Summary
Animal models are essential for understanding lymphoma biology and testing new treatments prior to human studies. Spontaneously arising lymphomas in pet dogs represent an underutilized resource that could be used to complement current mouse lymphoma models, which do not adequately represent all aspects of the human disease. Canine lymphoma resembles human lymphoma in many important ways, including characteristic translocations and molecular abnormalities and similar therapeutic responses to chemotherapy, radiation, and newer targeted therapies (e.g. ibrutinib). Given the large number of pet dogs and high incidence of lymphoma, particularly in susceptible breeds, dogs represent a largely untapped resource for advancing the understanding and treatment of human lymphoma. This review highlights similarities in molecular biology, diagnosis, treatment, and outcomes between human and canine lymphoma. It also describes resources that are currently available to study canine lymphoma, advantages to be gained by exploiting the genetic breed structure in dogs, and current and future challenges and opportunities to take full advantage of this resource for lymphoma studies.
Keywords: cancer, lymph nodes, comparative immunology, signal transduction, molecular biology, knockout mice
Rationale for a canine model of lymphoma
Representative animal models are critical for informing cancer biology research and developing new treatments. Research in lymphoma has benefited from traditional mouse models, but the lack of truly representative models has hindered full understanding of disease biology and new drug development. However, the advent of genomics technologies has now made nontraditional animal models more accessible than ever before, and leveraging these new opportunities represents a strategy that could result in more rapid disease research and new drug discovery. Growing numbers of studies demonstrate that spontaneously arising lymphoma in pet dogs could be an invaluable resource to study the biology and treatment of this disease.
Traditionally, mouse cancer models have been either xenografts of human tumors into immunocompromised mice or have been genetically engineered mouse models (GEMMs) with lesions that result in increased and/or accelerated lymphoma incidence (1, 2). Both types of models have advantages and disadvantages. In xenograft models, the usual tumor environment is often not representative. As the importance of microenvironment and tumor-stromal interactions becomes increasingly recognized in lymphoma, the deficiencies of this approach become more apparent. Another important limitation is the lack of a normal immune system, which is known to cause misrepresentative outcomes when treating any tumor type but which may be especially pertinent in the case of lymphoma, where cytokines and other lymphocyte signaling/survival factors are known to be involved in cell proliferation and survival. Therefore, while xenografts represent an excellent way to propagate human tumors, the ability to study detailed biologic mechanisms and treatment effects remains limited.
GEMMs are an improvement over xenograft models in that tumors arise in their native location and in the context of a normal murine immune system. However, GEMMs are limited by the necessity of knowing the specific genetic lesion(s) required to create the lymphoma-prone mouse, making them less useful for discovering the genetic origins of lymphoma. It also makes the mouse tumors more genetically homogeneous, which differs significantly from human tumors. Furthermore, mimicking particular lymphoma subtypes has been challenging with GEMM models. Hallmark translocations (IgH; CND1 in MCL and IgH; MYC in Burkitt lymphoma) have either not created increased rates of lymphoma and/or have not consistently produced the corresponding human lymphoma subtype in mice (1–3). Additional research should eventually overcome this problem, but until this can be achieved, complementary strategies are needed.
Pet dogs with spontaneously occurring lymphoma overcome many of the aforementioned challenges of murine models and also present additional advantages (4, 5). One advantage, from a drug development perspective, is that a large-animal model is essential for representative pharmacokinetic/pharmacodynamic (PK/PD) studies. It is well known that drug testing in mice and other small animals is often misleading since there are significant differences between rodent and human metabolism (6). Dogs, in contrast, are much larger, long-lived animals that are evolutionarily more closely related to humans than rodents (7). Lymphoma-bearing dogs would have the advantage of being able to ascertain PK/PD information while also getting information about efficacy of new drugs.
Another potential advantage of canine lymphoma is that their tumors arise spontaneously. This provides many opportunities not available with murine xenograft or GEMM models. First, the genetic diversity of canine tumors more accurately represents human tumors, as does the (relative) age of onset. Therefore, the biology of canine tumors is more similar to spontaneously arising tumors in humans, allowing questions to be answered about tumor initiation and promotion that are impossible in engineered animal models. Second, the canine model harnesses the power of evolutionary conservation to identify similarities between canine and human lymphomagenesis, for example in identifying important ‘driver’ gene mutations that are common to both species.
Advantages of a canine model extend beyond the biological advantages of a spontaneously arising tumor in a large animal. First, since pet dogs share our environment, environmental risk factors can be studied in dogs as well as humans, strengthening the evidence for associations found in both species. Second, regulatory hurdles are less stringent for animal studies, allowing some procedures, such as serial tumor sampling, in dogs that would be difficult or impossible in humans. Third, the accelerated life cycle of dogs relative to humans allow studies to be completed more quickly, since lymphomas arise and recur in a shorter time frame. Finally, the breed structure of dogs, in association with well-annotated multi-generational pedigrees kept by many breeders, presents a distinct genetic advantage that allows the genetic mapping of lymphoma predisposition genes with strategies that are not possible in humans.
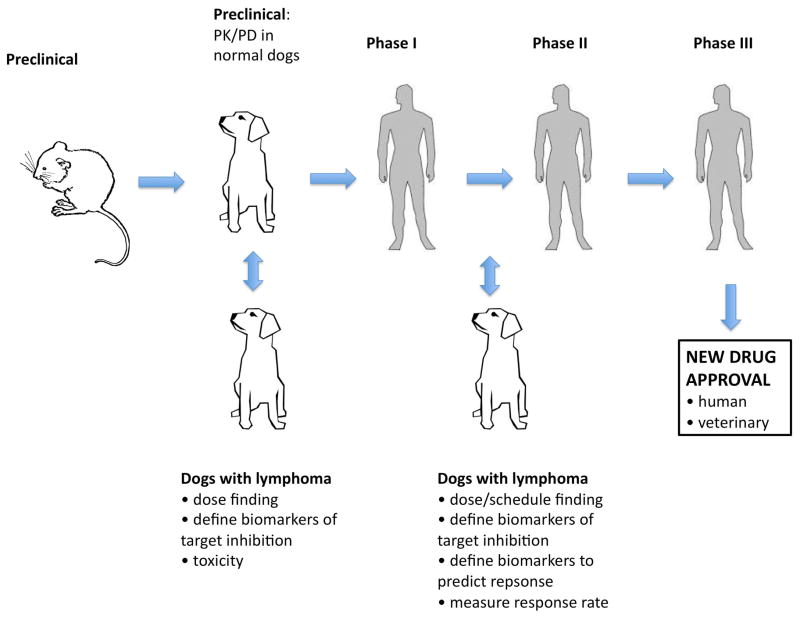
The final rationale for developing and using dogs as a model organism is a pragmatic one. The tumors that arise in dogs will continue to occur, whether we choose to study them or not. Exploiting this model may spare the creation and use of laboratory models, creating a ‘win-win’ strategy to find improved treatments for pet dogs that can both improve understanding of the human disease via cross-species oncogenomics while improving veterinary cancer care (Fig. 1). This dual benefit to both humans and their best friends provides a powerful rationale for further development and study of the canine lymphoma model. In this review, we discuss in detail the mounting evidence that dogs are an accurate model for human lymphomagenesis and treatment to encourage the broader use and acceptance of this valuable resource for improving lymphoma care in both dogs and people.
Fig. 1. Veterinary oncology.
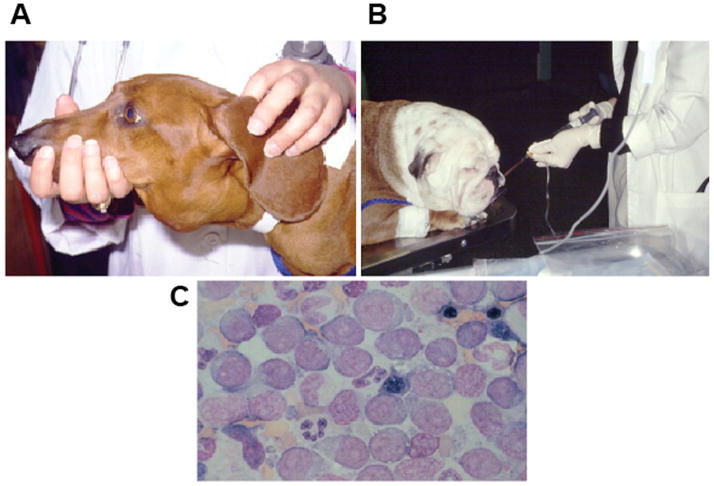
(A). A dachshund with severely enlarged submandibular lymph nodes. This is one of the most common sites that CL presents clinically. (B). An English bulldog receiving intravenous chemotherapy. Most dogs do not need sedation during this procedure. (C). A diagnostic fine needle aspirate of the enlarged lymph nodes of the dog in (A), showing a monomorphic population of large, immature cells commonly seen in high-grade CL.
Epidemiology of canine lymphoma and the growth of veterinary oncology
Similar to human non-Hodgkin lymphoma (NHL), canine lymphoma (CL) is a heterogeneous group of lymphoid malignancies that have different cells of origin and biological behaviors. The true incidence of CL is difficult to ascertain, since most dogs are diagnosed and treated by their local veterinarian and few are reported in the veterinary literature. In addition, most veterinary cancer registries have been short lived, with only four registries worldwide, to our knowledge, currently collecting information (8). Therefore, an estimate of the current incidence of the disease in the United States is based primarily on studies performed in the 1960s in California (9, 10). In these reports, CL accounted for ~90% of all canine hematopoietic cancers. The incidence of CL was estimated at 21.7 cases per 100,000 dogs at risk, although more recent data from the U.K. suggest an increasing estimated age-adjusted incidence of up to 107 cases per 100,000 dogs at risk (11). With ~75 million owned dogs in the United States, this suggests there are ~16–80,000 newly diagnosed cases of CL per year in the United States alone. These newer data imply that the incidence of CL is higher in dogs than in the human population, where the SEER age adjusted incidence is 20.2 cases per 100,000 in 2011 (12). Indeed, the lifetime risk for CL in golden retrievers in the United States is ~1:8, compared with a lifetime risk for people of ~1:50 (13). Importantly, the incidence of CL seems to be rising (14); it is now the second most frequent among all canine cancers after breast cancer. Supporting that these trends are also present in the U.S., a study of the Veterinary Medical Data Base Program (VMDP) at Purdue University found the rates of CL patients presenting to 27 North American veterinary teaching hospitals more than doubled between 1964 and 2002 (15). This mirrors human NHL, which has more than doubled in incidence since 1970, for reasons that are still unclear (12, 16, 17).
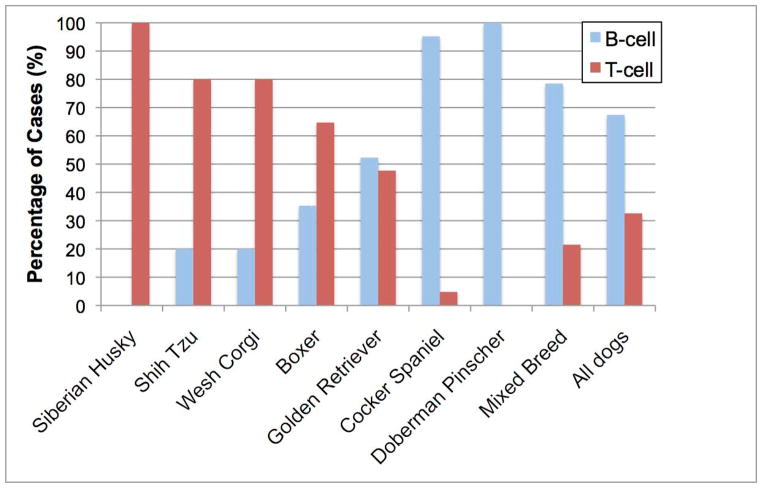
A variety of studies worldwide shows that certain dog breeds have a statistically significant increased risk of developing CL when compared to the average risk of all dogs (13, 15, 18–22). This suggests that heritable risk factors for CL were introduced and/or perpetuated as specific dog breeds were developed over the past ~2800 years. Other evidence supporting this notion is the familial clustering observed in specific dog breeds, including Bullmastiffs, Rottweilers, and Scottish terrier lines (19, 21). In addition, there is a predilection for some dog breeds to develop specific subtypes of CL (Fig. 2). For example, although canine T-cell lymphomas (cTCL) account for 25%–35% of all CL, ~85% of all CL in boxers is comprised of cTCL, with >50% of these malignancies being CD3+CD4+ T cell in origin (23, 24). In addition, the risk of a boxer to develop CL is ~4 fold higher than the average risk of any dog. Most Asian dog breeds and the modern Spitz also develop cTCL almost exclusively. In contrast, basset hounds and cocker spaniels almost always develop B-cell lymphomas (cBCL), while golden retrievers tend to develop cTCL and cBCL at equal frequencies. Finally, mixed breed dogs develop cBCL and cTCL at a frequency (~70% cBCL and ~30% cTCL) similar to all purebred dogs when they are considered as a single group (25). This unintentional selection of deleterious genes that increase the susceptibility of purebred dogs to a variety of diseases, including CL, provides a unique and powerful opportunity to utilize modern molecular techniques to identify mutations that contribute to canine lymphomagenesis that may have direct applicability to human NHL.
Fig. 2. B-cell vs. T-cell lymphoma prevalence varies by breed.
CL were phenotyped by BCR or TCR rearrangement, and percentages of each subtype (B vs. T) are shown. Breeds shown statistically significantly differ from one or both of the reference populations (mixed breed dogs or all study dogs as a group). Data are from Modiano et al. (13)
Another important contribution to the field of canine CL (and canine cancer in general) has been the expansion of veterinary specialty care. There are currently >350 board-certified veterinary oncologists in the United States who have undergone 4 years of additional training after completing the standard veterinary curriculum. This has led to expansion in both the diagnostic capabilities for veterinary cancer patients and the available treatments. Most specialty practices have access to CT scans, MRIs, radioactive scanning, fluoroscopy, digital radiography, ultrasonagraphy, flow cytometry, clinical pathology, anatomic pathology, and, at some institutions, PET/CT scans and DEXA scanning to aid in diagnoses (26). Veterinary patients are able to tolerate chemotherapy regimens that are used in human patients, although the protocols are generally less dose-intense to ensure an adequate quality of life. Surgical oncology is a rapidly growing subspecialty in the veterinary field, and radiation therapy is available at many private practice facilities and academic institutions. As such, the expansion of the number of highly trained veterinary medical oncologists, surgical oncologists, and radiation oncologists has led to a multidisciplinary approach to veterinary cancer care that is very similar to physician-based medicine. The availability of sophisticated diagnostics has led to a dramatic increase in ability to stage veterinary patients and, importantly, monitor treatment outcomes. Since veterinary cancer patients can be treated with the same treatment modalities that human patients are, they represent attractive large animal models to investigate novel treatment strategies.
Canine lymphoma subtypes and classification
Lymphomas in humans and dogs have many distinctive morphologic and immunophenotypic variants. A variety of histologic systems have been used to classify human NHL, and some of these have been applied to CL. Both the Kiel and Working Formulation (WF) systems categorize tumors as low-grade, intermediate-grade, or high-grade (27, 28). Based on these two systems, the majority of CL are considered intermediate or high grade, although geographical differences were found; diffuse immunoblastic forms predominated in the United States, while follicular large cell variants predominated in Europe. Using both systems, low-grade tumors are characterized by small cells, a low mitotic rate, a slow clinical progression, and are considered incurable, while intermediate to high-grade tumors are characterized by large cells, a high mitotic rate, a rapid clinical progression, and are potentially curable (in humans).
In 1994, the Revised European American Classification of Lymphoid Neoplasms (REAL) was rapidly accepted and incorporated into The World Health Organization (WHO) classification of hematopoietic and lymphoid malignancies. The REAL-WHO system has also been adapted to include tumors in domestic animals, including CL (29). The REAL-WHO system includes anatomic, histologic, and immunophenotypic data in an effort to provide an accurate and reproducible diagnosis for specific diseases. An additional benefit is an increased ability to do cross-species comparisons, detailing which histologic and immunophenotypic markers are conserved and which lymphoma subtypes are shared across species.
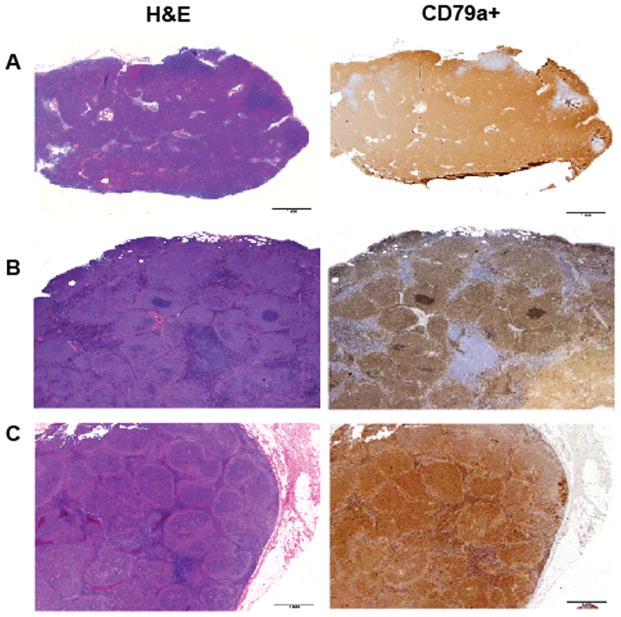
Based on the REAL-WHO system, the majority of CL are described as diffuse large B-cell lymphomas (DLBCL) (79%), with marginal zone lymphomas (MZL) a distant second (17%)(20). In contrast to human lymphomas, follicular lymphoma (B-cell) in dogs is very rare (Fig. 3). With regards to T-cell malignancies, the two main subtypes are peripheral T-cell lymphoma-not otherwise specified (PTCL-NOS) (high-grade) and T-zone lymphoma (TZL)(low-grade)(30). A wide variety of other B- and T-cell neoplasms exist, although they make up a small proportion of tumors that are commonly treated in the veterinary setting. Overall, ~65–75% of CLs are B-cell malignancies, and ~25%–35% are T-cell malignancies (20, 31). This mirrors the situation in human NHL, where the majority are B-cell malignancies, with DLBCL being the most common subtype, and <15% are T-cell malignancies (32, 33).
Fig. 3. Canine B-cell lymphoma histologies.
H&E and CD79a staining of canine lymph nodes showing different morphological patterns. CD79a is an intracellular antigen expressed by normal and neoplastic B cells. (A). Diffuse, large, B-cell lymphoma, the most common subtype of NHL in dogs. (B). Marginal zone lymphoma characterized morphologically by ‘fading germinal centers’. (C). Follicular lymphoma is rare in dogs.
Some human lymphoma subtypes are associated with viral infections, including human immunodeficiency virus (HIV), hepatitis C virus (HCV), human T-cell lymphotropic virus (HTLV), Kaposi sarcoma herpes virus (KSHV), and Epstein-Barr virus (EBV). There is mounting evidence that pet dogs may be infected with either EBV or an EBV-like gammaherpesvirus. A latent EBV or EBV-like gammaherpesvirus was detected in the peripheral blood and bone marrow of normal pet dogs in Taiwan (34), and antibodies to EBV or an EBV-like virus were detected in 38% of United Kingdom dog sera and 64% of United States dog sera (35). More recently, the expression of EBV transcripts were detected in multiple canine tumors and the presence of classic herpesvirus virions were detected via electron microscopy in various tumors, including BL, in two separate reports (36, 37). Using PCR primers directed against a variety of specific EBV transcripts, our laboratory has also found that both canine lymphoid cell lines and clinical cases of CL harbor EBV or EBV-like genomic sequences (authors’ unpublished data). These combined data suggest that either EBV or an EBV-like virus has infected dogs and may be contributing, at least in part, to canine lymphomagenesis. If ultimately proven, canine BL could provide an extremely valuable animal model to better understand EBV-induced lymphomagenesis in both species.
Treatment of CL and clinical trials
Treatment modalities for CL are similar to those used for human lymphoma (radiation, corticosteroids, chemotherapy), but there are also important differences, both in available treatment modalities and in factors dictating treatment decisions. Incorporation of canine lymphoma trials into the human drug development pipeline will require an appreciation of these factors to appropriately design informative canine lymphoma trials.
Chemotherapy
The front-line treatment of choice for advanced stage CL, similar to human NHL, is CHOP-based systemic chemotherapy (cyclophosphamide, doxorubicin, vincristine, and prednisone). Because FDA-approved chemotherapeutic agents are generally not labeled for use in dogs (so far there have only been two such drugs developed solely for a veterinary indication, neither known to be active in CL), the vast majority of drugs used by veterinary oncologists are based on human protocols and used off-label. Veterinary cancer chemotherapy in general has been developed only over the past 25 years with CL being the major focus, since it is one of the most chemo-sensitive malignancies in dogs (38) (Fig. 1).
There are a wide variety of studies showing that companion animals can tolerate most chemotherapeutic drugs given to human patients, and a complete review of these is beyond the scope of this article. However, some important general observations about companion animal chemotherapy based on these studies are notable. First, while the goal of most human chemotherapy protocols for aggressive lymphoma is to cure the patient (at the expense of significant toxicity), the usual goal of veterinary chemotherapy protocols is to extend an animal’s life and, at the same time, ensure a reasonable quality of life (QOL) during treatment. Therefore, veterinary protocols are less dose-intense, often only ~50% of the corresponding weight-based human dose, and are often given in smaller, more frequent doses (e.g. weekly instead of every 3 weeks) to decrease the associated toxicities. The vast majority of canine patients will not develop alopecia (except a few breeds such as poodles and Shih Tzus), and most dogs (and cats) enjoy an excellent QOL, with only minimal adverse events such a mild vomiting, diarrhea, anorexia, and/or febrile neutropenic episodes. With these less dose-intense protocols, malignancies such as CL are treatable, clearly extending life span, but incurable. Dogs with high-grade B-cell lymphoma have a median overall median of ~10–14 months (39, 40), whereas with no treatment, survival is ~6 weeks (41). The survival rate of CL using CHOP was ~20% of dogs at 2 years in one small study. (42)
Dogs with high-grade T-cell lymphoma (PTCL-NOS), which is often associated with paraneoplastic hypercalcemia, tend to fare worse with CHOP-based chemotherapy protocols. In spite of achieving clinical remission rates with induction chemotherapy similar to dogs with B-cell CL (~85%), they have overall median survivals of only ~6–9 months (42). These worse outcomes in T- versus B-cell lymphomas mirrors outcome data in human T- and B-cell lymphoma (32, 33).
The only clinically relevant low-grade lymphoid malignancy in dogs is T-zone lymphoma (TZL), which has been described morphologically (30), clinically (43), and with gene expression profiling (GEP)(44). In one study examining canine TZL, the median survival time was 33.5 months, with no statistically significant difference between dogs treated with chlorambucil/prednisone or CHOP (43). This is a statistically significant longer median survival than in dogs with high-grade B- and T-cell tumors treated with CHOP.
In the same study, the authors suggest that canine B-cell marginal zone lymphoma (MZL) is also an indolent disease, as it is in human MZL, since a median survival of 21.2 months was reported. However, only 15 cases were identified, many of the cases were treated differently (or not at all), and this category included both splenic and nodal MZL. Recent GEP and immunohistochemistry analysis suggests that canine nodal MZL and DLBCL may, indeed, be the same disease (44, 45). In any case, MZL may be a misnomer since there are many dissimilarities between canine MZL and human MZL (e.g. canine MZLs are often CD10-positive, and cells are large in size). Additional studies including larger numbers of dogs with nodal MZL are needed to clarify the origin of this lymphoma subtype.
Radiation
There are a variety of studies suggesting irradiation may be useful for the treatment of CL (46–48). More recently, a few non-randomized studies suggest the addition of external beam irradiation to a CHOP-based chemotherapy protocol is safe and, although not curative, may extend the lives of dogs with CL beyond the use of chemotherapy alone. All reported protocols utilize staged half-body irradiation given with a 2–4 week inter-fraction interval. The inter-fraction ‘rest’ allows recovery of the irradiated bone marrow so the other half of the body can be irradiated. Gustafson et al. (49) treated 6 dogs with CL using a half-body irradiation protocol (4 Gy two days in a row with a 4 week inter-fraction interval) interposed in a 25 week CHOP-based chemotherapy protocol and reported median remission and survival times of 455 and 560 days, respectively. Several other studies also suggested benefit from similar chemo/radiation approaches (50–52). Although protocols vary considerably, a variety of academic and private practice veterinary oncology practices offer half-body irradiation in conjunction with CHOP-based chemotherapy for the treatment of CL.
Monoclonal antibodies
Rituximab, a monoclonal antibody that recognizes the CD20 antigen universally present on mature B-cells, has been a major therapeutic advance in the treatment of all human B-cell NHL (53). However, rituximab is not useful to treat canine lymphomas because it does not recognize the canine CD20 protein (54). A murine monoclonal antibody raised using canine CD20 extracellular domain peptide sequence specifically recognizes canine B cells and B-cell lymphomas (55). Another canine anti-CD20 ‘caninized’ monoclonal antibody, AT-004, received a conditional license from the USDA for manufacture and distribution for the treatment of B-cell lymphoma in dogs (56). After preliminary studies showing improved overall survival compared to controls when given following chemotherapy, Novartis has licensed the product for commercialization. Another monoclonal antibody in the later stages of clinical development is AT-005. It recognizes CD52, the same antigen recognized by alemtuzumab (57), an antibody used to treat human TCL, and also has conditional approval from the USDA for the treatment of canine TCL (58). Other anti-lymphoma monoclonal antibodies recognizing antigens on lymphoma cells have demonstrated complement-dependent cytotoxicity and antibody mediated cytotoxicity, as well as in vivo growth inhibition of lymphoma cells, indicating that therapeutic potential exists (55, 59–61). Several examples of cross-species epitope recognition by therapeutic monoclonal antibodies of both the canine and human orthologs have been demonstrated (60, 62), which demonstrate that in these cases, dogs could be used as pre-clinical models for human monoclonal antibody development.
Autologous transplantation
The pioneering canine experiments utilizing autologous (progenitor cells harvested from the patient) or allogeneic [progenitor cells harvested from a dog leukocyte antigen (DLA)-matched donor] bone marrow transplants (BMT) to treat CL were performed in a research setting at the Fred Hutchinson Cancer Research Center. Drs. Joseph Murray and E. Donnall Thomas, who received the Nobel Prize in Medicine or Physiology in 1990 for this work, were utilizing dogs to study organ and cell transplantation for the treatment of human diseases. The large body of literature documenting these advances is beyond the scope of this review, nevertheless, based on this seminal work, the vast majority of human BMT protocols used today were perfected using normal dogs and dogs with CL. These early experiments showed that dogs could tolerate total body irradiation (TBI), that peripheral blood CD34+ cells could be harvested using sophisticated cell separator machines (63, 64), and that dogs given TBI could achieve complete hematologic reconstitution using either autologous (65, 66) or allogeneic (67) peripheral blood CD34+ cells. These studies also showed that dogs with CL could benefit from the addition of BMT to chemotherapy protocols (68, 69).
In more recent years, as veterinary care has become more sophisticated and cell separator machines have become available for clinical veterinary use, both autologous and allogeneic BMT are now options to treat CL. A variety of cell separator machines are able to harvest adequate numbers of canine peripheral blood CD34+ cells for BMT (70, 71), and dogs with CL will undergo complete hematologic reconstitution after otherwise lethal myeloablative therapy if given an appropriate dose of these cells (72, 73). Lupu et al. (70) described allogeneic BMT to treat a dog with relapsed T-cell CL that survived >19 months after diagnosis. Our group (74) treated 24 dogs with B-cell CL using autologous BMT and reported a median disease-free interval (DFI) and overall survival (OS) of all dogs from the time of BMT as 271 and 463 days, respectively. Thirty-three percent of these dogs lived > 2 years post-BMT. Our group (75) also treated 15 dogs with T-cell CL using autologous BMT and reported a median DFI and OS of the 13 dogs transplanted in first remission from the time of BMT as 184 and 240 days, respectively. Two of 13 (15%) dogs were alive 741 and 772 days post-BMT. Finally, Frimberger et al. (76) used a CHOP-based protocol that utilized 3 different high-doses of cyclophosphamide and autologous BMT (to reduce bone marrow toxicities) to treat dogs with B- and T-cell CL and reported a median survival time for dogs receiving the highest dose of cyclophosphamide as 139 weeks, compared with 43 weeks and 68 weeks for dogs in dogs receiving the lowest and intermediated dose of cyclophosphamide, respectively. At North Carolina State University, we have treated one dog with B-cell CL and one dog with acute large, granular lymphocytic leukemia using allogeneic BMT, and both dogs remain alive at 19 months and 26 months post-BMT, respectively (authors’ unpublished data). Both autologous and allogeneic BMT are currently offered by 4 private veterinary practices and 1 academic veterinary institution, although the costs of the procedure, at $16,000-$25,000, can be prohibitive to many pet owners.
Clinical trials
Comparing the efficacy of various veterinary chemotherapy protocols is limited, because most studies are statistically underpowered and/or do not utilize randomized, placebo controlled, prospective study designs. In addition, many published studies have inconsistent staging, inclusion, and response criteria. Finally, the majority of veterinary cancer patients do not die from their disease but are euthanized by their owners due to poor quality of life. This makes overall survival analysis much less robust than in human trials.
One final important observation is that the treatment of veterinary cancer patients and, therefore, prospective randomized trials, are dramatically influenced by finances. The vast majority of veterinary costs are paid out of pocket by owners, since veterinary insurance is rarely used. Therefore, veterinary oncologists must offer a variety of treatment protocols for a particular type of cancer based on cost rather than efficacy alone. Although this can be a difficult decision for pet owners, cost many times trumps efficacy. Another consideration is that most effective drugs used in veterinary oncology are unregistered off-label human generic (i.e. off-patent) drugs, so the incentive for sufficiently powered, randomized trials to study these agents is low.
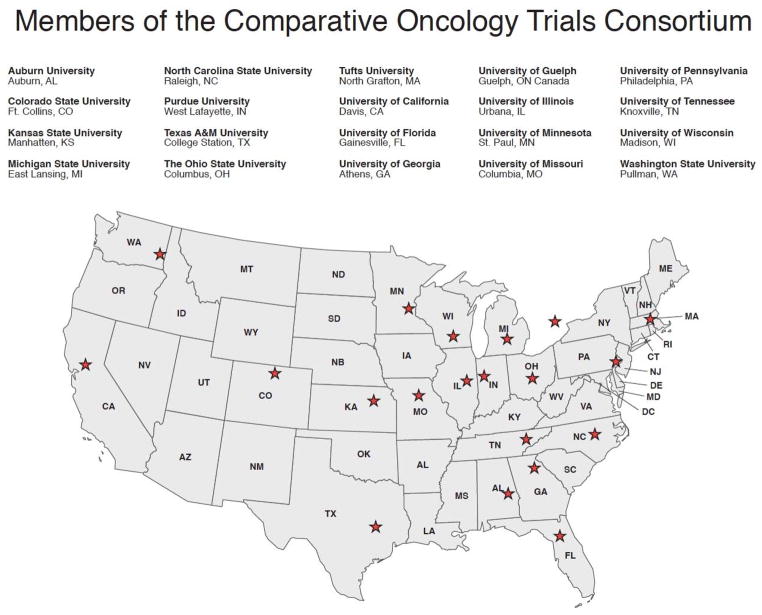
To address some of these issues, the veterinary oncology community has moved forward with some important initiatives. In an effort to standardize response criteria and outcome reporting for future trials, the Veterinary Cooperative Oncology Group (VCOG) has published response evaluation criteria (77–79). In addition, to enable larger trials the Comparative Oncology Trials Consortium (COTC) was launched in 2009 through the intramural NCI’s Center for Cancer Research–Comparative Oncology Program (80, 81). The COTC, which is supported by the NCI, operates as a collaborative effort between the NCI and 20 extramural academic comparative oncology centers (Fig. 4). It functions to design and execute clinical trials in dogs with cancer in collaboration with the pharmaceutical industry and nongovernmental groups interested in cancer drug development. To ensure the integration of such biological endpoints in these studies the COTC Pharmacodynamic (PD) Core was also developed to provide infrastructure to support the development, validation, and assessment of pharmacokinetic, pharmacodynamic, and biological endpoints within COTC trials (82). The first completed consortium trial published in 2009 (80) and a 12th trial is currently under development. Table 1 lists some examples of successful clinical trials with novel agents performed in dogs with CL.
Fig. 4. COTC member institutions.
The 20 current COTC sites are shown. They form a cooperative group network that conducts clinical trials in dogs with cancer (154).
Table 1.
Clinical trials using novel agents in CL.
| Agent Tested | Trial Type | Size (n=) | Results | Reference |
|---|---|---|---|---|
| Elsamitrucin (topoisomerase II inhibitor) | phase I | 20 (10 with CL) | MTD 0.08mg/kg; no objective responses | Fiocchi et al, 2011(137) |
| Telomerase vaccine | phase III | 42 | OS >76.1 wks with vaccine + chemo vs. 29.3 weeks with chemo alone | Gavazza et al, 2013(138) |
| Maitake mushroom extract | phase II | 15 | no objective responses | Griessmayr et al, 2007(139) |
| Nemo binding domain peptide (NF-KB inhibitor) | phase I | 11 | 7 dogs with CR; 1mg/kg maximally inhibits NF-KB without undue toxicity | Habineza Ndikuyeze et al, 2014(110) |
| Autologous tumor microbead vaccine | phase I | 15 | no change in PFS | Henson et al, 2011(140) |
| KPT-335 (exportin 1 inhibitor) | phase I | 23 (20 with CL) | stable disease or PR in 11/20 dogs | London et al, 2014(141) |
| Heat shock protein peptide complex vaccine | phase III | 19 | TTP 304 days with vaccine + chemo vs. 41 days with chemo alone | Marconato et al, 2014(142) |
| Patupilone (epothilone B) | phase I | 20 (6 with CL) | MTD 2.76 mg/m2; no responses in CL | Meier et al, 2013(143) |
| VDC-1101 (nucleotide analog, formerly GS-9219) | phase I/II | 38 | ORR 79%; median TTP 128 days | Vail et al, 2009(60, 144) |
| IMMU-114 (anti-HLA-DR antibody) | pilot | 6 | 2 of 6 dogs with transient response | Stein et al, 2011(60) |
| ABT-751 (targets microtubules) | phase I | 30 | MTD 350mg/m2; 3/15 (20%) ORR in expansion cohort | Silver et al, 2012(145) |
| ABT-526 (thrombospondin peptide mimetic) | phase III | 94 | ORR equal in two arms, but TTP in chemo + ABT-526 longer than chemo alone (41 vs. 15 days) | Rusk et al, 2006(146) |
| PCI-32765 (ibrutinib, BTK inhibitor) | pilot/phase I | 8 | 3 PR, 3 SD; 20mg/kg dose tolerated | Honigberg et al, 2010(113) |
MTD: maximum tolerated dose; OS: overall survival; CR: complete response; PR: partial response; ORR overall response rate (CR+PR); TTP: time to progression
As in human oncology, the clinical research organization (CRO) model aims to provide efficiency and facilitate partnering of pharmaceutical companies with veterinary hospital trial sites. The first example of a CRO in veterinary medicine is Animal Clinical Investigation (www.animalci.com). ACI provides regulatory strategy and preclinical planning, protocol design, and trial conduct at a multi-site network of oncology practices in the U.S. and Canada. They have performed over 30 clinical trials and are currently conducting trials with AT-005, an anti-CD52 antibody, with thalidomide, and with a thrombospondin-mimetic peptide (83).
Molecular similarities to human lymphoma
In addition to known clinical and histologic similarities, it is becoming increasingly apparent that canine lymphoma bears a striking resemblance to human lymphoma at the molecular level as well. These comparisons have become more nuanced as genomics tools have advanced, and many comparisons remain to be made. Still, findings to date reinforce the notion that human and canine lymphomas share significant similarity, while highlighting some differences as well.
Cytogenetics
Some of the first molecular comparisons between canine and human lymphoma were based on cytogenetic analyses. It is known that certain chromosomal alterations are recurrent in human lymphoma (84); some of these are highly enriched or even pathognomonic of the lymphoma subtype. These include t(8;14) in BL, t(11;14) in MCL, or t(14;18) in FL and DLBCL. Breen et al chose three regions of recurrent cytogenetic abnormalities and used the orthologous region in dogs to probe by fluorescence in situ hybridization (FISH).(85) Two of these abnormalities were hallmarks of B-cell malignancies [the t(8;14) in BL and deletion of p16 in chronic lymphocytic leukemia (CLL)], and the third was the Philadelphia chromosome [t(9;22), found in chronic myelogenous leukemia (CML)]. The Philadelphia chromosome [t(9;26) in dogs] was found in 2 dogs with CML and one with acute lymphoblastic leukemia (ALL). The translocation corresponding to t(8;14) was found in a BL case, and a p16Δ was in a CLL case, demonstrating for the first time genetic conservation between canine and human hematologic malignancies, both in the hallmark genetic aberrations and in the diseases associated with them.
DNA copy number
To comprehensively compare DNA copy number changes, array comparative genomic hybridization (aCGH) results were compared between canine and human lymphoma (86). There were notable differences in the degree of genomic instability between canine BCLs and TCLs; TCLs had more gains and losses than BCLs did. Interestingly, canine BCLs had many fewer areas of recurrent copy number changes than human BCLs. Recurrent canine BCL gains were seen on chromosome 13 and 31, corresponding to human chromosome 8 and 21, respectively. Losses were seen on chromosome 26. TCLs had gains on chromosomes 6, 9,13, 20, 29, 31, and 36, and losses of CFAs 11, 17, 22, 28, and 38. Gains on chromosomes 13 and 31 were common to both TCLs and BCLs. Importantly, these data were combined with human aCGH data to refine the results, narrowing the region on chromosome 13 to the syntenic region on human chromosome 8 containing the MYC gene, amplified in B-cell lymphomas from both species. This demonstrates that using two or more species, combined with mapping regions of synteny (87) allows finer resolution of important regions of gain/loss than are possible to resolve using single species data.
Gene expression profiling
Gene expression profiling (GEP) has played a major role in elucidating the biology of lymphoma subgroups as well as highlighting important prognostic signatures. In human DLBCL, GEP distinguishes two major subcategories based on cell of origin, the germinal-center B-cell (GCB) and activated B-cell (ABC) subtypes (88). The ABC subtype is characterized by upregulation of the NF-KB pathway and poorer survival than the GCB subtype, which has a GEP profile reminiscent of other germinal-center derived cells, and a better prognosis than the ABC subtype. GEP platforms are now commercially available (e.g. Affymetrix, Agilent) and are being used to comprehensively characterize canine lymphomas.
In the first report demonstrating the utility of GEP in canine lymphoma, Frantz et al. (45) demonstrated that GEP could separate low-grade T-cell lymphomas, high-grade T-cell lymphomas, and B-cell lymphomas into separate groups. With a larger number of B-cell lymphoma samples, our group was able to separate canine B-cell lymphomas into two groups reminiscent of human ABC/GCB subtypes, in which the ABC-like subtype had a worse prognosis, similar to human DLBCL (44). We also showed that the ABC-subtype had higher expression of B-cell receptor and NF-KB pathway genes, as in human DLBCL. Similarly, Mudaliar et al. (89) found a signature of the NF-KB canonical pathway when comparing canine and human B-cell lymphomas. Additional studies with larger sample sizes and perhaps breed-specific studies will be needed to confirm and elaborate on these data, but initial similarities to human DLBCL are promising.
Another powerful use of canine GEP data is to combine it with human data in search of evolutionarily conserved similarities in the two data sets. This has been done in canine osteosarcoma, yielding predictive markers discovered only in the canine GEP data, which were nonetheless predictive when applied to human osteosarcoma samples. (90, 91) Our group demonstrated the power of cross-species oncogenomics in lymphoma when they combined gene expression data from cBCL and human DLBCL in a bivariate mixture model, yielding a robust small set (n=21) of differentially expressed genes common to both species that predicted survival in human DLBCL as accurately as a much larger set of genes (n=190) derived when human expression data alone was used (92) (Fig. 5). These examples demonstrate that cross-species comparisons can focus attention to the most biologically relevant genes in large data sets.
Fig. 5. Combined species data shows equivalent prognostic power with many fewer genes.
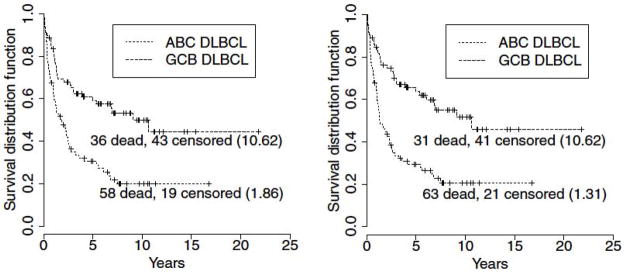
The panel on the left uses human gene expression data only (190 genes) to distinguish groups with different survival times; on the right, human and canine data are combined to produce a more focused gene list (21 genes) with equivalent power to predict survival in human DLBCL. Numbers in parentheses are median survival times, in years. Figure reproduced from Su et al. (92)
Cellular markers: flow cytometry/IHC
A combination of cell surface markers, assessed by flow cytometry, and intracellular markers, assessed by immunohistochemistry, are used in both human and canine lymphoma to subclassify lymphomas. Identifying the correct subtype of lymphoma has important implications, both for further understanding biology, as well as clinically, to ensure the optimal treatment is administered. For the most part, canine lymphoma flow markers are those that have been validated in humans as clinically and/or biologically useful (e.g. CD 3, CD79a, CD4, CD8, CD21) to define cell of origin (lymphoid vs. non-lymphoid, B-cell vs. T-cell, mature vs. immature, etc.) for diagnostic purposes (93, 94).
One important use of IHC in human lymphoma is to subclassify DLBCL ABC and GCB subtypes. The Hans criteria were developed as a surrogate for GEP classification; by staining for CD10, BCL6, and MUM1 proteins, DLBCL can be classified into GCB and non-GCB subtypes (95). This classification has been shown to predict survival in numerous studies and has even been used to demonstrate differential outcomes in different treatment arms in several clinical trials (96–99). However, variability between laboratories makes this classification system unreliable. Several additional classification systems based on IHC have subsequently been developed attempting to improve robustness, but there are still significant barriers to using any of these in clinical practice (100). In canine lymphoma, both BCL6 and MUM1 are infrequently expressed. (44, 101) This makes application of the Hans criteria difficult, so alternative IHC schema will likely need to be implemented.
Another immunohistochemical prognostic system shown by several groups to be informative in DLBCL is staining for both MYC and BCL2. It has been recognized for years that double-hit lymphomas, with both c-MYC and BCL2 translocations, have an exceptionally poor prognosis. In addition, several recent studies have demonstrated that combined overexpression (an ‘immunohistochemical double hit’) is also a poor prognostic sign, albeit not as bad as the true cytogenetic double hit (102). This has been examined in canine lymphoma and also found to be true (103), strengthening the importance of these markers as indicators of lymphoma aggressiveness in both species.
Immunoglobulin genes
The immunoglobulin genes, encoding the B-cell and T-cell receptors, provide a unique way to identify clonality in lymphoma. Each individual cell has a unique immunoglobulin receptor that is produced (in B cells) by V-D-J gene selection and recombination, isotype switching, and somatic hypermutation (SHM). Studies of human lymphoma have not definitively demonstrated a bias in IGHV gene usage in DLBCL. However, cBCLs do have a bias in their IGHV usage; 31/52 cBCL tested used VH1-44, which is significantly different than in normal canine B lymphocytes. (104) Furthermore, dogs with cBCLs that use VH1-44 have a longer overall survival. Intriguingly, VH1-44 is most closely homologous to human VH3-23, which is a commonly used VH gene in human DLBCL (10–15% of cases). It is not known whether usage of this gene is correlated with survival in human DLBCL.
Another feature of the immunoglobulin genes that is reported to be relevant to human DLBCL is the presence or absence of ongoing SHM. Nearly all human DLBCL have undergone SHM, indicating they traversed the germinal center prior to lymphomagenesis. However, some DLBCL have high levels of SHM and intraclonal variability, leading to the hypothesis that the SHM machinery remains active, with ongoing SHM in these cells. Lossos et al. (105) analyzed a small number of DLBCL patients (n=14) and suggested that ongoing SHM is associated with the GCB subtype of DLBCL, while lack of ongoing SHM is associated with the ABC subtype. With a larger sample size, this correlation was not as strong in cBCL. More provocatively, in cBCL, presence or absence of ongoing SHM predicted survival more accurately than ABC/GCB subtype (44). Larger numbers of human DLBCL will need to be tested for ongoing SHM status to see whether this is also true in human DLBCL.
Oncogenic pathways
Aberrations in oncogenic pathways are the fundamental underpinnings of cancer phenotypes. These are shared in canine lymphomas in many cases. As more targeted therapies become available, understanding which genes are dysregulated and which are ‘Achilles heels’ for a particular type of cancer becomes increasingly important. Conservation of mechanism is a particularly powerful strategy to identify these critical oncogenic changes. Several such changes have already been identified, demonstrating proof of principle.
FLT3
FLT3 is an important driver mutation in human acute myelogenous leukemia (AML) and is a negative prognostic marker (106). Rare cases of human ALL are also FLT3 positive. Similar to pediatric oncology incidence rates, canine AML is rare but ALL is more common. FLT3 mutations have been discovered in canine ALL, in the same characteristic internal tandem duplication that is mutated in humans ALL and AML (107). Furthermore, the same downstream signaling pathways are activated, and FLT3 inhibitors are efficacious against FLT3 mutant cells. Importantly, this establishes not only the same gene is affected in both species but also that the identical mutational mechanism occurs in both dogs and humans. This allows further study into both mutational mechanisms and their functional outcomes on aberrant oncogenic signaling.
NF-κB
The importance of the NF-κB pathway in human lymphoma is well established; NF-κB pathway members are constitutively upregulated in many malignancies and have been shown to be important for growth and survival of lymphoma cells (108). Cross-species conservation of aberrant NF-κB activation also supports its importance in lymphomagenesis and lymphoma growth/survival. Several studies have implicated the NF-κB pathway in canine lymphoma.
In the first large-scale study of gene expression profiling in canine lymphoma, we found two classes of canine B-cell lymphoma, based on human ABC and GCB subtypes in DLBCL (44). Separation into these two groups was improved when a ‘canine-specific’ ABC/GCB classifier was derived. The GCB-like subtype defined by this classifier had improved survival, analogous to human DLBCL. Furthermore, in the ABC-like subtype, genes in the B-cell receptor pathway and the NF-KB pathway were overexpressed relative to the GCB-like subtype, mirroring human DLBCL.
Mudaliar et al. (89) also examined gene expression profiling data, comparing normal and DLBCL-bearing lymph nodes in both dogs and humans, with a focus on NF-κB-related genes. Using expression levels of a literature-derived set of 120 NF-κB target genes, they demonstrated the ability in both human and canine samples to separate normal from tumor samples. Furthermore, using immunohistochemistry for p52 and p65 they demonstrated activation of both the alternative and canonical NF-κB pathway, respectively, in both canine and human DLBCL. Interestingly, the canine lymphoma samples had higher p52 (non-canonical pathway) levels than p65 (canonical pathway) by immunohistochemistry, while in humans this was reversed. Drug sensitivity studies with these cell lines demonstrated sensitivity to an IKK inhibitor in both human and canine cells in vitro.
Functional relevance of NF-KB activation in canine lymphoma was demonstrated in two clinical trials of NEMO-binding domain peptide, a drug that inhibits NF-κB signaling (109, 110). Eleven dogs with DLBCL and constitutive canonical NF-KB activity were treated with intravenous NBD peptide. NBD peptide was safe and blocked NF-KB activity in 6/10 dogs. Five dogs had >20% shrinkage after a single dose.
BCR pathway
Some of the most promising new drugs to treat human B-cell malignancies in recent years target the B-cell receptor pathway (111). One such agent, ibrutinib, has received expedited FDA approval for both mantle cell lymphoma and CLL (112). As part of the drug development pathway for this agent, it was tested in a veterinary trial for dogs with CBL (113). This study demonstrated responses in 3 of 8 dogs tested, with stable disease in an additional three dogs. No information about ABC/GCB status was available for these dogs, but it would be of interest to determine whether responses occur more often in the ABC subtype as has been shown in human DLBCL (99).
Bax/BCL2
Resistance to chemotherapeutic drugs is most likely due to resistance to apoptosis, since this is usually how neoplastic cells are killed by these agents (114). Since apoptosis is controlled by a family of both pro- and anti-apoptotic proteins of the BCL-2 family, it is possible that TCL cells have altered pro-/anti-apoptotic protein ratios that contribute to increased chemotherapy resistance and worse outcomes relative to BCL. Indeed, our laboratory has found that canine PTCL-NOS cells have a statistically significant reduced Bax (pro-apoptotic):BCL-2 (anti-apoptotic) ratio when compared to neoplastic B-cells (manuscript in preparation). In addition, neoplastic canine T-cells have >300% increase in the expression of a microRNA (miRNA) cluster called miR-19 a+b when compared to normal canine peripheral blood mononuclear cells (115). MiR-19 a+b is part of the miR17-92 cluster (called oncomir-1), an oncogene acting primarily through inhibition of apoptosis (116). Interestingly, decreased expression of Bax has been identified in human TCL, CLL (114), and lymphoblastic leukemia, and reduced levels of Bax have also been implicated in resistance to therapy (117, 118).
p16/RB
The p16/RB tumor suppressor pathway is inactivated in many tumor types in humans, including 50% of FL (119) and up to 66% of DLBCL (120), leading to RB phosphorylation and increased proliferation. Fosmire et al. (121) tested both T-cell and B-cell CL for deletion or promoter methylation of the p16 gene and RB phosphorylation. While deletion of p16 was restricted to high-grade TCL, high-grade BCL also showed increased RB phosphorylation (without p16 deletion), indicating alternative pathways of disrupting the p16/RB axis in these two tumor types. Likewise, aCGH results also confirm loss of the RB region, on canine chromosome 11, solely in cTCL (86). Furthermore, p16 loss and RB phosphorylation were both statistically significantly associated with survival in these dogs, which may partly reflect tumor type (T vs. B) and grade (high vs. low) (122).
Advantages unique to the canine model
To this point, we have stressed strategies to leverage the similarities between CL and human lymphoma. However, there are some advantages provided by canine models that are impossible to achieve in humans. These add to the already powerful rationale for utilizing the canine model in the study of human lymphoma.
One major advantage of dogs is the particularly high cancer susceptibility in certain breeds, implying a predisposition that can be genetically mapped (123). The genetic bottleneck and relatively short evolutionary time since breed divergence also creates much longer linkage disequilibrium blocks, resulting in a genome requiring many fewer SNPs to cover the genome, while maintaining very high power with fewer subjects than are needed in human genome wide association studies. It has been simulated that a simple Mendelian dominant trait could be found using only ~15,000 SNPs and 100 affected and 100 unaffected dogs, with >99% power (7). We have begun such a project to map the T-cell lymphoma susceptibility inherent in the boxer breed, with the hope that identification of the gene(s) will lead to greater insights about T-cell lymphomagenesis and potentially identify new treatments. Other phenotypes, such as particular cytogenetic abnormalities, lymphoma subtypes, or gene expression profiles, may also vary by breed, implying an underlying genetic predisposition that could be mapped, lending biologic insights that are not possible from a more heterogeneous human population.
Another advantage of studying lymphoma in dogs is that while it is similar in many ways to humans (spontaneously arising tumors, ability to perform clinical trials), ethical considerations differ. For instance, if needed, there is the capability to test biologic actions of drugs and other physiologically relevant phenomena in research dogs, using experimentally controlled conditions in a laboratory setting. Cadaver tissue from normal dogs, collected from dogs euthanized at shelters, is also more accessible, relative to humans. Permission for clinical trials comes from institutional animal use committees rather than institutional review boards governing human trials. This allows the focus to remain on the humane treatment of trial participants while eliminating some of the regulatory issues to which human trials are subject. Also important in this calculus is the fact that lymphoma in dogs is currently not curable, which creates a different risk/benefit ratio than in humans. This allows newer drugs to be moved earlier in the disease course and more flexibility in experimental design (drug/chemo combinations, drug/drug combinations, window trials, etc.).
Resources available for further development of the CL model
One of the necessities for development of a new model organism is the development of research tools to accompany it. Genomics tools have seen rapid development over the past 10 years, since the publication of the complete canine genome sequence. In combination with resources allowing wider access to tumor tissue and the ability to conduct clinical trials, the stage is set for rapid advancement of knowledge about canine lymphoma.
Experimental reagents
Canine lymphoid cell lines have been historically difficult to establish, and the number of well-characterized, available cell lines remains limited (Table 2). Even more limited are DLBCL cell lines. Only four of the available cell lines are reportedly of B-cell origin; GL-1 was derived from a dog with B-cell ALL, 17-71 was not phenotyped initially and does not express typical B-cell lymphoma markers, and 3132 is likely not of B-cell origin despite initial reports of surface immunoglobulin (authors’ manuscript in preparation). Therefore only CBLB-1 appears to be potentially of DLBCL origin. One advantage to small numbers of cell lines is that they are well characterized (124), since they are repeatedly used within the canine research community. Better methods of propagating cells in vitro are also being developed. This will enhance our ability to perform short-term in vitro experiments using primary tumor cells and also be a potential route to improved rates of establishing immortalized canine lymphoma cell lines. One promising method has been the use of CD40 ligand, allowing cell to proliferate up to 40 days (125).
Table 2.
Canine cell lines.
| Cell Line/Reference | Derived From | Phenotype | Notable Features |
|---|---|---|---|
| GL-1 (107, 124, 147) | B-cell leukemia | IgH+, CD79a+, CD3− | FLT3 ITD |
| UL-1 (132) | ascites from renal lymphoma patient | TCR+, IgH−, CD3−, CD21− | |
| CL-1 (124, 148) | pleural fluid from T-cell lymphoma patient | TCR+, IgH−, CD3−, CD21− | homozygous PTEN deletion |
| CLBL-1 (149) | FNA from B-cell lymphoma patient | TCR−, IgH+, CD79a+, CD3− | |
| Nody-1 (132) | ascites from alimentary lymphoma patient | TCR+, IgH−, CD3+, CD21− | KIT overexpression, also called TL-1 |
| Ema (132) | pleural fluid from T-cell lymphoma patient | TCR+, IgH−, CD3+, CD21− | |
| CLK (132) | ascites from T-cell lymphoma patient | TCR+, IgH−, CD3−, CD21− | |
| CLC (132) | pleural fluid from T-cell lymphoma patient | TCR−, IgH−, CD3−, CD21− | |
| 3132 (150, 151) | ascites from malignant lymphoma patient | IgH+ | produces retroviral particles? |
| CLGL-90 (124, 152) | LGL T-cell leukemia | TCR+, CD3+, CD79a− | homozygous CDKN2A deletion |
| 17–71 (124, 152, 153) | lymph node from B-cell lymphoma patient | TCR−, CD79a+, CD3−, CD21− | near tetraploid |
| OSW (130) | pleural fluid from T-cell lymphoma patient | TCR+, IgH−, CD3−, CD21− | MYC amplification; PTEN, p16, and RB1 deletion |
| CLL-1390 (124, 152) | primitive T-cell leukemia | TCR+, CD34+, CD3−, CD21− | homozygous PTEN deletion |
| DLC 01 (128) | lymph node from a Sezary syndrome patient | CD3+, CD4−, CD8+ | produces retroviral particles? |
| DLC 02 (128) | peripheral blood lymphocytes from an LGL patient | CD4−, CD8+ | produces retroviral particles? |
LGL: large granulocytic leukemia
The full genomic sequence of Canis lupus familiaris was published in 2005, and it was recently updated to cover 99.8% of the euchromatic region of the genome (7, 126). Having the full genomic sequence has allowed various sequence-based platforms to be commercially developed, including gene expression profiling chips, aCGH chips, SNP genotyping chips, and capture probes for whole exome sequencing (127).
Because lymphoid cell lines have been difficult to establish, alternative methods of propagating tumors have been developed. Xenografts into immunocompromised mice allow propagation of lymphoma specimens even in cases where cell lines cannot be established. Patient-derived xenografts are increasingly used to study human lymphoma, and are also being used in the study of canine lymphoma (128–132). This can potentially provide a wider variety of CL tumor types for in vivo studies.
The canine comparative oncology and genomics consortium (CCOGC) was formed to facilitate collaborations focused on understanding canine cancer. They formed a biospecimen repository in 2007, with current collection sites at six veterinary schools that provide samples from patients with seven common cancers, including lymphoma (5). They currently have over 2000 samples, which are available for scientific use.
One Medicine partnerships
The One Medicine concept proposes that human and veterinary research are complementary approaches to the same problem. Although this idea is not new, human and veterinary medical collaborations embracing the joint advancement of medical knowledge is currently enjoying a renaissance, particularly in cancer research. The NCI has a Comparative Oncology Program (http://ccr.cancer.gov/resources/cop/default.asp), and most of the major veterinary schools have comparative oncology programs that partner with nearby medical schools for joint research possibilities. The growth of comparative oncology is also reflected in the growing number of journals, websites, and scientific meetings dedicated to the concept (133). The first meeting of the European canine lymphoma group was held in 2013, as a satellite meeting to the 12th International Conference on Malignant Lymphoma in Lugano, Switzerland, bringing together canine and human lymphoma experts (134).
Future challenges
Although our understanding of and interest in canine cancer models has increased tremendously, especially over the past 10 years, and infrastructure has been established, there are still challenges remaining. Continued research and development, as well as changing some well-established paradigm will be necessary to take full advantage this valuable resource for lymphoma research.
Drug development pipelines worldwide currently follow a common strategy: preclinical testing in cell lines and in mice, PK/PD testing in large animals (usually normal research dogs), and then phase I–III testing in human clinical trials. Under this paradigm, 85% of new agents fail in early stage clinical trials, and half that succeed fail to win FDA approval (135). Utilizing clinical trials in dogs as part of preclinical testing or in parallel with early stage human trials would make this process more efficient (Fig. 6). PK/PD studies routinely performed in normal research dogs could instead be performed in dogs with lymphoma, and efficacy data could be collected at the same time. Most academic veterinary institutions have the study personnel and facilities to perform phase I–III testing. These studies are potentially less expensive and more accurate than studies done with research dogs. Efficacy and toxicity data from canine trials could provide an early signal to complement data from human trials. Robust companion diagnostics and biomarkers of response could be developed in dogs, allowing earlier insertion into human clinical trials. One potential hindrance in this scenario is that some toxicities may be specific to dogs and not seen in humans. However, if this is monitored appropriately, more information from the canine model should augment rather than hinder drug development in humans (136).
Fig. 6. Augmenting the drug development pipeline with lymphoma-bearing dogs.
The current drug development pipeline uses normal research dogs for PK/PD studies. Pet dogs with lymphoma could be studied in earlier phase studies to refine PK/PD data and define biomarkers to be used for companion diagnostics (both predictive and target assessment biomarkers). Efficacy in both dogs and humans would reinforce the utility of agent, and earlier dog studies would allow more rapid adaptation if dosing/schedule/endpoints needed to be modified.
Perhaps the largest hurdle to the widespread use of canine lymphoma as a model for the human disease is the lack of biologic/translational research data. As described in this article, research into the molecular underpinnings of canine lymphoma has just begun. Many more detailed studies are needed to determine which aspects of canine lymphoma biology will be best suited for modeling human lymphoma for drug development and other research purposes.
Another hurdle (as in human trials) is the accessibility of clinical trials. This problem is exacerbated in veterinary medicine, since there are fewer than half as many veterinary schools (n=29) than there are NCI-designated cancer centers (n=68). Furthermore, many veterinary schools are located in rural areas; therefore the small animal caseload is limiting. To engage a large proportion of the U.S. pet population, creative ways to engage private practice veterinary oncologists will need to be explored. In addition, the advantages of participation in clinical trials (subsidized cost of treatment, access to novel agents, advancement of medical care for dogs and humans) will need to be disseminated to pet owners to encourage their support and participation.
Summary
Canine lymphoma is strikingly similar to the human disease in many respects and is currently an underutilized resource in the study of human lymphoma, particularly in the drug development pipeline. Recent advancements in our understanding of the molecular biology of canine lymphomas reinforce the cross-species conservation that we had expected based on histologic and clinical similarities. Exceptions to this, for example in immunohistochemical staining and gene expression profiling signatures, are being elucidated and will be important to understand both for advancing cancer biology knowledge and for accurate and appropriate use in targeted drug development. The appropriate clinical and biological resources are now in place to harness this resource, and increased utilization will depend on buy-in by pharmaceutical companies, community veterinary oncologists, and most importantly pet owners. The phrase ‘man’s best friend’ is now poised to take on a whole new meaning in the fight against cancer.
Acknowledgments
KLR is supported by a Mentored Research Scholar Grant in Applied and Clinical Research (MSRG-12-086-01-TBG) from the American Cancer Society and R01-CA185372-01 from the National Cancer Institute. SES and KLR thank the pet owners whose dogs contributed tissues for the studies described in this review.
Footnotes
The authors have no conflicts of interest.
References
- 1.Zullo K, Amengual JE, O’Connor OA, Scotto L. Murine models in mantle cell lymphoma. Best practice & research Clinical haematology. 2012;25(2):153–63. doi: 10.1016/j.beha.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 2.Macor P, Secco E, Zorzet S, Tripodo C, Celeghini C, Tedesco F. An update on the xenograft and mouse models suitable for investigating new therapeutic compounds for the treatment of B-cell malignancies. Current pharmaceutical design. 2008;14(21):2023–39. doi: 10.2174/138161208785294591. [DOI] [PubMed] [Google Scholar]
- 3.Harris AW, Pinkert CA, Crawford M, Langdon WY, Brinster RL, Adams JM. The E mu-myc transgenic mouse. A model for high-incidence spontaneous lymphoma and leukemia of early B cells. The Journal of experimental medicine. 1988;167(2):353–71. doi: 10.1084/jem.167.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ito D, Frantz AM, Modiano JF. Canine lymphoma as a comparative model for human non-Hodgkin lymphoma: recent progress and applications. Veterinary immunology and immunopathology. 2014 doi: 10.1016/j.vetimm.2014.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khanna C, Lindblad-Toh K, Vail D, London C, Bergman P, Barber L, et al. The dog as a cancer model. Nature biotechnology. 2006;24(9):1065–6. doi: 10.1038/nbt0906-1065b. [DOI] [PubMed] [Google Scholar]
- 6.Kararli TT. Comparison of the gastrointestinal anatomy, physiology, and biochemistry of humans and commonly used laboratory animals. Biopharmaceutics & drug disposition. 1995;16(5):351–80. doi: 10.1002/bdd.2510160502. [DOI] [PubMed] [Google Scholar]
- 7.Lindblad-Toh K, Wade CM, Mikkelsen TS, Karlsson EK, Jaffe DB, Kamal M, et al. Genome sequence, comparative analysis and haplotype structure of the domestic dog. Nature. 2005;438(7069):803–19. doi: 10.1038/nature04338. [DOI] [PubMed] [Google Scholar]
- 8.Bronden LB, Flagstad A, Kristensen AT. Veterinary cancer registries in companion animal cancer: a review. Veterinary and comparative oncology. 2007;5(3):133–44. doi: 10.1111/j.1476-5829.2007.00126.x. [DOI] [PubMed] [Google Scholar]
- 9.Dorn CR, Taylor DO, Schneider R. The epidemiology of canine leukemia and lymphoma. Bibliotheca haematologica. 1970;(36):403–15. doi: 10.1159/000391733. [DOI] [PubMed] [Google Scholar]
- 10.Dorn CR, Taylor DO, Schneider R, Hibbard HH, Klauber MR. Survey of animal neoplasms in Alameda and Contra Costa Counties, California. II. Cancer morbidity in dogs and cats from Alameda County. Journal of the National Cancer Institute. 1968;40(2):307–18. [PubMed] [Google Scholar]
- 11.Dobson JM, Samuel S, Milstein H, Rogers K, Wood JL. Canine neoplasia in the UK: estimates of incidence rates from a population of insured dogs. The Journal of small animal practice. 2002;43(6):240–6. doi: 10.1111/j.1748-5827.2002.tb00066.x. [DOI] [PubMed] [Google Scholar]
- 12.Shiels MS, Engels EA, Linet MS, Clarke CA, Li J, Hall HI, et al. The epidemic of non-Hodgkin lymphoma in the United States: disentangling the effect of HIV, 1992–2009. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2013;22(6):1069–78. doi: 10.1158/1055-9965.EPI-13-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Modiano JF, Breen M, Burnett RC, Parker HG, Inusah S, Thomas R, et al. Distinct B-cell and T-cell lymphoproliferative disease prevalence among dog breeds indicates heritable risk. Cancer research. 2005;65(13):5654–61. doi: 10.1158/0008-5472.CAN-04-4613. [DOI] [PubMed] [Google Scholar]
- 14.Merlo DF, Rossi L, Pellegrino C, Ceppi M, Cardellino U, Capurro C, et al. Cancer incidence in pet dogs: findings of the Animal Tumor Registry of Genoa, Italy. Journal of veterinary internal medicine/American College of Veterinary Internal Medicine. 2008;22(4):976–84. doi: 10.1111/j.1939-1676.2008.0133.x. [DOI] [PubMed] [Google Scholar]
- 15.Villamil JA, Henry CJ, Hahn AW, Bryan JN, Tyler JW, Caldwell CW. Hormonal and sex impact on the epidemiology of canine lymphoma. Journal of cancer epidemiology. 2009;2009:591753. doi: 10.1155/2009/591753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garber K. Lymphoma rate rise continues to baffle researchers. Journal of the National Cancer Institute. 2001;93(7):494–6. doi: 10.1093/jnci/93.7.494. [DOI] [PubMed] [Google Scholar]
- 17.Jemal A, Clegg LX, Ward E, Ries LA, Wu X, Jamison PM, et al. Annual report to the nation on the status of cancer, 1975–2001, with a special feature regarding survival. Cancer. 2004;101(1):3–27. doi: 10.1002/cncr.20288. [DOI] [PubMed] [Google Scholar]
- 18.Dorn CR, Taylor DO, Hibbard HH. Epizootiologic characteristics of canine and feline leukemia and lymphoma. American journal of veterinary research. 1967;28(125):993–1001. [PubMed] [Google Scholar]
- 19.Edwards DS, Henley WE, Harding EF, Dobson JM, Wood JL. Breed incidence of lymphoma in a UK population of insured dogs. Veterinary and comparative oncology. 2003;1(4):200–6. doi: 10.1111/j.1476-5810.2003.00025.x. [DOI] [PubMed] [Google Scholar]
- 20.Ponce F, Marchal T, Magnol JP, Turinelli V, Ledieu D, Bonnefont C, et al. A morphological study of 608 cases of canine malignant lymphoma in France with a focus on comparative similarities between canine and human lymphoma morphology. Veterinary pathology. 2010;47(3):414–33. doi: 10.1177/0300985810363902. [DOI] [PubMed] [Google Scholar]
- 21.Priester WA, McKay FW. The occurrence of tumors in domestic animals. National Cancer Institute monograph. 1980;(54):1–210. [PubMed] [Google Scholar]
- 22.Teske E. Canine malignant lymphoma: a review and comparison with human non-Hodgkin’s lymphoma. The Veterinary quarterly. 1994;16(4):209–19. doi: 10.1080/01652176.1994.9694451. [DOI] [PubMed] [Google Scholar]
- 23.Lurie DM, Lucroy MD, Griffey SM, Simonson E, Madewell BR. T-cell-derived malignant lymphoma in the boxer breed. Veterinary and comparative oncology. 2004;2(3):171–5. doi: 10.1111/j.1476-5810.2004.00047.x. [DOI] [PubMed] [Google Scholar]
- 24.Lurie DM, Milner RJ, Suter SE, Vernau W. Immunophenotypic and cytomorphologic subclassification of T-cell lymphoma in the boxer breed. Veterinary immunology and immunopathology. 2008;125(1–2):102–10. doi: 10.1016/j.vetimm.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 25.Modiano JF, Breen M, Avery AC, et al. Breed specific canine lymphoproliferative diseases. In: Ostrander EA, Giger U, Lindblad-Toh K, editors. The Dog and it’s Genome. Cold Spring Harbor: Cold Spring Harbor Press; 2010. pp. 439–50. [Google Scholar]
- 26.LeBlanc AK. Cancer and comparative imaging. ILAR journal/National Research Council, Institute of Laboratory Animal Resources. 2014;55(1):164–8. doi: 10.1093/ilar/ilu014. [DOI] [PubMed] [Google Scholar]
- 27.National Cancer Institute sponsored study of classifications of non-Hodgkin’s lymphomas: summary and description of a working formulation for clinical usage. The Non-Hodgkin’s Lymphoma Pathologic Classification Project. Cancer. 1982;49:2112–35. doi: 10.1002/1097-0142(19820515)49:10<2112::aid-cncr2820491024>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 28.Lennert KFA. Histopathology of non-Hodkin’s lymphomas (based on the Updated Kiel Classification) 2. Berlin: Springer-Verlag; 1990. [Google Scholar]
- 29.Valli VE, JR, Parodi AL, et al. Histologic classification of hematopoietic tumors of domestic animals. Washington DC: Armed Forces Institute of Pathology and The World Health Organization; 2002. [Google Scholar]
- 30.Valli VE, Kass PH, San Myint M, Scott F. Canine lymphomas: association of classification type, disease stage, tumor subtype, mitotic rate, and treatment with survival. Veterinary pathology. 2013;50(5):738–48. doi: 10.1177/0300985813478210. [DOI] [PubMed] [Google Scholar]
- 31.Vezzali EPA, Marcato PS, et al. Histopathological classification of 171 cases of canine and feline non-Hodgkin lymphoma according to the WHO. Veterinary and comparative oncology. 2009;8:38–49. doi: 10.1111/j.1476-5829.2009.00201.x. [DOI] [PubMed] [Google Scholar]
- 32.Rizvi MA, Evens AM, Tallman MS, Nelson BP, Rosen ST. T-cell non-Hodgkin lymphoma. Blood. 2006;107(4):1255–64. doi: 10.1182/blood-2005-03-1306. [DOI] [PubMed] [Google Scholar]
- 33.Wang SS, Vose JM. Epidemiology and Prognosis of T-Cell Lymphoma. In: Foss F, editor. T-cell Lymphomas. Contemporary Hematology. XV. New York: Springer Science+Business Media; 2013. pp. 25–39. [Google Scholar]
- 34.Chiou SH, Chow KC, Yang CH, Chiang SF, Lin CH. Discovery of Epstein-Barr virus (EBV)-encoded RNA signal and EBV nuclear antigen leader protein DNA sequence in pet dogs. The Journal of general virology. 2005;86(Pt 4):899–905. doi: 10.1099/vir.0.80792-0. [DOI] [PubMed] [Google Scholar]
- 35.Milman G, Smith KC, Erles K. Serological detection of Epstein-Barr virus infection in dogs and cats. Veterinary microbiology. 2011;150(1–2):15–20. doi: 10.1016/j.vetmic.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 36.Chiu HC, Chow KC, Fan YH, Chang SC, Chiou SH, Chiang SF, et al. Expression of EBV-encoded oncogenes and EBV-like virions in multiple canine tumors. Veterinary microbiology. 2013;163(1–2):79–89. doi: 10.1016/j.vetmic.2012.12.022. [DOI] [PubMed] [Google Scholar]
- 37.Huang SH, Kozak PJ, Kim J, Habineza-Ndikuyeze G, Meade C, Gaurnier-Hausser A, et al. Evidence of an oncogenic gammaherpesvirus in domestic dogs. Virology. 2012;427(2):107–17. doi: 10.1016/j.virol.2012.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.MacEwen EG. Spontaneous tumors in dogs and cats: models for the study of cancer biology and treatment. Cancer metastasis reviews. 1990;9(2):125–36. doi: 10.1007/BF00046339. [DOI] [PubMed] [Google Scholar]
- 39.Daters AT, Mauldin GE, Mauldin GN, Brodsky EM, Post GS. Evaluation of a multidrug chemotherapy protocol with mitoxantrone based maintenance (CHOP-MA) for the treatment of canine lymphoma. Veterinary and comparative oncology. 2010;8(1):11–22. doi: 10.1111/j.1476-5829.2009.00199.x. [DOI] [PubMed] [Google Scholar]
- 40.Garrett LD, Thamm DH, Chun R, Dudley R, Vail DM. Evaluation of a 6-month chemotherapy protocol with no maintenance therapy for dogs with lymphoma. Journal of veterinary internal medicine/American College of Veterinary Internal Medicine. 2002;16(6):704–9. doi: 10.1892/0891-6640(2002)016<0704:eoacpw>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 41.MacEwen EG, Patnaik AK, Wilkins RJ. Diagnosis and treatment of canine hematopoietic neoplasms. The Veterinary clinics of North America. 1977;7(1):105–18. doi: 10.1016/s0091-0279(77)50009-3. [DOI] [PubMed] [Google Scholar]
- 42.MacDonald VS, Thamm DH, Kurzman ID, Turek MM, Vail DM. Does L-asparaginase influence efficacy or toxicity when added to a standard CHOP protocol for dogs with lymphoma? Journal of veterinary internal medicine/American College of Veterinary Internal Medicine. 2005;19(5):732–6. doi: 10.1892/0891-6640(2005)19[732:dlieot]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 43.Flood-Knapik KE, Durham AC, Gregor TP, Sanchez MD, Durney ME, Sorenmo KU. Clinical, histopathological and immunohistochemical characterization of canine indolent lymphoma. Veterinary and comparative oncology. 2013;11(4):272–86. doi: 10.1111/j.1476-5829.2011.00317.x. [DOI] [PubMed] [Google Scholar]
- 44.Richards KL, Motsinger-Reif AA, Chen HW, Fedoriw Y, Fan C, Nielsen DM, et al. Gene profiling of canine B-cell lymphoma reveals germinal center and postgerminal center subtypes with different survival times, modeling human DLBCL. Cancer research. 2013;73(16):5029–39. doi: 10.1158/0008-5472.CAN-12-3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Frantz AM, Sarver AL, Ito D, Phang TL, Karimpour-Fard A, Scott MC, et al. Molecular profiling reveals prognostically significant subtypes of canine lymphoma. Veterinary pathology. 2013;50(4):693–703. doi: 10.1177/0300985812465325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brown EM, Ruslander DM, Azuma C, Moore AS, Bengtson AE, Quesenberry PJ, et al. A feasibility study of low-dose total body irradiation for relapsed canine lymphoma. Veterinary and comparative oncology. 2006;4(2):75–83. doi: 10.1111/j.1476-5810.2006.00095.x. [DOI] [PubMed] [Google Scholar]
- 47.Laing EJ, Fitzpatrick PJ, Binnington AG, Norris AM, Mosseri A, Rider WD, et al. Half-body radiotherapy in the treatment of canine lymphoma. Journal of veterinary internal medicine/American College of Veterinary Internal Medicine. 1989;3(2):102–8. doi: 10.1111/j.1939-1676.1989.tb03087.x. [DOI] [PubMed] [Google Scholar]
- 48.Rassnick KM, McEntee MC, Erb HN, Burke BP, Balkman CE, Flory AB, et al. Comparison of 3 protocols for treatment after induction of remission in dogs with lymphoma. Journal of veterinary internal medicine/American College of Veterinary Internal Medicine. 2007;21(6):1364–73. doi: 10.1892/07-057.1. [DOI] [PubMed] [Google Scholar]
- 49.Gustafson NR, Lana SE, Mayer MN, LaRue SM. A preliminary assessment of whole-body radiotherapy interposed within a chemotherapy protocol for canine lymphoma. Veterinary and comparative oncology. 2004;2(3):125–31. doi: 10.1111/j.1476-5810.2004.00046.x. [DOI] [PubMed] [Google Scholar]
- 50.Lurie DM, Gordon IK, Theon AP, Rodriguez CO, Suter SE, Kent MS. Sequential low-dose rate half-body irradiation and chemotherapy for the treatment of canine multicentric lymphoma. Journal of veterinary internal medicine/American College of Veterinary Internal Medicine. 2009;23(5):1064–70. doi: 10.1111/j.1939-1676.2009.0353.x. [DOI] [PubMed] [Google Scholar]
- 51.Lurie DM, Kent MS, Fry MM, Theon AP. A toxicity study of low-dose rate half-body irradiation and chemotherapy in dogs with lymphoma. Veterinary and comparative oncology. 2008;6(4):257–67. doi: 10.1111/j.1476-5829.2008.00164.x. [DOI] [PubMed] [Google Scholar]
- 52.Williams LE, Johnson JL, Hauck ML, Ruslander DM, Price GS, Thrall DE. Chemotherapy followed by half-body radiation therapy for canine lymphoma. Journal of veterinary internal medicine/American College of Veterinary Internal Medicine. 2004;18(5):703–9. doi: 10.1892/0891-6640(2004)18<703:cfbhrt>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 53.Coiffier B. Rituximab therapy in malignant lymphoma. Oncogene. 2007;26(25):3603–13. doi: 10.1038/sj.onc.1210376. [DOI] [PubMed] [Google Scholar]
- 54.Jubala CM, Wojcieszyn JW, Valli VE, Getzy DM, Fosmire SP, Coffey D, et al. CD20 expression in normal canine B cells and in canine non-Hodgkin lymphoma. Veterinary pathology. 2005;42(4):468–76. doi: 10.1354/vp.42-4-468. [DOI] [PubMed] [Google Scholar]
- 55.Ito D, Brewer S, Modiano JF, Beall MJ. Development of a novel anti-canine CD20 monoclonal antibody with diagnostic and therapeutic potential. Leukemia & lymphoma. 2014 doi: 10.3109/10428194.2014.914193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Aratana Therapeutics I. Financial Statements and Exhibits, Ex-99.1. 2014 [Google Scholar]
- 57.Zinzani PL, Corradini P, Gallamini A, Grossi A, Lazzarino M, Marchetti M, et al. Overview of alemtuzumab therapy for the treatment of T-cell lymphomas. Leukemia & lymphoma. 2012;53(5):789–95. doi: 10.3109/10428194.2011.629701. [DOI] [PubMed] [Google Scholar]
- 58.Aratana Therapeutics Initiates and Doses First Patient in Market Development Studies of AT-005 for Treating Canine T-cell Lymphoma [press release] PR Newswire. 2014 [Google Scholar]
- 59.Rosales C, Jeglum KA, Obrocka M, Steplewski Z. Cytolytic activity of murine anti-dog lymphoma monoclonal antibodies with canine effector cells and complement. Cellular immunology. 1988;115(2):420–8. doi: 10.1016/0008-8749(88)90194-3. [DOI] [PubMed] [Google Scholar]
- 60.Stein R, Balkman C, Chen S, Rassnick K, McEntee M, Page R, et al. Evaluation of anti-human leukocyte antigen-DR monoclonal antibody therapy in spontaneous canine lymphoma. Leukemia & lymphoma. 2011;52(2):273–84. doi: 10.3109/10428194.2010.535182. [DOI] [PubMed] [Google Scholar]
- 61.Steplewski Z, Rosales C, Jeglum KA, McDonald-Smith J. In vivo destruction of canine lymphoma mediated by murine monoclonal antibodies. In vivo. 1990;4(4):231–4. [PubMed] [Google Scholar]
- 62.Singer J, Fazekas J, Wang W, Weichselbaumer M, Matz M, Mader A, et al. Generation of a Canine Anti-EGFR (ErbB-1) Antibody for Passive Immunotherapy in Dog Cancer Patients. Molecular cancer therapeutics. 2014 doi: 10.1158/1535-7163.MCT-13-0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Buckner D, Eisel R, Perry S. Blood cell separation in the dog by continuous flow centrifugation. Blood. 1968;31(5):653–72. [PubMed] [Google Scholar]
- 64.Lee R, Storb R, Little MT, Joslyn A, Spector M, Kuhr CS. Percutaneous central dual-lumen catheter for apheresis in the canine. Journal of investigative surgery: the official journal of the Academy of Surgical Research. 2002;15(6):337–41. doi: 10.1080/08941930290086155. [DOI] [PubMed] [Google Scholar]
- 65.Deeg HJ, Appelbaum FR, Weiden PL, Hackman RC, Graham TC, Storb RC. Autologous marrow transplantation as consolidation therapy for canine lymphoma: efficacy and toxicity of various regimens of total body irradiation. American journal of veterinary research. 1985;46(9):2016–8. [PubMed] [Google Scholar]
- 66.Weiden PL, Storb R, Deeg HJ, Graham TC. Total body irradiation and autologous marrow transplantation as consolidation therapy for spontaneous canine lymphoma in remission. Experimental hematology. 1979;7 (Suppl 5):160–3. [PubMed] [Google Scholar]
- 67.Weiden PL, Storb R, Sale GE, Graham TC, Thomas ED. Allogeneic hematopoietic grafts after total-body irradiation in dogs with spontaneous tumors. Journal of the National Cancer Institute. 1978;61(2):353–7. [PubMed] [Google Scholar]
- 68.Appelbaum FR, Deeg HJ, Storb R, Graham TC, Charrier K, Bensinger W. Cure of malignant lymphoma in dogs with peripheral blood stem cell transplantation. Transplantation. 1986;42(1):19–22. doi: 10.1097/00007890-198607000-00004. [DOI] [PubMed] [Google Scholar]
- 69.Weiden PL, Storb R, Deeg HJ, Graham TC, Thomas ED. Prolonged disease-free survival in dogs with lymphoma after total-body irradiation and autologous marrow transplantation consolidation of combination-chemotherapy-induced remissions. Blood. 1979;54(5):1039–49. [PubMed] [Google Scholar]
- 70.Lupu M, Gooley T, Zellmer E, Graves SS, Storb R. Principles of peripheral blood mononuclear cell apheresis in a preclinical canine model of hematopoietic cell transplantation. Journal of veterinary internal medicine/American College of Veterinary Internal Medicine. 2008;22(1):74–82. doi: 10.1111/j.1939-1676.2007.0016.x. [DOI] [PubMed] [Google Scholar]
- 71.Suter SE. Collection of peripheral blood CD34+ progenitor cells from healthy dogs and dogs diagnosed with lymphoproliferative diseases using a Baxter-Fenwal CS-3000 Plus blood cell separator. Journal of veterinary internal medicine/American College of Veterinary Internal Medicine. 2011;25(6):1406–13. doi: 10.1111/j.1939-1676.2011.00827.x. [DOI] [PubMed] [Google Scholar]
- 72.Bodenberger U, Kolb HJ, Rieder I, Netzel B, Schaffer E, Kolb H, et al. Fractionated total body irradiation and autologous bone marrow transplantation in dogs: hemopoietic recovery after various marrow cell doses. Experimental hematology. 1980;8(4):384–94. [PubMed] [Google Scholar]
- 73.Escobar C, Grindem C, Neel JA, Suter SE. Hematologic changes after total body irradiation and autologous transplantation of hematopoietic peripheral blood progenitor cells in dogs with lymphoma. Veterinary pathology. 2012;49(2):341–3. doi: 10.1177/0300985811410721. [DOI] [PubMed] [Google Scholar]
- 74.Willcox JL, Pruitt A, Suter SE. Autologous peripheral blood hematopoietic cell transplantation in dogs with B-cell lymphoma. Journal of veterinary internal medicine/American College of Veterinary Internal Medicine. 2012;26(5):1155–63. doi: 10.1111/j.1939-1676.2012.00980.x. [DOI] [PubMed] [Google Scholar]
- 75.Warry EE, Willcox JL, Suter SE. Autologous peripheral blood hematopoietic cell transplantation in dogs with T-cell lymphoma. Journal of veterinary internal medicine/American College of Veterinary Internal Medicine. 2014;28(2):529–37. doi: 10.1111/jvim.12302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Frimberger AE, Moore AS, Rassnick KM, Cotter SM, O’Sullivan JL, Quesenberry PJ. A combination chemotherapy protocol with dose intensification and autologous bone marrow transplant (VELCAP-HDC) for canine lymphoma. Journal of veterinary internal medicine/American College of Veterinary Internal Medicine. 2006;20(2):355–64. doi: 10.1892/0891-6640(2006)20[355:accpwd]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 77.Veterinary cooperative oncology group - common terminology criteria for adverse events (VCOG-CTCAE) following chemotherapy or biological antineoplastic therapy in dogs and cats v1.1. Veterinary and comparative oncology. 2011 doi: 10.1111/vco.283. [DOI] [PubMed] [Google Scholar]
- 78.Nguyen SM, Thamm DH, Vail DM, London CA. Response evaluation criteria for solid tumours in dogs (v1.0): a Veterinary Cooperative Oncology Group (VCOG) consensus document. Veterinary and comparative oncology. 2013 doi: 10.1111/vco.12032. [DOI] [PubMed] [Google Scholar]
- 79.Vail DM, Michels GM, Khanna C, Selting KA, London CA Veterinary Cooperative Oncology G. Response evaluation criteria for peripheral nodal lymphoma in dogs (v1.0)--a Veterinary Cooperative Oncology Group (VCOG) consensus document. Veterinary and comparative oncology. 2010;8(1):28–37. doi: 10.1111/j.1476-5829.2009.00200.x. [DOI] [PubMed] [Google Scholar]
- 80.Gordon I, Paoloni M, Mazcko C, Khanna C. The Comparative Oncology Trials Consortium: using spontaneously occurring cancers in dogs to inform the cancer drug development pathway. PLoS medicine. 2009;6(10):e1000161. doi: 10.1371/journal.pmed.1000161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Paoloni MC, Tandle A, Mazcko C, Hanna E, Kachala S, Leblanc A, et al. Launching a novel preclinical infrastructure: comparative oncology trials consortium directed therapeutic targeting of TNFalpha to cancer vasculature. PloS one. 2009;4(3):e4972. doi: 10.1371/journal.pone.0004972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Paoloni M, Lana S, Thamm D, Mazcko C, Withrow S. The creation of the Comparative Oncology Trials Consortium Pharmacodynamic Core: Infrastructure for a virtual laboratory. Veterinary journal. 2010;185(1):88–9. doi: 10.1016/j.tvjl.2010.04.019. [DOI] [PubMed] [Google Scholar]
- 83.Vail DM. Comparative oncology - the North American experience. BMC proceedings. 2013;7(Suppl 2):K5. doi: 10.1186/1753-6561-7-S2-K5. Sao Paulo Advanced School of Comparative Oncology: AbstractsClaudia Aparecida Rainho, Patricia Pintor dos Reis and Silvia Regina RogattoPublication of this supplement was funded by the Sao Paulo Research Foundation (FAPESP)) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hartmann EM, Ott G, Rosenwald A. Molecular biology and genetics of lymphomas. Hematology/oncology clinics of North America. 2008;22(5):807–23. vii. doi: 10.1016/j.hoc.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 85.Breen M, Modiano JF. Evolutionarily conserved cytogenetic changes in hematological malignancies of dogs and humans--man and his best friend share more than companionship. Chromosome research: an international journal on the molecular, supramolecular and evolutionary aspects of chromosome biology. 2008;16(1):145–54. doi: 10.1007/s10577-007-1212-4. [DOI] [PubMed] [Google Scholar]
- 86.Thomas R, Seiser EL, Motsinger-Reif A, Borst L, Valli VE, Kelley K, et al. Refining tumor-associated aneuploidy through ‘genomic recoding’ of recurrent DNA copy number aberrations in 150 canine non-Hodgkin lymphomas. Leukemia & lymphoma. 2011;52(7):1321–35. doi: 10.3109/10428194.2011.559802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Derrien T, Andre C, Galibert F, Hitte C. AutoGRAPH: an interactive web server for automating and visualizing comparative genome maps. Bioinformatics. 2007;23(4):498–9. doi: 10.1093/bioinformatics/btl618. [DOI] [PubMed] [Google Scholar]
- 88.Alizadeh AA, Eisen MB, Davis RE, Ma C, Lossos IS, Rosenwald A, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000;403(6769):503–11. doi: 10.1038/35000501. [DOI] [PubMed] [Google Scholar]
- 89.Mudaliar MA, Haggart RD, Miele G, Sellar G, Tan KA, Goodlad JR, et al. Comparative gene expression profiling identifies common molecular signatures of NF-kappaB activation in canine and human diffuse large B cell lymphoma (DLBCL) PloS one. 2013;8(9):e72591. doi: 10.1371/journal.pone.0072591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Paoloni M, Davis S, Lana S, Withrow S, Sangiorgi L, Picci P, et al. Canine tumor cross-species genomics uncovers targets linked to osteosarcoma progression. BMC genomics. 2009;10:625. doi: 10.1186/1471-2164-10-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Scott MC, Sarver AL, Gavin KJ, Thayanithy V, Getzy DM, Newman RA, et al. Molecular subtypes of osteosarcoma identified by reducing tumor heterogeneity through an interspecies comparative approach. Bone. 2011;49(3):356–67. doi: 10.1016/j.bone.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Su Y, Nielsen D, Zhu L, Richards K, Suter S, Breen M, et al. Gene selection and cancer type classification of diffuse large-B-cell lymphoma using a bivariate mixture model for two-species data. Human genomics. 2013;7:2. doi: 10.1186/1479-7364-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Comazzi S, Gelain ME. Use of flow cytometric immunophenotyping to refine the cytological diagnosis of canine lymphoma. Veterinary journal. 2011;188(2):149–55. doi: 10.1016/j.tvjl.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 94.Wilkerson MJ, Dolce K, Koopman T, Shuman W, Chun R, Garrett L, et al. Lineage differentiation of canine lymphoma/leukemias and aberrant expression of CD molecules. Veterinary immunology and immunopathology. 2005;106(3–4):179–96. doi: 10.1016/j.vetimm.2005.02.020. [DOI] [PubMed] [Google Scholar]
- 95.Hans CP, Weisenburger DD, Greiner TC, Gascoyne RD, Delabie J, Ott G, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103(1):275–82. doi: 10.1182/blood-2003-05-1545. [DOI] [PubMed] [Google Scholar]
- 96.Nowakowski GS, LaPlant B, Macon WR, Reeder CB, Foran JM, Nelson GD, et al. Effect of lenalidomide combined with R-CHOP (R2CHOP) on negative prognostic impact of nongerminal center (non-GCB) phenotype in newly diagnosed diffuse large B-cell lymphoma: A phase 2 study. J Clin Oncol; 2014 ASCO Annual Meeting; Chicago, IL. 2014. [DOI] [PubMed] [Google Scholar]
- 97.Ruan J, Martin P, Furman RR, Lee SM, Cheung K, Vose JM, et al. Bortezomib plus CHOP-rituximab for previously untreated diffuse large B-cell lymphoma and mantle cell lymphoma. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2011;29(6):690–7. doi: 10.1200/JCO.2010.31.1142. [DOI] [PubMed] [Google Scholar]
- 98.Thieblemont C, Briere J, Mounier N, Voelker HU, Cuccuini W, Hirchaud E, et al. The germinal center/activated B-cell subclassification has a prognostic impact for response to salvage therapy in relapsed/refractory diffuse large B-cell lymphoma: a bio-CORAL study. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2011;29(31):4079–87. doi: 10.1200/JCO.2011.35.4423. [DOI] [PubMed] [Google Scholar]
- 99.Wilson WH. Treatment strategies for aggressive lymphomas: what works? Hematology/the Education Program of the American Society of Hematology American Society of Hematology Education Program. 2013;2013:584–90. doi: 10.1182/asheducation-2013.1.584. [DOI] [PubMed] [Google Scholar]
- 100.Coutinho R, Clear AJ, Owen A, Wilson A, Matthews J, Lee A, et al. Poor concordance among nine immunohistochemistry classifiers of cell-of-origin for diffuse large B-cell lymphoma: implications for therapeutic strategies. Clinical cancer research: an official journal of the American Association for Cancer Research. 2013;19(24):6686–95. doi: 10.1158/1078-0432.CCR-13-1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sato M, Kanemoto H, Kagawa Y, Kobayashi T, Goto-Koshino Y, Mochizuki H, et al. Evaluation of the prognostic significance of BCL6 gene expression in canine high-grade B-cell lymphoma. Veterinary journal. 2012;191(1):108–14. doi: 10.1016/j.tvjl.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 102.Li S, Lin P, Young KH, Kanagal-Shamanna R, Yin CC, Medeiros LJ. MYC/BCL2 double-hit high-grade B-cell lymphoma. Advances in anatomic pathology. 2013;20(5):315–26. doi: 10.1097/PAP.0b013e3182a289f2. [DOI] [PubMed] [Google Scholar]
- 103.Nguyen F, Fourel JAA, Moreau A, Le Gouill S, Davodeau F, editors. Canine lymphoma as a spontaneous model in oncology: Special emphasis on DLBCLs. INCa Workshop, Spontaneous tumours in dogs: Nosology conference for 4 tumour types; 2014; Nantes, France. [Google Scholar]
- 104.Chen HW, Small GW, Motsinger-Reif A, Suter SE, Richards KL. VH1-44 gene usage defines a subset of canine B-cell lymphomas associated with better patient survival. Veterinary immunology and immunopathology. 2014;157(3–4):125–30. doi: 10.1016/j.vetimm.2013.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lossos IS, Alizadeh AA, Eisen MB, Chan WC, Brown PO, Botstein D, et al. Ongoing immunoglobulin somatic mutation in germinal center B cell-like but not in activated B cell-like diffuse large cell lymphomas. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(18):10209–13. doi: 10.1073/pnas.180316097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Small D. Targeting FLT3 for the treatment of leukemia. Seminars in hematology. 2008;45(3 Suppl 2):S17–21. doi: 10.1053/j.seminhematol.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Suter SE, Small GW, Seiser EL, Thomas R, Breen M, Richards KL. FLT3 mutations in canine acute lymphocytic leukemia. BMC cancer. 2011;11:38. doi: 10.1186/1471-2407-11-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gasparini C, Celeghini C, Monasta L, Zauli G. NF-kappaB pathways in hematological malignancies. Cellular and molecular life sciences: CMLS. 2014;71(11):2083–102. doi: 10.1007/s00018-013-1545-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gaurnier-Hausser A, Patel R, Baldwin AS, May MJ, Mason NJ. NEMO-binding domain peptide inhibits constitutive NF-kappaB activity and reduces tumor burden in a canine model of relapsed, refractory diffuse large B-cell lymphoma. Clinical cancer research: an official journal of the American Association for Cancer Research. 2011;17(14):4661–71. doi: 10.1158/1078-0432.CCR-10-3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Habineza Ndikuyeze G, Gaurnier-Hausser A, Patel R, Baldwin AS, May MJ, Flood P, et al. A Phase I Clinical Trial of Systemically Delivered NEMO Binding Domain Peptide in Dogs with Spontaneous Activated B-Cell like Diffuse Large B-Cell Lymphoma. PloS one. 2014;9(5):e95404. doi: 10.1371/journal.pone.0095404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Niemann CU, Wiestner A. B-cell receptor signaling as a driver of lymphoma development and evolution. Seminars in cancer biology. 2013;23(6):410–21. doi: 10.1016/j.semcancer.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Maddocks K, Blum KA. Ibrutinib in B-cell Lymphomas. Current treatment options in oncology. 2014;15(2):226–37. doi: 10.1007/s11864-014-0274-8. [DOI] [PubMed] [Google Scholar]
- 113.Honigberg LA, Smith AM, Sirisawad M, Verner E, Loury D, Chang B, et al. The Bruton tyrosine kinase inhibitor PCI-32765 blocks B-cell activation and is efficacious in models of autoimmune disease and B-cell malignancy. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(29):13075–80. doi: 10.1073/pnas.1004594107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Voutsadakis IA. Apoptosis and the pathogenesis of lymphoma. Acta oncologica. 2000;39(2):151–6. doi: 10.1080/028418600430707. [DOI] [PubMed] [Google Scholar]
- 115.Uhl E, Krimer P, Schliekelman P, Tompkins SM, Suter S. Identification of altered MicroRNA expression in canine lymphoid cell lines and cases of B- and T-Cell lymphomas. Genes, chromosomes & cancer. 2011;50(11):950–67. doi: 10.1002/gcc.20917. [DOI] [PubMed] [Google Scholar]
- 116.He L, Thomson JM, Hemann MT, Hernando-Monge E, Mu D, Goodson S, et al. A microRNA polycistron as a potential human oncogene. Nature. 2005;435(7043):828–33. doi: 10.1038/nature03552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Pepper C, Bentley P, Hoy T. Regulation of clinical chemoresistance by bcl-2 and bax oncoproteins in B-cell chronic lymphocytic leukaemia. British journal of haematology. 1996;95(3):513–7. doi: 10.1046/j.1365-2141.1996.d01-1927.x. [DOI] [PubMed] [Google Scholar]
- 118.Thomas A, El Rouby S, Reed JC, Krajewski S, Silber R, Potmesil M, et al. Drug-induced apoptosis in B-cell chronic lymphocytic leukemia: relationship between p53 gene mutation and bcl-2/bax proteins in drug resistance. Oncogene. 1996;12(5):1055–62. [PubMed] [Google Scholar]
- 119.Oricchio E, Ciriello G, Jiang M, Boice MH, Schatz JH, Heguy A, et al. Frequent disruption of the RB pathway in indolent follicular lymphoma suggests a new combination therapy. The Journal of experimental medicine. 2014;211(7):1379–91. doi: 10.1084/jem.20132120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Monti S, Chapuy B, Takeyama K, Rodig SJ, Hao Y, Yeda KT, et al. Integrative analysis reveals an outcome-associated and targetable pattern of p53 and cell cycle deregulation in diffuse large B cell lymphoma. Cancer cell. 2012;22(3):359–72. doi: 10.1016/j.ccr.2012.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fosmire SP, Thomas R, Jubala CM, Wojcieszyn JW, Valli VE, Getzy DM, et al. Inactivation of the p16 cyclin-dependent kinase inhibitor in high-grade canine non-Hodgkin’s T-cell lymphoma. Veterinary pathology. 2007;44(4):467–78. doi: 10.1354/vp.44-4-467. [DOI] [PubMed] [Google Scholar]
- 122.Modiano JF, Breen M, Valli VE, Wojcieszyn JW, Cutter GR. Predictive value of p16 or Rb inactivation in a model of naturally occurring canine non-Hodgkin’s lymphoma. Leukemia. 2007;21(1):184–7. doi: 10.1038/sj.leu.2404392. [DOI] [PubMed] [Google Scholar]
- 123.Parker HG, Kim LV, Sutter NB, Carlson S, Lorentzen TD, Malek TB, et al. Genetic structure of the purebred domestic dog. Science. 2004;304(5674):1160–4. doi: 10.1126/science.1097406. [DOI] [PubMed] [Google Scholar]
- 124.Seiser EL, Thomas R, Richards KL, Kelley MK, Moore P, Suter SE, et al. Reading between the lines: molecular characterization of five widely used canine lymphoid tumour cell lines. Veterinary and comparative oncology. 2013;11(1):30–50. doi: 10.1111/j.1476-5829.2011.00299.x. [DOI] [PubMed] [Google Scholar]
- 125.Ito D, Frantz AM, Williams C, Thomas R, Burnett RC, Avery AC, et al. CD40 ligand is necessary and sufficient to support primary diffuse large B-cell lymphoma cells in culture: a tool for in vitro preclinical studies with primary B-cell malignancies. Leukemia & lymphoma. 2012;53(7):1390–8. doi: 10.3109/10428194.2011.654337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hoeppner MP, Lundquist A, Pirun M, Meadows JR, Zamani N, Johnson J, et al. An improved canine genome and a comprehensive catalogue of coding genes and non-coding transcripts. PloS one. 2014;9(3):e91172. doi: 10.1371/journal.pone.0091172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Emenaker N, Kagan J. Biomarkers and “Omics”. In: Ettinger SJ, Feldman EC, editors. Textbook of Veterinary Internal Medicine: Diseases of the Dog and Cat. 7. Vol. 1. St. Louis, Missouri: Saunders; 2010. pp. 2117–20. [Google Scholar]
- 128.Ghernati I, Auger C, Chabanne L, Corbin A, Bonnefont C, Magnol JP, et al. Characterization of a canine long-term T cell line (DLC 01) established from a dog with Sezary syndrome and producing retroviral particles. Leukemia. 1999;13(8):1281–90. doi: 10.1038/sj.leu.2401480. [DOI] [PubMed] [Google Scholar]
- 129.Ito D, Endicott MM, Jubala CM, Helm KM, Burnett RC, Husbands BD, et al. A tumor-related lymphoid progenitor population supports hierarchical tumor organization in canine B-cell lymphoma. Journal of veterinary internal medicine/American College of Veterinary Internal Medicine. 2011;25(4):890–6. doi: 10.1111/j.1939-1676.2011.0756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kisseberth WC, Nadella MV, Breen M, Thomas R, Duke SE, Murahari S, et al. A novel canine lymphoma cell line: a translational and comparative model for lymphoma research. Leukemia research. 2007;31(12):1709–20. doi: 10.1016/j.leukres.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 131.Rutgen BC, Willenbrock S, Reimann-Berg N, Walter I, Fuchs-Baumgartinger A, Wagner S, et al. Authentication of primordial characteristics of the CLBL-1 cell line prove the integrity of a canine B-cell lymphoma in a murine in vivo model. PloS one. 2012;7(6):e40078. doi: 10.1371/journal.pone.0040078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Umeki S, Ema Y, Suzuki R, Kubo M, Hayashi T, Okamura Y, et al. Establishment of five canine lymphoma cell lines and tumor formation in a xenotransplantation model. The Journal of veterinary medical science/the Japanese Society of Veterinary Science. 2013;75(4):467–74. doi: 10.1292/jvms.12-0448. [DOI] [PubMed] [Google Scholar]
- 133.Argyle DJ, Madewell BR. A landmark in Veterinary and Comparative Oncology. Veterinary and comparative oncology. 2003;1(1):1–2. doi: 10.1046/j.1476-5829.2003.00011.x. [DOI] [PubMed] [Google Scholar]
- 134.Comazzi S, Guscetti F, Marconato L. First meeting of the European canine lymphoma group. Hematological oncology; Workshop: state of the art and comparative aspects in canine lymphoma; CH-Lugano. 22 June 2013; 2013. [DOI] [PubMed] [Google Scholar]
- 135.Ledford H. Translational research: 4 ways to fix the clinical trial. Nature. 2011;477(7366):526–8. doi: 10.1038/477526a. [DOI] [PubMed] [Google Scholar]
- 136.Mack GS. Clinical trials going to the dogs: canine program to study tumor treatment, biology. Journal of the National Cancer Institute. 2006;98(3):161–2. doi: 10.1093/jnci/djj061. [DOI] [PubMed] [Google Scholar]
- 137.Fiocchi SC, Selting KA, Rosenberg MP, Kolli P, Lenaz G, Henry C. An open-label, dose-escalating phase I study of elsamitrucin (SPI 28090) in treatment of malignant solid tumors in dogs. Journal of veterinary internal medicine/American College of Veterinary Internal Medicine. 2011;25(4):897–902. doi: 10.1111/j.1939-1676.2011.0752.x. [DOI] [PubMed] [Google Scholar]
- 138.Gavazza A, Lubas G, Fridman A, Peruzzi D, Impellizeri JA, Luberto L, et al. Safety and efficacy of a genetic vaccine targeting telomerase plus chemotherapy for the therapy of canine B-cell lymphoma. Human gene therapy. 2013;24(8):728–38. doi: 10.1089/hum.2013.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Griessmayr PC, Gauthier M, Barber LG, Cotter SM. Mushroom-derived maitake PETfraction as single agent for the treatment of lymphoma in dogs. Journal of veterinary internal medicine/American College of Veterinary Internal Medicine. 2007;21(6):1409–12. doi: 10.1892/07-070.1. [DOI] [PubMed] [Google Scholar]
- 140.Henson MS, Curtsinger JM, Larson VS, Klausner JS, Modiano JF, Mescher MF, et al. Immunotherapy with autologous tumour antigen-coated microbeads (large multivalent immunogen), IL-2 and GM-CSF in dogs with spontaneous B-cell lymphoma. Veterinary and comparative oncology. 2011;9(2):95–105. doi: 10.1111/j.1476-5829.2010.00234.x. [DOI] [PubMed] [Google Scholar]
- 141.London CA, Bernabe LF, Barnard S, Kisseberth WC, Borgatti A, Henson M, et al. Preclinical evaluation of the novel, orally bioavailable Selective Inhibitor of Nuclear Export (SINE) KPT-335 in spontaneous canine cancer: results of a phase I study. PloS one. 2014;9(2):e87585. doi: 10.1371/journal.pone.0087585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Marconato L, Frayssinet P, Rouquet N, Comazzi S, Leone VF, Laganga P, et al. Randomized, placebo-controlled, double-blinded chemoimmunotherapy clinical trial in a pet dog model of diffuse large B-cell lymphoma. Clinical cancer research: an official journal of the American Association for Cancer Research. 2014;20(3):668–77. doi: 10.1158/1078-0432.CCR-13-2283. [DOI] [PubMed] [Google Scholar]
- 143.Meier V, Geigy C, Grosse N, McSheehy P, Rohrer Bley C. Use of epothilone B (patupilone) in refractory lymphoma and advanced solid tumors in dogs. Journal of veterinary internal medicine/American College of Veterinary Internal Medicine. 2013;27(1):120–5. doi: 10.1111/jvim.12019. [DOI] [PubMed] [Google Scholar]
- 144.Vail DM, Thamm DH, Reiser H, Ray AS, Wolfgang GH, Watkins WJ, et al. Assessment of GS-9219 in a pet dog model of non-Hodgkin’s lymphoma. Clinical cancer research: an official journal of the American Association for Cancer Research. 2009;15(10):3503–10. doi: 10.1158/1078-0432.CCR-08-3113. [DOI] [PubMed] [Google Scholar]
- 145.Silver M, Rusk A, Phillips B, Beck E, Jankowski M, Philibert J, et al. Evaluation of the oral antimitotic agent (ABT-751) in dogs with lymphoma. Journal of veterinary internal medicine/American College of Veterinary Internal Medicine. 2012;26(2):349–54. doi: 10.1111/j.1939-1676.2012.00892.x. [DOI] [PubMed] [Google Scholar]
- 146.Rusk A, Cozzi E, Stebbins M, Vail D, Graham J, Valli V, et al. Cooperative activity of cytotoxic chemotherapy with antiangiogenic thrombospondin-I peptides, ABT-526 in pet dogs with relapsed lymphoma. Clinical cancer research: an official journal of the American Association for Cancer Research. 2006;12(24):7456–64. doi: 10.1158/1078-0432.CCR-06-0110. [DOI] [PubMed] [Google Scholar]
- 147.Nakaichi M, Taura Y, Kanki M, Mamba K, Momoi Y, Tsujimoto H, et al. Establishment and characterization of a new canine B-cell leukemia cell line. The Journal of veterinary medical science/the Japanese Society of Veterinary Science. 1996;58(5):469–71. doi: 10.1292/jvms.58.469. [DOI] [PubMed] [Google Scholar]
- 148.Momoi Y, Okai Y, Watari T, Goitsuka R, Tsujimoto H, Hasegawa A. Establishment and characterization of a canine T-lymphoblastoid cell line derived from malignant lymphoma. Veterinary immunology and immunopathology. 1997;59(1–2):11–20. doi: 10.1016/s0165-2427(97)00053-6. [DOI] [PubMed] [Google Scholar]
- 149.Rutgen BC, Hammer SE, Gerner W, Christian M, de Arespacochaga AG, Willmann M, et al. Establishment and characterization of a novel canine B-cell line derived from a spontaneously occurring diffuse large cell lymphoma. Leukemia research. 2010;34(7):932–8. doi: 10.1016/j.leukres.2010.01.021. [DOI] [PubMed] [Google Scholar]
- 150.Holmes MA, Duffus WP, Gorman NT. Natural cytotoxicity in the dog: description of two new allogeneic tumour targets. Veterinary immunology and immunopathology. 1989;23(1–2):161–70. doi: 10.1016/0165-2427(89)90118-9. [DOI] [PubMed] [Google Scholar]
- 151.Strandstrom HV, Rimaila-Parnanen E. Canine atypical malignant lymphoma. American journal of veterinary research. 1979;40(7):1033–4. [PubMed] [Google Scholar]
- 152.Suter SE, Chein MB, von Messling V, Yip B, Cattaneo R, Vernau W, et al. In vitro canine distemper virus infection of canine lymphoid cells: a prelude to oncolytic therapy for lymphoma. Clinical cancer research: an official journal of the American Association for Cancer Research. 2005;11(4):1579–87. doi: 10.1158/1078-0432.CCR-04-1944. [DOI] [PubMed] [Google Scholar]
- 153.Steplewski Z, Jeglum KA, Rosales C, Weintraub N. Canine lymphoma-associated antigens defined by murine monoclonal antibodies. Cancer immunology, immunotherapy: CII. 1987;24(3):197–201. doi: 10.1007/BF00205629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.COTC. 2014 https://ccrod.cancer.gov/confluence/display/CCRCOPWeb/Comparative+Oncology+Trials+Consortium.