Abstract
Importance
Cognitive decline is a common and feared aspect of aging. Mild Cognitive Impairment (MCI) is defined as the “symptomatic pre-dementia stage” on the continuum of cognitive decline, characterized by objective impairment in cognition that is not severe enough to require help with usual activities of daily living.
Objective
To present evidence on the diagnosis,treatment, and prognosis of MCI, and to provide physicians with an evidence-based framework for caring for older MCI patients and their caregivers.
Evidence Acquisition
We searched PubMed for English-language articles in peer-reviewed journals and the Cochrane Library database through July 2014. Relevant references from retrieved articles were also evaluated.
Findings
The prevalence of MCI in adults aged ≥65 years is 10- 20%; risk increases with age, and men appear to be at higher risk than women. In older MCI patients, clinicians shouldconsider depression, polypharmacy, and uncontrolled cardiovascular risk factors, all of which may increase risk for cognitive impairment and other negative outcomes. Currently, no medications have proven effective for MCI; treatments and interventions should be aimed at reducing cardiovascular risk factors and prevention of stroke. Aerobic exercise, mental activity, and social engagement may help decrease risk of further cognitive decline. Although patients with MCI are at greater risk of developing dementia compared withthe general population, there is currently substantial variation in risk estimates (from <5% to 20% annual conversion rates), depending on the population studied.Current research is aimed at improving early detection and treatment of MCI, particularly in patients at high risk for progression to dementia.
Conclusions and Relevance
Cognitive decline and MCI have important implications for patients and their families, and will require that primary care clinicians be skilled in identifying and managing this common disorder as the number of older adults increases in coming decades. Current evidence supports aerobic exercise, mental activity, and cardiovascular risk factor control in patients with MCI.
The Patient's Story
Mrs J, age 81 years, with hypertension and hyperlipidemia, requested a referral to a neurologist,stating: “I am forgetting things I just heard.”
Mrs J and her husband began noticing mild memory problems 1.5 years earlier, and report slow progression since. Her husband noticed changes in problem solving and time management. Mrs Jwas easily distracted and had difficulty remembering recent conversations.She misplaced objects and spent time looking for them;she read and wrote less than before. She repeatedly askedhow to do things on her computer and cell phone. Her husband reported that she exhibitedno initiative, and that their home seemed more disorganized. She had difficulty planning dinnerandher cooking wassimpler. Both denied changes in language or speech. She continued to drive locallywithoutaccidents but had difficulty remembering directions to familiar places. Mrs J had no hallucinations or delusions. She slept well,her mood was fine, and she exhibitedno behavioral problems or personality changes.
Functionally, she remained independent in all activities of daily living (ADLs). She had urinary frequency and over the past couple of months she had a few incidents of incontinence, especially when awakening from a nap. Ininstrumental activities of daily living (IADLs), Mr J had recently taken over paying bills. Finally, even with a compartmentalized pill-box, she occasionally forgot to take her medications (amlodipine 5 mg daily; losartan 50 mg twice daily; and ergocalciferol 1,000 units daily.)
Perspectives
Mrs J: (Asked when her memory first became a concern)….Would you believe I'm about to say: “I forget?”…It's just been gradual….I was asked to play my monthly bridge game … and I declined….I thought: “I'll never remember the cards.”
Mild Cognitive Impairment (MCI) is a clinical stage on the continuum of cognitive decline between “normal aging” and dementia. It ischaracterized by impairment in cognition that is not severe enough to require help with ADLs / IADLs. MrsJ's declining memory has clearly affected daily life in ways that both she and her husband have noticed, but she has remained generally independent, and therefore has MCI.
Methods
We searched PubMed for English-language articles in peer-reviewed journals through July 2014 using: “Mild Cognitive Impairment / Diagnosis [MeSH]” or “Mild Cognitive Impairment / Treatment [MeSH]”or “Mild Cognitive Impairment / Therapy [MeSH]”.We also searched the CochraneLibrary database using “Mild Cognitive Impairment”; we reviewed the updated 2011 National Institute on Aging (NIA)—Alzheimer's Association (AA) diagnostic guidelines for dementia1, MCI2, and pre-clinical Alzheimer's disease (AD).3 Finally, we reviewed a recent analysis of diagnostic testing for ADfrom the Institute for Clinical and Economic Review4, and a Centers for Medicare and Medicaid Services (CMS) decision memo regarding beta-amyloid Positron Emission Tomography (PET) imaging for dementia.5 Our PubMed search yielded 4,977 unique articles; our Cochrane Library search yielded 22 systematic reviews, the titles and abstracts of which were examined for relevance. For each of the relevant articles identified, we then screened the references and checked related citations in PubMed. Randomized double-blind placebo-controlled trials (RCTs) with results reported as intention-to-treat analyses were considered highest quality data. Large prospective cohort studies, meta-analyses, and systematic literature reviews were also included as appropriate for supplementing the RCT results.Our results and discussion cite the articles that one of the authors found most relevant to the diagnosis and management of MCI. We developed recommendations using evidence from these sources, as well as our clinical experience.
Definitions and Diagnostic Criteria
In 2011, the NIA and the AA convened workgroups to revise the 1984 diagnostic criteria for dementia, as well as dementia due to AD.Diagnostic criteria for MCI,the “symptomatic, pre-dementia phase” of the trajectory of cognitive decline, wereestablished(Box 1).2 The key criteria that distinguish MCI from dementia are preservation of independence in functional abilities (i.e., ADLs and IADLs), and lack of significant impairment in social or occupational functioning. MCI sub-types are sometimes defined based on presence or absence of memory difficulties (amnestic vs. non-amnestic MCI)6 and the number of affected cognitive domains.2
Box 1.
Criteria for the Diagnosis of Mild Cognitive Impairment (MCI)2
Concern regarding a change in cognition from the patient, knowledgeable informant, or from a skilled clinician observing the patient
Objective evidence of impairment (from cognitive testing)in one or more cognitive domains, including memory, executive function, attention, language, or visuospatial skills
Preservation of independence in functional abilities (although individuals may be less efficient and make more errors at performing ADLs / IADLs than in the past)
No evidence of a significant impairment in social or occupational functioning (i.e., “not demented”)
Clinical Characteristics Suggestive that MCI is due to Alzheimer's Disease2
Memory impairment present
Progressive decline in cognition over months to years (very rapid decline may suggest prion disease, neoplasm, or metabolic disorders)
Lack of Parkinsonism and visual hallucinations (suggestive of dementia with Lewybodies)
Lack of vascular risk factors and extensive cerebrovascular disease on brain imaging (suggestive of vascular cognitive impairment)
Lack of prominent behavioral or language disorders (suggestive of frontotemporal lobar degeneration)
The NIA-AA criteria define “MCI due to Alzheimer's disease,” as “those symptomatic but non-demented individuals whose primary underlying pathophysiology is AD.”2 MCI due to AD is characterized by memory impairment,longitudinal decline in cognitive function, and lack of evidence for vascular, traumatic, or other medical causes of cognitive decline (Box 1). The NIA-AA guidelines also proposed research criteria for the use of biomarkers—measures ofamyloid-beta (Aβ) deposition and of neuronal injury—to further refine the likelihood that a patient's MCIis due to AD, but these tests are not yet recommendedfor routine clinical use.2
Two other recently-developed clinical classification systems identify a symptomatic but non-demented stage of cognitive decline, but use different terminology than the NIA-AA criteria. The International Working Group (IWG) criteria use the terms “prodromal AD” or “predementia AD” to refer to individuals with cognitive impairment that is not severe enough to significantly affect activities of daily living7, while the new DSM-5 refers to this stage as “mild neurocognitive disorder.”8
Epidemiology and Risk Factors
Recent clinical and population-based samples suggest an MCI prevalence of 10-20% for adults aged ≥65 years,6,9 although lack of standardized diagnostic criteria and differences in sample characteristics across studieshave led to significant uncertainty around these estimates.Importantly, the likelihood that MCI will progress to dementia depends on the specific diagnostic criteria used and the setting in which the diagnosis is made (e.g., primary care, specialist clinic, or general population.10Prevalence of MCI increases with age, and men appear to be at higher risk.9,11 Additional risk factors identified in some studies include lower educational level, vascular risk factors (e.g., diabetes and hypertension), Apolipoprotein E (APOE) e4 genotype, Vitamin D deficiency, sleep-disordered breathing12, and prior critical illness (eg, sepsis).13
Evaluation of the Patient with Suspected MCI
Dr F: Somebody who tells me that their memory has always been bad… [is] less worrying to me than a patient like [Mrs J] who told me that over the past 1.5 years there's been a discrete and distinct decline in her ability to remember things. And, she had an informant…who agreed with that.
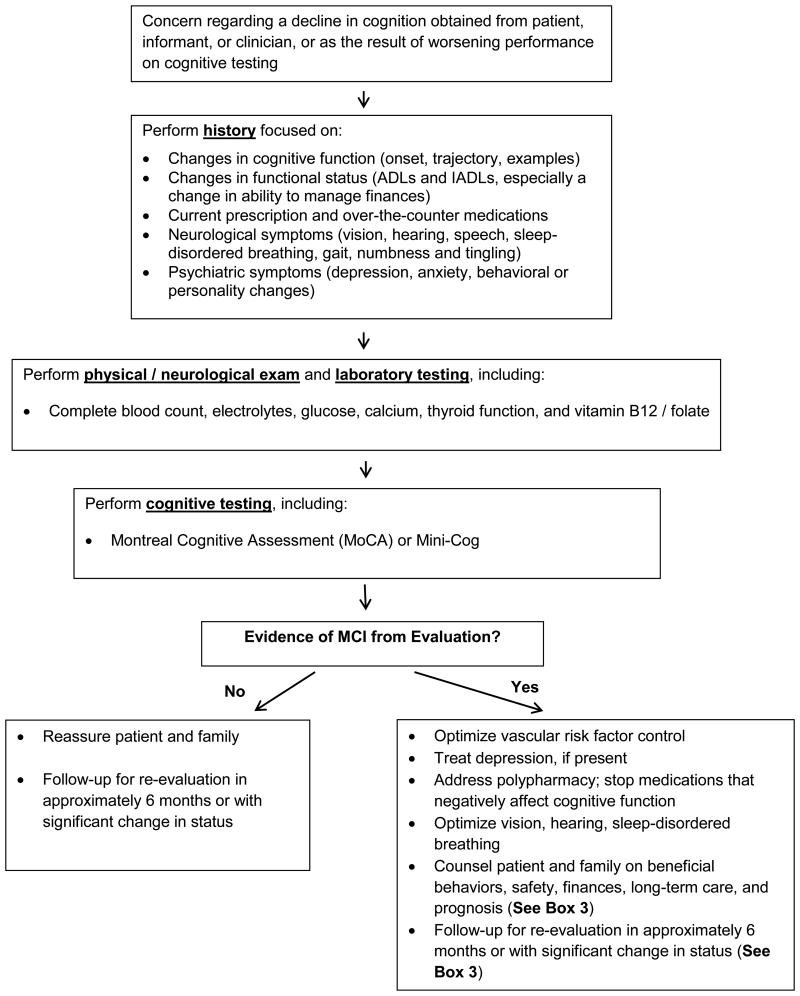
All patients with suspected MCI should undergo a comprehensive history and physical examination focusing on cognitive function, functional status, medications, neurological or psychiatric abnormalities, and laboratory testing. The main goals are to distinguish MCI from normal aging or dementia and to identifypotentially reversible forms of MCI due to other conditions (e.g., depression, medication effects, thyroid disease, and B12 / folate deficiency) (Figure).
Figure. Suggested Approach to the Diagnosis and Management of Mild Cognitive Impairment.
Cognitive Function
A history of cognitive changes over time, verified by knowledgeable informants, if available, is important for identifying the first diagnostic criterion, decline in cognitive function (Box 1).14,15 Critical features that may elucidate a cause are onset, trajectory, time-course, and nature of the cognitive symptoms. Very rapid cognitive decline (e.g., weeks to months) is not typical of MCI due to AD, and should raise concerns for other causes, such as neoplasm, metabolic disorders, or prion disease (Box 1). Patients and informants (such as family members) may report conflicting views regarding the presence and severity of cognitive symptoms, either from lack of insight, or because cognitive decline can be emotionally-charged andsymptom report may beminimizedto avoid difficult or “disrespectful” discussions.14
Patients with suspected MCI should have cognitive functionassessments at baseline and follow-up visits. A recent USPSTF systematic review on screening for cognitive impairment in older adults examined a number of instruments for primary care settings.16 The review concluded that brief cognitive assessments can successfully detect dementia in primary care, but the sensitivity of those instruments for detecting MCI is generally lower, and it is still unclear whether early diagnosis of cognitive impairment improves important patient or caregiver outcomes.16
The Montreal Cognitive Assessment (MoCA) is a screening tool that was developed specifically for detection of MCI and takes about 10 minutes to administer.17 Using a cut-point of 25/26, the MoCA has a sensitivity of 80 to 100% and specificity of 50 to 76% for detecting MCI.16The Mini-Mental State Exam (MMSE) has a sensitivity of45 to 60% and specificity of 65 to 90% for detecting MCI using cut-points of 27 or 28.16A recent study directly comparing the MoCA and MMSE found the MoCA to be more sensitive for accurately differentiating individuals with MCI from those with normal cognition.18Clinicians may also consider the Mini-cog test (which combines the Clock Drawing Test with a 3-word recall test), as it also hasacceptable test performance characteristics, and can be performed in ≤3 minutes.16 Referral for formalneuropsychological testing may helpdiagnose MCI in patients with subtle cognitive decline.Although Mrs. J scored in the normal range on the MMSE (29 out of 30), her formal neuropsychological testing showed objective deficits in memory function.
Clinicians can collect standardized information on cognitive function from informants using the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE),19 the Dementia Severity Rating Scale (DSRS),20 and the AD8.21The NIA-AA MCI criteria note that scores on cognitive tests for individuals with MCI are typically 1 to 1.5 standard deviations below age- and education- adjusted normative means. However, it is emphasized that these score ranges should be considered guidelines, rather than firm cutoffs, for making an MCI diagnosis.2
Functional Status
Assessment of functional status determines whether a patient is independent (MCI), or whether cognitive decline is severe enough to require consistent help with daily activities (dementia). The Functional Activities Questionnaire22 is a brief standardized instrument for clinicians to obtain IADL information from an informant.6When using the FAQ, patients with MCI have more IADLs that “require assistance” compared to those with normal cognition (2.7 vs. 0.1, p<.01)23, and using a cut-point of ≥6 points on the FAQ was found to have an 85% accuracy for distinguishing patients with MCI from those with dementia.24Mrs. J was still generally independent, but had become slower and less efficient with customary activities (e.g., cooking and driving); increasing difficulties with finances may be anothersensitive indicator of early cognitive decline.25
Medication Review
Certain classes and combinations of medicationscan contribute to cognitive impairment,26 so all current prescription and over-the-counter medications should be reviewed. Classes most likely to contribute to cognitive impairment include: 1) anticholinergics; 2) opiates; 3) benzodiazepines and nonbenzodiazepine hypnotics (e.g., zolpidem); 4) digoxin; 5)antihistamines; 6) tricyclic anti-depressants; 7) skeletal muscle relaxants; and 8) antiepileptics. Hormonal therapy (estrogen alone or estrogen plus progestin) for menopause has been shown to increase risk for the combined endpoint of MCI or dementia.27In addition, hypotension related to intensive treatment of hypertension28and hypoglycemia related to intensive treatment of diabetes29 may also contribute to cognitive decline.
Neurological and Psychiatric Evaluation
Clinicians should perform a focused review of neurologic and psychiatric symptoms, a complete neurological exam, and a depression assessment in patients with suspected MCI. Review of symptomsshould probe vision and hearing problems, sleep-disordered breathing, behavioral or personality changes (which may suggest depression, thyroid disease, or frontotemporal dementia), visual hallucinations (Lewy-Body dementia, depression with psychotic features), numbness or tingling in the extremities (neuropathy), dizziness upon standing (orthostatic hypotension), changes in speech (stroke, Parkinson's disease [PD]), and changes in gait (stroke, normal pressure hydrocephalus [NPH], PD).14A complete neurological exam, including orthostatic hypotension, extraocular movements, vision, hearing, speech, focal weakness, ability to stand from a chair, and gait,isuseful for identifying potential contributors to cognitive decline, including stroke, PD, NPH or neuropathy due to toxins or vitamin deficiency.14
Depression is associated with cognitive impairment in older adults, and the relationship is likely bi-directional.Depression can bescreened in older adults using assessment tools such as the Geriatric Depression Scale, on which a score of ≥6 is suggestive of depression.30Clinicians can query the informant about a patient's depressive and behavioral symptoms using the neuropsychiatric inventory.31
Diagnostic Testing
Neuroimaging
Structural
The NIA-AA diagnostic guidelines do not recommend routine neuroimaging in the typical clinical assessment of MCI, but do propose research criteria in which neuroimaging may help in determining MCI etiology and prognosis.2Some studies suggestthat structural magnetic resonance imaging (MRI) may be useful for identifying MCI and those at greater risk for progression from MCI to dementia.6,32Volumetric measures of the hippocampus that show atrophy, for instance, are suggestive of MCI and have been shown to correlate with likelihood of progression to dementia.33 However, lack of standardization and validation for these measures limits their usefulness in clinical practice,34 and they are not currently recommended for informing prognosis. Structural brain MRI may rule out other potential causes for cognitive decline, such as subdural hematoma, stroke, NPH, or tumor, so should be considered if the history, physical, or laboratory studies suggest one of these causes.35
Functional and Amyloid Imaging
Fluorodeoxyglucose positron emission tomography (FDG-PET) can detect regions of hypometabolism in the brain which may be characteristic of MCI due to AD, AD dementia, or other causes for cognitive impairment.4,36Most recently, PET imaging of the extent of Aβ plaques in the brain has become more feasible with the radiopharmaceutical tracer florbetapir,37 and has been studied for its utility in identifying individuals with, or at high-risk for developing, AD.38A recent CMS review of PET amyloid imaging noted that this technology accurately identifies the presence of amyloid, but there is not yet sufficient evidence that the imaging results will affect medical decision-making or improve health outcomes for older adults with suspected MCI or AD.5 Use of the technology is therefore currently only recommended and covered by Medicare when used in the context of a research study.5
Laboratory Testing
Laboratory testing of complete blood count, electrolytes, glucose, calcium, thyroid function, vitamin B12, and folateis recommended to identify potentially reversible forms of MCI including infection, renal failure, hypo- or hypermagnesemia, hyperglycemia, hypo- or hypercalcemia, hypo- or hyperthyroidism, and B12 / folate deficiency. Laboratory testing for liver function, syphilis, Lyme titers (Borrelia), and HIV may revealrarer causes for cognitive impairment.14The proportion of dementia cases thought to be due to potentially reversible causes is about 9%.39While studies have suggested that levels of biomarkers in the cerebrospinal fluid (e.g., Aβ 42 and tau protein) may help identify patients with MCI who are more likely to progress to AD,40routine lumbar puncture is not generally recommended for clinical evaluation.41
Medical Therapy
We summarize potential interventions for MCI from recent clinical trials, systematic reviews, meta-analyses, and observational studies (Table). Participants in MCI clinical trials have often had more education, healthier behaviors, and less comorbidity (including lower rates of smoking, hypertension, and heart disease) compared withage-matched general populations.More trials in MCI patients with less favorable demographic and health profiles are needed to increase generalizability, especially regarding prognosis.
Table. Selected Randomized Controlled Trials of Interventions for MCI.
| Source | Patients | Mean Patient Age | Intervention | Trial Length | Primary Outcomes |
|---|---|---|---|---|---|
| Pharmacologic Treatments | |||||
| Cholinesterase inhibitors | |||||
| Salloway et al,82 2004 | N=270 adults with MCI. | 72 years | Donepezil 10 mg daily vs. placebo | 24 weeks | No significant differences between treatment groups in the two primary outcomes, New York University Paragraph Delayed Recall test and the ADCS-CGIC-MCI. |
| Petersen et al, 200583 | N=769 adults with amnestic MCI. | 73 years | Donepezil 10 mg daily vs. 2000 IU of vitamin E daily vs. placebo | 3 years | As compared with the placebo group, there were no significant differences in the probability of progression to Alzheimer's disease in the vitamin E group (hazard ratio, 1.02; 95 percent confidence interval, 0.74 to 1.41; P=0.91) or the donepezil group (hazard ratio, 0.80; 95 percent confidence interval, 0.57 to 1.13; P=0.42). |
| Doody et al,84 2009 | N=821 adults with amnestic MCI. | 70 years | Donepezil 10 mg daily vs. placebo | 48 weeks | The dual primary efficacy endpoint was not reached. At 48 weeks, there was a small, significant decrease in modified ADAS-cog (-0.90, SE, 0.37) favoring donepezil (P=0.01). Changes in CDR-SB scores were minimal and not significantly different between treatment groups. |
| Koontz et al,85 2005 | N=19 men with MCI. | 71 years | Galantamine 12 mg twice daily vs. placebo | 16 weeks | The primary outcome was the CANTAB. At 16 weeks, only one of the 6 sub-tests of the CANTAB (stockings of Cambridge) differed significantly between galantamine and placebo groups (8.3 ± 1.9 vs. 7.0 ± 1.4; P=0.023). |
| Behavioral Interventions | |||||
| Cognitive intervention | |||||
| Buschert et al,86 2011 | N=39 adults with MCI or mild AD. | 73 years | Group-based multi-component cognitive intervention vs. active control | 6 months | There were significant improvements in the ADAS-cog (P=0.02) and non-significant improvements in the MMSE (P=0.07) favoring the intervention MCI group. |
| Barnes et al,87 2009 | N=37 adults with MCI. | 74 years | Intensive, computer-based cognitive training vs. passive computer activities | 6 weeks | The primary outcome, Repeatable Battery for Assessment of Neuropsychological Status total scores, improved 0.36 standard deviations (SD) in the intervention group (P=0.097) compared with 0.03 SD in the control group (P=0.88) and the between-group difference was 0.33 SD (P=0.26). |
| Barnes et al,63 2013 | N=126 adults with memory complaints. | 73 years | 2 × 2 factorial design 4 groups: mental activity intervention (MA-I; intensive computer)/exercise intervention (EX-I; aerobic), MA-I/exercise control (EX-C; stretching and toning), mental activity control (MA-C; educational DVDs)/EX-1, MA-C/EX-C. | 12 weeks | Global cognitive scores improved significantly over time (mean, 0.16 SD; P < .001) but did not differ between groups in the comparison between the mental activity groups (P =0.17), the exercise groups (P =0.74), or across all 4 randomization groups (P=0.26). |
| Physical activity | |||||
| Lautenschlager et al,88 2008 | N=170 adults with memory complaints, 60% of whom had MCI. | 69 years | Home-based physical activity program vs. education and usual care | 24 weeks | At 18 months: 0.73-Point improvement on the ADAS-cog among patients in the intervention group vs. a 0.04-point improvement for those receiving placebo (0.69-point treatment difference, P=0.04); At 6 months: 0.26-Point improvement on the ADAS-cog among patients in the intervention group vs. a 1.04-point decrease for those receiving placebo (1.3-point treatment difference, P<0.001). Results were similar in sub-group of patients with MCI. The intervention group also showed modest improvements in word list delayed recall (verbal memory) and CDR sum of boxes (functional impairment due to cognition). |
| Baker et al,89 2010 | 33 adults with amnestic MCI. | 70 years | High-intensity aerobic exercise vs. stretching control group | 6 months | Compared with stretching, high-intensity aerobic exercise significantly improved performance on tests of executive function with stronger effects for women than men. |
| Suzuki et al,90 2012 | N=50 adults with amnestic MCI. | 75 years | Multi-component exercise program vs. educational control | 12 months | Patients in the exercise group showed superior improvements of cognitive function at treatment end for the Mini-Mental State Examination (group-by-time interaction P=0.04), the logical memory subtest of the Wechsler memory scale-revised (group-by-time interaction P=0.03), and the letter verbal fluency test (group-by-time interaction P=0.02). |
| Nagamatsu et al,91 2012 | N=86 women with subjective memory complaints. | 75 years | Resistant training twice-weekly, aerobic training twice-weekly, or balanceand tone training twice-weekly (control group) | 26 weeks | Compared with the balance and tone training (control) group, the resistance training group significantly improved performance on the Stroop Test of executive function (mean change 1.4 seconds vs. 9.1 seconds, P=0.04). Changes in Stroop test performance did not differ significantly between the aerobic training and balance and tone training groups (mean change 1.4 seconds vs. 8.8 seconds, P-value not given). |
| Barnes et al,63 2013 | N=126 adults with memory complaints. | 73 years | 2 × 2 factorial design 4 groups: mental activity intervention (MA-I; intensive computer)/exercise intervention (EX-I; aerobic), MA-I/exercise control (EX-C; stretching and toning), mental activity control (MA-C; educational DVDs)/EX-1, MA-C/EX-C. | 12 weeks | Global cognitive scores improved significantly over time (mean, 0.16 SD; P < .001) but did not differ between groups in the comparison between the mental activity groups (P =0.17), the exercise groups (P =0.74), or across all 4 randomization groups (P=0.26). |
| Multi-disciplinary care | |||||
| Woolfs et al,92 2008 | N=235adults with a suspected diagnosis of dementia or a cognitive disorder, >15% of whom had MCI. | 78 years | Integrated multidisciplinary diagnostic clinic vs. usual care | 52 weeks | At 12 months, no significant difference between groupson change in mean score on the visual analogue scale of the EuroQd measure EQ–5D (5.2 points; 95%CI, -0.58 to 10.94 points). |
| Psychotherapeutic interventions | |||||
| Joosten-WeynBanningh et al,66 2011 | N=93adults with MCI. | 70 years | Group cognitive behavioral therapy for patients vs. assignment to waiting list (control group) | 10 weeks | Primary outcome for patients: Acceptance assessed using a subscale of the Illness Cognition Questionnaire increased more in the intervention group compared to the waiting-list period (P=0.03) with an estimated between group difference of 3.49 (95%CI, –6.21 to –0.73; P=0.01). |
| Joosten-WeynBanningh et al,67 2013 | N=88 significant others of adults with MCI. | 69 years | Group cognitive behavioral therapy for significant others vs. assignment to waiting list (control group) | 10 weeks | Primary outcome for significant others: Sense of competence assessed with the Sense of Competence Questionnaire was not significantly different between the waiting list period and the interventionperiod (P=0.59), |
Abbreviations: ADAS-cog, 11-item (70-point) Alzheimer Disease Assessment Scale-cognitive subscale (higher scores indicating greater severity of cognitive impairment). ADCS-CGIC-MCI, Alzheimer Disease Cooperative Study Clinician's Global Impression of Change for MCI.CDR-SB. Clinical Dementia Rating Sum of Boxes. CANTAB, Cambridge Automated Neuropsychiatric Test Assessment Battery.
Pharmacologic Treatment of MCI
Currently, no drug has proven effective in treatment of MCI. Cholinesterase inhibitors have not been shown to decrease risk of progression from MCI to dementia at 1 and 3 years16, they have limited to nosignificant effects on cognitive function over the short-term (<12 months), and may substantially increase adverse effects (Table), based on a meta-analysis of 4 trials (n=1,960) and another of 9 trials (n=5,149).16,42 Consequently, cholinesterase inhibitors and memantineare not recommended for MCI treatment and there are currently no FDA-approved medications for MCI.16,42Ginkgo biloba, a widely-used herbal supplement to improve cognition and memory, has not been shown in randomized trials to prevent cognitive decline in those with MCI or normal cognition.43Similarly, testosterone supplementation in older men showed no benefit for cognitive function in a randomized controlled trial.44
Vascular Risk Factor Control
Stroke prevention and vascular risk factor control may reduce risk of progression from MCI to dementia, regardless of radiographicevidence of cerebrovascular injury.45An acute stroke and subclinical infarcts canaccelerate cognitive declineand precipitate dementia in patients with MCI.46,47Vascular contributions to cognitive impairment are common,and many MCI patients have pathological evidence of neurodegenerative and cerebrovascular disease.48Strategies for primary or secondary stroke prevention include blood pressure control, smoking cessation, statin therapy, anti-platelet therapy, and anticoagulation or antithrombotic therapy for atrial fibrillation.49,50
Independent of clinical stroke prevention, blood pressure control may reduce dementia risk. In the Systolic Hypertension in Europe (Syst-Eur) trial of 2,418 adults (mean age 70 years), treatment of isolated systolic hypertension reduced incidence of dementia by 50% (3.8 vs. 7.7 cases per 1000 patient-years; P=0.05) over 2 years.51A meta-analysis of 4 placebo-controlled trials (n=16,595) also suggested that anti-hypertensive treatment may reduce incident dementia(HR 0.87, 0.76–1.00; p=0.045).52The robustness of the evidence base for antihypertensive therapy to prevent cognitive decline, particularly in the oldest old, is debated because several RCTs (including HYVET-COG)52 and meta-analyses have been negative. Elevated or worsening systolic blood pressureand cigarette smoking eachincrease the risk of cerebral white matter lesion progression, which is associated with cognitive decline in the domains of information processing speed and executive function.53,54 Trials have not established that statins or intensive glycemic control reduce the risk of dementia independent of stroke prevention.
The Eighth Joint National Committee (JNC 8) recently recommended treating hypertensive adults aged ≥60 years to a blood pressure goal of <150/90 mm Hg.55 Given that higher variability in systolic blood pressure is associated with stroke, cerebral white matter lesion progression, lower hippocampal volume, and cognitive impairment,clinicians may consider medications that reduce BP variability (calcium channel blockers, thiazide diuretics) when selecting anti-hypertensive regimens, particularly in patients with marked BP variability,56,57although this is unsettled.58It is important to avoid over-treatment of hypertension and diabetes because hypotension and hypoglycemia may increase the risk for cognitive decline and other patient harms.28,29
Treatment of the Patient
Although there are no drugs proven or approved to treat MCI, optimizing patients' general medical and functional status, and providing counseling regarding issues such as driving and home safety, can maximize patient and caregiver well-being, and reduce risk for negative outcomes(Box 3). Individuals with MCI are at increased risk for gait dysfunction, mobility decline, and falls.59Gait assessment while performing an attention-demanding task (dual-task testing) may identify motor control and performance problems that couldbenefit from tailored interventions.59Optimizing visual and auditory acuity may enhance functioning as untreated vision and hearing problems are associated with cognitive decline.60,61CPAP for patients with sleep-disordered breathing may also reduce risk for progression of cognitive decline,12 although definitive clinical trials are still necessary.
While depressive and vegetative symptoms may cause or contribute to MCI, there is mixed evidence as to whether treatment of depression improves cognitive impairment, or decreases risk for incident MCI or dementia. Given increasing evidence of the negative impact of anti-cholinergic medications on cognitive function in older adults62, treatment with anti-depressants that have significant anti-cholinergic properties (e.g., amitriptyline, nortriptyline, and paroxetine) should be avoided. A trial of withdrawing and simplifying medication regimens in older adults may lead to improvements in cognitive function.26
Counseling on Behaviors
Dr F: Sometimes a physical therapy referral to facilitate aerobic exercise is useful ….I also suggested that she stay mentally engaged…being out of the house and…around other people is a very important way of stimulating the brain…and helps preserve brain function.”
There is modest evidence from RCTs and clinical studies that various behavioral interventions, particularly aerobic exercise and mental activity,may have small, but beneficial effects on cognitive function in older adults with MCI (Table).16Several RCTs of community-dwelling adults with or at risk for MCI have shown that home-based or professionally-supervised programs of aerobic exercise or resistance training modestly improvecognition, particularly executive function, over 18-months of follow-up(Table). The combination of aerobic exercise and mental activity may benefit MCI patients. The Mental Activity and eXercise (MAX) RCT of126 inactive, older adults with memory complaints demonstrated that a 12-week program of combined physical plus mental activitywas associated with small, significant improvements in global cognitive function regardless of the types of physical activity (aerobic vs. stretching/toning) and mental activity (intensive vs. educational videos).63Observational studies suggest that the Mediterranean diet also may reduce the risk of converting from MCI to dementia.64
Observational studies suggest that social engagement may reduce the risk of cognitive decline and preserve memory, particularly in adults with <12 years of education or those with vascular disease.65Little is known about the effectiveness of multidisciplinary care programs or supportive care interventions (e.g., counseling, education, support groups) for patients with MCI or their families because well-designed RCTs are lackingand one RCT that included many patients with dementia was negative (Table).16A few, small RCTs found that psychotherapy may modestly increase patients' acceptance of an MCI diagnosis and also provide knowledge, insight, acceptance, and coping skills for significant others,66,67but larger RCTs are needed.
Small RCTs and clinical studies suggest that cognitive interventions may improve cognitive function moderately over 6 to 12 months for persons with MCI or mild dementia; however, improvements are often specific to targeted cognitive domains, may not be greater than active controls, and may not improve daily functioning(Table).16,68Moreover, it is difficult to recommend specific components of cognitive interventions because of heterogeneity across studies.
Anissue that often arises in patients with MCI is driving safety.69While there is general consensus among medical and transportation societies that those with moderate to severe dementia should not drive due to significantly increased risk for accidents, evidence of driving impairment for patients with MCI is less clear.69,70The clinician should probe patient and family for indicators of driving impairment, including recent motor vehicle accidents or “near misses,” changes in the patient's driving behaviors (e.g., speed, ability to stay in lane, road sign comprehension), or episodes of getting lost in familiar areas.69Testing for deficits in visuospatial and executive function (Clock Drawing Task and Trail-making tests),cognitive domains thought to be important for driving safely, or formal driving evaluation may provide useful information.70Patients are often reluctant to stop driving even onphysician and/or family recommendation, so early and repeated discussions suggesting that driving cessation may be necessary, and referral to public transportation or other options may be useful in early counseling sessions. There is no legal requirement for physicians to report a patient with MCI to a Department of Motor Vehicles, but some states have mandatory reporting for patients with diagnosed dementia(see Web Resources).69
Follow-Up
Serial assessments of cognition are recommended because “progressive cognitive decline provides additionalevidence that the individual has ‘MCI due to AD'.”2Longitudinal follow-up and serial cognitive assessments are also useful since they allow a clearer assessment of a patient's true baseline and trajectory of cognitive function over time, and decrease the risk that poor performance on a single assessment due to anxiety, fatigue, or acute illness leads to a false positive diagnosis of MCI. However, the optimal timing, choice, and cost-effectiveness of longitudinal cognitive assessments are unclear. Tests of episodic memory identify MCI patients with high likelihood of progressing to AD within a few years.2General cognitive screening instruments (e.g., MoCA) are recommended for detecting dementia in individuals with suspected MCI.71Serial assessments of daily functioning may identify MCI patients who are more likely to develop dementia72,may indicate incident dementia, and may identify need for additional resources.
No neuroimaging or laboratory test is currently recommended for predicting MCI progression to dementia in clinical practice.2Although specific brain imaging findings (e.g., Aβ deposition, medial temporal lobe atrophy, hippocampal atrophy, or hypoperfusion or hypometabolism in the temporoparietal cortex) are associated with an increased risk of progression, these findings currently lack specificity. Cerebrospinal fluid tests showing low levels of Aβ42, elevated levels of tau, or a low Aβ42 to tau ratio confer an increased likelihood of progressing to dementia; however, results may be ambiguous or contradictory for a given patient, may vary across sites, and diagnostic accuracy and positive predictive value are sub-optimal.40,73It is not recommended to perform routine genetic testing for mutations in amyloid precursor protein, presenilin 1, or presenilin2 in adults with cognitive changes presenting before age 65 or in the APOE e4 allelein older adults.
A common question is whether patients with suspected MCI requirespecialist consultations. Given the currentlimited evidence for effective MCI treatments, consultation would serve primarily to confirm the diagnosis and help identify reversible causes.
Prognosis
Mr J: You're worried… [whether] this thing is going to get worse, which it probably will, but at what rate? … It's uncertainty of the future regarding all of these things, whether it's safety or whether it's decision making. …. And doctors don't seem to know either. It's very frustrating.
Prognosis is uncertain for patients with MCI and, as articulated by Mr. J, uncertainty aboutthe futureisa major source of worry.Although patients with MCI have a greaterrisk of developing dementia compared withthe general population, studies report substantial variability. Reported annual rates of MCI conversion to dementia span<5%72 to 12-20%9, depending on the country and population studied. Patients can be counseled that a minority will progress to dementia annually, however many patients with MCI (40-70%) may not progress to dementia even after 10 years.74 Importantly, some MCI patients (15-20%) will have improved cognition1-2 years later, although the latter group likely remains at increased risk of future cognitive decline.9,75
Risk factors for MCI progressionincludeolder age, fewer years of education, stroke, diabetes,and amnestic MCI subtype.9,76 The presence of an APOE e4 allele may modestly increase the risk of progressing from MCI to ADdementia.77Conversely, factors associated with increased likelihood of reverting from MCI to normal cognition include younger age, more years of education, higher baseline cognitive function, and non-amnestic single MCI type.75 Ongoing research aims to identify those MCI patients most likely to progress to dementia, particularly AD dementia, in order to target interventions.
It is important to counsel that clinicians cannot confidently predict Mrs. J's individual risk of progressing to dementia. Guidelines recommend using deficit type (amnestic or non-amnestic MCI)6 and ruling out other causes of cognitive impairment (vascular, trauma, depression, medical comorbidity)2to estimate risk for progression. However, MCI is a clinical diagnosis that has variable predictive accuracy for AD dementia depending on the patient's age and the definition of MCI used. Moreover, MCI sub-type classification may not accurately predict brain pathology. Although amnestic MCI is thought to be a prodrome of AD dementia, 30% of amnestic MCI patients who develop dementia have a primary brain pathology that is not AD.78
Existing models to predict risk of MCI progression havelimitations, including poor discrimination and low positive predictive values. Overall, better prognostic models are needed that account for demographic, clinical, neuropsychiatric, biological and pathological heterogeneity ineldersat-risk for cognitive decline.79
Competing risks are another important consideration: althoughpatients with MCI have an increased risk of dementia, they also have greater mortality. Risk prediction models need to estimate the likelihood that MCI patients will develop dementia prior to dying from competing causes, especially cardiovascular disease and cancer. Unfortunately, clinicians currently have insufficient information regardingMrs. J'sabsolute risk of developing dementia compared toher risks of dying from a non-dementia cause, or experiencing stable or improved cognition in the next few years.
Future Directions
Older adults fear cognitive decline36,and most patients prefertesting that would indicate future AD risk.80 These factors, combined with changes in diagnostic technologies forrisk-stratification, portendthat guidelines for the diagnosis and management of MCI will likely be in flux, and debated, over the next decade.
The limited efficacy of currently available interventions to prevent or delay progression of MCI to dementia79increases the importance of clinicians discussing the balance of benefits and risks of interventions, as well as patients' goals and preferences regarding medical interventions in later life. Given that there can be significant financial costs associated with diagnostic testing for MCI, as well as emotional distress and social stigma, some patients and families may prefer to forego the diagnostic evaluation.81Clinical decision-making regarding when and how to diagnose and treat MCI (and pre-clinical AD)willlikely change significantly if and when a safe and effective disease-modifying agent for pre-clinical AD and dementia is identified. In the meantime, primary care clinicians can support patients like Mrs. J in practicing healthy lifestyle behaviors, minimizing risks from polypharmacy and comorbidities, and counseling patients and families about how best to plan for the future.
Supplementary Material
Table S1: Characteristics of Selected Cognitive Tests
Box 2. Descriptions of Cognitive Problems14,15.
Changes in memory (is the patient misplacing things more, using notes and reminders more, repeating questions, having trouble keeping track of dates and appointments?)
Changes in language (word-finding difficulties?)
Changes in visuospatial function (new driving difficulties, including being slow to identify roadway hazards, late to apply brakes, or difficulty staying in lane?)
Changes in attention/executive function (easily distracted, new difficulties preparing meals or using household appliances, new difficulty writing checks, new safety concerns from family members?)
Box 3. Treating and Counseling Patients with MCI.
| Control of vascular risk factors, and prevention of stroke and subclinical brain injury |
| Hypertension present: control blood pressure and avoid hypotension |
| Diabetes present: control severe hyperglycemia and avoid severe hypoglycemia |
| Statin if indicated for primary or secondary stroke prevention |
| Atrial fibrillation present: initiate anti-coagulant or anti-thrombotic therapy if no contraindications |
| Beneficial behaviors |
| Abstain from heavy alcohol or illicit drug use |
| Engage in mental activity |
| Engage in physical activity |
| Stop smoking |
| Social Needs |
| Encourage and facilitate social interactions |
| Discuss living will, durable power of attorney, financial and long-term care plans |
| Provide community resources for patient and caregivers |
| Discuss driving safety |
| Discuss home safety, including kitchen safety, firearms, poisons, and potential fall risks |
| Prognosis and Follow-up |
| Discuss current evidence and uncertainty regarding MCI prognosis with patient and family |
| Arrange follow-up approximately every 6 months to assess changes in cognitive function and potential evolving needs for social support |
Acknowledgments
Funding / Support: Dr. Langa was supported in part by NIH grant U01 AG009740. Dr. Levine was supported by NIH grant K23 AG040278. The Care of the Aging Patient series is made possible by funding from The SCAN Foundation.
Web Resources
Alzheimer's Association – http://www.alz.org
The Alzheimer's Association is a national advocacy organization that supports care and research related to Alzheimer's disease and other dementias. Their website provides information and links that can be useful for patients, caregivers, health care providers, and researchers who have questions regarding Mild Cognitive Impairment (MCI) and cognitive decline, including a telephone helpline (800-272-3900) and how to contact local Alzheimer's Association chapters.
Alzheimer's Disease Education and Referral Center -- http://www.nia.nih.gov/alzheimers
The Alzheimer's Disease Education and Referral Center (ADEAR) is an information clearinghouse sponsored by the National Institute on Aging (NIA), one of the institutes of the National Institutes of Health (NIH). ADEAR provides a wide range of free information on causes for cognitive decline, MCI, and dementia. The information is geared to patients, caregivers, and health care providers. There is information on ongoing research studies related to cognitive decline and dementia, including how to explore enrolling in NIH-sponsored research studies.
American Bar Association Commission on Law and Aging – http://www.americanbar.org/groups/law_aging.html
The American Bar Association Commission on Law and Aging provides extensive information on a wide range of legal issues that older adults, in general, and older adults with MCI, in particular, may need to face. These legal issues include: advance care planning, capacity assessment, health care decision-making, durable power of attorney, guardianship, and elder abuse. The website includes helpful charts on how relevant laws may differ by state.
Caring Connections – http://www.caringinfo.org
Caring Connections is a program and website run by the National Hospice and Palliative Care Organization (NHPCO) which aims to provide resources to improve care at the end of life. The website provides a wide range of information for people living with illness and their caregivers. State-specific information on advance directives and locating a hospice provider is available at the website, and there is also a telephone helpline (800-658-8898).
Administration on Aging – http://www.aoa.gov
The Administration on Aging (AoA) is a program run by the federal Department of Health and Human Services that provides home and community-based services for older adults. The AoA website has information and web links on government benefits, health and wellness programs, and long-term care services. An “eldercare locator” at the website can help identify local resources related to Alzheimer's disease, caregiver programs, food and nutrition, and transportation.
National Highway Traffic Safety Administration –http://www.nhtsa.gov
The National Highway Traffic Safety Administration maintains educational resources for older drivers, their families, and physicians, including the Physician's Guide to Assessing and Counseling Older Drivers (http://www.nhtsa.gov/people/injury/olddrive/olderdriversbook/pages/contents.html) which details individual state laws regarding mandatory physician reporting requirements for patients with a dementia diagnosis.
Cognitive Function Assessment Instruments
Patient
Montreal Cognitive Assessment (MoCA) – http://www.mocatest.org/pdf_files/test/MoCA-Test-English_7_1.pdf
Mini-Cog Test – http://www.alz.org/documents_custom/minicog.pdf
Mini-Mental State Examination (MMSE) – the MMSE is copyrighted by Psychological Assessment Resources, Inc. Information on obtaining the test and permission to administer it can be found at: http://www4.parinc.com/Products/Product.aspx?ProductID=MMSE-2.
Informant
Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE) – http://www.alz.org/documents_custom/shortiqcode_english.pdf
Dementia Severity Rating Scale (DSRS) – http://www.dementia-assessment.com.au/global/DSRS_Full.pdf
AD8 – http://knightadrc.wustl.edu/About_Us/PDFs/AD8form2005.pdf
References
- 1.McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's & dementia : the journal of the Alzheimer's Association. 2011;7(3):263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's & dementia : the journal of the Alzheimer's Association. 2011;7(3):270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's & dementia : the journal of the Alzheimer's Association. 2011;7(3):280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Institute for Clinical and Economic Review. Diagnostic tests for Alzheimer's disease: generating and evaluating evidence to inform insurance coverage policy. [Accessed August 8, 2014];2012 http://www.icer-review.org/alzheimers/
- 5.Centers for Medicare and Medicaid. [Accessed August 8, 2014]; http://www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId=265.
- 6.Petersen RC. Clinical practice. Mild cognitive impairment. The New England journal of medicine. 2011;364(23):2227–2234. doi: 10.1056/NEJMcp0910237. [DOI] [PubMed] [Google Scholar]
- 7.Dubois B, Feldman HH, Jacova C, et al. Revising the definition of Alzheimer's disease: a new lexicon. Lancet Neurol. 2010;9(11):1118–1127. doi: 10.1016/S1474-4422(10)70223-4. [DOI] [PubMed] [Google Scholar]
- 8.Diagnostic and Statistical Manual of Mental Disorders. DSM-5. 5th. Washington, DC: American Psychiatric Association; 2013. [Google Scholar]
- 9.Plassman BL, Langa KM, Fisher GG, et al. Prevalence of cognitive impairment without dementia in the United States. Ann Intern Med. 2008;148(6):427–434. doi: 10.7326/0003-4819-148-6-200803180-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matthews FE, Stephan BC, McKeith IG, et al. Two-year progression from mild cognitive impairment to dementia: to what extent do different definitions agree? Journal of the American Geriatrics Society. 2008;56(8):1424–1433. doi: 10.1111/j.1532-5415.2008.01820.x. [DOI] [PubMed] [Google Scholar]
- 11.Petersen RC, Roberts RO, Knopman DS, et al. Prevalence of mild cognitive impairment is higher in men. The Mayo Clinic Study of Aging. Neurology. 2010;75(10):889–897. doi: 10.1212/WNL.0b013e3181f11d85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yaffe K, Laffan AM, Harrison SL, et al. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA. 2011;306(6):613–619. doi: 10.1001/jama.2011.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iwashyna TJ, Ely EW, Smith DM, Langa KM. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304(16):1787–1794. doi: 10.1001/jama.2010.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCarten JR. Clinical evaluation of early cognitive symptoms. Clinics in geriatric medicine. 2013;29(4):791–807. doi: 10.1016/j.cger.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 15.Holsinger T, Deveau J, Boustani M, Williams JW., Jr Does this patient have dementia? JAMA. 2007;297(21):2391–2404. doi: 10.1001/jama.297.21.2391. [DOI] [PubMed] [Google Scholar]
- 16.Lin JS, O'Connor E, Rossom R, Perdue LA, Burda BU, Thompson M, Eckstrom E. Screening for Cognitive Impairment in Older Adults: An Evidence Update for the US Preventive Services Task Force. Rockville, MD: Agency for Healthcare Research and Quality; 2013. Evidence Report No. 107. AHRQ Publication No. 14-05198-EF-1. [PubMed] [Google Scholar]
- 17.Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. Journal of the American Geriatrics Society. 2005;53(4):695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 18.Roalf DR, Moberg PJ, Xie SX, Wolk DA, Moelter ST, Arnold SE. Comparative accuracies of two common screening instruments for classification of Alzheimer's disease, mild cognitive impairment, and healthy aging. Alzheimer's & dementia : the journal of the Alzheimer's Association. 2013;9(5):529–537. doi: 10.1016/j.jalz.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jorm AF. A short form of the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): development and cross-validation. Psychological medicine. 1994;24(1):145–153. doi: 10.1017/s003329170002691x. [DOI] [PubMed] [Google Scholar]
- 20.Clark CM, Ewbank DC. Performance of the dementia severity rating scale: a caregiver questionnaire for rating severity in Alzheimer disease. Alzheimer Dis Assoc Disord. 1996;10(1):31–39. [PubMed] [Google Scholar]
- 21.Galvin JE, Roe CM, Powlishta KK, et al. The AD8: a brief informant interview to detect dementia. Neurology. 2005;65(4):559–564. doi: 10.1212/01.wnl.0000172958.95282.2a. [DOI] [PubMed] [Google Scholar]
- 22.Pfeffer RI, Kurosaki TT, Harrah CH, Jr, Chance JM, Filos S. Measurement of functional activities in older adults in the community. Journal of gerontology. 1982;37(3):323–329. doi: 10.1093/geronj/37.3.323. [DOI] [PubMed] [Google Scholar]
- 23.Brown PJ, Devanand DP, Liu X, Caccappolo E. Alzheimer's Disease Neuroimaging I. Functional impairment in elderly patients with mild cognitive impairment and mild Alzheimer disease. Archives of general psychiatry. 2011;68(6):617–626. doi: 10.1001/archgenpsychiatry.2011.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Teng E, Becker BW, Woo E, Knopman DS, Cummings JL, Lu PH. Utility of the functional activities questionnaire for distinguishing mild cognitive impairment from very mild Alzheimer disease. Alzheimer Dis Assoc Disord. 2010;24(4):348–353. doi: 10.1097/WAD.0b013e3181e2fc84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Widera E, Steenpass V, Marson D, Sudore R. Finances in the older patient with cognitive impairment: “He didn't want me to take over”. JAMA. 2011;305(7):698–706. doi: 10.1001/jama.2011.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steinman MA, Hanlon JT. Managing medications in clinically complex elders: “There's got to be a happy medium”. JAMA. 2010;304(14):1592–1601. doi: 10.1001/jama.2010.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shumaker SA, Legault C, Kuller L, et al. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women's Health Initiative Memory Study. JAMA. 2004;291(24):2947–2958. doi: 10.1001/jama.291.24.2947. [DOI] [PubMed] [Google Scholar]
- 28.Liu H, Gao S, Hall KS, et al. Optimal blood pressure for cognitive function: findings from an elderly African-American cohort study. Journal of the American Geriatrics Society. 2013;61(6):875–881. doi: 10.1111/jgs.12259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yaffe K, Falvey CM, Hamilton N, et al. Association between hypoglycemia and dementia in a biracial cohort of older adults with diabetes mellitus. JAMA Intern Med. 2013;173(14):1300–1306. doi: 10.1001/jamainternmed.2013.6176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. Journal of psychiatric research. 1982;17(1):37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 31.Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44(12):2308–2314. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- 32.Jack CR, Jr, Wiste HJ, Vemuri P, et al. Brain beta-amyloid measures and magnetic resonance imaging atrophy both predict time-to-progression from mild cognitive impairment to Alzheimer's disease. Brain : a journal of neurology. 2010;133(11):3336–3348. doi: 10.1093/brain/awq277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jack CR, Jr, Lowe VJ, Weigand SD, et al. Serial PIB and MRI in normal, mild cognitive impairment and Alzheimer's disease: implications for sequence of pathological events in Alzheimer's disease. Brain : a journal of neurology. 2009;132(Pt 5):1355–1365. doi: 10.1093/brain/awp062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jack CR, Jr, Barkhof F, Bernstein MA, et al. Steps to standardization and validation of hippocampal volumetry as a biomarker in clinical trials and diagnostic criterion for Alzheimer's disease. Alzheimer's & dementia : the journal of the Alzheimer's Association. 2011;7(4):474–485 e474. doi: 10.1016/j.jalz.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ries ML, Carlsson CM, Rowley HA, et al. Magnetic resonance imaging characterization of brain structure and function in mild cognitive impairment: a review. Journal of the American Geriatrics Society. 2008;56(5):920–934. doi: 10.1111/j.1532-5415.2008.01684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Small GW, Bookheimer SY, Thompson PM, et al. Current and future uses of neuroimaging for cognitively impaired patients. Lancet Neurol. 2008;7(2):161–172. doi: 10.1016/S1474-4422(08)70019-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Clark CM, Schneider JA, Bedell BJ, et al. Use of florbetapir-PET for imaging beta-amyloid pathology. JAMA. 2011;305(3):275–283. doi: 10.1001/jama.2010.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Johnson KA, Sperling RA, Gidicsin CM, et al. Florbetapir (F18-AV-45) PET to assess amyloid burden in Alzheimer's disease dementia, mild cognitive impairment, and normal aging. Alzheimer's & dementia : the journal of the Alzheimer's Association. 2013;9(5 Suppl):S72–83. doi: 10.1016/j.jalz.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Clarfield AM. The decreasing prevalence of reversible dementias: an updated meta-analysis. Arch Intern Med. 2003;163(18):2219–2229. doi: 10.1001/archinte.163.18.2219. [DOI] [PubMed] [Google Scholar]
- 40.Mattsson N, Zetterberg H, Hansson O, et al. CSF biomarkers and incipient Alzheimer disease in patients with mild cognitive impairment. JAMA. 2009;302(4):385–393. doi: 10.1001/jama.2009.1064. [DOI] [PubMed] [Google Scholar]
- 41.Petersen RC, Trojanowski JQ. Use of Alzheimer disease biomarkers: potentially yes for clinical trials but not yet for clinical practice. JAMA. 2009;302(4):436–437. doi: 10.1001/jama.2009.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Russ TC, Morling JR. Cholinesterase inhibitors for mild cognitive impairment. Cochrane Database Syst Rev. 2012(9):CD009132. doi: 10.1002/14651858.CD009132.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Snitz BE, O'Meara ES, Carlson MC, et al. Ginkgo biloba for preventing cognitive decline in older adults: a randomized trial. JAMA. 2009;302(24):2663–2670. doi: 10.1001/jama.2009.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Emmelot-Vonk MH, Verhaar HJ, Nakhai Pour HR, et al. Effect of testosterone supplementation on functional mobility, cognition, and other parameters in older men: a randomized controlled trial. JAMA. 2008;299(1):39–52. doi: 10.1001/jama.2007.51. [DOI] [PubMed] [Google Scholar]
- 45.Bosch J, Yusuf S, Pogue J, et al. Use of ramipril in preventing stroke: double blind randomised trial. Bmj. 2002;324(7339):699–702. doi: 10.1136/bmj.324.7339.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Snowdon DA, Greiner LH, Mortimer JA, Riley KP, Greiner PA, Markesbery WR. Brain infarction and the clinical expression of Alzheimer disease. The Nun Study. JAMA. 1997;277(10):813–817. [PubMed] [Google Scholar]
- 47.Vermeer SE, Prins ND, den Heijer T, Hofman A, Koudstaal PJ, Breteler MM. Silent brain infarcts and the risk of dementia and cognitive decline. The New England journal of medicine. 2003;348(13):1215–1222. doi: 10.1056/NEJMoa022066. [DOI] [PubMed] [Google Scholar]
- 48.Schneider JA, Arvanitakis Z, Leurgans SE, Bennett DA. The neuropathology of probable Alzheimer disease and mild cognitive impairment. Ann Neurol. 2009 Aug;66(2):200–208. doi: 10.1002/ana.21706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Goldstein LB, Bushnell CD, Adams RJ, et al. Guidelines for the primary prevention of stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke; a journal of cerebral circulation. 2011;42(2):517–584. doi: 10.1161/STR.0b013e3181fcb238. [DOI] [PubMed] [Google Scholar]
- 50.Furie KL, Kasner SE, Adams RJ, et al. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the american heart association/american stroke association. Stroke; a journal of cerebral circulation. 2011;42(1):227–276. doi: 10.1161/STR.0b013e3181f7d043. [DOI] [PubMed] [Google Scholar]
- 51.Forette F, Seux ML, Staessen JA, et al. Prevention of dementia in randomised double-blind placebo-controlled Systolic Hypertension in Europe (Syst-Eur) trial. Lancet. 1998;352(9137):1347–1351. doi: 10.1016/s0140-6736(98)03086-4. [DOI] [PubMed] [Google Scholar]
- 52.Peters R, Beckett N, Forette F, et al. Incident dementia and blood pressure lowering in the Hypertension in the Very Elderly Trial cognitive function assessment (HYVET-COG): a double-blind, placebo controlled trial. Lancet Neurol. 2008;7(8):683–689. doi: 10.1016/S1474-4422(08)70143-1. [DOI] [PubMed] [Google Scholar]
- 53.van Dijk EJ, Prins ND, Vrooman HA, Hofman A, Koudstaal PJ, Breteler MM. Progression of cerebral small vessel disease in relation to risk factors and cognitive consequences: Rotterdam Scan study. Stroke; a journal of cerebral circulation. 2008;39(10):2712–2719. doi: 10.1161/STROKEAHA.107.513176. [DOI] [PubMed] [Google Scholar]
- 54.White WB, Wolfson L, Wakefield DB, et al. Average daily blood pressure, not office blood pressure, is associated with progression of cerebrovascular disease and cognitive decline in older people. Circulation. 2011;124(21):2312–2319. doi: 10.1161/CIRCULATIONAHA.111.037036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.James PA, Oparil S, Carter BL, et al. 2014 Evidence-Based Guideline for the Management of High Blood Pressure in Adults: Report From the Panel Members Appointed to the Eighth Joint National Committee (JNC 8) JAMA. 2014;311(5):507–20. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 56.Webb AJ, Fischer U, Mehta Z, Rothwell PM. Effects of antihypertensive-drug class on interindividual variation in blood pressure and risk of stroke: a systematic review and meta-analysis. Lancet. 2010;375(9718):906–915. doi: 10.1016/S0140-6736(10)60235-8. [DOI] [PubMed] [Google Scholar]
- 57.Sabayan B, Wijsman LW, Foster-Dingley JC, et al. Association of visit-to-visit variability in blood pressure with cognitive function in old age: prospective cohort study. Bmj. 2013;347:f4600. doi: 10.1136/bmj.f4600. [DOI] [PubMed] [Google Scholar]
- 58.Gao S, Hendrie HC, Wang C, et al. Redefined blood pressure variability measure and its association with mortality in elderly primary care patients. Hypertension. 2014;64(1):45–52. doi: 10.1161/HYPERTENSIONAHA.114.03576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Montero-Odasso M, Verghese J, Beauchet O, Hausdorff JM. Gait and cognition: a complementary approach to understanding brain function and the risk of falling. Journal of the American Geriatrics Society. 2012;60(11):2127–2136. doi: 10.1111/j.1532-5415.2012.04209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lin FR, Yaffe K, Xia J, et al. Hearing loss and cognitive decline in older adults. JAMA Intern Med. 2013;173(4):293–299. doi: 10.1001/jamainternmed.2013.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rogers MA, Langa KM. Untreated poor vision: a contributing factor to late-life dementia. American journal of epidemiology. 2010;171(6):728–735. doi: 10.1093/aje/kwp453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cai X, Campbell N, Khan B, Callahan C, Boustani M. Long-term anticholinergic use and the aging brain. Alzheimer's & dementia : the journal of the Alzheimer's Association. 2013;9(4):377–385. doi: 10.1016/j.jalz.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Barnes DE, Santos-Modesitt W, Poelke G, et al. The Mental Activity and eXercise (MAX) trial: a randomized controlled trial to enhance cognitive function in older adults. JAMA Intern Med. 2013;173(9):797–804. doi: 10.1001/jamainternmed.2013.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Feart C, Samieri C, Rondeau V, et al. Adherence to a Mediterranean diet, cognitive decline, and risk of dementia. JAMA. 2009;302(6):638–648. doi: 10.1001/jama.2009.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ertel KA, Glymour MM, Berkman LF. Effects of social integration on preserving memory function in a nationally representative US elderly population. Am J Public Health. 2008;98(7):1215–1220. doi: 10.2105/AJPH.2007.113654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Joosten-Weyn Banningh LW, Prins JB, Vernooij-Dassen MJ, Wijnen HH, Olde Rikkert MG, Kessels RP. Group therapy for patients with mild cognitive impairment and their significant others: results of a waiting-list controlled trial. Gerontology. 2011;57(5):444–454. doi: 10.1159/000315933. [DOI] [PubMed] [Google Scholar]
- 67.Banningh LW, Vernooij-Dassen MJ, Vullings M, Prins JB, Rikkert MG, Kessels RP. Learning to live with a loved one with mild cognitive impairment: effectiveness of a waiting list controlled trial of a group intervention on significant others' sense of competence and well-being. Am J Alzheimers Dis Other Demen. 2013;28(3):228–238. doi: 10.1177/1533317513481093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Martin M, Clare L, Altgassen AM, Cameron MH, Zehnder F. Cognition-based interventions for healthy older people and people with mild cognitive impairment. Cochrane Database Syst Rev. 2011(1):CD006220. doi: 10.1002/14651858.CD006220.pub2. [DOI] [PubMed] [Google Scholar]
- 69.Carr DB, Ott BR. The older adult driver with cognitive impairment: “It's a very frustrating life”. JAMA. 2010;303(16):1632–1641. doi: 10.1001/jama.2010.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rapoport MJ, Naglie G, Herrmann N, et al. Developing Physician Consensus on the Reporting of Patients with Mild Cognitive Impairment and Mild Dementia to Transportation Authorities in a Region with Mandatory Reporting Legislation [published online December 8, 2013] The American journal of geriatric psychiatry : official journal of the American Association for Geriatric Psychiatry. doi: 10.1016/j.jagp.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 71.Petersen RC, Stevens JC, Ganguli M, Tangalos EG, Cummings JL, DeKosky ST. Practice parameter: early detection of dementia: mild cognitive impairment (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001;56(9):1133–1142. doi: 10.1212/wnl.56.9.1133. [DOI] [PubMed] [Google Scholar]
- 72.Farias ST, Mungas D, Reed BR, Harvey D, DeCarli C. Progression of mild cognitive impairment to dementia in clinic- vs community-based cohorts. Archives of neurology. 2009;66(9):1151–1157. doi: 10.1001/archneurol.2009.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Petersen RC, Aisen P, Boeve BF, et al. Criteria for mild cognitive impairment due to Alzheimer's disease in the community. Ann Neurol. 2013;74(2):199–208. doi: 10.1002/ana.23931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mitchell AJ, Shiri-Feshki M. Rate of progression of mild cognitive impairment to dementia--meta-analysis of 41 robust inception cohort studies. Acta psychiatrica Scandinavica. 2009;119(4):252–265. doi: 10.1111/j.1600-0447.2008.01326.x. [DOI] [PubMed] [Google Scholar]
- 75.Koepsell TD, Monsell SE. Reversion from mild cognitive impairment to normal or near-normal cognition: risk factors and prognosis. Neurology. 79(15):1591–1598. doi: 10.1212/WNL.0b013e31826e26b7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Campbell NL, Unverzagt F, Lamantia MA, Khan BA, Boustani MA. Risk factors for the progression of mild cognitive impairment to dementia. Clinics in geriatric medicine. 2013;29(4):873–893. doi: 10.1016/j.cger.2013.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Elias-Sonnenschein LS, Viechtbauer W, Ramakers IH, Verhey FR, Visser PJ. Predictive value of APOE-epsilon4 allele for progression from MCI to AD-type dementia: a meta-analysis. J Neurol Neurosurg Psychiatry. 2011;82(10):1149–1156. doi: 10.1136/jnnp.2010.231555. [DOI] [PubMed] [Google Scholar]
- 78.Jicha GA, Parisi JE, Dickson DW, et al. Neuropathologic outcome of mild cognitive impairment following progression to clinical dementia. Archives of neurology. 2006;63(5):674–681. doi: 10.1001/archneur.63.5.674. [DOI] [PubMed] [Google Scholar]
- 79.Brayne C, Davis D. Making Alzheimer's and dementia research fit for populations. Lancet. 2012;380(9851):1441–1443. doi: 10.1016/S0140-6736(12)61803-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wikler EM, Blendon RJ, Benson JM. Would you want to know? Public attitudes on early diagnostic testing for Alzheimer's disease. Alzheimer's research & therapy. 2013;5(5):43. doi: 10.1186/alzrt206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Le Couteur DG, Doust J, Creasey H, Brayne C. Political drive to screen for pre-dementia: not evidence based and ignores the harms of diagnosis. Bmj. 2013;347:f5125. doi: 10.1136/bmj.f5125. [DOI] [PubMed] [Google Scholar]
- 82.Salloway S, Ferris S, Kluger A, et al. Efficacy of donepezil in mild cognitive impairment: a randomized placebo-controlled trial. Neurology. 2004;63(4):651–657. doi: 10.1212/01.wnl.0000134664.80320.92. [DOI] [PubMed] [Google Scholar]
- 83.Petersen RC, Thomas RG, Grundman M, et al. Vitamin E and donepezil for the treatment of mild cognitive impairment. The New England journal of medicine. 2005;352(23):2379–2388. doi: 10.1056/NEJMoa050151. [DOI] [PubMed] [Google Scholar]
- 84.Doody RS, Ferris SH, Salloway S, et al. Donepezil treatment of patients with MCI: a 48-week randomized, placebo-controlled trial. Neurology. 2009;72(18):1555–1561. doi: 10.1212/01.wnl.0000344650.95823.03. [DOI] [PubMed] [Google Scholar]
- 85.Koontz J, Baskys A. Effects of galantamine on working memory and global functioning in patients with mild cognitive impairment: a double-blind placebo-controlled study. Am J Alzheimers Dis Other Demen. 2005;20(5):295–302. doi: 10.1177/153331750502000502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Buschert VC, Friese U, Teipel SJ, et al. Effects of a newly developed cognitive intervention in amnestic mild cognitive impairment and mild Alzheimer's disease: a pilot study. J Alzheimers Dis. 2011;25(4):679–694. doi: 10.3233/JAD-2011-100999. [DOI] [PubMed] [Google Scholar]
- 87.Barnes DE, Yaffe K, Belfor N, et al. Computer-based cognitive training for mild cognitive impairment: results from a pilot randomized, controlled trial. Alzheimer Dis Assoc Disord. 2009;23(3):205–210. doi: 10.1097/WAD.0b013e31819c6137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lautenschlager NT, Cox KL, Flicker L, et al. Effect of physical activity on cognitive function in older adults at risk for Alzheimer disease: a randomized trial. JAMA. 2008;300(9):1027–1037. doi: 10.1001/jama.300.9.1027. [DOI] [PubMed] [Google Scholar]
- 89.Baker LD, Frank LL, Foster-Schubert K, et al. Effects of aerobic exercise on mild cognitive impairment: a controlled trial. Archives of neurology. 2010;67(1):71–79. doi: 10.1001/archneurol.2009.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Suzuki T, Shimada H, Makizako H, et al. Effects of multicomponent exercise on cognitive function in older adults with amnestic mild cognitive impairment: a randomized controlled trial. BMC Neurol. 2012;12:128. doi: 10.1186/1471-2377-12-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nagamatsu LS, Handy TC, Hsu CL, Voss M, Liu-Ambrose T. Resistance training promotes cognitive and functional brain plasticity in seniors with probable mild cognitive impairment. Arch Intern Med. 2012;172(8):666–668. doi: 10.1001/archinternmed.2012.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wolfs CA, Kessels A, Dirksen CD, Severens JL, Verhey FR. Integrated multidisciplinary diagnostic approach for dementia care: randomised controlled trial. The British journal of psychiatry : the journal of mental science. 2008;192(4):300–305. doi: 10.1192/bjp.bp.107.035204. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Characteristics of Selected Cognitive Tests