Yu X, Gilden DH, Burgoon MP, Owens GP. Infectious agents and multiple sclerosis. In: Raine CS, McFarland HF, Hohlfeld R, editors. Multiple Sclerosis: A comprehensive text. Saunders/Elsevier; 2008. p. 480. [Google Scholar]
- 20.Virtanen JO, et al. Oligoclonal bands in multiple sclerosis reactive against two herpesviruses and association with magnetic resonance imaging findings. Mult Scler. 2014;20(1):27–34. doi: 10.1177/1352458513490545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wuest SC, et al. A complex role of herpes viruses in the disease process of multiple sclerosis. PLoS One. 2014;9(8):e105434. doi: 10.1371/journal.pone.0105434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Soldan SS, et al. Association of human herpes virus 6 (HHV-6) with multiple sclerosis: increased IgM response to HHV-6 early antigen and detection of serum HHV-6 DNA [see comments] Nat Med. 1997;3(12):1394–7. doi: 10.1038/nm1297-1394. [DOI] [PubMed] [Google Scholar]
- 23.Berti R, et al. Increased detection of serum HHV-6 DNA sequences during multiple sclerosis (MS) exacerbations and correlation with parameters of MS disease progression. J Neurovirol. 2002;8(3):250–6. doi: 10.1080/13550280290049615-1. [DOI] [PubMed] [Google Scholar]
- 24.Khaki M, et al. Evaluation of viral antibodies in Iranian multiple sclerosis patients. Neurosciences (Riyadh) 2011;16(3):224–8. [PubMed] [Google Scholar]
- •25.Ramroodi N, et al. Monitoring of active human herpes virus 6 infection in Iranian patients with different subtypes of multiple sclerosis. J Pathog. 2013;2013:194932. doi: 10.1155/2013/194932. This comprehensive study of Iranian MS patients and controls examines IgM, IgG and viral sequences in PBMC, saliva and serum, and extends previous observations of a systemically reactivated HHV-6 infection in MS patients compared to controls. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Behzad-Behbahani A, et al. Human herpesvirus-6 viral load and antibody titer in serum samples of patients with multiple sclerosis. J Microbiol Immunol Infect. 2011;44 (4):247–51. doi: 10.1016/j.jmii.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 27.Simpson S, Jr, et al. Anti-HHV-6 IgG titer significantly predicts subsequent relapse risk in multiple sclerosis. Mult Scler. 2012;18(6):799–806. doi: 10.1177/1352458511428081. [DOI] [PubMed] [Google Scholar]
- 28.Ortega-Madueno I, et al. Anti-Human Herpesvirus 6A/B IgG Correlates with Relapses and Progression in Multiple Sclerosis. PLoS One. 2014;9(8):e104836. doi: 10.1371/journal.pone.0104836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •29.Nora-Krukle Z, et al. Human herpesvirus 6 and 7 reactivation and disease activity in multiple sclerosis. Medicina (Kaunas) 2011;47(10):527–31. This study correlates serum concentrations of IL-12 and TNF-alpha with active HHV-6 infection and relapse in RRMS and SPMS patients. [PubMed] [Google Scholar]
- 30.Uno H, et al. TNF-alpha as a useful predictor of human herpesvirus-6 reactivation and indicator of the disease process in drug-induced hypersensitivity syndrome (DIHS)/drug reaction with eosinophilia and systemic symptoms (DRESS) J Dermatol Sci. 2014;74(2):177–9. doi: 10.1016/j.jdermsci.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 31.Ben-Fredj N, et al. Prevalence of human herpesvirus U94/REP antibodies and DNA in Tunisian multiple sclerosis patients. J Neurovirol. 2013;19(1):42–7. doi: 10.1007/s13365-012-0138-6. [DOI] [PubMed] [Google Scholar]
- 32.Caselli E, et al. Detection of antibodies directed against human herpesvirus 6 U94/REP in sera of patients affected by multiple sclerosis. J Clin Microbiol. 2002;40(11):4131–7. doi: 10.1128/JCM.40.11.4131-4137.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •33.Alenda R, et al. Identification of the major HHV-6 antigen recognized by cerebrospinal fluid IgG in multiple sclerosis. Eur J Neurol. 2014;21(8):1096–101. doi: 10.1111/ene.12435. This study developed a method to purify and identify the HHV-6 antigens that are recognized by the IgG in MS patient CSF. [DOI] [PubMed] [Google Scholar]
- 34.Dhib-Jalbut S, Marks S. Interferon-beta mechanisms of action in multiple sclerosis. Neurology. 2010;74(Suppl 1):S17–24. doi: 10.1212/WNL.0b013e3181c97d99. [DOI] [PubMed] [Google Scholar]
- 35.Garcia-Montojo M, et al. Human herpesvirus 6 and effectiveness of interferon beta1b in multiple sclerosis patients. Eur J Neurol. 2011;18(8):1027–35. doi: 10.1111/j.1468-1331.2011.03410.x. [DOI] [PubMed] [Google Scholar]
- 36.Vandenbroeck K, et al. Validation of IRF5 as multiple sclerosis risk gene: putative role in interferon beta therapy and human herpes virus-6 infection. Genes Immun. 2011;12 (1):40–5. doi: 10.1038/gene.2010.46. [DOI] [PubMed] [Google Scholar]
- 37.Garcia-Montojo M, et al. Herpesvirus active replication in multiple sclerosis: a genetic control? J Neurol Sci. 2011;311(1–2):98–102. doi: 10.1016/j.jns.2011.09.001. [DOI] [PubMed] [Google Scholar]
- •38.Dominguez-Mozo MI, et al. MHC2TA mRNA levels and human herpesvirus 6 in multiple sclerosis patients treated with interferon beta along two-year follow-up. BMC Neurol. 2012;12:107. doi: 10.1186/1471-2377-12-107. This study found that the absence of HHV-6 viral DNA in serum and the increase of MHC2TA expression may serve as biomarkers predictive of response to interferon therapy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee AW, et al. Human cytomegalovirus decreases constitutive transcription of MHC class II genes in mature Langerhans cells by reducing CIITA transcript levels. Mol Immunol. 2011;48(9–10):1160–7. doi: 10.1016/j.molimm.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sullivan BM, Coscoy L. The U24 protein from human herpesvirus 6 and 7 affects endocytic recycling. J Virol. 2010;84(3):1265–75. doi: 10.1128/JVI.01775-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tejada-Simon MV, et al. Cross-reactivity with myelin basic protein and human herpesvirus-6 in multiple sclerosis. Ann Neurol. 2003;53(2):189–97. doi: 10.1002/ana.10425. [DOI] [PubMed] [Google Scholar]
- 42.Cheng W, et al. Cross-reactivity of autoreactive T cells with MBP and viral antigens in patients with MS. Front Biosci (Landmark Ed) 2012;17:1648–58. doi: 10.2741/4010. [DOI] [PubMed] [Google Scholar]
- 43.Hon GM, Erasmus RT, Matsha T. Low prevalence of human herpesvirus-6 and varicella zoster virus in blood of multiple sclerosis patients, irrespective of inflammatory status or disease progression. J Clin Neurosci. 2014;21(8):1437–40. doi: 10.1016/j.jocn.2013.10.027. [DOI] [PubMed] [Google Scholar]
- 44.Gustafsson R, et al. Incidence of human herpesvirus 6 in clinical samples from Swedish patients with demyelinating diseases. J Microbiol Immunol Infect. 2013 doi: 10.1016/j.jmii.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 45.Simpson S, Jr, et al. EBV & HHV6 reactivation is infrequent and not associated with MS clinical course. Acta Neurol Scand. 2014 doi: 10.1111/ane.12268. [DOI] [PubMed] [Google Scholar]
- •46.Ablashi D, et al. Classification of HHV-6A and HHV-6B as distinct viruses. Arch Virol. 2014;159(5):863–70. doi: 10.1007/s00705-013-1902-5. This review highlights the distinctions between HHV-6A and HHV-6B (biological, immunological, epidemiological) that support their recent reclassifcation from viral variants to distinct viral species. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Leibovitch EC, et al. Coinfection of human herpesviruses 6A (HHV-6A) and HHV-6B as demonstrated by novel digital droplet PCR assay. PLoS One. 2014;9(3):e92328. doi: 10.1371/journal.pone.0092328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Akhyani N, et al. Tissue distribution and variant characterization of human herpesvirus (HHV)-6: increased prevalence of HHV-6A in patients with multiple sclerosis. J Infect Dis. 2000;182(5):1321–5. doi: 10.1086/315893. [DOI] [PubMed] [Google Scholar]
- 49.Higashimoto Y, et al. Development of a human herpesvirus 6 species-specific immunoblotting assay. J Clin Microbiol. 2012;50(4):1245–51. doi: 10.1128/JCM.05834-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Haahr S, et al. A role of late Epstein-Barr virus infection in multiple sclerosis. Acta Neurol Scand. 2004;109(4):270–5. doi: 10.1046/j.1600-0404.2003.00221.x. [DOI] [PubMed] [Google Scholar]
- 51.Yea C, et al. Epstein-Barr virus in oral shedding of children with multiple sclerosis. Neurology. 2013;81(16):1392–9. doi: 10.1212/WNL.0b013e3182a841e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ryschkewitsch CF, Jensen PN, Major EO. Multiplex qPCR assay for ultra sensitive detection of JCV DNA with simultaneous identification of genotypes that discriminates non-virulent from virulent variants. J Clin Virol. 2013;57(3):243–8. doi: 10.1016/j.jcv.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]