Abstract
Two separate protein factors that markedly stimulate DNA-dependent RNA polymerase have been partially purified from ribosome-free extracts of E. coli Q13 cells. These proteins appear to differ from sigma, M, H, psi, and the factor described by Mahadik and Srinivasan. The stimulation by either factor requires the presence of the sigma protein. Using RNA-DNA hybridization competition analyses, we also demonstrate that neither of the new factors preferentially stimulates the synthesis of ribosomal RNA. The purification, properties, and mechanism of action of these new factors are examined.
Keywords: RNA polymerase, ribosomal RNA, template specificity
Full text
PDF
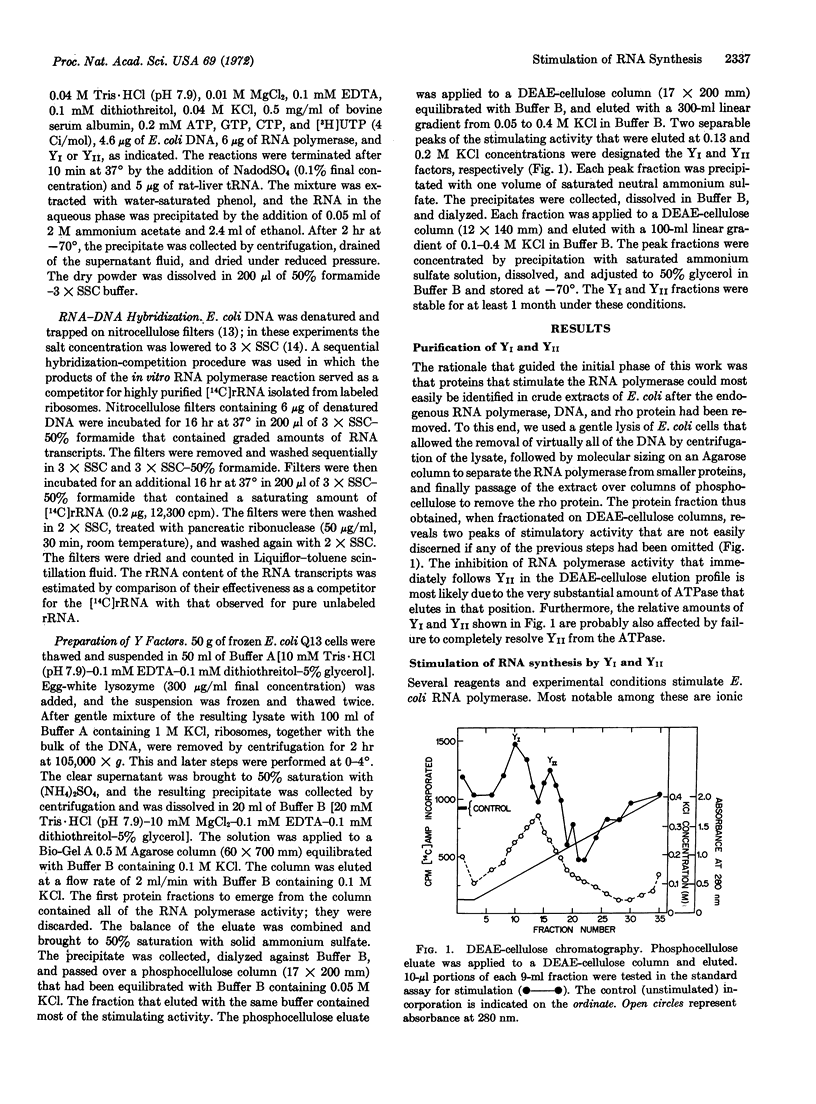
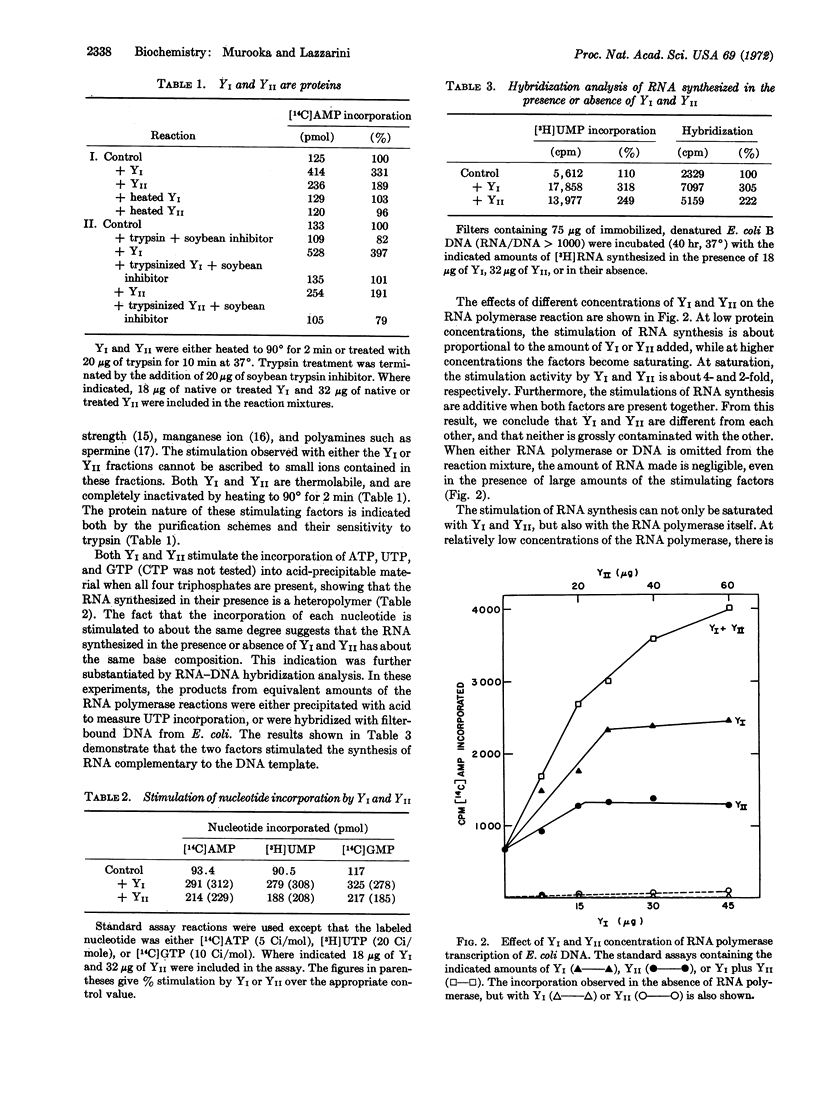
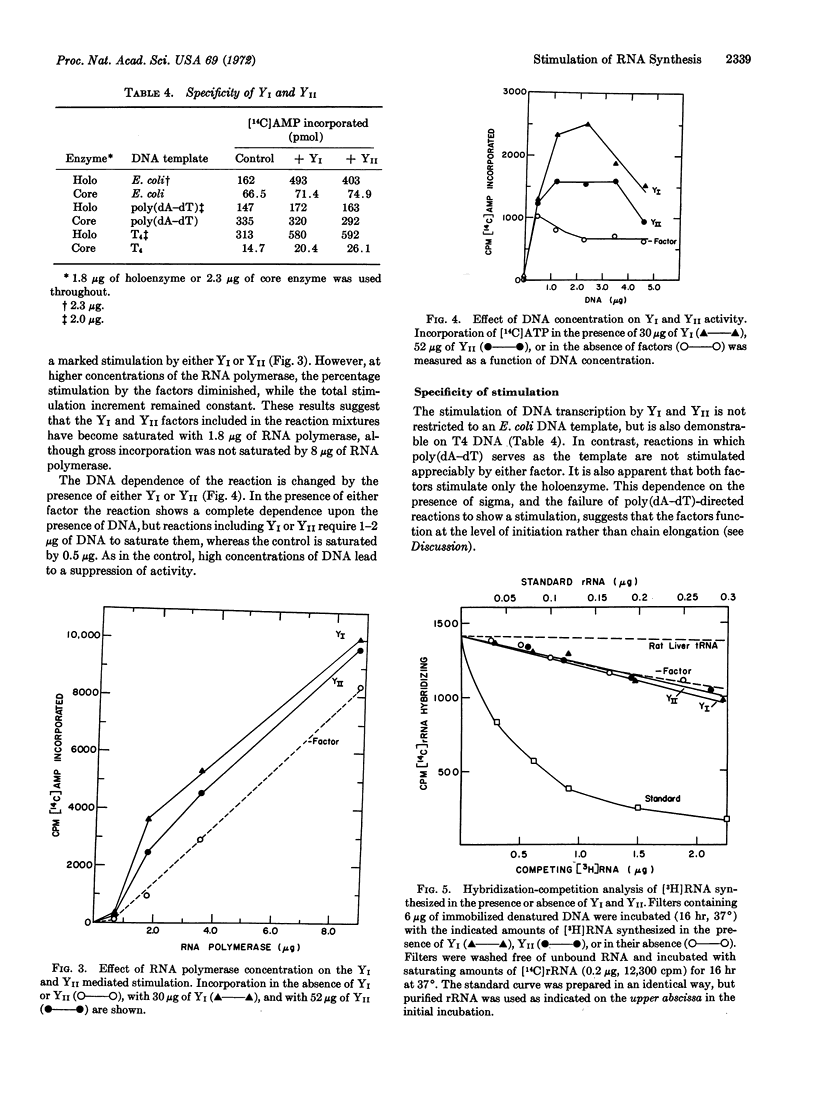

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bautz E. K., Bautz F. A., Dunn J. J. E. coli sigma factor: a positive control element in phage T4 development. Nature. 1969 Sep 6;223(5210):1022–1024. doi: 10.1038/2231022a0. [DOI] [PubMed] [Google Scholar]
- Burgess R. R., Travers A. A., Dunn J. J., Bautz E. K. Factor stimulating transcription by RNA polymerase. Nature. 1969 Jan 4;221(5175):43–46. doi: 10.1038/221043a0. [DOI] [PubMed] [Google Scholar]
- Davison J., Pilarski L. M., Echols H. A factor that stimulates RNA synthesis by purified RNA polymerase. Proc Natl Acad Sci U S A. 1969 May;63(1):168–174. doi: 10.1073/pnas.63.1.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Crombrugghe B., Chen B., Anderson W., Nissley P., Gottesman M., Pastan I., Perlman R. Lac DNA, RNA polymerase and cyclic AMP receptor protein, cyclic AMP, lac repressor and inducer are the essential elements for controlled lac transcription. Nat New Biol. 1971 Jun 2;231(22):139–142. doi: 10.1038/newbio231139a0. [DOI] [PubMed] [Google Scholar]
- Echols H., Pilarski L., Xcheng P. Y. In vitro repression of phage lambda DNA transcription by a partially purified repressor from lysogenic cells. Proc Natl Acad Sci U S A. 1968 Mar;59(3):1016–1023. doi: 10.1073/pnas.59.3.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FURTH J. J., HURWITZ J., ANDERS M. The role of deoxyribonucleic acid in ribonucleic acid synthesis. I. The purification and properties of ribonucleic acid polymerase. J Biol Chem. 1962 Aug;237:2611–2619. [PubMed] [Google Scholar]
- Gillespie S., Gillespie D. Ribonucleic acid-deoxyribonucleic acid hybridization in aqueous solutions and in solutions containing formamide. Biochem J. 1971 Nov;125(2):481–487. doi: 10.1042/bj1250481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenblatt J., Schleif R. Arabinose C protein: regulation of the arabinose operon in vitro. Nat New Biol. 1971 Oct 6;233(40):166–170. doi: 10.1038/newbio233166a0. [DOI] [PubMed] [Google Scholar]
- Jacquet M., Cukier-Kahn R., Pla J., Gros F. A thermostable protein factor acting on in vitro DNA transcription. Biochem Biophys Res Commun. 1971 Dec 17;45(6):1597–1607. doi: 10.1016/0006-291x(71)90204-x. [DOI] [PubMed] [Google Scholar]
- KRAKOW J. S. RIBONUCLEIC ACID POLYMERASE OF AZOTOBACTER VINELANDII. III. EFFECT OF POLYAMINES. Biochim Biophys Acta. 1963 Aug 20;72:566–571. [PubMed] [Google Scholar]
- Lazzarini R. A., Dahlberg A. E. The control of ribonucleic acid synthesis during amino acid deprivation in Escherichia coli. J Biol Chem. 1971 Jan 25;246(2):420–429. [PubMed] [Google Scholar]
- Mahadik S. P., Srinivasan P. R. Stimulation of DNA-dependent RNA synthesis by a protein associated with ribosomes. Proc Natl Acad Sci U S A. 1971 Aug;68(8):1898–1901. doi: 10.1073/pnas.68.8.1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J. W. Termination factor for RNA synthesis. Nature. 1969 Dec 20;224(5225):1168–1174. doi: 10.1038/2241168a0. [DOI] [PubMed] [Google Scholar]
- So A. G., Davie E. W., Epstein R., Tissières A. Effects of cations on DNA-dependent RNA polymerase. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1739–1746. doi: 10.1073/pnas.58.4.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura M., Okamoto T., Takanami M. RNA polymerase sigma-factor and the selection of initiation site. Nature. 1970 Feb 14;225(5233):598–600. doi: 10.1038/225598a0. [DOI] [PubMed] [Google Scholar]
- Travers A. A., Kamen R. I., Schleif R. F. Factor necessary for ribosomal RNA synthesis. Nature. 1970 Nov 21;228(5273):748–751. doi: 10.1038/228748a0. [DOI] [PubMed] [Google Scholar]
- Vogt V. Breaks in DNA stimulate transcription by core RNA polymerase. Nature. 1969 Aug 23;223(5208):854–855. doi: 10.1038/223854a0. [DOI] [PubMed] [Google Scholar]
- Zubay G., Gielow L., Englesberg E. Cell-free studies on the regulation of the arabinose operon. Nat New Biol. 1971 Oct 6;233(40):164–165. doi: 10.1038/newbio233164a0. [DOI] [PubMed] [Google Scholar]



