Abstract
New supportive evidence is advanced in favor of the hypothesis that the enzymic coagulation of guinea pig semen involves transamidase reactions that result in the formation of γ-glutamyl-ε-lysine intermolecular cross linkages between molecules of a basic protein in seminal vesicle secretion. The dipeptide γ-glutamyl-ε-lysine was isolated in large quantities from proteolytic digests of the coagulated basic vesicular secretion protein that comprises the seminal clot.
Keywords: anterior prostate clotting enzyme, cross linkages
Full text
PDF
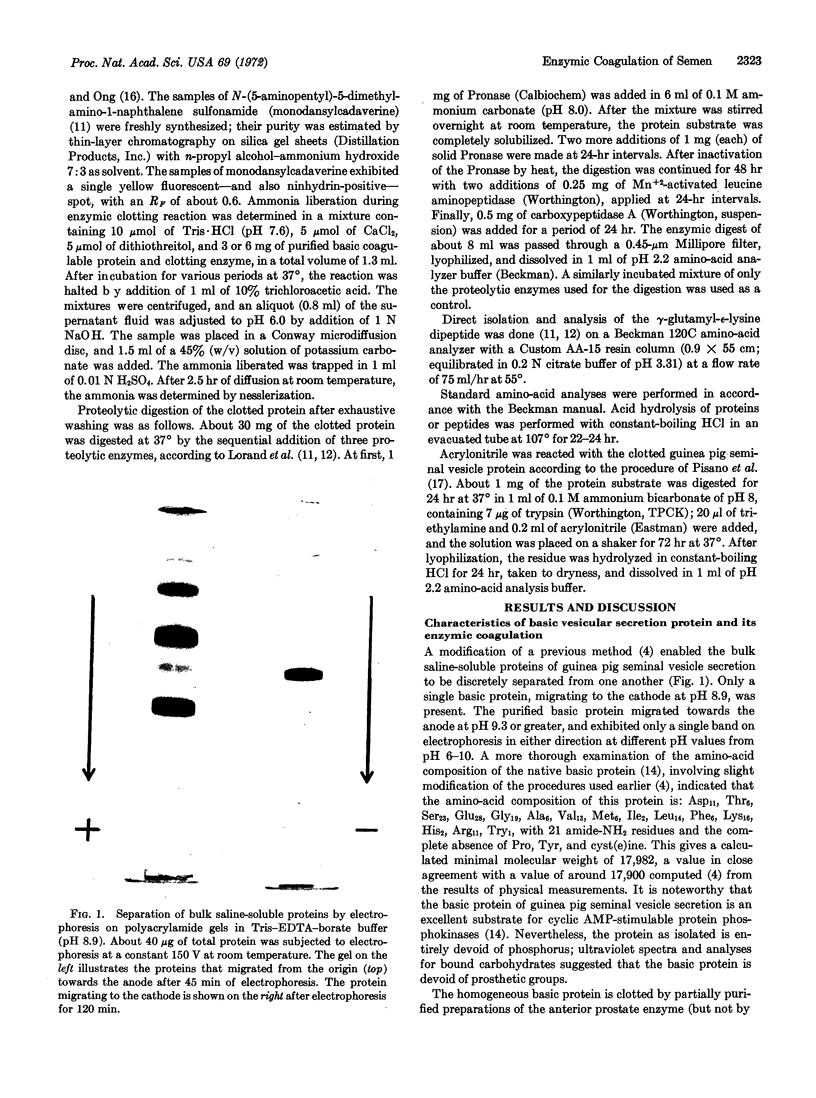
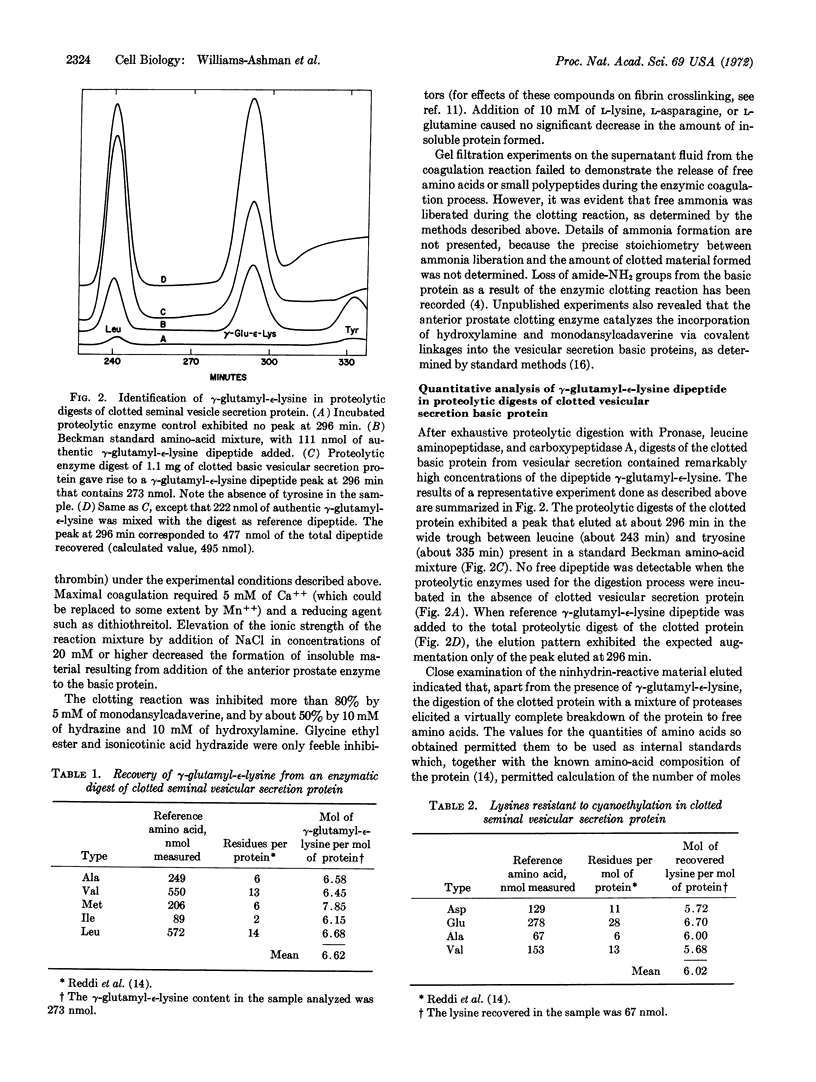

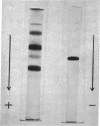
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BALLARD P., WILLIAMS-ASHMAN H. G. FRACTIONATION OF THE BULK PROTEINS OF SEMINAL VESICLE SECRETIONS. Invest Urol. 1964 Jul;2:38–46. [PubMed] [Google Scholar]
- GOTTERER B., GINSBERG D., SCHULMAN T., BANKS J., WILLIAMS-ASHMAN H. G. Enzymatic coagulation of semen. Nature. 1955 Dec 24;176(4495):1209–1211. doi: 10.1038/1761209a0. [DOI] [PubMed] [Google Scholar]
- GOTTERER G. S., WILLIAMS-ASHMAN H. G. Some factors which influence vesiculase action. Proc Soc Exp Biol Med. 1957 Jan;94(1):60–64. doi: 10.3181/00379727-94-22855. [DOI] [PubMed] [Google Scholar]
- Harding H. W., Rogers G. E. Epsilon-(gamma-glutamyl)lysine cross-linkage in citrulline-containing protein fractions from hair. Biochemistry. 1971 Feb 16;10(4):624–630. doi: 10.1021/bi00780a013. [DOI] [PubMed] [Google Scholar]
- Hart R. G. Cowper's gland secretion in rat semen coagulation. I. Isolation and amino acid analysis of the seminal vesicle substrate. Biol Reprod. 1970 Dec;3(3):347–352. doi: 10.1093/biolreprod/3.3.347. [DOI] [PubMed] [Google Scholar]
- Lorand L., Downey J., Gotoh T., Jacobsen A., Tokura S. The transpeptidase system which crosslinks fibrin by gamma-glutamyle-episilon-lysine bonds. Biochem Biophys Res Commun. 1968 Apr 19;31(2):222–230. doi: 10.1016/0006-291x(68)90734-1. [DOI] [PubMed] [Google Scholar]
- Lorand L., Ong H. H. Labeling of amine-acceptor cross-linking sites of fibrin by transpeptidation. Biochemistry. 1966 May;5(5):1747–1753. doi: 10.1021/bi00869a043. [DOI] [PubMed] [Google Scholar]
- Lorand L., Rule N. G., Ong H. H., Furlanetto R., Jacobsen A., Downey J., Oner N., Bruner-Lorand J. Amine specificity in transpeptidation. Inhibition of fibrin cross-linking. Biochemistry. 1968 Mar;7(3):1214–1223. doi: 10.1021/bi00843a043. [DOI] [PubMed] [Google Scholar]
- MANYAI S. ISOLATION OF THE CLOTTABLE PROTEIN FROM THE SECRETION OF THE RAT'S SEMINAL VESICLE. Acta Physiol Acad Sci Hung. 1964;24:419–432. [PubMed] [Google Scholar]
- Mányai S., Beney L., Czuppon A. Some characteristics of the clottable protein secreted by the seminal vesicles of the rat. Acta Physiol Acad Sci Hung. 1965;28(2):105–115. [PubMed] [Google Scholar]
- Mányai S. Interaction of the proteins of the rat's seminal vesicle secretion with macromolecular polyanions. Acta Physiol Acad Sci Hung. 1965;28(4):295–307. [PubMed] [Google Scholar]
- Notides A. C., Williams-Ashman H. G. The basic protein responsible for the clotting of guinea pig semen. Proc Natl Acad Sci U S A. 1967 Nov;58(5):1991–1995. doi: 10.1073/pnas.58.5.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisano J. J., Finlayson J. S., Peyton M. P. Chemical and enzymic detection of protein cross-links. Measurement of epsilon-(gamma-glutamyl)lysine in fibrin polymerized by factor XIII. Biochemistry. 1969 Mar;8(3):871–876. doi: 10.1021/bi00831a016. [DOI] [PubMed] [Google Scholar]
- Platz R. D., Wolfe H. G. Mouse seminal vesicle proteins. The inheritance of electrophoretic variants. J Hered. 1969 Jul-Aug;60(4):187–192. doi: 10.1093/oxfordjournals.jhered.a107969. [DOI] [PubMed] [Google Scholar]
- Reddi A. H., Ewing L. L., Williams-Ashman H. G. Protein phospholinase reactions in mammalian testis. Stimulatory effects of adenosine 3':5'-cyclic monophosphate on the phosphorylation of basic proteins. Biochem J. 1971 Apr;122(3):333–345. doi: 10.1042/bj1220333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tóth M., Zakár T. Extraction of a secretory protein from the tissues of the seminal vesicle of the rat. Acta Biochim Biophys Acad Sci Hung. 1971;6(3):231–242. [PubMed] [Google Scholar]