Abstract
Axonal transport is essential for neuronal function, and many neurodevelopmental and neurodegenerative diseases result from mutations in the axonal transport machinery. Anterograde transport supplies distal axons with newly synthesized proteins and lipids, including synaptic components required to maintain presynaptic activity. Retrograde transport is required to maintain homeostasis by removing aging proteins and organelles from the distal axon for degradation and recycling of components. Retrograde axonal transport also plays a major role in neurotrophic and injury response signaling. This review provides an overview of the axonal transport pathway and discusses its role in neuronal function.
Keywords: Fast axonal transport, slow axonal transport, organelle transport, vesicular transport, cytoplasmic dynein, kinesin, microtubules, signaling endosomes, autophagosomes, lysosomes, mitochondria, organelle motility
The active transport of organelles, proteins, and RNA along the extended axons of neurons has long fascinated scientists. The remarkable fact that the axon depends on the biosynthetic and degradative activities of the soma, located up to a meter away, highlights the importance of active transport. Genetic evidence confirms an essential role for active transport in the neuron, as defects in many of the proteins involved are sufficient to cause either neurodevelopmental or neurodegenerative disease (Table I).
TABLE 1.
Neurodevelopmental and neurodegenerative diseases caused by mutations in the axonal transport machinery
Abbreviations: AD, Alzheimer’s Disease; ARID, Autosomal Recessive Intellectual Disability; CDCBM3, complex cortical dysplasia with other brain malformations-3; CFEOM, congenital fibrosis of the extraocular muscles; CMT, Charcot-Marie-Tooth disease; FTD, frontotemporal dementia; HD, Huntington’s Disease; HMN, hereditary motor neuropathy; HSN, hereditary sensory neuropathy; HSP, Hereditary Spastic Paraplegia; ID, Intellectual Disability; MCD, Malformations of Cortical Development; PD, Parkinson’s Disease; MR, Mental Retardation; SMA, Spinal Muscular Atrophy; SMA-LED, SMA-Lower Extremity Dominant; SPAX, spastic ataxia.
Metabolic cell-labeling experiments in the 1960s demonstrated the rapid movement of newly synthesized proteins along the axon in a process once termed “cellulifugal transport” (Weiss, 1967). Experiments with drugs that disrupt the cellular cytoskeleton demonstrated that microtubules are required for active transport along the axon (Kreutzberg, 1969). Pulse-chase labeling experiments led to the discovery of multiple phases of transport. (reviewed in (Griffin et al., 1976)). Organelles were observed to move outward from the cell body at “fast” speeds of up to 400 mm/day, or ~1 μm/s, while cytoskeletal proteins and some soluble proteins were observed to move via “slow” transport, at speeds of <8 mm/day, or <0.1 μm/s. Outward-bound, anterograde (also known as orthograde) transport was most clearly defined by these metabolic labeling approaches. However, the retrograde transport of organelles from the distal axon back toward the cell body was also observed (Griffin et al., 1976). The development of live-cell imaging allowed the direct observation of organelle motility (Allen et al., 1982; Brady et al., 1982). These observations led to the discovery of the microtubule motor kinesin (Vale et al., 1985), now known as kinesin-1 (see Table I); cytoplasmic dynein was discovered soon after (Paschal et al., 1987). Breakthrough experiments using nerve ligation assays identified kinesin as a major motor for anterograde transport along the axon (Hirokawa et al., 1991), and dynein as the motor for retrograde transport (Hirokawa et al., 1990).
Since these initial discoveries there has been considerable progress in understanding the mechanisms regulating the transport of organelles including mitochondria, lysosomes, autophagosomes, and endosomes (Figure 1), as well as the transport mechanisms involved in neurotrophic and injury signaling. Together, these studies support a model in which the regulation of transport is compartment-specific. The complement of motors, adaptors and scaffolding proteins bound to each cargo are organelle-specific, leading to distinct patterns of motility and localization along the axon. Thus, while broad themes emerge, the specific mechanisms regulating the transport of each organelle or protein complex may be unique. Further, there is increasing evidence for the localized regulation of trafficking in key zones along the axon, such as the axon initial segment or in the distal axon.
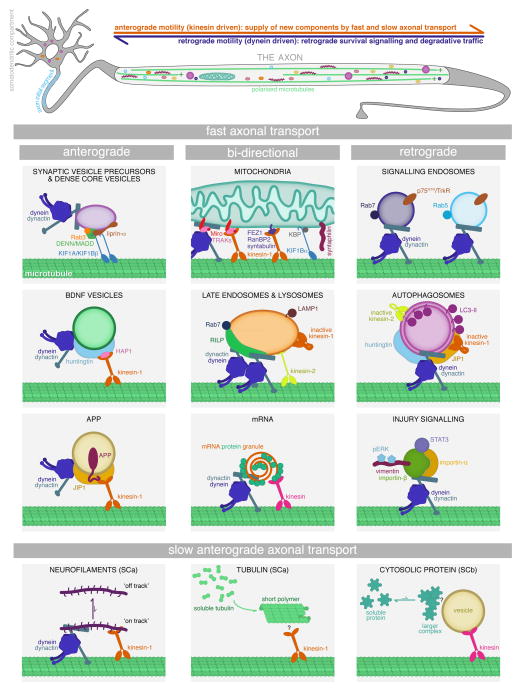
FIGURE 1. Molecular mechanisms of axonal transport.
Microtubule motor proteins kinesin and dynein drive the movement of organelles, vesicles, RNA granules, and proteins along the axon. Kinesins drive anterograde transport outward from the soma, and dynein drives retrograde transport back from distal axon. However, most cargos may have both motor types bound simultaneously. Cargo-bound motors are regulated by organelle-specific complements of scaffolding and adaptor proteins. To avoid either distal accumulation or distal depletion of cellular components, anterograde and retrograde axonal transport must be in balance.
Here, we discuss both general themes and specific mechanisms involved in axonal transport. We will review recent progress and highlight some of the critical questions that remain, focusing on the mechanisms that regulate the dynamic trafficking of organelles along the axon.
MOLECULAR MOTORS DRIVE TRANSPORT ALONG THE NEURONAL CYTOSKELETON
The neuronal cytoskeleton
Microtubules, actin filaments, and intermediate filaments all contribute to the morphology and function of neurons, but axonal transport depends almost entirely on microtubules. Microtubules are polarized tubulin polymers with fast growing plus ends and more stable minus ends, organized in a generally radial array in the soma with plus ends directed toward the cortex. In the axon, parallel microtubules form a unipolar array with plus-ends oriented outward (Burton and Paige, 1981; Stepanova et al., 2003), while in dendrites microtubule organization is more complex, with microtubules often organized in arrays with mixed polarity (Baas et al., 1988; Kleele et al., 2014; Kwan et al., 2008). In the cell body, microtubule minus-ends may be rooted near the centrosome, but microtubules along axons are likely to be capped at their minus-ends by a mechanism that is not yet understood (Kuijpers and Hoogenraad, 2011).
Microtubule-associated proteins, or MAPs, are bound along the length of axonal and dendritic microtubules. The canonical role for MAPs is to promote microtubule polymerization and stabilization; because of the high expression levels of MAPs in neurons, microtubules are generally more stable in these cells than in other cell types. MAPs may also function to regulate transport, as in vitro studies indicate they modulate the interaction of motors with the microtubule (Dixit et al., 2008b; Vershinin et al., 2007). The discovery of a specific class of MAPs, known as plus-end interacting proteins or +TIPs, has shown that microtubules in axons can be dynamic. Live-cell imaging with GFP-labeled +TIPs that bind selectively to actively growing microtubule plus ends has shown that axonal microtubules exhibit the parameters of dynamic instability observed in non-neuronal cells, including slow growth and rapid shortening, punctuated by catastrophe and rescue events, respectively (Stepanova et al., 2003; Stepanova et al., 2010). The +TIPS EB1 and EB3 recruit additional binding partners to microtubule ends, many of which have a role in the localized regulation of axonal transport (Moughamian et al., 2013).
Direct post-translational modification of tubulin is widespread in neurons (Janke and Bulinski, 2011). Microtubule modifications directly modulate the activities of motor proteins (Sirajuddin et al., 2014), potentially contributing to the polarized trafficking of motors into axons (Hammond et al., 2010; Jacobson et al., 2006; Konishi and Setou, 2009). The nucleotide state of microtubules can also affect motor activity and contribute to polarized vesicle transport (Nakata et al., 2011).
Kinesin and dynein motors drive axonal transport
The kinesin superfamily constitutes 45 genes in the human genome, 38 of which are expressed in brain (Miki et al., 2001). The neuronal motor proteome is more complex than that expressed in most other cell types, likely reflecting the enhanced importance of regulated and specific intracellular transport in neurons with their highly polarized morphology (Kuta et al., 2010; Silverman et al., 2010). A standardized nomenclature (Lawrence et al., 2004) groups kinesin genes into 14 sub-families that share structural and functional similarities; motors from the kinesin-1, kinesin-2, and kinesin-3 families all contribute to axonal transport dynamics.
Members of the kinesin-1 family drive the transport of a wide range of cargos along the axon at velocities of ~0.5–1 μm/s, including vesicles, organelles, proteins, and RNA particles (Hirokawa et al., 2010) (Figure 1). Active kinesin-1 motors are formed from a dimer of kinesin heavy chains (encoded by three mammalian genes, KIF5A, B and C); a dimer of kinesin light chains (KLCs) is often but not always part of the complex (Sun et al., 2011) and contributes to the autoinhibitory mechanism of the motor.
Kinesin-2 and kinesin-3 motors are also critical for normal axonal transport (Figure 1). Kinesin-2 members can assemble into either homodimeric or heterotrimeric motors (Scholey, 2013), while kinesin-3 motors undergo cargo-mediated dimerization resulting in the formation of highly processive motors when bound to intracellular organelles (Soppina et al., 2014). Kinesin-2 motors drive the anterograde motility of fodrin-positive plasma membrane precursors (Takeda et al., 2000), N-cadherin and β-catenin (Teng et al., 2005) and choline acetyltransferase (Ray et al., 1999), and are also associated with Rab7-positive late endosome-lysosome compartments in the neuron (Castle et al., 2014; Hendricks et al., 2010). Kinesin-3 motors drive the motility of synaptic vesicle precursors and dense core vesicles (Hall and Hedgecock, 1991; Lo et al., 2011; Okada et al., 1995).
Cytoplasmic dynein is the major motor driving retrograde transport. In contrast to the diversity of the kinesin superfamily, the motor subunit of cytoplasmic dynein is encoded by a single gene (reviewed in (Roberts et al., 2013)). Two dynein heavy chains (DHCs) dimerize by their N-terminal tail domains; additional intermediate chains, light intermediate chains, and light chains associate with the tails of the heavy chains to form a cargo-binding domain. Together, these proteins serve as the binding site for many of the proteins regulating dynein function in the cell. While there is a single gene encoding the motor domain of cytoplasmic dynein, there is more diversity in the other subunits of the dynein complex – for example, there are two genes encoding dynein intermediate chains, one of which is neuron-specific (DIC1) and two genes encoding dynein light intermediate chains (Kuta et al., 2010). There is evidence that these subunits can either co-assemble (Zhang et al., 2013) or alternatively assemble into distinct complexes with specialized functions (Mitchell et al., 2012; Salata et al., 2001) which may allow for organelle-specific recruitment or regulation.
Most dynein functions in the cell require the dynein activator, dynactin. Dynactin is a highly conserved multi-protein complex (Schroer, 2004) that is essential for normal neuronal function (LaMonte et al., 2002; Moughamian and Holzbaur, 2012). The base of dynactin is formed from a 37 nm-long actin-like polymer; both the Arp1 subunit that forms this polymer and additional dynactin subunits including p25 and p27 have been implicated in cargo binding (Holleran et al., 1996; Yeh et al., 2012; Zhang et al., 2011). Projecting from this base is a dimer of the subunit p150Glued (Holzbaur et al., 1991). This subunit binds directly to dynein intermediate chain (Karki and Holzbaur, 1995; Vaughan and Vallee, 1995), and also binds directly to microtubules via a CAP-Gly (Cytoskeletal Associated Protein-Glycine-rich) domain (Waterman-Storer et al., 1995) and a lower affinity basic domain found in neuronal isoforms of p150Glued (Culver-Hanlon et al., 2006; Dixit et al., 2008a). In vitro assays demonstrate that these independent microtubule-binding domains increase the processivity of the dynein-dynactin motor complex (King and Schroer, 2000; Ross et al., 2006) by enhancing the association of the motor with the microtubule (Ayloo et al., 2014). In neurons, the CAP-Gly domain of dynactin has a key role in the initiation of retrograde transport in the distal axon (Lloyd et al., 2012; Moughamian and Holzbaur, 2012).
The properties of kinesin and dynein motors have been explored in vitro at the single molecule level. Kinesin-1 motors move in a highly processive manner toward the plus-end of the microtubule, taking 8 nm steps in a straight path along a single protofilament. A single kinesin-1 motor has a stall force of 5–6 pN (Svoboda and Block, 1994), sufficient to move an organelle through the cytoplasm. Kinesin-2 motors also drive organelle motility along axons, and have a stall force of similar magnitude (5 pN). However, kinesin-2 exhibits force-dependent detachment from the microtubule (Schroeder et al., 2012), indicating that this motor may be less likely to win a tug-of-war interaction with an opposing motor such as dynein. Stall forces of kinesin-3 motors have not yet been determined. However, recent work has shown that kinesin-3 motors become super-processive following cargo-mediated dimerization (Soppina et al., 2014).
Studies with purified mammalian dynein indicate that dynein is a fast motor, with velocities from 0.5 to 1 μm/sec. Unlike the highly processive unidirectional motility of kinesin-1, kinesin-2, and kinesin-3 motors, single mammalian dynein motors take frequent back- and side-steps during movement along the microtubule (Mallik et al., 2005; Ross et al., 2006). However, either the coordinated activities of multiple dynein motors (Mallik et al., 2005) or the binding of activators such as BICD2 (McKenney et al., 2014; Schlager et al., 2014) convert dynein to a unidirectional and highly processive motor. Dynein is a much weaker motor than kinesin-1 or kinesin-2; there is general although not complete consensus that the stall force for mammalian cytoplasmic dynein is ~1 pN (Mallik et al., 2004; Schroeder et al., 2010).
While these observations might suggest that dynein is a less effective motor than kinesin, both the flexible nature of dynein and its ability to move backwards and sideways along a microtubule may allow the motor to function effectively in teams (Mallik et al., 2013), and to navigate around obstacles along its path (Dixit et al., 2008b). In contrast, kinesin-1 motors are much less capable of effectively working in teams (Mallik et al., 2013). Kinesin-1 motors are also more likely than dynein to detach from the microtubule track upon encountering obstacles (Dixit et al., 2008b; Vershinin et al., 2007), although recent work has shown that kinesin-2 motors are more robust (Hoeprich et al., 2014).
Opposing motors bind simultaneously to cargos along the axon
Many axonal cargos have multiple motor types bound simultaneously (Figure 1). For example, late endosomes/lysosomes co-purify with kinesin-1, kinesin-2, and dynein motors (Hendricks et al., 2010). Similarly, kinesin-1 and dynein colocalize on single prion-positive vesicles undergoing transport along the axon (Encalada et al., 2011). Even cargos that move processively in a single direction over long distances, such as autophagosomes, co-purify with opposing dynein and kinesin motors (Maday et al., 2012). Quantitative analyses and live-cell trapping experiments suggest that 1–2 kinesins and 6–12 dyneins may act together to move a single organelle along the microtubule (Hendricks et al., 2012; Hendricks et al., 2010; Rai et al., 2013).
Thus, it is essential to consider how multiple motors, and multiple types of motors may interact either cooperatively or competitively to yield effective motility. Multiple models have been put forth (Fu and Holzbaur, 2014; Gross, 2004; Gross et al., 2007; Muller et al., 2008; Welte, 2004). The simplest model posits an unregulated tug-of-war between opposing kinesin and dynein motors. In a contrasting model, motors are coordinately regulated so that only a single motor type is active at any given time. Intermediate models suggest that one motor, such as kinesin, might be tightly regulated while the activity of dynein might be less carefully controlled; as dynein is a weaker motor than kinesin-1 it might simply be overpowered in situations where both motors are active simultaneously.
The motility of some axonal cargos, such as late endosomes/lysosomes, can be effectively modeled, at least to a first approximation, as a tug-of-war between opposing kinesin and dynein motors (Hendricks et al., 2010; Muller et al., 2008). In contrast, the motility of other cargos in the degradative pathway such as autophagosomes exhibit strongly unidirectional motility indicating that kinesin motor activity can be effectively down-regulated (Fu et al., 2014; Maday et al., 2012). Growing evidence suggests that the activities of opposing motors bound to the same cargo are regulated by scaffolding proteins (reviewed in (Fu and Holzbaur, 2014)).
The autoinhibition of kinesin-1 is key to this regulation. The binding of kinesin tail to the motor domain blocks motor function (Kaan et al., 2011); inhibition is relieved by specific binding partners such as the scaffolding proteins JIP1 and JIP3 (Blasius et al., 2007; Fu and Holzbaur, 2013; Sun et al., 2011). In the mechanisms explored in detail to date, tight regulation of kinesin-1 activation by scaffolding proteins allows for sustained axonal transport of organelles in either the anterograde or retrograde directions. The regulation of other kinesin sub-families is less well studied.
Regulation of dynein motors is also important to maintain axonal transport, but the mechanisms involved are not as well understood. Lis1 is a critical and conserved effector of dynein function. Structural studies indicate that Lis1 binds directly to the dynein motor domain, and uncouples ATP hydrolysis from force production, leading to sustained attachment of the motor to the microtubule (Huang et al., 2012). While induction of tight binding might be expected to block effective transport, instead it has been found that depletion of Lis1 inhibits the dynein-driven transport of late endosomes and lysosomes along the axon (Moughamian et al., 2013; Pandey and Smith, 2011); as well as mitochondrial motility in axons (Shao et al., 2013). Nde1 (also known as NudE) and Ndel1 (also known as NudE-like or NudEL) form a complex with Lis1, and are similarly required for normal axonal transport of at least some dynein cargos (Pandey and Smith, 2011; Shao et al., 2013).
The Bicaudal D homolog (BICD) proteins are also key dynein effectors. BICD1 and BICD2 recruit dynein-dynactin to Rab6 positive Golgi and cytoplasmic vesicles (Matanis et al., 2002) as well as mRNAs including Fragile X Mental Retardation Protein (FMRP; (Bianco et al., 2010)). Recently BICD1 was shown to control the trafficking of activated neurotrophin receptors to degradation routes in order to balance the neuronal response to neurotrophin stimulation (Terenzio et al 2014). In vitro studies have shown that an N-terminal fragment of BICD2 induces highly processive dynein motility (McKenney et al., 2014; Schlager et al., 2014).
Multiple additional mechanisms have been proposed to regulate motor activity on cargos moving along the axon. Rab GTPases have been shown to regulate motor recruitment to several cargos (reviewed in (Akhmanova and Hammer, 2010)). Scaffolding proteins are also key: huntingtin is involved in the regulation of BDNF-positive vesicles (Gauthier et al., 2004) and autophagosomes (Wong and Holzbaur, 2014); JIP1 is involved in the regulation of APP-positive vesicles; JIP3 regulates the injury-signaling pathway in mammalian cells and lysosomal motility in zebrafish (Drerup and Nechiporuk, 2013); and the Miro/TRAK complex regulates motors bound to mitochondria (Macaskill et al., 2009b; Wang and Schwarz, 2009). Finally, there is clear evidence implicating upstream kinases in the regulation of transport including Cdk5, JNK, and p38MAPK (Fu and Holzbaur, 2013; Horiuchi et al., 2007; Morfini et al., 2013; Pandey and Smith, 2011), but the mechanisms involved have not yet been fully elucidated.
Both common themes and cargo-specific mechanisms operate in the axonal transport of diverse axonal cargos
Live-cell and in vivo imaging of fluorescently-tagged organelles moving along axons have revealed a surprising diversity in the movement of specific populations, indicating that the regulation of the motors that drive transport likely occurs primarily at the level of the organelle, rather than reflecting an overall regulatory environment within the axon. While the observed patterns of motility are diverse, some common themes are emerging:
Motors remain stably associated with a cargo during transport along the axon, even when they are inactive.
Only a small complement of motors is necessary to effectively move even large (>1 μm) organelles along the microtubule. These motors function in groups that usually include opposing motor activities.
Motors are regulated by mechanisms that may include Rab-specific recruitment, upstream regulation by kinases and phosphatases, and scaffolding proteins that control motor activity.
Mutations in motors, their adaptors, or their regulators can lead to neurodegeneration or neuronal cell death (Table I), consistent with an essential role for axonal transport in maintaining neuronal homeostasis.
Despite these common themes, accumulating evidence suggests that the motility of each cargo actively transported along the axon is regulated by a distinct mechanism.
FAST ANTEROGRADE TRANSPORT: AXONAL PROTEINS AND SYNAPTIC COMPONENTS
APP-positive vesicles
APP-positive vesicles are a canonical cargo of kinesin-1 motors (Kamal et al., 2000). APP-positive vesicles are transported in a highly processive manner at rapid speeds (~1 μm/s), primarily in the anterograde direction although rapid retrograde motility is also observed (Falzone et al., 2009; Kaether et al., 2000). APP binds to the scaffolding protein JIP1 (Matsuda et al., 2001; Scheinfeld et al., 2002). JIP1 is a JNK-binding scaffolding protein implicated in the regulation of constitutive axonal transport in Drosophila (Horiuchi et al., 2005). The C-terminus of JIP1 binds to KLC (Verhey et al., 2001) and this binding contributes to the regulation of the kinesin-1 motor in concert with FEZ1 (Blasius et al., 2007). JIP1 also binds directly to kinesin-1 heavy chain (KHC) and the p150Glued subunit of dynactin (Fu and Holzbaur, 2013). JIP1 binding to KHC activates the motor by relieving auto-inhibition, while the binding of JIP1 to dynactin competitively blocks this activation (Fu and Holzbaur, 2013).
In the neuron, the relative affinity of JIP1 for kinesin-1 or dynactin is controlled by a JNK-dependent phosphorylation site, which acts as a molecular switch to control the directionality of APP transport – when S421 in JIP1 is phosphorylated, anterograde transport of APP is favored, while dephosphorylation of S421 favors the retrograde motility of JIP1 (Fu and Holzbaur, 2013). Regulation of this activation is likely to involve JNK, and possibly upstream kinases such as Wallenda/DLK (Horiuchi et al., 2007).
Synaptic vesicle precursors and dense core vesicles
A large fraction of vesicular organelles in the axon are components destined for the pre-synapse, namely synaptic vesicle precursors (SVPs) and dense core vesicles (DCVs) packed with neuropeptides and neurotrophins.
Anterograde transport of SVPs is driven by motors from the kinesin-3 family, Unc-104 in C. elegans and KIF1A in mammals (Hall and Hedgecock, 1991; Okada et al., 1995). Neurons from unc-104 mutants and KIF1A knockout mice (Yonekawa et al., 1998) fail to develop normal synapses; synaptic precursors accumulate in the soma consistent with a transport defect. Conversely, overexpression of KIF1A promotes the formation of pre-synaptic boutons (Kondo et al., 2012). Kinesin-3 motors undergo cargo-mediated dimerization, which leads to the formation of highly processive anterograde motors to drive efficient delivery of synaptic components (Klopfenstein and Vale, 2004; Soppina et al., 2014).
Two adaptors have been proposed to couple kinesin-3 motors to SVPs, liprin-α and DENN/MADD. Liprin-α is a multifunctional scaffolding protein that binds directly to KIF1A and many other neuronal scaffolding proteins (Shin et al., 2003); mutations in liprin-α perturb SVP transport (Miller et al., 2005). The protein DENN/MADD is required for the transport of SVPs and binds directly to the stalk domain of kinesin-3 motors (Niwa et al., 2008). DENN/MADD can differentiate between GTP and GDP forms of Rab3, a marker for SVPs, suggesting a mechanism for regulation of motor recruitment.
Once delivered to the pre-synaptic site, SVPs can be recycled locally. However, DCVs can only be packaged in the soma and must be continuously supplied, targeted to axon and/or dendrites depending on their content. DCV transport is also dependent on Unc-104/KIF1A motors, suggesting the mechanisms involved are similar to those driving SVP transport (Lo et al., 2011). Upstream regulation of kinesin-3 transport is regulated by Cdk5, which promotes the Unc-104-dependent transport of dense core vesicles into axons and inhibits the dynein-dependent transport of these vesicles into dendrites (Goodwin et al., 2012).
The current exception to the paradigm of kinesin-3-dependent transport of DCVs is BDNF transport. The neurotrophin BDNF is stored in DCVs and trafficked within axons to the pre-synaptic site (Altar et al., 1997; Dieni et al., 2012). However, the axonal transport of BDNF is regulated by huntingtin (Gauthier et al., 2004), which scaffolds both kinesin-1 and dynein motors (Caviston and Holzbaur, 2009). The phosphorylation of huntingtin through the IGF-1/Akt pathway acts as a molecular switch to regulate the transport of BDNF-containing vesicles in axons (Colin et al., 2008; Zala et al., 2008). Phosphorylation of huntingtin at S421 promotes anterograde transport while dephosphorylation of huntingtin promotes retrograde transport (Colin et al., 2008). Biochemical studies indicate that phosphorylation of S421 enhances the recruitment of kinesin-1 to BDNF transport vesicles and enhances the association of kinesin-1 motors with microtubules, leading to increased anterograde flux and BDNF release (Colin et al., 2008).
FAST RETROGRADE TRANSPORT: SIGNALING ENDOSOMES AND AUTOPHAGOSOMES
Signaling endosomes
The balance between neuronal survival and death is regulated by neurotrophin secretion from target tissues to modulate the connection with innervating neurons (Chowdary et al., 2012; Harrington and Ginty, 2013). Neurotrophins bind to receptors on the presynaptic membrane and are transported from the distal axon toward the cell soma to effect changes in gene expression. Since these signals must be relayed over distances of up to 1 meter, robust mechanisms must exist to preserve the fidelity of information being carried.
Neurotrophins (NGF, BDNF, NT3/4) bind to and activate neurotrophin receptors (TrkA, TrkB, TrkC, p75NTR). Following receptor-mediated endocytosis, these receptor-ligand complexes are sorted into compartments called signaling endosomes for transport toward the cell soma (Chowdary et al., 2012; Harrington and Ginty, 2013). There is evidence for an early endosomal lineage for signaling endosomes, since these organelles are positive for EEA1 and Rab5B (Cui et al., 2007; Deinhardt et al., 2006; Delcroix et al., 2003), but they may mature to Rab7-positive compartments (Deinhardt et al., 2006; Sandow et al., 2000). Ligand-receptor complexes can be sustained during transport, resulting in activated Trk receptors (pTrks) and downstream signaling molecules (e.g. pERK1/2, B-Raf and p-p38) in both the axon and cell body (Bhattacharyya et al., 2002; Cui et al., 2007; Delcroix et al., 2003; Grimes et al., 1997).
To relay information from the distal axon to the cell soma, signaling endosomes undergo robust retrograde transport. Ligation of the sciatic nerve results in the accumulation of activated neurotrophin receptors and signaling molecules distal to the ligation site, demonstrating a robust retrograde flux of signaling endosomes along the axon (Bhattacharyya et al., 2002; Delcroix et al., 2003; Ehlers et al., 1995). Precise spatial and temporal resolution of signaling endosome dynamics was revealed with NGF-coated quantum dots, which exhibited pronounced unidirectional motility toward the cell soma interspersed with frequent pauses; average speeds ranged from 0.2 μm/sec to 3 μm/sec (Cui et al., 2007). This retrograde transport depends on dynein-dynactin as inhibition of this motor complex prevents activated neurotrophin receptors from exiting the distal axon, thereby decreasing neuron viability (Heerssen et al., 2004).
Autophagosomes
Maintaining protein and organelle quality across the extended distance of the axon poses a unique challenge to the neuronal degradation machinery. Autophagy is an essential lysosomal degradation pathway in neurons (Hara et al., 2006; Komatsu et al., 2006; Komatsu et al., 2007), required to maintain cellular homeostasis. Autophagosomes are preferentially generated in the distal axon (Hollenbeck, 1993; Maday and Holzbaur, 2014; Maday et al., 2012). For a short period after the compartment is formed, autophagosomes exhibit bidirectional motility, likely driven by both kinesin-1 and dynein motors, but they soon switch to robust retrograde transport along the length of the axon (Maday et al., 2012). Both live imaging and biochemical analysis have shown that dynein and kinesin-1 motors remain tightly bound to autophagosomes during retrograde movement along the axon, despite primarily unidirectional movement with few reversals and pauses (Maday et al., 2012). Two scaffolding proteins, JIP1 and huntingtin, regulate autophagosome motility by interacting with both kinesin-1 and the retrograde dynein-dynactin motor complex (Fu et al., 2014; Wong and Holzbaur, 2014). JIP1 binding to LC3 is required to effectively block the activation of kinesin-1 on these organelles, leading to the robust retrograde motility of autophagosomes along the axon (Fu et al., 2014).
As autophagosomes transit along the axon, they undergo maturation to form autolysosomes (Lee et al., 2011; Maday et al., 2012). Initial fusion with late endosomes occurs upon exit from the distal region of the axon but full acidification occurs as they approach the soma (Maday et al., 2012), consistent with a gradient of degradative function along the axon (Lee et al., 2011). Transport along the axon likely facilitates additional fusion events with lysosomes encountered en route to the cell soma, as inhibition of transport leads to defective acidification and accumulation of undigested contents within the lumen of the autolysosome (Fu et al., 2014; Wong and Holzbaur, 2014).
While some degradation may occur locally within the axon (Ashrafi et al., 2014), >80% of axonal autophagosomes formed by constitutive autophagy travel toward the cell soma (Maday et al., 2012) indicating a dependence on long-range axonal transport for clearance pathways. Delivery of autophagosomes to the cell soma may ensure efficient recycling of the amino acids generated to primary sites of protein synthesis. The pronounced retrograde motility characteristic of constitutive autophagy in neurons could also balance the net outward flow of organelles and proteins via fast and slow anterograde transport (Maday and Holzbaur, 2014).
BIDIRECTIONAL TRANSPORT: MITOCHONDRIA AND LYSOSOMES
Mitochondria
Localized regions within the neuron such as growth cones and synapses experience significant energetic demands. This requirement for ATP cannot be sustained by diffusion from the cell soma and must be handled locally within the neuron. Mitochondria, the organelles responsible for ATP production and intracellular calcium buffering, are actively shuttled and positioned within the neuron to meet the localized needs of the cell. Thus mitochondrial motility facilitates a dynamic response to balance environmental demands. In axons of hippocampal neurons grown in vitro, ~20–30% of mitochondria are motile, moving equally in both anterograde and retrograde directions; the remaining ~70–80% are stationary (reviewed in (Hollenbeck and Saxton, 2005)). In vivo, axonal mitochondria are ~10% motile and exhibit a greater bias in flux in the anterograde direction as compared to in vitro studies; ~70% are anterograde and ~30% are retrograde (Misgeld et al., 2007; Pilling et al., 2006).
Mitochondrial transport is regulated by neuronal activity (Sajic et al., 2013). Elevated intracellular calcium levels resulting from enhanced synaptic activity arrest mitochondrial motility in a highly localized fashion, since mitochondria as little as 15 μm away from the stimulation site remain motile (Li et al., 2004; Macaskill et al., 2009b; Wang and Schwarz, 2009). Passing mitochondria become immobilized in areas of locally high [Ca2+] at active synapses where demands for energy and calcium buffering are high. The distribution of mitochondria at synapses in turn affects synaptic transmission and strength. Stable positioning of mitochondria at presynaptic boutons maintains a steady release of synaptic vesicles (SV), resulting in steady amplitudes of excitatory postsynaptic currents (EPSCs) (Sun et al., 2013).
Mitochondrial distribution is also coupled to the balance between mitochondrial fission and fusion. Mutations in the mitochondrial fission protein dynamin-related protein (DRP1) result in the accumulation of mitochondria in the soma of both Drosophila motor neurons (Verstreken et al., 2005) and cultured hippocampal neurons (Li et al., 2004). The resulting decrease in mitochondrial density at pre-synaptic terminals of the neuromuscular junction impairs SV release, a defect rescued with exogenous ATP (Verstreken et al., 2005).
The calcium-dependent arrest of mitochondrial motility is mediated by Mitochondrial Rho GTPase (Miro) (Fransson et al., 2003; Guo et al., 2005). Miro has two Ca2+-binding EF-hand domains and two GTPase domains, and binds the kinesin-1 adaptors, TRAK1 and TRAK2, also known as Milton in Drosophila (Fransson et al., 2006; MacAskill et al., 2009a). Ca2+ binding to Miro induces mitochondrial arrest, however controversy still surrounds the mechanism. One model proposes that high levels of calcium promote binding of Miro1 to the motor domain of kinesin-1, thereby sterically inhibiting access to the microtubule (Wang and Schwarz, 2009). A second model posits that elevated calcium levels cause the dissociation of kinesin-1 from mitochondria and the Miro/TRAK complex (Macaskill et al., 2009b). Differences between axonal versus dendritic modes of regulation may underlie some of these observations. Syntaphilin is enriched on stationary mitochondria in the axon and knockout mice show enhanced axonal mitochondrial motility, with no effect observed on the motility of dendritic mitochondria (Kang et al., 2008). Calcium promotes binding of syntaphilin to both microtubules and kinesin-1, thereby decreasing the ATPase rate of kinesin-1 and acting as a brake on motility (Chen and Sheng, 2013), but only in the axon. Thus, the differing models may reflect cell-compartment specific regulatory mechanisms for mitochondrial movement.
In addition to the Miro/TRAK complex, syntabulin (Cai et al., 2005), FEZ1 (Fujita et al., 2007; Ikuta et al., 2007) and RanBP2 (Cho et al., 2007; Patil et al., 2013) have all been shown to recruit kinesin-1 to mitochondria to regulate mitochondrial motility. Whether these proteins can interact with the Miro1 complex or act independently remains to be established. However, in the absence of kinesin-1, a small population of mitochondria are still motile (Pilling et al., 2006), indicating that other kinesins also drive mitochondrial motility. There is evidence that both KIF1Bα (Nangaku et al., 1994) and KLP6 (Tanaka et al., 2011) contribute to the intracellular transport of mitochondria.
The role of dynein in mitochondrial trafficking is less well studied. Mutations in kinesin-1 and the Ca2+-dependent inactivation of kinesin-1 arrest mitochondrial motion in both anterograde as well as retrograde directions (Chen and Sheng, 2013; Macaskill et al., 2009b; Pilling et al., 2006; Wang and Schwarz, 2009), suggesting that the activity of oppositely-directed motors is coordinated (Pilling et al., 2006). The TRAK proteins interact with the dynein/dynactin complex and may modulate this coordination (van Spronsen et al., 2013). Loss of Miro affects both anterograde and retrograde transport (Russo et al., 2009), also consistent with an integrated regulatory mechanism.
Late endosomes and lysosomes
Approximately half of the late endosomes/lysosomes in the axon undergo bidirectional motility characterized by frequent directional changes and pauses while the remaining half undergo either anterograde or retrograde directed transport in approximately equal proportion (Hendricks et al., 2010; Moughamian and Holzbaur, 2012). Dynein is necessary for the proper positioning of both late endosomes and lysosomes (Harada et al., 1998). Dynein is recruited to late endosomes and lysosomes via the Rab7 effector RILP (Rab7-interacting lysosomal protein), which interacts directly with the C-terminus of the p150Glued subunit of dynactin (Johansson et al., 2007; Jordens et al., 2001). ORP1L, another Rab7 effector, then facilitates transport by recruiting the RILP-Rab7-dynactin-dynein complex onto βIII spectrin-associated membranes via an interaction with the Arp1 subunit of dynactin (Holleran et al., 2001; Johansson et al., 2007). Dynein light intermediate chain (DLIC) may also function, independently of RILP, to recruit dynein to late endosomes and lysosomes (Tan et al., 2011). Snapin has also been proposed to regulate the recruitment of dynein to late endosomes through a direct interaction with the dynein-intermediate chain (DIC); this interaction may facilitate the fusion of late endosomes and lysosomes (Cai et al., 2010).
The anterograde transport of lysosomes is mediated by SKIP (SifA and kinesin-interacting protein), which links Arl8, a mature lysosome Arf-like G protein, directly to the light chain of kinesin-1 (Rosa-Ferreira and Munro, 2011). Kinesin-2 motors are also associated with late endosomes and lysosomes (Brown et al., 2005; Castle et al., 2014; Hendricks et al., 2010), but the regulatory mechanisms that may control kinesin-2 activity, or that coordinate kinesin-2 function with the other lysosome-bound motors, remain to be determined.
SLOW AXONAL TRANSPORT OF CYTOSKELETAL POLYMERS AND SOLUBLE PROTEINS
While organelles and vesicles are transported relatively rapidly along the axon, the delivery of hundreds of different types of newly synthesized cytosolic proteins and cytoskeletal polymers occurs more slowly. The slow anterograde axonal transport of protein is subdivided into two speed categories: slow component a (SCa, mainly tubulin and neurofilaments) at rates of 0.2 to 1mm/day and slow component b (SCb, cytosolic proteins) which is around 10 fold faster at 1–10mm/day (reviewed in (Roy, 2014)). Due in large part to the difficulty in visualizing slow axonal transport in real time, it has remained the enigmatic cousin of fast axonal transport. Advances in imaging technologies and fluorescent probes, as well as computational modeling (Li et al., 2012; Scott et al., 2011), have allowed significant conceptual advances in understanding the processes at work, although specific molecular mechanisms and often the motor proteins involved are not yet understood.
Movement of neurofilaments by slow axonal transport has been well characterized. The vast majority of neurofilament protein is transported as assembled units of oligomers (Brown, 2000; Wang et al., 2000; Yan and Brown, 2005), moving in both the forward and reverse direction by engaging kinesin-1 and dynein motors (Shah et al., 2000; Uchida et al., 2009; Wagner et al., 2004; Yabe et al., 1999). Neurofilament subunit M binds directly to dynein (Wagner et al., 2004); KIF5A appears to be the primary kinesin-1 isoform for neurofilament transport (Wang, 2010), but the mechanisms regulating the recruitment of this motor remain unknown. In a breakthrough study, Brown and colleagues determined that the overall slow net rate of transport of neurofilaments along the axon is a result of short-lived motor-driven movements punctuated by extended pauses (Wang et al., 2000). How the activities of dynein and kinesin-1 are regulated in the context of neurofilament transport to result in such a disparate rate of transport compared to vesicular motility is unknown.
The slow axonal transport of the two other key cytoskeletal families, actin and tubulin, is more ambiguous. Analysis is complicated by the rapid polymerization and depolymerization rates of the polymers. Analogous to neurofilament transport, the movement of short microtubule fragments may be driven by motor proteins (Wang and Brown, 2002), although there is also evidence for the transport of soluble tubulin dimers in a kinesin-dependent manner (Terada et al., 2000). In contrast, the slow transport of actin occurs in growth-cone like waves that support neurite growth during development (Flynn et al., 2009), but how actin is replenished in mature neurons is unknown.
Slow axonal transport also carries a large and diverse pool of cytosolic proteins, with more than 200 distinct components although the complete proteome is unknown (Roy, 2014). A handful of examples have been studied so far. Current models suggest that proteins in this pool, such as synapsin, form spontaneously aggregated complexes (Scott et al., 2011) that undergo ‘dynamic recruitment’ to allow short bursts of anterograde transport by hitching a ride on passing vesicles (Tang et al., 2013).
Both the dynamic recruitment model for soluble proteins and the stop-and-go model for neurofilament transport (Brown and Jung, 2013; Roy, 2014) rely on the same microtubule motors that power fast axonal transport. The major differences in transport rates observed arise from differences in the time spent actively engaged in transport. Thus, slow axonal transport is a balance between long pauses and short bouts of motility. Despite the apparent inefficiency of this mechanism, it is worth stating that the amount of protein delivered to the pre-synapse by slow axonal transport outweighs that of fast axonal transport by at least 3 to 1 (Garner and Mahler, 1987; McEwen and Grafstein, 1968; Roy, 2014). The persistent and constitutive delivery of new material to the axon terminal by slow transport is critical to synapse survival.
An important outcome of slow axonal transport as it relates to neuronal function is the age of proteins conveyed by this method. Proteins that reach the axon terminus of a 1 m axon could be anywhere from four to twelve months old and might persist for as long as another 100 days (Garner and Mahler, 1987). Recent work suggests that mitochondria are also aged in distal neurites (Ferree et al., 2013). This fact highlights the specific axonal requirement for distal quality control (Maday and Holzbaur, 2014) and for chaperones (Song et al., 2013; Terada et al., 2010) that maintain the integrity of the proteome in the distal axon.
REGIONAL SPECIFICITY OF AXONAL TRANSPORT
Axonal transport is not uniform along the axon, as cargos exhibit different motility patterns within distinct regions of the axon. Both the axon initial segment and the distal axon are key sites for regulatory control. Site-specific organization of the microtubule cytoskeleton may provide a structural basis for the motility differences observed.
The axon initial segment
The AIS has a highly specialized cytoskeletal architecture. Microtubules are stabilized at the AIS by +TIPS EB1 and EB3 interacting with ankyrin G (Leterrier et al., 2011). The AIS has been proposed to act as a selective filter to exclude somatodendritic vesicular cargos from entering the axon. Live imaging studies indicate that axonal cargos move from cell body to axon with no change in velocity, while dendritic cargos that enter the base of the axon are specifically arrested at the start of the AIS (Petersen et al., 2014). Multiple mechanisms have been proposed to explain the underlying mechanism. One model posits that a dense actin meshwork at the AIS is key (Song et al., 2009; Watanabe et al., 2012), although neither polarized actin arrays nor dense actin meshworks were seen in recent platinum replica EM analysis (Jones et al., 2014) nor by super-resolution imaging (Xu et al., 2013). Alternatively, differences in the microtubule cytoskeleton may be critical in mediating axonal/dendritic sorting. It has been proposed that the mixed microtubule polarity of dendrites may be sufficient to allow dynein motors to selectively steer dendritic cargos to this compartment (Kapitein et al., 2010). Or, post-translational modifications to the microtubule cytoskeleton may contribute to the regulation of axonal vs. dendritic cargo sorting (Hammond et al., 2010; Nakata and Hirokawa, 2003; Setou et al., 2002). The recent observation that axo-dendritic selectivity precedes the establishment of both the AIS and mixed microtubule polarity in dendrites (Petersen et al., 2014) favors the interpretation that kinesin motors driving axonal cargos are responding to microtubule-based cues (Jacobson et al., 2006), but more work is required to fully establish this model.
Intriguingly, there is some evidence that the AIS also affects retrograde transport, as dense core vesicles in Drosophila circulating through the axon reverse at both the distal and the proximal axon, further implicating these regions of the axon as specialized zones for transport regulation (Wong et al., 2012).
Distal initiation of retrograde transport
Cargos undergoing retrograde transport often initiate motility very far from the soma, in the distal axon. Microtubules in the distal axon display enhanced dynamicity, with an enriched population of actively growing microtubule plus ends (Moughamian et al., 2013). Efficient initiation of retrograde transport from the distal axon requires a set of microtubule plus-end interacting proteins, or +TIPs (Lloyd et al., 2012; Moughamian and Holzbaur, 2012; Moughamian et al., 2013). The CAP-Gly domain of the p150Glued subunit of dynactin interacts with additional +TIP proteins, CLIP-170 and the end-binding proteins EB1 and EB3. Ordered recruitment of these plus-end binding proteins has been proposed to facilitate the active loading of the dynein-dynactin motor complex onto dynamic microtubule ends (Moughamian et al., 2013). This mechanism enhances retrograde transport initiation for multiple cargos, including early endosomes, late endosomes and lysosomes, and mitochondria (Moughamian et al., 2013).
The dynein-binding protein Lis1 is also a +TIP, and has been proposed to act as an initiation factor for dynein-mediated transport in fungi (Lenz et al., 2006). In neurons, however, Lis1 is required for transport all along the axon, not just in regions of increased microtubule dynamicity (Moughamian et al., 2013; Pandey and Smith, 2011). Lis1 likely acts directly on dynein, priming the motor for transport (Huang et al., 2012) and/or recruiting the dynein/dynactin complex onto certain cargos (Dix et al., 2013). In sensory neurons, the spectraplakin BPAG1n4 and the endosomal protein retrolinkin have also been reported to be required for sustained retrograde transport along the axon, in a mechanism that also depends on dynamic microtubule plus ends (Kapur et al., 2014).
mRNA TRANSPORT, LOCAL PROTEIN SYNTHESIS, AND INJURY SIGNALING
To carry out domain-specific tasks, neurons can locally regulate the proteome in response to dynamic changes in the environment. Local translation of mRNAs has been well characterized in dendrites (Holt and Schuman, 2013), but local translation in the axon is less well understood. Some direct evidence for this process comes from metabolic labeling studies to measure newly synthesized protein from severed axons (Merianda et al., 2009; Willis et al., 2005).
Sequences within the 5′ and 3′ UTR of mRNAs, are recognized by RNA-binding proteins and direct transport to either dendrites or the axon (Holt and Schuman, 2013; Merianda et al., 2013). mRNA is transported as translationally-repressed RNA granules along with RNA-binding proteins and ribosomes (Kanai et al., 2004; Knowles et al., 1996; Krichevsky and Kosik, 2001). These RNA granules exhibit bidirectional as well as confined oscillatory movement dependent on plus- and minus-end directed microtubule-based motors (Alami et al., 2014; Davidovic et al., 2007; Gumy et al., 2014; Kanai et al., 2004; Ling et al., 2004; Zhang et al., 2001). While mechanistic details are lacking, motor recruitment may be regulated by 3′ UTR localization signals (Amrute-Nayak and Bullock, 2012; Serano and Cohen, 1995).
Two prominent mechanisms initiate mRNA transport and local translation in the axon - axonal injury and chemotrophic signals. Injury in the distal axon induces local translation of importin-β, which heterodimerizes with importin-α to bind dynein (Hanz et al., 2003). These α/β dimers have high affinity for binding to the nuclear localization signals of transcription factors (e.g., STAT3; also translated locally upon injury). Thus, injury-induced local translation of importin-β assembles a retrograde signaling complex that delivers transcription factors to the nucleus to initiate a pro-regenerative transcriptional program (Hanz et al., 2003; Perry et al., 2012; Rishal and Fainzilber, 2014). Conditional disruption of the axon targeting sequence within the 3′ UTR of importin-β depletes importin-β mRNA and protein from the axon, causing delayed axon regeneration in vivo (Perry et al., 2012). Local translation of vimentin links pERK to importin-βmediated retrograde signaling to further modulate the transcriptional program (Perlson et al., 2005).
Axonal injury also induces the formation of the retrograde signaling complex DLK-pJNK3-JIP3-dynein/dynactin. Injury-induced calcium influx activates the mitogen-activated protein kinase kinase kinase dual leucine zipper kinase (DLK), which in turn activates c-Jun NH2-terminal kinase (JNK3) (Rishal and Fainzilber, 2014). JNK3 is linked to axonal transport vesicles via the JNK-interacting protein JIP3 (Cavalli et al., 2005). While JIP3 can interact with both kinesin and dynactin, injury induces preferential association with dynactin (Cavalli et al., 2005). Thus, upon injury, a complex is assembled of DLK-pJNK3-JIP3-dynein/dynactin that transports activated transcription factors (e.g., pSTAT3) to the nucleus to initiate axonal regeneration (Cavalli et al., 2005; Rishal and Fainzilber, 2014; Shin et al., 2012). In the absence of DLK, retrograde transport of pSTAT3 and JIP3 is blocked, resulting in delayed axonal regeneration (Shin et al., 2012).
Enhanced calcium influx at the injury site also back-propogates along the axon toward the cell soma to elicit changes in gene expression (Cho et al., 2013). Elevated intracellular calcium in the soma induces PKCμ-dependent export of HDAC5 from the nucleus, resulting in enhanced histone acetylation and activation of a pro-regenerative transcriptional program (Cho et al., 2013). Export of HDAC5 from the nucleus serves a dual function, as subsequent anterograde transport of HDAC5 to the injury site increases tubulin deacetylation, promoting growth cone dynamics and axon regeneration (Cho and Cavalli, 2012).
Chemotrophic signals can also induce mRNA transport and local translation in the distal axon and growth cone (reviewed in (Rishal and Fainzilber, 2014)). Treatment with neurotrophins localizes β-actin mRNA to the distal axon and growth cone; increased β-actin mRNA is concomitant with increased β-actin protein and forward protrusion of the growth cone (Bassell et al., 1998; Zhang et al., 2001). Interference with the 3′ UTR axonal targeting signal prevents distal accumulation of β-actin mRNA and protein, resulting in growth cone retraction (Zhang et al., 2001). Transport and translation of β-actin mRNA in the axon can be induced by NGF added exclusively to the axonal compartment, indicating an efficient relay of information from the distal axon to the cell soma and back (Willis et al., 2005). Translocation of β-actin mRNA into axons has also been observed in vivo upon axonal injury (Willis et al., 2011).
THE ENERGY REQUIREMENTS OF AXONAL TRANSPORT
Axonal transport is an energetically costly process as molecular motors hydrolyze ATP to carry out the work of stepping along microtubules. The conventional kinesin-1 motor consumes one molecule of ATP for every 8 nm step taken (Hackney, 1994). Measurements to date indicate a typical vesicle has one to two kinesins bound and exerting force at any one time (Encalada et al., 2011; Hendricks et al., 2012; Hendricks et al., 2010; Rai et al., 2013; Soppina et al., 2009). Taking the example of an average axon in the rat cortex, 40 mm in length, a single vesicle traversing this axon in the anterograde direction would require ~5 × 106 ATP molecules to do so: assuming no tug-of-war or switch events occur, which can be frequent in vivo (Hendricks et al., 2010; Soppina et al., 2009). In the 1 m-long axons of human motor neurons, the minimum ATP consumed per anterograde transport event reaches ~1.25 × 108 ATP molecules.
Unlike the consistent unidirectional stepping of kinesin-1 motors, the step size of single cytoplasmic dynein motors purified from mammalian brain ranges from 8 to 32 nm in length and can include backsteps (Mallik et al., 2004; Ross et al., 2006). However, recent in vitro and in vivo measurements show that dynein acts in teams of 6–12 motors per vesicle to produce persistent retrograde motility, and under these conditions motor teams show a step size of 8 nm (Hendricks et al., 2010; Rai et al., 2013; Soppina et al., 2009). Thus, a single vesicle traversing a human motor neuron from neuromuscular junction back to the soma would require a minimum of ~7.5 × 108 ATP molecules.
Strikingly, however, the amount of ATP hydrolyzed during axonal transport is relatively inconsequential compared to the amount of ATP consumed by those same neurons to fire action potentials and maintain resting potentials. A single action potential propagated along a 40 mm-long axon would require ~1 ×108 ATP molecules, and thus, axonal transport likely amounts to a fraction of the 25% of energy allocated to the housekeeping budget of the grey matter (Harris and Attwell, 2012).
One mechanism proposed to specifically address the energy demands of axonal transport is based on the finding that glycolytic enzymes are bound to the surface of vesicles moving along the axon, and can serve as an independent source of ATP for the motors driving transport of these vesicles (Zala et al., 2013). The identification of an energy source independent of mitochondria that can power vesicular transport is intriguing, and may allow cargos to transit any gaps in ATP gradients between unevenly dispersed mitochondria along the axon (MacAskill and Kittler, 2010; Zala et al., 2013). However, it remains unclear whether on-board energy production by glycolysis is required for axonal transport in vivo, as the energetic life of glia and neurons are intimately linked (Saab et al., 2013). Glia supply neurons with lactate under conditions of glucose shortage, bypassing glycolysis in the axon. Indeed, myelinated axons can survive for extended periods with only lactate, while fast axonal transport would be predicted to stop under these conditions if solely dependent on glycolysis. Further, there are several forms of axonal transport not associated with vesicular membranes including slow axonal transport and the movement of RNA granules. Without an onboard ATP supplier, these transport processes would experience regions of slow to no motility in the hypothesized low ATP regions. Alternatively, diffusion may be sufficient to maintain consistent levels of ATP along the axon. In either case, an onboard mechanism of glycolysis might become more relevant in situations of fast action potential firing – a high-energy task that increases local ATP demands, potentially restricting the ATP available for housekeeping tasks.
COMMON THEMES AND OUTSTANDING QUESTIONS
The compartmentalized nature of neurons requires active mechanisms of transport to distribute organelles to localized regions of demand. The differing patterns of motility observed for distinct organelles may reflect underlying functional differences. For example, mitochondrial motility facilitates distribution to sites of need, where these organelles become tethered to supply local needs for energy production and calcium buffering. Similarly, the bidirectional movement of mRNA granules may effectively distribute these particles to sites of local synthesis. Upon arrival, mRNA granules remain poised in a translationally-repressed state to rapidly respond to stimuli such as axonal injury. Other organelles, such as signaling endosomes, must relay information across the extended distance of the axon and thus undergo long journeys with highly processive, unidirectional motility to efficiently move from distal axon to cell soma. And degradative organelles such as autophagosomes must efficiently clear damaged organelles and aggregated proteins, recycling components back to the cell body for reuse.
Many major outstanding questions remain unanswered. How is organelle movement in the axon choreographed? How is the complement of motors associated with each organelle regulated? Since most organelles may have opposing motors bound simultaneously, future work will determine how oppositely-directed motors are coordinated to achieve organelle-specific differences in motility patterns. Further work is also required to uncover regional-specific differences in organelle transport within the neuron. Advances in imaging technology will continue to facilitate the study of these pathways both in cells and in vivo and will provide insights into the alteration of these pathways in stress and disease. A growing number of human diseases, both neurodevelopmental and neurodegenerative, are caused by mutations in the axonal transport machinery (Table I). Further, axonal transport is misregulated in many of the major neurodegenerative diseases affecting human populations, including ALS, Alzheimer’s, Huntington’s and Parkinson’s diseases (Millecamps and Julien, 2013). Thus, continued research into the molecular mechanisms involved in axonal transport and its regulation should provide new insights pointing toward development of novel therapeutic approaches in future.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- The Huntington’s Disease Collaborative Research Group. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. The Huntington’s Disease Collaborative Research Group. Cell. 1993;72:971–983. doi: 10.1016/0092-8674(93)90585-e. [DOI] [PubMed] [Google Scholar]
- Abdollahi MR, Morrison E, Sirey T, Molnar Z, Hayward BE, Carr IM, Springell K, Woods CG, Ahmed M, Hattingh L, et al. Mutation of the variant alpha-tubulin TUBA8 results in polymicrogyria with optic nerve hypoplasia. Am J Hum Genet. 2009;85:737–744. doi: 10.1016/j.ajhg.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhmanova A, Hammer JA., 3rd Linking molecular motors to membrane cargo. Curr Opin Cell Biol. 2010;22:479–487. doi: 10.1016/j.ceb.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alami NH, Smith RB, Carrasco MA, Williams LA, Winborn CS, Han SS, Kiskinis E, Winborn B, Freibaum BD, Kanagaraj A, et al. Axonal transport of TDP-43 mRNA granules is impaired by ALS-causing mutations. Neuron. 2014;81:536–543. doi: 10.1016/j.neuron.2013.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkuraya FS, Cai X, Emery C, Mochida GH, Al-Dosari MS, Felie JM, Hill RS, Barry BJ, Partlow JN, Gascon GG, et al. Human mutations in NDE1 cause extreme microcephaly with lissencephaly [corrected] Am J Hum Genet. 2011;88:536–547. doi: 10.1016/j.ajhg.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen RD, Metuzals J, Tasaki I, Brady ST, Gilbert SP. Fast axonal transport in squid giant axon. Science. 1982;218:1127–1129. doi: 10.1126/science.6183744. [DOI] [PubMed] [Google Scholar]
- Altar CA, Cai N, Bliven T, Juhasz M, Conner JM, Acheson AL, Lindsay RM, Wiegand SJ. Anterograde transport of brain-derived neurotrophic factor and its role in the brain. Nature. 1997;389:856–860. doi: 10.1038/39885. [DOI] [PubMed] [Google Scholar]
- Amrute-Nayak M, Bullock SL. Single-molecule assays reveal that RNA localization signals regulate dynein-dynactin copy number on individual transcript cargoes. Nat Cell Biol. 2012;14:416–423. doi: 10.1038/ncb2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki E, Tsuboi Y, Daechsel J, Milnerwood A, Vilarino-Guell C, Fujii N, Mishima T, Oka T, Hara H, Fukae J, et al. A Novel DCTN1 mutation with late-onset parkinsonism and frontotemporal atrophy. Movement disorders: official journal of the Movement Disorder Society. 2014 doi: 10.1002/mds.25833. [DOI] [PubMed] [Google Scholar]
- Ashrafi G, Schlehe JS, LaVoie MJ, Schwarz TL. Mitophagy of damaged mitochondria occurs locally in distal neuronal axons and requires PINK1 and Parkin. J Cell Biol. 2014;206:655–670. doi: 10.1083/jcb.201401070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayloo S, Lazarus JE, Dodda A, Tokito M, Ostap EM, Holzbaur EL. Dynactin functions as both a dynamic tether and brake during dynein-driven motility. Nature Communications. 2014 doi: 10.1038/ncomms5807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baas PW, Deitch JS, Black MM, Banker GA. Polarity orientation of microtubules in hippocampal neurons: uniformity in the axon and nonuniformity in the dendrite. Proc Natl Acad Sci U S A. 1988;85:8335–8339. doi: 10.1073/pnas.85.21.8335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakircioglu M, Carvalho OP, Khurshid M, Cox JJ, Tuysuz B, Barak T, Yilmaz S, Caglayan O, Dincer A, Nicholas AK, et al. The essential role of centrosomal NDE1 in human cerebral cortex neurogenesis. Am J Hum Genet. 2011;88:523–535. doi: 10.1016/j.ajhg.2011.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassell GJ, Zhang H, Byrd AL, Femino AM, Singer RH, Taneja KL, Lifshitz LM, Herman IM, Kosik KS. Sorting of beta-actin mRNA and protein to neurites and growth cones in culture. J Neurosci. 1998;18:251–265. doi: 10.1523/JNEUROSCI.18-01-00251.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya A, Watson FL, Pomeroy SL, Zhang YZ, Stiles CD, Segal RA. High-resolution imaging demonstrates dynein-based vesicular transport of activated Trk receptors. J Neurobiol. 2002;51:302–312. doi: 10.1002/neu.10062. [DOI] [PubMed] [Google Scholar]
- Bianco A, Dienstbier M, Salter HK, Gatto G, Bullock SL. Bicaudal-D regulates fragile X mental retardation protein levels, motility, and function during neuronal morphogenesis. Curr Biol. 2010;20:1487–1492. doi: 10.1016/j.cub.2010.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasius TL, Cai D, Jih GT, Toret CP, Verhey KJ. Two binding partners cooperate to activate the molecular motor Kinesin-1. J Cell Biol. 2007;176:11–17. doi: 10.1083/jcb.200605099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady ST, Lasek RJ, Allen RD. Fast axonal transport in extruded axoplasm from squid giant axon. Science. 1982;218:1129–1131. doi: 10.1126/science.6183745. [DOI] [PubMed] [Google Scholar]
- Brown A. Slow axonal transport: stop and go traffic in the axon. Nat Rev Mol Cell Biol. 2000;1:153–156. doi: 10.1038/35040102. [DOI] [PubMed] [Google Scholar]
- Brown A, Jung P. A critical reevaluation of the stationary axonal cytoskeleton hypothesis. Cytoskeleton (Hoboken) 2013;70:1–11. doi: 10.1002/cm.21083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CL, Maier KC, Stauber T, Ginkel LM, Wordeman L, Vernos I, Schroer TA. Kinesin-2 is a motor for late endosomes and lysosomes. Traffic. 2005;6:1114–1124. doi: 10.1111/j.1600-0854.2005.00347.x. [DOI] [PubMed] [Google Scholar]
- Burton PR, Paige JL. Polarity of axoplasmic microtubules in the olfactory nerve of the frog. Proc Natl Acad Sci U S A. 1981;78:3269–3273. doi: 10.1073/pnas.78.5.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Q, Gerwin C, Sheng ZH. Syntabulin-mediated anterograde transport of mitochondria along neuronal processes. J Cell Biol. 2005;170:959–969. doi: 10.1083/jcb.200506042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Q, Lu L, Tian JH, Zhu YB, Qiao H, Sheng ZH. Snapin-regulated late endosomal transport is critical for efficient autophagy-lysosomal function in neurons. Neuron. 2010;68:73–86. doi: 10.1016/j.neuron.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caroppo P, Le Ber I, Clot F, Rivaud-Pechoux S, Camuzat A, De Septenville A, Boutoleau-Bretonniere C, Mourlon V, Sauvee M, Lebouvier T, et al. DCTN1 mutation analysis in families with progressive supranuclear palsy-like phenotypes. JAMA neurology. 2014;71:208–215. doi: 10.1001/jamaneurol.2013.5100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castle MJ, Perlson E, Holzbaur EL, Wolfe JH. Long-distance axonal transport of AAV9 is driven by dynein and kinesin-2 and is trafficked in a highly motile Rab7-positive compartment. Molecular therapy: the journal of the American Society of Gene Therapy. 2014;22:554–566. doi: 10.1038/mt.2013.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalli V, Kujala P, Klumperman J, Goldstein LS. Sunday Driver links axonal transport to damage signaling. J Cell Biol. 2005;168:775–787. doi: 10.1083/jcb.200410136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caviston JP, Holzbaur EL. Huntingtin as an essential integrator of intracellular vesicular trafficking. Trends Cell Biol. 2009;19:147–155. doi: 10.1016/j.tcb.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Sheng ZH. Kinesin-1-syntaphilin coupling mediates activity-dependent regulation of axonal mitochondrial transport. J Cell Biol. 2013;202:351–364. doi: 10.1083/jcb.201302040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew S, Balasubramanian R, Chan WM, Kang PB, Andrews C, Webb BD, MacKinnon SE, Oystreck DT, Rankin J, Crawford TO, et al. A novel syndrome caused by the E410K amino acid substitution in the neuronal beta-tubulin isotype 3. Brain: a journal of neurology. 2013;136:522–535. doi: 10.1093/brain/aws345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho KI, Cai Y, Yi H, Yeh A, Aslanukov A, Ferreira PA. Association of the kinesin-binding domain of RanBP2 to KIF5B and KIF5C determines mitochondria localization and function. Traffic. 2007;8:1722–1735. doi: 10.1111/j.1600-0854.2007.00647.x. [DOI] [PubMed] [Google Scholar]
- Cho Y, Cavalli V. HDAC5 is a novel injury-regulated tubulin deacetylase controlling axon regeneration. EMBO J. 2012;31:3063–3078. doi: 10.1038/emboj.2012.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho Y, Sloutsky R, Naegle KM, Cavalli V. Injury-induced HDAC5 nuclear export is essential for axon regeneration. Cell. 2013;155:894–908. doi: 10.1016/j.cell.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdary PD, Che DL, Cui B. Neurotrophin signaling via long-distance axonal transport. Annual review of physical chemistry. 2012;63:571–594. doi: 10.1146/annurev-physchem-032511-143704. [DOI] [PubMed] [Google Scholar]
- Colin E, Zala D, Liot G, Rangone H, Borrell-Pages M, Li XJ, Saudou F, Humbert S. Huntingtin phosphorylation acts as a molecular switch for anterograde/retrograde transport in neurons. EMBO J. 2008;27:2124–2134. doi: 10.1038/emboj.2008.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui B, Wu C, Chen L, Ramirez A, Bearer EL, Li WP, Mobley WC, Chu S. One at a time, live tracking of NGF axonal transport using quantum dots. Proc Natl Acad Sci U S A. 2007;104:13666–13671. doi: 10.1073/pnas.0706192104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culver-Hanlon TL, Lex SA, Stephens AD, Quintyne NJ, King SJ. A microtubule-binding domain in dynactin increases dynein processivity by skating along microtubules. Nat Cell Biol. 2006;8:264–270. doi: 10.1038/ncb1370. [DOI] [PubMed] [Google Scholar]
- Davidovic L, Jaglin XH, Lepagnol-Bestel AM, Tremblay S, Simonneau M, Bardoni B, Khandjian EW. The fragile X mental retardation protein is a molecular adaptor between the neurospecific KIF3C kinesin and dendritic RNA granules. Hum Mol Genet. 2007;16:3047–3058. doi: 10.1093/hmg/ddm263. [DOI] [PubMed] [Google Scholar]
- de Ligt J, Willemsen MH, van Bon BW, Kleefstra T, Yntema HG, Kroes T, Vulto-van Silfhout AT, Koolen DA, de Vries P, Gilissen C, et al. Diagnostic exome sequencing in persons with severe intellectual disability. The New England journal of medicine. 2012;367:1921–1929. doi: 10.1056/NEJMoa1206524. [DOI] [PubMed] [Google Scholar]
- Deinhardt K, Salinas S, Verastegui C, Watson R, Worth D, Hanrahan S, Bucci C, Schiavo G. Rab5 and Rab7 control endocytic sorting along the axonal retrograde transport pathway. Neuron. 2006;52:293–305. doi: 10.1016/j.neuron.2006.08.018. [DOI] [PubMed] [Google Scholar]
- Delcroix JD, Valletta JS, Wu C, Hunt SJ, Kowal AS, Mobley WC. NGF signaling in sensory neurons: evidence that early endosomes carry NGF retrograde signals. Neuron. 2003;39:69–84. doi: 10.1016/s0896-6273(03)00397-0. [DOI] [PubMed] [Google Scholar]
- des Portes V, Francis F, Pinard JM, Desguerre I, Moutard ML, Snoeck I, Meiners LC, Capron F, Cusmai R, Ricci S, et al. doublecortin is the major gene causing X-linked subcortical laminar heterotopia (SCLH) Hum Mol Genet. 1998a;7:1063–1070. doi: 10.1093/hmg/7.7.1063. [DOI] [PubMed] [Google Scholar]
- des Portes V, Pinard JM, Billuart P, Vinet MC, Koulakoff A, Carrie A, Gelot A, Dupuis E, Motte J, Berwald-Netter Y, et al. A novel CNS gene required for neuronal migration and involved in X-linked subcortical laminar heterotopia and lissencephaly syndrome. Cell. 1998b;92:51–61. doi: 10.1016/s0092-8674(00)80898-3. [DOI] [PubMed] [Google Scholar]
- Dieni S, Matsumoto T, Dekkers M, Rauskolb S, Ionescu MS, Deogracias R, Gundelfinger ED, Kojima M, Nestel S, Frotscher M, et al. BDNF and its pro-peptide are stored in presynaptic dense core vesicles in brain neurons. J Cell Biol. 2012;196:775–788. doi: 10.1083/jcb.201201038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dix CI, Soundararajan HC, Dzhindzhev NS, Begum F, Suter B, Ohkura H, Stephens E, Bullock SL. Lissencephaly-1 promotes the recruitment of dynein and dynactin to transported mRNAs. J Cell Biol. 2013;202:479–494. doi: 10.1083/jcb.201211052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixit R, Levy JR, Tokito M, Ligon LA, Holzbaur EL. Regulation of dynactin through the differential expression of p150Glued isoforms. J Biol Chem. 2008a;283:33611–33619. doi: 10.1074/jbc.M804840200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixit R, Ross JL, Goldman YE, Holzbaur EL. Differential regulation of dynein and kinesin motor proteins by tau. Science. 2008b;319:1086–1089. doi: 10.1126/science.1152993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobyns WB, Reiner O, Carrozzo R, Ledbetter DH. Lissencephaly. A human brain malformation associated with deletion of the LIS1 gene located at chromosome 17p13. JAMA: the journal of the American Medical Association. 1993;270:2838–2842. doi: 10.1001/jama.270.23.2838. [DOI] [PubMed] [Google Scholar]
- Dor T, Cinnamon Y, Raymond L, Shaag A, Bouslam N, Bouhouche A, Gaussen M, Meyer V, Durr A, Brice A, et al. KIF1C mutations in two families with hereditary spastic paraparesis and cerebellar dysfunction. Journal of medical genetics. 2014;51:137–142. doi: 10.1136/jmedgenet-2013-102012. [DOI] [PubMed] [Google Scholar]
- Drerup CM, Nechiporuk AV. JNK-interacting protein 3 mediates the retrograde transport of activated c-Jun N-terminal kinase and lysosomes. PLoS Genet. 2013;9:e1003303. doi: 10.1371/journal.pgen.1003303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebbing B, Mann K, Starosta A, Jaud J, Schols L, Schule R, Woehlke G. Effect of spastic paraplegia mutations in KIF5A kinesin on transport activity. Hum Mol Genet. 2008;17:1245–1252. doi: 10.1093/hmg/ddn014. [DOI] [PubMed] [Google Scholar]
- Ehlers MD, Kaplan DR, Price DL, Koliatsos VE. NGF-stimulated retrograde transport of trkA in the mammalian nervous system. J Cell Biol. 1995;130:149–156. doi: 10.1083/jcb.130.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Encalada SE, Szpankowski L, Xia CH, Goldstein LS. Stable kinesin and dynein assemblies drive the axonal transport of mammalian prion protein vesicles. Cell. 2011;144:551–565. doi: 10.1016/j.cell.2011.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlich Y, Edvardson S, Hodges E, Zenvirt S, Thekkat P, Shaag A, Dor T, Hannon GJ, Elpeleg O. Exome sequencing and disease-network analysis of a single family implicate a mutation in KIF1A in hereditary spastic paraparesis. Genome research. 2011;21:658–664. doi: 10.1101/gr.117143.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falzone TL, Stokin GB, Lillo C, Rodrigues EM, Westerman EL, Williams DS, Goldstein LS. Axonal stress kinase activation and tau misbehavior induced by kinesin-1 transport defects. J Neurosci. 2009;29:5758–5767. doi: 10.1523/JNEUROSCI.0780-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrer MJ, Hulihan MM, Kachergus JM, Dachsel JC, Stoessl AJ, Grantier LL, Calne S, Calne DB, Lechevalier B, Chapon F, et al. DCTN1 mutations in Perry syndrome. Nature genetics. 2009;41:163–165. doi: 10.1038/ng.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferree AW, Trudeau K, Zik E, Benador IY, Twig G, Gottlieb RA, Shirihai OS. MitoTimer probe reveals the impact of autophagy, fusion, and motility on subcellular distribution of young and old mitochondrial protein and on relative mitochondrial protein age. Autophagy. 2013;9:1887–1896. doi: 10.4161/auto.26503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorillo C, Moro F, Yi J, Weil S, Brisca G, Astrea G, Severino M, Romano A, Battini R, Rossi A, et al. Novel dynein DYNC1H1 neck and motor domain mutations link distal spinal muscular atrophy and abnormal cortical development. Human mutation. 2014;35:298–302. doi: 10.1002/humu.22491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn KC, Pak CW, Shaw AE, Bradke F, Bamburg JR. Growth cone-like waves transport actin and promote axonogenesis and neurite branching. Dev Neurobiol. 2009;69:761–779. doi: 10.1002/dneu.20734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson A, Ruusala A, Aspenstrom P. Atypical Rho GTPases have roles in mitochondrial homeostasis and apoptosis. J Biol Chem. 2003;278:6495–6502. doi: 10.1074/jbc.M208609200. [DOI] [PubMed] [Google Scholar]
- Fransson S, Ruusala A, Aspenstrom P. The atypical Rho GTPases Miro-1 and Miro-2 have essential roles in mitochondrial trafficking. Biochem Biophys Res Commun. 2006;344:500–510. doi: 10.1016/j.bbrc.2006.03.163. [DOI] [PubMed] [Google Scholar]
- Fu M-M, Holzbaur EL. Integrated regulation of motor-driven organelle transport by scaffolding proteins. Trends Cell Biol. 2014:76–72. doi: 10.1016/j.tcb.2014.05.002. [DOI] [PMC free article] [PubMed]
- Fu MM, Nirschl JJ, Holzbaur ELF. LC3 binding to the scaffolding protein JIP1 regulates processive dynein-driven transport of autophagosomes. Dev Cell. 2014;29:577–590. doi: 10.1016/j.devcel.2014.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu MM, Holzbaur EL. JIP1 regulates the directionality of APP axonal transport by coordinating kinesin and dynein motors. J Cell Biol. 2013;202:495–508. doi: 10.1083/jcb.201302078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita T, Maturana AD, Ikuta J, Hamada J, Walchli S, Suzuki T, Sawa H, Wooten MW, Okajima T, Tatematsu K, et al. Axonal guidance protein FEZ1 associates with tubulin and kinesin motor protein to transport mitochondria in neurites of NGF-stimulated PC12 cells. Biochem Biophys Res Commun. 2007;361:605–610. doi: 10.1016/j.bbrc.2007.07.050. [DOI] [PubMed] [Google Scholar]
- Garner JA, Mahler HR. Biogenesis of presynaptic terminal proteins. Journal of neurochemistry. 1987;49:905–915. doi: 10.1111/j.1471-4159.1987.tb00979.x. [DOI] [PubMed] [Google Scholar]
- Gauthier LR, Charrin BC, Borrell-Pages M, Dompierre JP, Rangone H, Cordelieres FP, De Mey J, MacDonald ME, Lessmann V, Humbert S, et al. Huntingtin controls neurotrophic support and survival of neurons by enhancing BDNF vesicular transport along microtubules. Cell. 2004;118:127–138. doi: 10.1016/j.cell.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Gleeson JG, Allen KM, Fox JW, Lamperti ED, Berkovic S, Scheffer I, Cooper EC, Dobyns WB, Minnerath SR, Ross ME, et al. Doublecortin, a brain-specific gene mutated in human X-linked lissencephaly and double cortex syndrome, encodes a putative signaling protein. Cell. 1998;92:63–72. doi: 10.1016/s0092-8674(00)80899-5. [DOI] [PubMed] [Google Scholar]
- Goodwin PR, Sasaki JM, Juo P. Cyclin-dependent kinase 5 regulates the polarized trafficking of neuropeptide-containing dense-core vesicles in Caenorhabditis elegans motor neurons. J Neurosci. 2012;32:8158–8172. doi: 10.1523/JNEUROSCI.0251-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin JW, Price DL, Drachman DB, Engel WK. Axonal transport to and from the motor nerve ending. Annals of the New York Academy of Sciences. 1976;274:31–45. doi: 10.1111/j.1749-6632.1976.tb47674.x. [DOI] [PubMed] [Google Scholar]
- Grimes ML, Beattie E, Mobley WC. A signaling organelle containing the nerve growth factor-activated receptor tyrosine kinase, TrkA. Proc Natl Acad Sci U S A. 1997;94:9909–9914. doi: 10.1073/pnas.94.18.9909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross SP. Hither and yon: a review of bi-directional microtubule-based transport. Physical biology. 2004;1:R1–11. doi: 10.1088/1478-3967/1/2/R01. [DOI] [PubMed] [Google Scholar]
- Gross SP, Vershinin M, Shubeita GT. Cargo transport: two motors are sometimes better than one. Curr Biol. 2007;17:R478–486. doi: 10.1016/j.cub.2007.04.025. [DOI] [PubMed] [Google Scholar]
- Gumy LF, Katrukha EA, Kapitein LC, Hoogenraad CC. New insights into mRNA trafficking in axons. Dev Neurobiol. 2014;74:233–244. doi: 10.1002/dneu.22121. [DOI] [PubMed] [Google Scholar]
- Guo X, Macleod GT, Wellington A, Hu F, Panchumarthi S, Schoenfield M, Marin L, Charlton MP, Atwood HL, Zinsmaier KE. The GTPase dMiro is required for axonal transport of mitochondria to Drosophila synapses. Neuron. 2005;47:379–393. doi: 10.1016/j.neuron.2005.06.027. [DOI] [PubMed] [Google Scholar]
- Hackney DD. Evidence for alternating head catalysis by kinesin during microtubule-stimulated ATP hydrolysis. Proc Natl Acad Sci U S A. 1994;91:6865–6869. doi: 10.1073/pnas.91.15.6865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall DH, Hedgecock EM. Kinesin-related gene unc-104 is required for axonal transport of synaptic vesicles in C. elegans. Cell. 1991;65:837–847. doi: 10.1016/0092-8674(91)90391-b. [DOI] [PubMed] [Google Scholar]
- Hamdan FF, Gauthier J, Araki Y, Lin DT, Yoshizawa Y, Higashi K, Park AR, Spiegelman D, Dobrzeniecka S, Piton A, et al. Excess of de novo deleterious mutations in genes associated with glutamatergic systems in nonsyndromic intellectual disability. Am J Hum Genet. 2011;88:306–316. doi: 10.1016/j.ajhg.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond JW, Huang CF, Kaech S, Jacobson C, Banker G, Verhey KJ. Posttranslational modifications of tubulin and the polarized transport of kinesin-1 in neurons. Mol Biol Cell. 2010;21:572–583. doi: 10.1091/mbc.E09-01-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanz S, Perlson E, Willis D, Zheng JQ, Massarwa R, Huerta JJ, Koltzenburg M, Kohler M, van-Minnen J, Twiss JL, et al. Axoplasmic importins enable retrograde injury signaling in lesioned nerve. Neuron. 2003;40:1095–1104. doi: 10.1016/s0896-6273(03)00770-0. [DOI] [PubMed] [Google Scholar]
- Hara T, Nakamura K, Matsui M, Yamamoto A, Nakahara Y, Suzuki-Migishima R, Yokoyama M, Mishima K, Saito I, Okano H, et al. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature. 2006;441:885–889. doi: 10.1038/nature04724. [DOI] [PubMed] [Google Scholar]
- Harada A, Takei Y, Kanai Y, Tanaka Y, Nonaka S, Hirokawa N. Golgi vesiculation and lysosome dispersion in cells lacking cytoplasmic dynein. J Cell Biol. 1998;141:51–59. doi: 10.1083/jcb.141.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms MB, Ori-McKenney KM, Scoto M, Tuck EP, Bell S, Ma D, Masi S, Allred P, Al-Lozi M, Reilly MM, et al. Mutations in the tail domain of DYNC1H1 cause dominant spinal muscular atrophy. Neurology. 2012;78:1714–1720. doi: 10.1212/WNL.0b013e3182556c05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington AW, Ginty DD. Long-distance retrograde neurotrophic factor signalling in neurons. Nat Rev Neurosci. 2013;14:177–187. doi: 10.1038/nrn3253. [DOI] [PubMed] [Google Scholar]
- Harris JJ, Attwell D. The energetics of CNS white matter. J Neurosci. 2012;32:356–371. doi: 10.1523/JNEUROSCI.3430-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazan J, Fonknechten N, Mavel D, Paternotte C, Samson D, Artiguenave F, Davoine CS, Cruaud C, Durr A, Wincker P, et al. Spastin, a new AAA protein, is altered in the most frequent form of autosomal dominant spastic paraplegia. Nature genetics. 1999;23:296–303. doi: 10.1038/15472. [DOI] [PubMed] [Google Scholar]
- Heerssen HM, Pazyra MF, Segal RA. Dynein motors transport activated Trks to promote survival of target-dependent neurons. Nat Neurosci. 2004;7:596–604. doi: 10.1038/nn1242. [DOI] [PubMed] [Google Scholar]
- Hendricks AG, Holzbaur EL, Goldman YE. Force measurements on cargoes in living cells reveal collective dynamics of microtubule motors. Proc Natl Acad Sci U S A. 2012;109:18447–18452. doi: 10.1073/pnas.1215462109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks AG, Perlson E, Ross JL, Schroeder HW, 3rd, Tokito M, Holzbaur EL. Motor coordination via a tug-of-war mechanism drives bidirectional vesicle transport. Curr Biol. 2010;20:697–702. doi: 10.1016/j.cub.2010.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirokawa N, Niwa S, Tanaka Y. Molecular motors in neurons: transport mechanisms and roles in brain function, development, and disease. Neuron. 2010;68:610–638. doi: 10.1016/j.neuron.2010.09.039. [DOI] [PubMed] [Google Scholar]
- Hirokawa N, Sato-Yoshitake R, Kobayashi N, Pfister KK, Bloom GS, Brady ST. Kinesin associates with anterogradely transported membranous organelles in vivo. J Cell Biol. 1991;114:295–302. doi: 10.1083/jcb.114.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirokawa N, Sato-Yoshitake R, Yoshida T, Kawashima T. Brain dynein (MAP1C) localizes on both anterogradely and retrogradely transported membranous organelles in vivo. J Cell Biol. 1990;111:1027–1037. doi: 10.1083/jcb.111.3.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeprich GJ, Thompson AR, McVicker DP, Hancock WO, Berger CL. Kinesin’s neck-linker determines its ability to navigate obstacles on the microtubule surface. Biophys J. 2014;106:1691–1700. doi: 10.1016/j.bpj.2014.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollenbeck PJ. Products of endocytosis and autophagy are retrieved from axons by regulated retrograde organelle transport. J Cell Biol. 1993;121:305–315. doi: 10.1083/jcb.121.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollenbeck PJ, Saxton WM. The axonal transport of mitochondria. J Cell Sci. 2005;118:5411–5419. doi: 10.1242/jcs.02745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holleran EA, Ligon LA, Tokito M, Stankewich MC, Morrow JS, Holzbaur EL. beta III spectrin binds to the Arp1 subunit of dynactin. J Biol Chem. 2001;276:36598–36605. doi: 10.1074/jbc.M104838200. [DOI] [PubMed] [Google Scholar]
- Holleran EA, Tokito MK, Karki S, Holzbaur EL. Centractin (ARP1) associates with spectrin revealing a potential mechanism to link dynactin to intracellular organelles. J Cell Biol. 1996;135:1815–1829. doi: 10.1083/jcb.135.6.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt CE, Schuman EM. The central dogma decentralized: new perspectives on RNA function and local translation in neurons. Neuron. 2013;80:648–657. doi: 10.1016/j.neuron.2013.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzbaur EL, Hammarback JA, Paschal BM, Kravit NG, Pfister KK, Vallee RB. Homology of a 150K cytoplasmic dynein-associated polypeptide with the Drosophila gene Glued. Nature. 1991;351:579–583. doi: 10.1038/351579a0. [DOI] [PubMed] [Google Scholar]
- Horiuchi D, Barkus RV, Pilling AD, Gassman A, Saxton WM. APLIP1, a kinesin binding JIP-1/JNK scaffold protein, influences the axonal transport of both vesicles and mitochondria in Drosophila. Curr Biol. 2005;15:2137–2141. doi: 10.1016/j.cub.2005.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiuchi D, Collins CA, Bhat P, Barkus RV, Diantonio A, Saxton WM. Control of a kinesin-cargo linkage mechanism by JNK pathway kinases. Curr Biol. 2007;17:1313–1317. doi: 10.1016/j.cub.2007.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Roberts AJ, Leschziner AE, Reck-Peterson SL. Lis1 acts as a “clutch” between the ATPase and microtubule-binding domains of the dynein motor. Cell. 2012;150:975–986. doi: 10.1016/j.cell.2012.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton M, Lendon CL, Rizzu P, Baker M, Froelich S, Houlden H, Pickering-Brown S, Chakraverty S, Isaacs A, Grover A, et al. Association of missense and 5′-splice-site mutations in tau with the inherited dementia FTDP-17. Nature. 1998;393:702–705. doi: 10.1038/31508. [DOI] [PubMed] [Google Scholar]
- Ikuta J, Maturana A, Fujita T, Okajima T, Tatematsu K, Tanizawa K, Kuroda S. Fasciculation and elongation protein zeta-1 (FEZ1) participates in the polarization of hippocampal neuron by controlling the mitochondrial motility. Biochem Biophys Res Commun. 2007;353:127–132. doi: 10.1016/j.bbrc.2006.11.142. [DOI] [PubMed] [Google Scholar]
- Jacobson C, Schnapp B, Banker GA. A change in the selective translocation of the Kinesin-1 motor domain marks the initial specification of the axon. Neuron. 2006;49:797–804. doi: 10.1016/j.neuron.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Jaglin XH, Poirier K, Saillour Y, Buhler E, Tian G, Bahi-Buisson N, Fallet-Bianco C, Phan-Dinh-Tuy F, Kong XP, Bomont P, et al. Mutations in the beta-tubulin gene TUBB2B result in asymmetrical polymicrogyria. Nature genetics. 2009;41:746–752. doi: 10.1038/ng.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janke C, Bulinski JC. Post-translational regulation of the microtubule cytoskeleton: mechanisms and functions. Nat Rev Mol Cell Biol. 2011;12:773–786. doi: 10.1038/nrm3227. [DOI] [PubMed] [Google Scholar]
- Johansson M, Rocha N, Zwart W, Jordens I, Janssen L, Kuijl C, Olkkonen VM, Neefjes J. Activation of endosomal dynein motors by stepwise assembly of Rab7-RILP-p150Glued, ORP1L, and the receptor betalll spectrin. J Cell Biol. 2007;176:459–471. doi: 10.1083/jcb.200606077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SL, Korobova F, Svitkina T. Axon initial segment cytoskeleton comprises a multiprotein submembranous coat containing sparse actin filaments. J Cell Biol. 2014;205:67–81. doi: 10.1083/jcb.201401045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordens I, Fernandez-Borja M, Marsman M, Dusseljee S, Janssen L, Calafat J, Janssen H, Wubbolts R, Neefjes J. The Rab7 effector protein RILP controls lysosomal transport by inducing the recruitment of dynein-dynactin motors. Curr Biol. 2001;11:1680–1685. doi: 10.1016/s0960-9822(01)00531-0. [DOI] [PubMed] [Google Scholar]
- Kaan HY, Hackney DD, Kozielski F. The structure of the kinesin-1 motor-tail complex reveals the mechanism of autoinhibition. Science. 2011;333:883–885. doi: 10.1126/science.1204824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaether C, Skehel P, Dotti CG. Axonal membrane proteins are transported in distinct carriers: a two-color video microscopy study in cultured hippocampal neurons. Mol Biol Cell. 2000;11:1213–1224. doi: 10.1091/mbc.11.4.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamal A, Stokin GB, Yang Z, Xia CH, Goldstein LS. Axonal transport of amyloid precursor protein is mediated by direct binding to the kinesin light chain subunit of kinesin-I. Neuron. 2000;28:449–459. doi: 10.1016/s0896-6273(00)00124-0. [DOI] [PubMed] [Google Scholar]
- Kanai Y, Dohmae N, Hirokawa N. Kinesin transports RNA: isolation and characterization of an RNA-transporting granule. Neuron. 2004;43:513–525. doi: 10.1016/j.neuron.2004.07.022. [DOI] [PubMed] [Google Scholar]
- Kang JS, Tian JH, Pan PY, Zald P, Li C, Deng C, Sheng ZH. Docking of axonal mitochondria by syntaphilin controls their mobility and affects short-term facilitation. Cell. 2008;132:137–148. doi: 10.1016/j.cell.2007.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapitein LC, Schlager MA, Kuijpers M, Wulf PS, van Spronsen M, MacKintosh FC, Hoogenraad CC. Mixed microtubules steer dynein-driven cargo transport into dendrites. Curr Biol. 2010;20:290–299. doi: 10.1016/j.cub.2009.12.052. [DOI] [PubMed] [Google Scholar]
- Kapur M, Maloney MT, Wang W, Chen X, Millan I, Mooney T, Yang J, Yang Y. A SxIP motif interaction at the microtubule plus end is important for processive retrograde axonal transport. Cell Mol Life Sci. 2014 doi: 10.1007/s00018-014-1611-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karki S, Holzbaur EL. Affinity chromatography demonstrates a direct binding between cytoplasmic dynein and the dynactin complex. J Biol Chem. 1995;270:28806–28811. doi: 10.1074/jbc.270.48.28806. [DOI] [PubMed] [Google Scholar]
- Keays DA, Tian G, Poirier K, Huang GJ, Siebold C, Cleak J, Oliver PL, Fray M, Harvey RJ, Molnar Z, et al. Mutations in alpha-tubulin cause abnormal neuronal migration in mice and lissencephaly in humans. Cell. 2007;128:45–57. doi: 10.1016/j.cell.2006.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King SJ, Schroer TA. Dynactin increases the processivity of the cytoplasmic dynein motor. Nat Cell Biol. 2000;2:20–24. doi: 10.1038/71338. [DOI] [PubMed] [Google Scholar]
- Klebe S, Lossos A, Azzedine H, Mundwiller E, Sheffer R, Gaussen M, Marelli C, Nawara M, Carpentier W, Meyer V, et al. KIF1A missense mutations in SPG30, an autosomal recessive spastic paraplegia: distinct phenotypes according to the nature of the mutations. European journal of human genetics: EJHG. 2012;20:645–649. doi: 10.1038/ejhg.2011.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleele T, Marinkovic P, Williams PR, Stern S, Weigand EE, Engerer P, Naumann R, Hartmann J, Karl RM, Bradke F, et al. An assay to image neuronal microtubule dynamics in mice. Nat Commun. 2014;5:4827. doi: 10.1038/ncomms5827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klopfenstein DR, Vale RD. The lipid binding pleckstrin homology domain in UNC-104 kinesin is necessary for synaptic vesicle transport in Caenorhabditis elegans. Mol Biol Cell. 2004;15:3729–3739. doi: 10.1091/mbc.E04-04-0326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles RB, Sabry JH, Martone ME, Deerinck TJ, Ellisman MH, Bassell GJ, Kosik KS. Translocation of RNA granules in living neurons. J Neurosci. 1996;16:7812–7820. doi: 10.1523/JNEUROSCI.16-24-07812.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu M, Waguri S, Chiba T, Murata S, Iwata J, Tanida I, Ueno T, Koike M, Uchiyama Y, Kominami E, et al. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature. 2006;441:880–884. doi: 10.1038/nature04723. [DOI] [PubMed] [Google Scholar]
- Komatsu M, Wang QJ, Holstein GR, Friedrich VL, Jr, Iwata J, Kominami E, Chait BT, Tanaka K, Yue Z. Essential role for autophagy protein Atg7 in the maintenance of axonal homeostasis and the prevention of axonal degeneration. Proc Natl Acad Sci U S A. 2007;104:14489–14494. doi: 10.1073/pnas.0701311104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo M, Takei Y, Hirokawa N. Motor protein KIF1A is essential for hippocampal synaptogenesis and learning enhancement in an enriched environment. Neuron. 2012;73:743–757. doi: 10.1016/j.neuron.2011.12.020. [DOI] [PubMed] [Google Scholar]
- Konishi Y, Setou M. Tubulin tyrosination navigates the kinesin-1 motor domain to axons. Nat Neurosci. 2009;12:559–567. doi: 10.1038/nn.2314. [DOI] [PubMed] [Google Scholar]
- Kreutzberg GW. Neuronal dynamics and axonal flow. IV. Blockage of intra-axonal enzyme transport by colchicine. Proc Natl Acad Sci U S A. 1969;62:722–728. doi: 10.1073/pnas.62.3.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krichevsky AM, Kosik KS. Neuronal RNA granules: a link between RNA localization and stimulation-dependent translation. Neuron. 2001;32:683–696. doi: 10.1016/s0896-6273(01)00508-6. [DOI] [PubMed] [Google Scholar]
- Kuijpers M, Hoogenraad CC. Centrosomes, microtubules and neuronal development. Mol Cell Neurosci. 2011;48:349–358. doi: 10.1016/j.mcn.2011.05.004. [DOI] [PubMed] [Google Scholar]
- Kuta A, Deng W, Morsi El-Kadi A, Banks GT, Hafezparast M, Pfister KK, Fisher EM. Mouse cytoplasmic dynein intermediate chains: identification of new isoforms, alternative splicing and tissue distribution of transcripts. PLoS One. 2010;5:e11682. doi: 10.1371/journal.pone.0011682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan AC, Dombeck DA, Webb WW. Polarized microtubule arrays in apical dendrites and axons. Proc Natl Acad Sci U S A. 2008;105:11370–11375. doi: 10.1073/pnas.0805199105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaMonte BH, Wallace KE, Holloway BA, Shelly SS, Ascano J, Tokito M, Van Winkle T, Howland DS, Holzbaur EL. Disruption of dynein/dynactin inhibits axonal transport in motor neurons causing late-onset progressive degeneration. Neuron. 2002;34:715–727. doi: 10.1016/s0896-6273(02)00696-7. [DOI] [PubMed] [Google Scholar]
- Larti F, Kahrizi K, Musante L, Hu H, Papari E, Fattahi Z, Bazazzadegan N, Liu Z, Banan M, Garshasbi M, et al. A defect in the CLIP1 gene (CLIP-170) can cause autosomal recessive intellectual disability. European journal of human genetics: EJHG. 2014 doi: 10.1038/ejhg.2014.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence CJ, Dawe RK, Christie KR, Cleveland DW, Dawson SC, Endow SA, Goldstein LS, Goodson HV, Hirokawa N, Howard J, et al. A standardized kinesin nomenclature. J Cell Biol. 2004;167:19–22. doi: 10.1083/jcb.200408113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Sato Y, Nixon RA. Lysosomal proteolysis inhibition selectively disrupts axonal transport of degradative organelles and causes an Alzheimer’s-like axonal dystrophy. J Neurosci. 2011;31:7817–7830. doi: 10.1523/JNEUROSCI.6412-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz JH, Schuchardt I, Straube A, Steinberg G. A dynein loading zone for retrograde endosome motility at microtubule plus-ends. EMBO J. 2006;25:2275–2286. doi: 10.1038/sj.emboj.7601119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leterrier C, Vacher H, Fache MP, d’Ortoli SA, Castets F, Autillo-Touati A, Dargent B. End-binding proteins EB3 and EB1 link microtubules to ankyrin G in the axon initial segment. Proc Natl Acad Sci U S A. 2011;108:8826–8831. doi: 10.1073/pnas.1018671108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Jung P, Brown A. Axonal transport of neurofilaments: a single population of intermittently moving polymers. J Neurosci. 2012;32:746–758. doi: 10.1523/JNEUROSCI.4926-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Okamoto K, Hayashi Y, Sheng M. The importance of dendritic mitochondria in the morphogenesis and plasticity of spines and synapses. Cell. 2004;119:873–887. doi: 10.1016/j.cell.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Ling SC, Fahrner PS, Greenough WT, Gelfand VI. Transport of Drosophila fragile X mental retardation protein-containing ribonucleoprotein granules by kinesin-1 and cytoplasmic dynein. Proc Natl Acad Sci U S A. 2004;101:17428–17433. doi: 10.1073/pnas.0408114101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd TE, Machamer J, O’Hara K, Kim JH, Collins SE, Wong MY, Sahin B, Imlach W, Yang Y, Levitan ES, et al. The p150(Glued) CAP-Gly domain regulates initiation of retrograde transport at synaptic termini. Neuron. 2012;74:344–360. doi: 10.1016/j.neuron.2012.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo KY, Kuzmin A, Unger SM, Petersen JD, Silverman MA. KIF1A is the primary anterograde motor protein required for the axonal transport of dense-core vesicles in cultured hippocampal neurons. Neurosci Lett. 2011;491:168–173. doi: 10.1016/j.neulet.2011.01.018. [DOI] [PubMed] [Google Scholar]
- MacAskill AF, Brickley K, Stephenson FA, Kittler JT. GTPase dependent recruitment of Grif-1 by Miro1 regulates mitochondrial trafficking in hippocampal neurons. Mol Cell Neurosci. 2009a;40:301–312. doi: 10.1016/j.mcn.2008.10.016. [DOI] [PubMed] [Google Scholar]
- MacAskill AF, Kittler JT. Control of mitochondrial transport and localization in neurons. Trends Cell Biol. 2010;20:102–112. doi: 10.1016/j.tcb.2009.11.002. [DOI] [PubMed] [Google Scholar]
- Macaskill AF, Rinholm JE, Twelvetrees AE, Arancibia-Carcamo IL, Muir J, Fransson A, Aspenstrom P, Attwell D, Kittler JT. Miro1 is a calcium sensor for glutamate receptor-dependent localization of mitochondria at synapses. Neuron. 2009b;61:541–555. doi: 10.1016/j.neuron.2009.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maday S, Holzbaur EL. Autophagosome biogenesis in primary neurons follows an ordered and spatially regulated pathway. Dev Cell. 2014;30:71–85. doi: 10.1016/j.devcel.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maday S, Wallace KE, Holzbaur EL. Autophagosomes initiate distally and mature during transport toward the cell soma in primary neurons. J Cell Biol. 2012;196:407–417. doi: 10.1083/jcb.201106120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallik R, Carter BC, Lex SA, King SJ, Gross SP. Cytoplasmic dynein functions as a gear in response to load. Nature. 2004;427:649–652. doi: 10.1038/nature02293. [DOI] [PubMed] [Google Scholar]
- Mallik R, Petrov D, Lex SA, King SJ, Gross SP. Building complexity: an in vitro study of cytoplasmic dynein with in vivo implications. Curr Biol. 2005;15:2075–2085. doi: 10.1016/j.cub.2005.10.039. [DOI] [PubMed] [Google Scholar]
- Mallik R, Rai AK, Barak P, Rai A, Kunwar A. Teamwork in microtubule motors. Trends Cell Biol. 2013;23:575–582. doi: 10.1016/j.tcb.2013.06.003. [DOI] [PubMed] [Google Scholar]
- Matanis T, Akhmanova A, Wulf P, Del Nery E, Weide T, Stepanova T, Galjart N, Grosveld F, Goud B, De Zeeuw CI, et al. Bicaudal-D regulates COPI-independent Golgi-ER transport by recruiting the dynein-dynactin motor complex. Nat Cell Biol. 2002;4:986–992. doi: 10.1038/ncb891. [DOI] [PubMed] [Google Scholar]
- Matsuda S, Yasukawa T, Homma Y, Ito Y, Niikura T, Hiraki T, Hirai S, Ohno S, Kita Y, Kawasumi M, et al. c-Jun N-terminal kinase (JNK)-interacting protein-1b/islet-brain-1 scaffolds Alzheimer’s amyloid precursor protein with JNK. J Neurosci. 2001;21:6597–6607. doi: 10.1523/JNEUROSCI.21-17-06597.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Grafstein B. Fast and slow components in axonal transport of protein. J Cell Biol. 1968;38:494–508. doi: 10.1083/jcb.38.3.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenney RJ, Huynh W, Tanenbaum ME, Bhabha G, Vale RD. Activation of cytoplasmic dynein motility by dynactin-cargo adapter complexes. Science. 2014;345:337–341. doi: 10.1126/science.1254198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merianda TT, Gomes C, Yoo S, Vuppalanchi D, Twiss JL. Axonal localization of neuritin/CPG15 mRNA in neuronal populations through distinct 5′ and 3′ UTR elements. J Neurosci. 2013;33:13735–13742. doi: 10.1523/JNEUROSCI.0962-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merianda TT, Lin AC, Lam JS, Vuppalanchi D, Willis DE, Karin N, Holt CE, Twiss JL. A functional equivalent of endoplasmic reticulum and Golgi in axons for secretion of locally synthesized proteins. Mol Cell Neurosci. 2009;40:128–142. doi: 10.1016/j.mcn.2008.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mersiyanova IV, Perepelov AV, Polyakov AV, Sitnikov VF, Dadali EL, Oparin RB, Petrin AN, Evgrafov OV. A new variant of Charcot-Marie-Tooth disease type 2 is probably the result of a mutation in the neurofilament-light gene. Am J Hum Genet. 2000;67:37–46. doi: 10.1086/302962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki H, Setou M, Kaneshiro K, Hirokawa N. All kinesin superfamily protein, KIF, genes in mouse and human. Proc Natl Acad Sci U S A. 2001;98:7004–7011. doi: 10.1073/pnas.111145398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millecamps S, Julien JP. Axonal transport deficits and neurodegenerative diseases. Nat Rev Neurosci. 2013;14:161–176. doi: 10.1038/nrn3380. [DOI] [PubMed] [Google Scholar]
- Miller KE, DeProto J, Kaufmann N, Patel BN, Duckworth A, Van Vactor D. Direct observation demonstrates that Liprin-alpha is required for trafficking of synaptic vesicles. Curr Biol. 2005;15:684–689. doi: 10.1016/j.cub.2005.02.061. [DOI] [PubMed] [Google Scholar]
- Misgeld T, Kerschensteiner M, Bareyre FM, Burgess RW, Lichtman JW. Imaging axonal transport of mitochondria in vivo. Nat Methods. 2007;4:559–561. doi: 10.1038/nmeth1055. [DOI] [PubMed] [Google Scholar]
- Mitchell DJ, Blasier KR, Jeffery ED, Ross MW, Pullikuth AK, Suo D, Park J, Smiley WR, Lo KW, Shabanowitz J, et al. Trk activation of the ERK1/2 kinase pathway stimulates intermediate chain phosphorylation and recruits cytoplasmic dynein to signaling endosomes for retrograde axonal transport. J Neurosci. 2012;32:15495–15510. doi: 10.1523/JNEUROSCI.5599-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morfini GA, Bosco DA, Brown H, Gatto R, Kaminska A, Song Y, Molla L, Baker L, Marangoni MN, Berth S, et al. Inhibition of fast axonal transport by pathogenic SOD1 involves activation of p38 MAP kinase. PLoS One. 2013;8:e65235. doi: 10.1371/journal.pone.0065235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moughamian AJ, Holzbaur EL. Dynactin is required for transport initiation from the distal axon. Neuron. 2012;74:331–343. doi: 10.1016/j.neuron.2012.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moughamian AJ, Osborn GE, Lazarus JE, Maday S, Holzbaur EL. Ordered recruitment of dynactin to the microtubule plus-end is required for efficient initiation of retrograde axonal transport. J Neurosci. 2013;33:13190–13203. doi: 10.1523/JNEUROSCI.0935-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller MJ, Klumpp S, Lipowsky R. Tug-of-war as a cooperative mechanism for bidirectional cargo transport by molecular motors. Proc Natl Acad Sci U S A. 2008;105:4609–4614. doi: 10.1073/pnas.0706825105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrell JR, Spillantini MG, Zolo P, Guazzelli M, Smith MJ, Hasegawa M, Redi F, Crowther RA, Pietrini P, Ghetti B, et al. Tau gene mutation G389R causes a tauopathy with abundant pick body-like inclusions and axonal deposits. J Neuropathol Exp Neurol. 1999;58:1207–1226. doi: 10.1097/00005072-199912000-00002. [DOI] [PubMed] [Google Scholar]
- Nakata T, Hirokawa N. Microtubules provide directional cues for polarized axonal transport through interaction with kinesin motor head. J Cell Biol. 2003;162:1045–1055. doi: 10.1083/jcb.200302175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakata T, Niwa S, Okada Y, Perez F, Hirokawa N. Preferential binding of a kinesin-1 motor to GTP-tubulin-rich microtubules underlies polarized vesicle transport. J Cell Biol. 2011;194:245–255. doi: 10.1083/jcb.201104034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nangaku M, Sato-Yoshitake R, Okada Y, Noda Y, Takemura R, Yamazaki H, Hirokawa N. KIF1B, a novel microtubule plus end-directed monomeric motor protein for transport of mitochondria. Cell. 1994;79:1209–1220. doi: 10.1016/0092-8674(94)90012-4. [DOI] [PubMed] [Google Scholar]
- Neveling K, Martinez-Carrera LA, Holker I, Heister A, Verrips A, Hosseini-Barkooie SM, Gilissen C, Vermeer S, Pennings M, Meijer R, et al. Mutations in BICD2, which encodes a golgin and important motor adaptor, cause congenital autosomal-dominant spinal muscular atrophy. Am J Hum Genet. 2013;92:946–954. doi: 10.1016/j.ajhg.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa S, Tanaka Y, Hirokawa N. KIF1Bbeta- and KIF1A-mediated axonal transport of presynaptic regulator Rab3 occurs in a GTP-dependent manner through DENN/MADD. Nat Cell Biol. 2008;10:1269–1279. doi: 10.1038/ncb1785. [DOI] [PubMed] [Google Scholar]
- Novarino G, Fenstermaker AG, Zaki MS, Hofree M, Silhavy JL, Heiberg AD, Abdellateef M, Rosti B, Scott E, Mansour L, et al. Exome sequencing links corticospinal motor neuron disease to common neurodegenerative disorders. Science. 2014;343:506–511. doi: 10.1126/science.1247363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oates EC, Rossor AM, Hafezparast M, Gonzalez M, Speziani F, MacArthur DG, Lek M, Cottenie E, Scoto M, Foley AR, et al. Mutations in BICD2 cause dominant congenital spinal muscular atrophy and hereditary spastic paraplegia. Am J Hum Genet. 2013;92:965–973. doi: 10.1016/j.ajhg.2013.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada Y, Yamazaki H, Sekine-Aizawa Y, Hirokawa N. The neuron-specific kinesin superfamily protein KIF1A is a unique monomeric motor for anterograde axonal transport of synaptic vesicle precursors. Cell. 1995;81:769–780. doi: 10.1016/0092-8674(95)90538-3. [DOI] [PubMed] [Google Scholar]
- Paciorkowski AR, Keppler-Noreuil K, Robinson L, Sullivan C, Sajan S, Christian SL, Bukshpun P, Gabriel SB, Gleeson JG, Sherr EH, et al. Deletion 16p13.11 uncovers NDE1 mutations on the non-deleted homolog and extends the spectrum of severe microcephaly to include fetal brain disruption. American journal of medical genetics Part A. 2013;161A:1523–1530. doi: 10.1002/ajmg.a.35969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey JP, Smith DS. A Cdk5-dependent switch regulates Lis1/Ndel1/dynein-driven organelle transport in adult axons. J Neurosci. 2011;31:17207–17219. doi: 10.1523/JNEUROSCI.4108-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paschal BM, Shpetner HS, Vallee RB. MAP 1C is a microtubule-activated ATPase which translocates microtubules in vitro and has dynein-like properties. J Cell Biol. 1987;105:1273–1282. doi: 10.1083/jcb.105.3.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil H, Cho KI, Lee J, Yang Y, Orry A, Ferreira PA. Kinesin-1 and mitochondrial motility control by discrimination of structurally equivalent but distinct subdomains in Ran-GTP-binding domains of Ran-binding protein 2. Open biology. 2013;3:120183. doi: 10.1098/rsob.120183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeters K, Litvinenko I, Asselbergh B, Almeida-Souza L, Chamova T, Geuens T, Ydens E, Zimon M, Irobi J, De Vriendt E, et al. Molecular defects in the motor adaptor BICD2 cause proximal spinal muscular atrophy with autosomal-dominant inheritance. Am J Hum Genet. 2013;92:955–964. doi: 10.1016/j.ajhg.2013.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlson E, Hanz S, Ben-Yaakov K, Segal-Ruder Y, Seger R, Fainzilber M. Vimentin-dependent spatial translocation of an activated MAP kinase in injured nerve. Neuron. 2005;45:715–726. doi: 10.1016/j.neuron.2005.01.023. [DOI] [PubMed] [Google Scholar]
- Perry RB, Doron-Mandel E, Iavnilovitch E, Rishal I, Dagan SY, Tsoory M, Coppola G, McDonald MK, Gomes C, Geschwind DH, et al. Subcellular knockout of importin beta1 perturbs axonal retrograde signaling. Neuron. 2012;75:294–305. doi: 10.1016/j.neuron.2012.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen JD, Kaech S, Banker G. Selective microtubule-based transport of dendritic membrane proteins arises in concert with axon specification. J Neurosci. 2014;34:4135–4147. doi: 10.1523/JNEUROSCI.3779-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilling AD, Horiuchi D, Lively CM, Saxton WM. Kinesin-1 and Dynein are the primary motors for fast transport of mitochondria in Drosophila motor axons. Mol Biol Cell. 2006;17:2057–2068. doi: 10.1091/mbc.E05-06-0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirier K, Keays DA, Francis F, Saillour Y, Bahi N, Manouvrier S, Fallet-Bianco C, Pasquier L, Toutain A, Tuy FP, et al. Large spectrum of lissencephaly and pachygyria phenotypes resulting from de novo missense mutations in tubulin alpha 1A (TUBA1A) Human mutation. 2007;28:1055–1064. doi: 10.1002/humu.20572. [DOI] [PubMed] [Google Scholar]
- Poirier K, Lebrun N, Broix L, Tian G, Saillour Y, Boscheron C, Parrini E, Valence S, Pierre BS, Oger M, et al. Mutations in TUBG1, DYNC1H1, KIF5C and KIF2A cause malformations of cortical development and microcephaly. Nature genetics. 2013;45:639–647. doi: 10.1038/ng.2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puls I, Jonnakuty C, LaMonte BH, Holzbaur EL, Tokito M, Mann E, Floeter MK, Bidus K, Drayna D, Oh SJ, et al. Mutant dynactin in motor neuron disease. Nature genetics. 2003;33:455–456. doi: 10.1038/ng1123. [DOI] [PubMed] [Google Scholar]
- Rai AK, Rai A, Ramaiya AJ, Jha R, Mallik R. Molecular adaptations allow dynein to generate large collective forces inside cells. Cell. 2013;152:172–182. doi: 10.1016/j.cell.2012.11.044. [DOI] [PubMed] [Google Scholar]
- Ray K, Perez SE, Yang Z, Xu J, Ritchings BW, Steller H, Goldstein LS. Kinesin-II is required for axonal transport of choline acetyltransferase in Drosophila. J Cell Biol. 1999;147:507–518. doi: 10.1083/jcb.147.3.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner O, Carrozzo R, Shen Y, Wehnert M, Faustinella F, Dobyns WB, Caskey CT, Ledbetter DH. Isolation of a Miller-Dieker lissencephaly gene containing G protein beta-subunit-like repeats. Nature. 1993;364:717–721. doi: 10.1038/364717a0. [DOI] [PubMed] [Google Scholar]
- Rishal I, Fainzilber M. Axon-soma communication in neuronal injury. Nat Rev Neurosci. 2014;15:32–42. doi: 10.1038/nrn3609. [DOI] [PubMed] [Google Scholar]
- Riviere JB, Ramalingam S, Lavastre V, Shekarabi M, Holbert S, Lafontaine J, Srour M, Merner N, Rochefort D, Hince P, et al. KIF1A, an axonal transporter of synaptic vesicles, is mutated in hereditary sensory and autonomic neuropathy type 2. Am J Hum Genet. 2011;89:219–230. doi: 10.1016/j.ajhg.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AJ, Kon T, Knight PJ, Sutoh K, Burgess SA. Functions and mechanics of dynein motor proteins. Nat Rev Mol Cell Biol. 2013;14:713–726. doi: 10.1038/nrm3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa-Ferreira C, Munro S. Arl8 and SKIP act together to link lysosomes to kinesin-1. Dev Cell. 2011;21:1171–1178. doi: 10.1016/j.devcel.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross JL, Wallace K, Shuman H, Goldman YE, Holzbaur EL. Processive bidirectional motion of dynein-dynactin complexes in vitro. Nat Cell Biol. 2006;8:562–570. doi: 10.1038/ncb1421. [DOI] [PubMed] [Google Scholar]
- Roy S. Seeing the unseen: the hidden world of slow axonal transport. The Neuroscientist: a review journal bringing neurobiology, neurology and psychiatry. 2014;20:71–81. doi: 10.1177/1073858413498306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo GJ, Louie K, Wellington A, Macleod GT, Hu F, Panchumarthi S, Zinsmaier KE. Drosophila Miro is required for both anterograde and retrograde axonal mitochondrial transport. J Neurosci. 2009;29:5443–5455. doi: 10.1523/JNEUROSCI.5417-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saab AS, Tzvetanova ID, Nave KA. The role of myelin and oligodendrocytes in axonal energy metabolism. Current opinion in neurobiology. 2013;23:1065–1072. doi: 10.1016/j.conb.2013.09.008. [DOI] [PubMed] [Google Scholar]
- Salata MW, Dillman JF, 3rd, Lye RJ, Pfister KK. Growth factor regulation of cytoplasmic dynein intermediate chain subunit expression preceding neurite extension. J Neurosci Res. 2001;65:408–416. doi: 10.1002/jnr.1168. [DOI] [PubMed] [Google Scholar]
- Sandow SL, Heydon K, Weible MW, 2nd, Reynolds AJ, Bartlett SE, Hendry IA. Signalling organelle for retrograde axonal transport of internalized neurotrophins from the nerve terminal. Immunology and cell biology. 2000;78:430–435. doi: 10.1046/j.1440-1711.2000.00924.x. [DOI] [PubMed] [Google Scholar]
- Scheinfeld MH, Roncarati R, Vito P, Lopez PA, Abdallah M, D’Adamio L. Jun NH2-terminal kinase (JNK) interacting protein 1 (JIP1) binds the cytoplasmic domain of the Alzheimer’s beta-amyloid precursor protein (APP) J Biol Chem. 2002;277:3767–3775. doi: 10.1074/jbc.M108357200. [DOI] [PubMed] [Google Scholar]
- Schlager MA, Hoang HT, Urnavicius L, Bullock SL, Carter AP. In vitro reconstitution of a highly processive recombinant human dynein complex. EMBO J. 2014 doi: 10.15252/embj.201488792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholey JM. Kinesin-2: a family of heterotrimeric and homodimeric motors with diverse intracellular transport functions. Annu Rev Cell Dev Biol. 2013;29:443–469. doi: 10.1146/annurev-cellbio-101512-122335. [DOI] [PubMed] [Google Scholar]
- Schroeder HW, 3rd, Hendricks AG, Ikeda K, Shuman H, Rodionov V, Ikebe M, Goldman YE, Holzbaur EL. Force-dependent detachment of kinesin-2 biases track switching at cytoskeletal filament intersections. Biophys J. 2012;103:48–58. doi: 10.1016/j.bpj.2012.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder HW, 3rd, Mitchell C, Shuman H, Holzbaur EL, Goldman YE. Motor number controls cargo switching at actin-microtubule intersections in vitro. Curr Biol. 2010;20:687–696. doi: 10.1016/j.cub.2010.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroer TA. Dynactin. Annu Rev Cell Dev Biol. 2004;20:759–779. doi: 10.1146/annurev.cellbio.20.012103.094623. [DOI] [PubMed] [Google Scholar]
- Scott DA, Das U, Tang Y, Roy S. Mechanistic logic underlying the axonal transport of cytosolic proteins. Neuron. 2011;70:441–454. doi: 10.1016/j.neuron.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serano TL, Cohen RS. A small predicted stem-loop structure mediates oocyte localization of Drosophila K10 mRNA. Development. 1995;121:3809–3818. doi: 10.1242/dev.121.11.3809. [DOI] [PubMed] [Google Scholar]
- Setou M, Seog DH, Tanaka Y, Kanai Y, Takei Y, Kawagishi M, Hirokawa N. Glutamate-receptor-interacting protein GRIP1 directly steers kinesin to dendrites. Nature. 2002;417:83–87. doi: 10.1038/nature743. [DOI] [PubMed] [Google Scholar]
- Shah JV, Flanagan LA, Janmey PA, Leterrier JF. Bidirectional translocation of neurofilaments along microtubules mediated in part by dynein/dynactin. Mol Biol Cell. 2000;11:3495–3508. doi: 10.1091/mbc.11.10.3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao CY, Zhu J, Xie YJ, Wang Z, Wang YN, Wang Y, Su LD, Zhou L, Zhou TH, Shen Y. Distinct functions of nuclear distribution proteins LIS1, Ndel1 and NudCL in regulating axonal mitochondrial transport. Traffic. 2013;14:785–797. doi: 10.1111/tra.12070. [DOI] [PubMed] [Google Scholar]
- Shin H, Wyszynski M, Huh KH, Valtschanoff JG, Lee JR, Ko J, Streuli M, Weinberg RJ, Sheng M, Kim E. Association of the kinesin motor KIF1A with the multimodular protein liprin-alpha. J Biol Chem. 2003;278:11393–11401. doi: 10.1074/jbc.M211874200. [DOI] [PubMed] [Google Scholar]
- Shin JE, Miller BR, Babetto E, Cho Y, Sasaki Y, Qayum S, Russler EV, Cavalli V, Milbrandt J, DiAntonio A. SCG10 is a JNK target in the axonal degeneration pathway. Proc Natl Acad Sci U S A. 2012;109:E3696–3705. doi: 10.1073/pnas.1216204109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman MA, Kaech S, Ramser EM, Lu X, Lasarev MR, Nagalla S, Banker G. Expression of kinesin superfamily genes in cultured hippocampal neurons. Cytoskeleton (Hoboken) 2010;67:784–795. doi: 10.1002/cm.20487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirajuddin M, Rice LM, Vale RD. Regulation of microtubule motors by tubulin isotypes and post-translational modifications. Nat Cell Biol. 2014;16:335–344. doi: 10.1038/ncb2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song AH, Wang D, Chen G, Li Y, Luo J, Duan S, Poo MM. A selective filter for cytoplasmic transport at the axon initial segment. Cell. 2009;136:1148–1160. doi: 10.1016/j.cell.2009.01.016. [DOI] [PubMed] [Google Scholar]
- Song Y, Nagy M, Ni W, Tyagi NK, Fenton WA, Lopez-Giraldez F, Overton JD, Horwich AL, Brady ST. Molecular chaperone Hsp110 rescues a vesicle transport defect produced by an ALS-associated mutant SOD1 protein in squid axoplasm. Proc Natl Acad Sci U S A. 2013;110:5428–5433. doi: 10.1073/pnas.1303279110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soppina V, Norris SR, Dizaji AS, Kortus M, Veatch S, Peckham M, Verhey KJ. Dimerization of mammalian kinesin-3 motors results in superprocessive motion. Proc Natl Acad Sci U S A. 2014;111:5562–5567. doi: 10.1073/pnas.1400759111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soppina V, Rai AK, Ramaiya AJ, Barak P, Mallik R. Tug-of-war between dissimilar teams of microtubule motors regulates transport and fission of endosomes. Proc Natl Acad Sci U S A. 2009;106:19381–19386. doi: 10.1073/pnas.0906524106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanova T, Slemmer J, Hoogenraad CC, Lansbergen G, Dortland B, De Zeeuw CI, Grosveld F, van Cappellen G, Akhmanova A, Galjart N. Visualization of microtubule growth in cultured neurons via the use of EB3-GFP (end-binding protein 3-green fluorescent protein) J Neurosci. 2003;23:2655–2664. doi: 10.1523/JNEUROSCI.23-07-02655.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanova T, Smal I, van Haren J, Akinci U, Liu Z, Miedema M, Limpens R, van Ham M, van der Reijden M, Poot R, et al. History-dependent catastrophes regulate axonal microtubule behavior. Curr Biol. 2010;20:1023–1028. doi: 10.1016/j.cub.2010.04.024. [DOI] [PubMed] [Google Scholar]
- Sun F, Zhu C, Dixit R, Cavalli V. Sunday Driver/JIP3 binds kinesin heavy chain directly and enhances its motility. EMBO J. 2011;30:3416–3429. doi: 10.1038/emboj.2011.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun T, Qiao H, Pan PY, Chen Y, Sheng ZH. Motile axonal mitochondria contribute to the variability of presynaptic strength. Cell reports. 2013;4:413–419. doi: 10.1016/j.celrep.2013.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svoboda K, Block SM. Force and velocity measured for single kinesin molecules. Cell. 1994;77:773–784. doi: 10.1016/0092-8674(94)90060-4. [DOI] [PubMed] [Google Scholar]
- Takeda S, Yamazaki H, Seog DH, Kanai Y, Terada S, Hirokawa N. Kinesin superfamily protein 3 (KIF3) motor transports fodrin-associating vesicles important for neurite building. J Cell Biol. 2000;148:1255–1265. doi: 10.1083/jcb.148.6.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan SC, Scherer J, Vallee RB. Recruitment of dynein to late endosomes and lysosomes through light intermediate chains. Mol Biol Cell. 2011;22:467–477. doi: 10.1091/mbc.E10-02-0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K, Sugiura Y, Ichishita R, Mihara K, Oka T. KLP6: a newly identified kinesin that regulates the morphology and transport of mitochondria in neuronal cells. J Cell Sci. 2011;124:2457–2465. doi: 10.1242/jcs.086470. [DOI] [PubMed] [Google Scholar]
- Tang Y, Scott D, Das U, Gitler D, Ganguly A, Roy S. Fast vesicle transport is required for the slow axonal transport of synapsin. J Neurosci. 2013;33:15362–15375. doi: 10.1523/JNEUROSCI.1148-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng J, Rai T, Tanaka Y, Takei Y, Nakata T, Hirasawa M, Kulkarni AB, Hirokawa N. The KIF3 motor transports N-cadherin and organizes the developing neuroepithelium. Nat Cell Biol. 2005;7:474–482. doi: 10.1038/ncb1249. [DOI] [PubMed] [Google Scholar]
- Terada S, Kinjo M, Aihara M, Takei Y, Hirokawa N. Kinesin-1/Hsc70-dependent mechanism of slow axonal transport and its relation to fast axonal transport. EMBO J. 2010;29:843–854. doi: 10.1038/emboj.2009.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terada S, Kinjo M, Hirokawa N. Oligomeric tubulin in large transporting complex is transported via kinesin in squid giant axons. Cell. 2000;103:141–155. doi: 10.1016/s0092-8674(00)00094-5. [DOI] [PubMed] [Google Scholar]
- Tischfield MA, Baris HN, Wu C, Rudolph G, Van Maldergem L, He W, Chan WM, Andrews C, Demer JL, Robertson RL, et al. Human TUBB3 mutations perturb microtubule dynamics, kinesin interactions, and axon guidance. Cell. 2010;140:74–87. doi: 10.1016/j.cell.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsurusaki Y, Saitoh S, Tomizawa K, Sudo A, Asahina N, Shiraishi H, Ito J, Tanaka H, Doi H, Saitsu H, et al. A DYNC1H1 mutation causes a dominant spinal muscular atrophy with lower extremity predominance. Neurogenetics. 2012;13:327–332. doi: 10.1007/s10048-012-0337-6. [DOI] [PubMed] [Google Scholar]
- Uchida A, Alami NH, Brown A. Tight functional coupling of kinesin-1A and dynein motors in the bidirectional transport of neurofilaments. Mol Biol Cell. 2009;20:4997–5006. doi: 10.1091/mbc.E09-04-0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale RD, Reese TS, Sheetz MP. Identification of a novel force-generating protein, kinesin, involved in microtubule-based motility. Cell. 1985;42:39–50. doi: 10.1016/s0092-8674(85)80099-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Spronsen M, Mikhaylova M, Lipka J, Schlager MA, van den Heuvel DJ, Kuijpers M, Wulf PS, Keijzer N, Demmers J, Kapitein LC, et al. TRAK/Milton Motor-Adaptor Proteins Steer Mitochondrial Trafficking to Axons and Dendrites. Neuron. 2013;77:485–502. doi: 10.1016/j.neuron.2012.11.027. [DOI] [PubMed] [Google Scholar]
- Vaughan KT, Vallee RB. Cytoplasmic dynein binds dynactin through a direct interaction between the intermediate chains and p150Glued. J Cell Biol. 1995;131:1507–1516. doi: 10.1083/jcb.131.6.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhey KJ, Meyer D, Deehan R, Blenis J, Schnapp BJ, Rapoport TA, Margolis B. Cargo of kinesin identified as JIP scaffolding proteins and associated signaling molecules. J Cell Biol. 2001;152:959–970. doi: 10.1083/jcb.152.5.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhoeven K, De Jonghe P, Coen K, Verpoorten N, Auer-Grumbach M, Kwon JM, FitzPatrick D, Schmedding E, De Vriendt E, Jacobs A, et al. Mutations in the small GTP-ase late endosomal protein RAB7 cause Charcot-Marie-Tooth type 2B neuropathy. Am J Hum Genet. 2003;72:722–727. doi: 10.1086/367847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vershinin M, Carter BC, Razafsky DS, King SJ, Gross SP. Multiple-motor based transport and its regulation by Tau. Proc Natl Acad Sci U S A. 2007;104:87–92. doi: 10.1073/pnas.0607919104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstreken P, Ly CV, Venken KJ, Koh TW, Zhou Y, Bellen HJ. Synaptic mitochondria are critical for mobilization of reserve pool vesicles at Drosophila neuromuscular junctions. Neuron. 2005;47:365–378. doi: 10.1016/j.neuron.2005.06.018. [DOI] [PubMed] [Google Scholar]
- Wagner OI, Ascano J, Tokito M, Leterrier JF, Janmey PA, Holzbaur EL. The interaction of neurofilaments with the microtubule motor cytoplasmic dynein. Mol Biol Cell. 2004;15:5092–5100. doi: 10.1091/mbc.E04-05-0401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Brown A. Rapid movement of microtubules in axons. Curr Biol. 2002;12:1496–1501. doi: 10.1016/s0960-9822(02)01078-3. [DOI] [PubMed] [Google Scholar]
- Wang L, Ho CL, Sun D, Liem RK, Brown A. Rapid movement of axonal neurofilaments interrupted by prolonged pauses. Nat Cell Biol. 2000;2:137–141. doi: 10.1038/35004008. [DOI] [PubMed] [Google Scholar]
- Wang X, Schwarz TL. The mechanism of Ca2+-dependent regulation of kinesin-mediated mitochondrial motility. Cell. 2009;136:163–174. doi: 10.1016/j.cell.2008.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K, Al-Bassam S, Miyazaki Y, Wandless TJ, Webster P, Arnold DB. Networks of polarized actin filaments in the axon initial segment provide a mechanism for sorting axonal and dendritic proteins. Cell reports. 2012;2:1546–1553. doi: 10.1016/j.celrep.2012.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterman-Storer CM, Karki S, Holzbaur EL. The p150Glued component of the dynactin complex binds to both microtubules and the actin-related protein centractin (Arp-1) Proc Natl Acad Sci U S A. 1995;92:1634–1638. doi: 10.1073/pnas.92.5.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weedon MN, Hastings R, Caswell R, Xie W, Paszkiewicz K, Antoniadi T, Williams M, King C, Greenhalgh L, Newbury-Ecob R, et al. Exome sequencing identifies a DYNC1H1 mutation in a large pedigree with dominant axonal Charcot-Marie-Tooth disease. Am J Hum Genet. 2011;89:308–312. doi: 10.1016/j.ajhg.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss P. Neuronal dynamics and axonal flow. 3. Cellulifugal transport of labeled neuroplasm in isolated nerve preparations. Proc Natl Acad Sci U S A. 1967;57:1239–1245. doi: 10.1073/pnas.57.5.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welte MA. Bidirectional transport along microtubules. Curr Biol. 2004;14:R525–537. doi: 10.1016/j.cub.2004.06.045. [DOI] [PubMed] [Google Scholar]
- Willemsen MH, Vissers LE, Willemsen MA, van Bon BW, Kroes T, de Ligt J, de Vries BB, Schoots J, Lugtenberg D, Hamel BC, et al. Mutations in DYNC1H1 cause severe intellectual disability with neuronal migration defects. Journal of medical genetics. 2012;49:179–183. doi: 10.1136/jmedgenet-2011-100542. [DOI] [PubMed] [Google Scholar]
- Willis D, Li KW, Zheng JQ, Chang JH, Smit AB, Kelly T, Merianda TT, Sylvester J, van Minnen J, Twiss JL. Differential transport and local translation of cytoskeletal, injury-response, and neurodegeneration protein mRNAs in axons. J Neurosci. 2005;25:778–791. doi: 10.1523/JNEUROSCI.4235-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis DE, Xu M, Donnelly CJ, Tep C, Kendall M, Erenstheyn M, English AW, Schanen NC, Kirn-Safran CB, Yoon SO, et al. Axonal Localization of transgene mRNA in mature PNS and CNS neurons. J Neurosci. 2011;31:14481–14487. doi: 10.1523/JNEUROSCI.2950-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong MY, Zhou C, Shakiryanova D, Lloyd TE, Deitcher DL, Levitan ES. Neuropeptide delivery to synapses by long-range vesicle circulation and sporadic capture. Cell. 2012;148:1029–1038. doi: 10.1016/j.cell.2011.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong YC, Holzbaur EL. The regulation of autophagosome dynamics by huntingtin and HAP1 is disrupted by expression of mutant huntingtin, leading to defective cargo degradation. J Neurosci. 2014;34:1293–1305. doi: 10.1523/JNEUROSCI.1870-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K, Zhong G, Zhuang X. Actin, spectrin, and associated proteins form a periodic cytoskeletal structure in axons. Science. 2013;339:452–456. doi: 10.1126/science.1232251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabe JT, Pimenta A, Shea TB. Kinesin-mediated transport of neurofilament protein oligomers in growing axons. J Cell Sci. 1999;112(Pt 21):3799–3814. doi: 10.1242/jcs.112.21.3799. [DOI] [PubMed] [Google Scholar]
- Yamada K, Andrews C, Chan WM, McKeown CA, Magli A, de Berardinis T, Loewenstein A, Lazar M, O’Keefe M, Letson R, et al. Heterozygous mutations of the kinesin KIF21A in congenital fibrosis of the extraocular muscles type 1 (CFEOM1) Nature genetics. 2003;35:318–321. doi: 10.1038/ng1261. [DOI] [PubMed] [Google Scholar]
- Yan Y, Brown A. Neurofilament polymer transport in axons. J Neurosci. 2005;25:7014–7021. doi: 10.1523/JNEUROSCI.2001-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh TY, Quintyne NJ, Scipioni BR, Eckley DM, Schroer TA. Dynactin’s pointed-end complex is a cargo-targeting module. Mol Biol Cell. 2012;23:3827–3837. doi: 10.1091/mbc.E12-07-0496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonekawa Y, Harada A, Okada Y, Funakoshi T, Kanai Y, Takei Y, Terada S, Noda T, Hirokawa N. Defect in synaptic vesicle precursor transport and neuronal cell death in KIF1A motor protein-deficient mice. J Cell Biol. 1998;141:431–441. doi: 10.1083/jcb.141.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zala D, Colin E, Rangone H, Liot G, Humbert S, Saudou F. Phosphorylation of mutant huntingtin at S421 restores anterograde and retrograde transport in neurons. Hum Mol Genet. 2008;17:3837–3846. doi: 10.1093/hmg/ddn281. [DOI] [PubMed] [Google Scholar]
- Zala D, Hinckelmann MV, Yu H, Lyra da Cunha MM, Liot G, Cordelieres FP, Marco S, Saudou F. Vesicular glycolysis provides on-board energy for fast axonal transport. Cell. 2013;152:479–491. doi: 10.1016/j.cell.2012.12.029. [DOI] [PubMed] [Google Scholar]
- Zhang HL, Eom T, Oleynikov Y, Shenoy SM, Liebelt DA, Dictenberg JB, Singer RH, Bassell GJ. Neurotrophin-induced transport of a beta-actin mRNP complex increases beta-actin levels and stimulates growth cone motility. Neuron. 2001;31:261–275. doi: 10.1016/s0896-6273(01)00357-9. [DOI] [PubMed] [Google Scholar]
- Zhang J, Twelvetrees AE, Lazarus JE, Blasier KR, Yao X, Inamdar NA, Holzbaur EL, Pfister KK, Xiang X. Establishing a novel knock-in mouse line for studying neuronal cytoplasmic dynein under normal and pathologic conditions. Cytoskeleton (Hoboken) 2013;70:215–227. doi: 10.1002/cm.21102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Yao X, Fischer L, Abenza JF, Penalva MA, Xiang X. The p25 subunit of the dynactin complex is required for dynein-early endosome interaction. J Cell Biol. 2011;193:1245–1255. doi: 10.1083/jcb.201011022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Takita J, Tanaka Y, Setou M, Nakagawa T, Takeda S, Yang HW, Terada S, Nakata T, Takei Y, et al. Charcot-Marie-Tooth disease type 2A caused by mutation in a microtubule motor KIF1Bbeta. Cell. 2001;105:587–597. doi: 10.1016/s0092-8674(01)00363-4. [DOI] [PubMed] [Google Scholar]