Abstract
It is recognized that diaphragm muscle plasticity occurs with mechanical overloads, yet less is known regarding synergistic parasternal intercostal muscle fiber remodeling. We conducted overload training with intrinsic transient tracheal occlusion (ITTO) exercises in conscious animals. We hypothesized ITTO would yield significant fiber hypertrophy and myogenic activation that would parallel diaphragm fiber remodeling. Sprague-Dawley rats underwent placement of a tracheal cuff and were randomly assigned to receive daily ten-minute sessions of conscious ITTO or observation (SHAM) over two weeks. After training, fiber morphology, myosin heavy chain isoform composition, cross-sectional area, proportion of Pax7-positive nuclei, and presence of embryonic myosin (eMHC) were quantified. Type IIx/b fibers were 20% larger after ITTO training than with SHAM training (ITTO: 4431±676 μm2, SHAM: 3689±400 μm2, p<0.05), and type I fibers were more prevalent after ITTO (p<0.01). Expression of Pax7 was increased in ITTO parasternals and diaphragm (p<0.05). In contrast, the proportion of eMHC-positive fibers was increased only in ITTO parasternals (1.2 (3.4-0.6)%, SHAM: 0 (0.6-0%, p<0.05). Although diaphragm and parasternal type II fibers hypertrophy to a similar degree, myogenic remodeling appears to differ between the two muscles.
Introduction
The diaphragm contracts the earliest among the muscles of inspiration [1], and its displacement accounts for approximately 70% of the change in resting tidal volume [2]. Agonist intercostals in the parasternal region and lower external intercostals also have a large inspiratory mechanical advantage [3,4], and they contract in a predictable manner at rest [1,5]. Consequently, clinical weakness of either the diaphragm or intercostals results in paradoxical movements between the thorax and the abdomen [6] and impedes compensatory increases in ventilation during periods of elevated drive [7]. Rehabilitation with inspiratory muscle strength training (IMST) may counteract respiratory muscle insufficiency by improving maximal inspiratory pressure [8,9] and enhancing flow and volume compensations to loaded breathing [10]. However, the cellular remodeling responses of the inspiratory muscles to respiratory overloads are less understood, and their timing and magnitude could differ from those of the limb muscles.
It has been noted that the inspiratory intercostals appear to remodel with chronic changes in motor demands. Of the intercostal segments, the mid-thoracic external intercostals are best understood, and they have been found to atrophy [11] and hypertrophy [12] in clinical diseases and conditions that often occur in conjunction with chronic changes in inspiratory motor activity. Single airway occlusions in anesthetized animals also induce respiratory load compensation responses of the diaphragm and parasternal intercostals that include a prolonged inspiratory time and increased peak EMG [13]. Our laboratory found that acute bouts of intrinsic, transient tracheal occlusion (ITTO) in anesthetized animals appeared to induce progressive load compensation responses throughout the respiratory pump, as measured by an elevated diaphragm EMG activity, increased inspiratory time, and increased esophageal pressure [14]. When repeated for several days in conscious animals, ITTO load compensation responses also promote neural plasticity [15] and diaphragmatic fast fiber hypertrophy [16]. It is not known whether the plasticity to ITTO observed in diaphragmatic muscle fibers can be similarly achieved in the parasternal intercostals.
The model of ITTO developed in our laboratory [14,17] provides a reversible, inescapable load to the respiratory muscles that is high intensity with short trial durations and resembles the initial occlusion-load phase of clinical IMST [9]. ITTO is advantageous, because it eliminates the need for permanent tracheostomies or tracheal banding techniques [15]. We recently reported the ITTO training model and its resultant hypertrophy in diaphragmatic type IIx/b fibers [16]. Mechanical strain promotes muscle fiber hypertrophy by facilitating protein synthesis pathways [18], and it also can activate satellite cells, muscle progenitors anatomically and functionally distinct from other myonuclei [19,20]. Pax7 is a transcription factor expressed by quiescent, activated, and proliferating satellite cells [21]. Satellite cell proliferation and differentiation facilitates myofiber growth and repair, and supplies new myonuclei to the tissue. Developmental myosin heavy chain isoforms, which are typically absent in mature skeletal muscle, reappear transiently in regenerating myotubes [22]. Since intramuscular tension is particularly high within intercostal muscle segments [23], we reasoned that these markers of myogenic activity could be present following ITTO.
In the current study, we investigated myogenic activity and morphological remodeling of parasternal muscle fibers in conscious rats, after ITTO. The purposes of the study were two-fold: to examine whether ITTO facilitated muscle fiber hypertrophy and myogenic activation in the parasternal muscles, and then to contrast the myogenic responses of the parasternals to the diaphragm. We hypothesized that respiratory loads generated during ITTO would result in significant hypertrophy and myogenic activity in the parasternal intercostals, when compared to a surgical control group. Additionally, we hypothesized that the type and degree of myogenic activation of the parasternal intercostals of ITTO animals would be similar to diaphragm regenerative activity.
Materials and Methods
Two experiments were conducted. First, we investigated whether daily sessions of ITTO loading would facilitate fiber hypertrophy and regeneration in the parasternal intercostals. Next, we compared the presence of myogenic biomarkers in the parasternal intercostals to the diaphragm after either ITTO or sham training. These experiments were an extension of our initial work on the ITTO model of inspiratory training and its use to study diaphragmatic hypertrophy [16]. The experimental procedures were approved and overseen by the University of Florida Institutional Animal Care and Use Committee.
Animals
Nineteen young adult male Sprague-Dawley rats (Table 1) were studied (Harlan Laboratories, Indianapolis, IN). Animals were maintained in standard housing in the University of Florida animal care facility, in accordance with National Institute of Health’s “Guide for the Care and Use of Laboratory Animals.” A 12:12 hour reverse light: dark cycle and ad libitum diet of animal chow and water were supplied. All animals underwent placement of a tracheal cuff and were randomly assigned to training groups.
Table 1.
Weight and age in sham-occluded animals and animals treated with 10 sessions of intrinsic, transient tracheal occlusion (ITTO). Two animals did not complete the experimental protocol due to infection (n=1, SHAM group) or occluder failure (n=1, ITTO group). There were no significant group differences.
| ITTO n=10 | SHAM n=9 | |
|---|---|---|
| Age in days, median (IQR) | ||
| study onset | 69 (60-101) | 67 (60-80) |
| Body mass (gm) | ||
| study onset | 284 ± 56 | 283 ± 46 |
| study conclusion | 318 ± 31 | 316 ± 31 |
Occluder Placement
To place the tracheal occluder cuff, spontaneously breathing animals were anesthetized with isoflurane gas (2-5% in O2) and breathed room air. After an absence of corneal and paw-pinch reflexes was confirmed, the trachea was exposed using a ventral incision. A vascular occluder cuff (Fine Science Tools, Foster City, CA) was sutured around the trachea, and the actuating line was externalized between the scapulae. To inflate the cuff bladder, the actuating line was connected to a saline-filled syringe. The inflated cuff closed the trachea and elicited ITTO. Deflation of the cuff fully restored the patency of the airway and removed the breathing load. The actuator line was stitched in place, and the tracheal incision sutured.
During surgery and recovery, the body temperature was maintained at 37 ° C using a thermal pad. Animals received doses of buprenorphine (0.01-0.05 mg/kg body weight) and carprofen (5mg/kg BW) for analgesia and were rehydrated (0.9% saline, 0.01-0.02 mL/g body weight) prior to anesthesia withdrawal. Animals were closely monitored for respiratory distress, infection, or pain. During the first four post-operative days, routine analgesics and anti-inflammatory medications were administered (buprenorphine 0.01-0.05 mg/kg body weight every 12-24 hours and carprofen 5mg/kg body weight every 24 hours).
Training Protocol
After a recovery period of 5-7 days, animals were assigned to an 11-session training intervention similar to our previous reports [15,16,24]. Experimental sessions occurred five days per week in the morning, and lasted approximately 20 minutes. On the first session, each animal was acclimatized to the study observation chamber for approximately 20 minutes. During observation, no occlusions were administered.
SHAM Training
In the subsequent daily experimental sessions (sessions 2-11), animals in the sham-trained (SHAM) group (N=9) were placed in the observation chamber for 15 minutes of observation. The tracheal cuff was never inflated during observation sessions of the SHAM animals.
ITTO Training
Animals in the ITTO group (N=10) completed daily training sessions of 2.5 minutes of unobstructed breathing, 10 minutes of ITTO, followed by 2.5 minutes of unobstructed breathing. A cuff pressure of 400-600 mm Hg was utilized to completely and reversibly occlude the trachea of rats. During ITTO, the tracheal cuff was inflated for approximately 5-8 seconds to elicit approximately 8 respiratory attempts, and then deflated for approximately 20-25 seconds. The onset and removal of ITTO was confirmed with analogue tracings of the cuff pressure and plethysmograph recordings.
Tissue Analysis
One day after the last session, animals were anesthetized with isoflurane gas (2-5% in O2). Anesthesia was confirmed by absent paw pinch and corneal reflexes, and then animals were euthanized by decapitation. The right 3rd parasternal intercostal muscle was placed in saline-moistened gauze and chilled for 3-5 minutes [25,26], mounted with pins at resting length [27], then embedded in mounting medium (OCT) and quick-frozen with liquid nitrogen-chilled isopentane. In a subset of animals, the right mid-costal diaphragm (n=14: ITTO=7, SHAM=7) was preserved for immunofluorescent analysis of regeneration markers. An additional subset of left parasternal tissue (n=8: ITTO=4, SHAM=4) was flash-frozen in liquid nitrogen for Western blot analysis. Specimens were stored in a -80° C freezer until analysis. For all immunofluorescence analyses, tissue was sectioned transversely at the mid-belly (10 μm) with a cryostat microtome (Microm HM505, Walldorf, Germany). Sections were air-dried at 25° C for 30 minutes prior to histological processing.
Experiment #1: Effect of ITTO training sessions on parasternal morphology, hypertrophy, and myogenic immunofluorescence
CSA, fiber composition, and cellular morphology in the 3rd parasternal muscles were compared from animals that received 10 minutes of ITTO daily and SHAM-trained animals.
Experiment #2: Effect of ITTO on myogenic activation of the respiratory muscles
The presence of Pax7-positive nuclei, and embryonic myosin heavy chain (eMHC) expression in the parasternals was compared to the diaphragm muscles, after ITTO or SHAM conditioning.
Myosin Heavy Chain Immunofluorescence
Slides were first permeabilized with 0.5% Triton-X100 in phosphate-buffered saline. The samples were incubated with primary antibodies for laminin (1:200, Lab Vision, Fremont, CA), type I myosin heavy chain (A4.840, 1:15, Developmental Studies, Iowa City, IA), and type IIa myosin heavy chain (SC-71, 1:50, Developmental Studies), followed by secondary antibody incubation with rhodamine, Alexa Fluor 350 and Alexa Fluor 488 (Invitrogen, Carlsbad, CA). Cover slips were mounted with Vectashield medium (Vector Labs, Burlingame, CA). Samples were visualized using fluorescence microscopy at 10x magnification (Leica DM LB, Solms, Germany), using Leica N21 (BP 515-560 nm), GFP (BP 470/40 nm), and A4 (BP 360/40 nm) filter cubes. The fluorescence exposure times, gain, and gamma settings were maintained across specimens. Single layer images were captured with a digital camera, and the scope software (LAS software, Leica, v3) produced an overlay image. An investigator blinded to the study quantified CSA, using laminin-fluoresced images of fibers (Scion Image software, NIH). The CSA results were then superimposed onto the overlay image provided by the microscope. To calculate the fiber type and the area fraction (AA) occupied by each fiber type, at least 250 fibers were counted from each muscle.
Myogenic immunofluorescence
We identified satellite cells in muscle specimens by labeling Pax7 (1:50, Developmental Studies), a transcription factor specific for quiescent, activated and proliferating satellite cells. Separately, we also labeled eMHC (1:50, Developmental Studies), expressed in differentiating myotubes. Slides were air-dried at 25° C, fixated with 1:1 acetone-methanol solution, and then rinsed in phosphate-buffered saline. Next, slides were blocked with Pierce’s Superblock (Pierce Biotechnology, Rockford, IL) for 60 minutes at 25° C. Sections were incubated in primary antibodies for laminin (1:200, LabVision, Fremont, CA) and either eMHC (1:50, F1.652) or Pax7 (1:50) in 10% normal goat serum and phosphate-buffered saline, overnight at 4° C. Secondary antibody incubation occurred with rhodamine (1:500) and Alexa Fluor 488 (1:300) antibodies (Invitrogen) in 10% normal goat serum and phosphate-buffered saline, for two hours at 25° C in the dark. Specimens were mounted in Vectashield medium with DAPI (Vector Labs, Burlingame, CA).
Pax7 and eMHC samples were visualized separately with fluorescence microscopy at 20x magnification, using Leica N21 (BP 515-560 nm), GFP (BP 470/40 nm), and A4 (BP 360/40 nm) filter cubes. The scope (Leica DM LB, Solms, Germany) then provided an overlay image that merged the blue, green and red fluorescence (LAS software, Leica, v3). The fluorescence exposure times, gain, and gamma settings were maintained across specimens. Images that contained background non-specific fluorescence in any layer were thrown out and the processing was repeated. The overlay images were de-identified with a random number and the proportions of Pax7 nuclei or eMHC-positive fibers were calculated from a minimum of 250 fibers per muscle specimen. Pax7 identification was confirmed among three investigators blinded to group assignment. Two separate investigators blinded to group assignment confirmed positive eMHC fiber identification.
Muscle fiber morphology
Ten-micron transverse serial sections were acquired with a cryostat cooled to −20° C (Microm HM505, Walldorf, Germany). Sections were stained with hematoxylin and eosin, and cover slips were mounted with Permount medium (Fisher Scientific). Specimens were visualized with brightfield microscopy (Leica DM LB, Solms Germany) at 40x magnification. Twenty randomly selected images were analyzed from each right 3rd parasternal.
An analysis of the area of tissue submitted to damage-regeneration phenomena was undertaken using a systematic point-counting technique [28,29]. Point-counting was conducted by overlaying a 63-point grid over each of the 20 images (a total of 1,260 points per muscle, per animal), using Adobe Photoshop CS3 software (Adobe Corporation, San Jose CA). The tissue underneath each point on the grid was classified into one of nine morphological categories and then grouped as normal muscle, connective tissue, or remodeled tissue. Connective tissue was identified as endomysial or perimysial tissue or fibroblasts. Remodeled tissue included small or angular fibers, internally-nucleated fibers, inflamed or necrotic fibers, abnormal cytoplasm, inflammatory cells, or adipocytes. In a sample of 3 animals, two study investigators blinded to group assignment separately analyzed all images, and then each reviewer's classifications were compared. The level of agreement (intraclass correlation coefficient) of the fiber classifications was 0.95. An area fraction (AA) was calculated for each category of tissue.
Western blot
The presence of eMHC was confirmed in the ITTO parasternal tissue, using a subset of eight animals (ITTO=4, SHAM=4). To extract protein, frozen left intercostal muscle (~30 mg) was triturated in a radio-immunoprecipitation assay buffer and protease inhibitor cocktail (1:1000, SigmaFast, Sigma-Aldrich, St. Louis, MO). Samples were vortexed and centrifuged, and the supernatant was carefully removed. The concentration of protein in the supernatant was calculated against known protein assay reagents (BioRad, Hercules, CA).
Equal concentrations of the isolated protein were combined with lithium dodecyl sulfate buffer and reducing agent. One-dimensional sodium dodecyl sulfate polyacrylamide gel electrophoresis was conducted with the Nu-Page mini-gel system and bis-tris 4-12% mini-gel (Invitrogen, Carlsbad, CA). Gels were transferred to a nitrocellulose membrane, and Ponceau S stain identified molecular mass markers. Membranes were blocked with 5% milk in TBS-tris-buffered saline with Tween and incubated with eMHC (1.652, 1:500 dilution, Developmental Studies) primary antibody and milk buffer at 4° C overnight. The gel was rinsed and then incubated for one hour in peroxidase-conjugated secondary antibody (Rockland laboratories, Gilbertsville, PA). After further rinses in tris-buffered saline, we applied enhanced chemiluminescence reagents (Bio-Rad), and exposed the protein bands with x-ray film. The intensity of the bands was compared to a dilution curve and quantified with Image J software (NIH).
Statistical Analysis
Statistical analysis was conducted using SPSS Statistics 20 software (IBM-SPSS, Armonk, NY). Kolmogorov-Smirnov and Shapiro-Wilk tests were used to examine the assumptions of normality and homogeneity of variance. Animal characteristics were assessed using independent samples t-tests. For each animal, the mean CSA of each fiber type was calculated, for each muscle. Parasternal intercostal muscle fiber typing and morphology were then analyzed with two-way ANOVAs (MHC x group), and then Holm-Sidak-corrected pair-wise contrasts when needed. Myogenic activation was analyzed with Mann-Whitney U tests. Non-parametric data are presented as median ± interquartile range (IQR), while parametric data are provided as mean ± standard deviation. The level of significance was p<0.05.
Results
Rats tolerated the ITTO sessions well, and >95% of sessions were successfully completed. Two animals did not complete the experimental protocol due to infection (n=1, SHAM) or occluder failure (n=1, ITTO), leaving 17 animals available for analysis. Table 1 depicts the age and body mass of each group of animals. The age and weight of animals was similar between groups. Animals gained weight during the experiment (R-M ANOVA, main effect, p<0.01), and the weight gain did not differ between groups.
ITTO and parasternal intercostal muscle fiber CSA
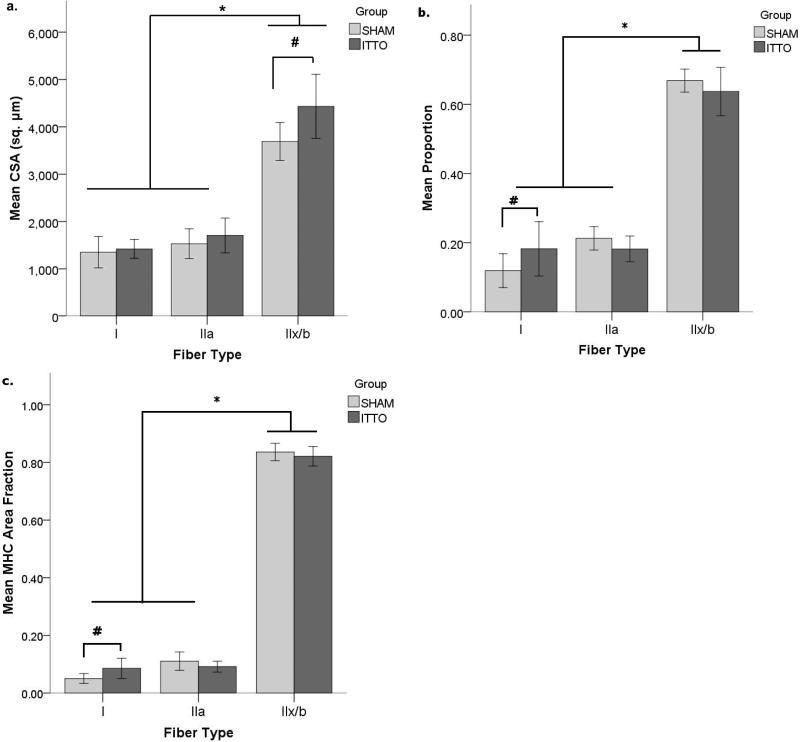
The CSA of the parasternal intercostal muscles (Figure 1A) differed according to both the training group and the fiber type. The CSA of type IIx/b fibers was 20% larger in the ITTO group than the SHAM group (ITTO: 4431±676 μm2, SHAM: 3689±400 μm2, ANOVA interaction effect, p<0.05). Type I and IIa CSA did not differ by training group. In both groups, type IIx/b fibers were significantly larger than type IIa or type I fibers (p<0.001).
Figure 1. Remodeling of the third parasternal intercostals after 10-minute ITTO conditioning sessions.
After 10 training sessions, (a.) CSA of type IIx/b fibers was greater in the ITTO group than in the SHAM group (# p<0.05). Regardless of group assignment, type IIx/b fibers were larger than type I or IIa fibers (* p<0.001). (b.) ITTO animals contained a larger proportion of type I fibers (# p<0.05). Type IIx/b fibers were more prevalent than type I or type IIa (* p<0.001). (c.) Type I MHC AA was greater in the ITTO group (# p<0.05). In addition, type IIx/b fibers occupied the largest AA (* p<0.001). AA: Area fraction
Fiber-type proportions of the third parasternal intercostal muscles
The third parasternal intercostal muscle contained a majority of fast type IIx/b fibers, (Figure 2). The proportion (ITTO: 0.18±0.08, SHAM: 0.12±0.05) and fiber area fraction (MHC AA, ITTO: 0.09±0.04, SHAM: 0.05±0.02) of type I fibers was greater in ITTO animals (ANOVA interactions, p<0.05) (Figures 1B and 1C). Regardless of the group, the intercostals contained more type IIx/b fibers and occupied a larger area, when compared to either type IIa or type I fibers (ANOVA main effects, p<0.001).
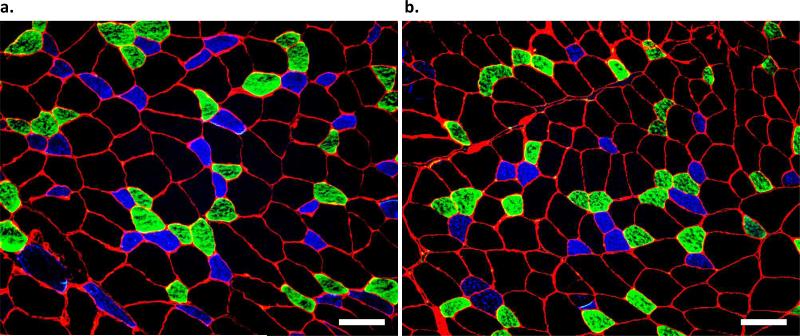
Figure 2. Immunofluorescence analysis of myosin heavy chain isoform in the third parasternal intercostal muscle.
Parasternal intercostal muscles from (a.) an ITTO animal and (b.) a SHAM animal. Type I fibers fluoresced blue, type IIa illuminated green, and type IIx/b fibers remained free of fluorescence. The green and red layers were acquired with a exposure times between 150-220 msec, while the blue layer required exposure times of 350-450 msec. The gain was 2.0. Images were captured with 10X eyepiece and 10X magnification. The average CSA of each fiber type in a muscle sample was calculated from at least 250 fibers. Scale bar = 100 μm.
Morphological assessment of the third parasternal intercostal muscles
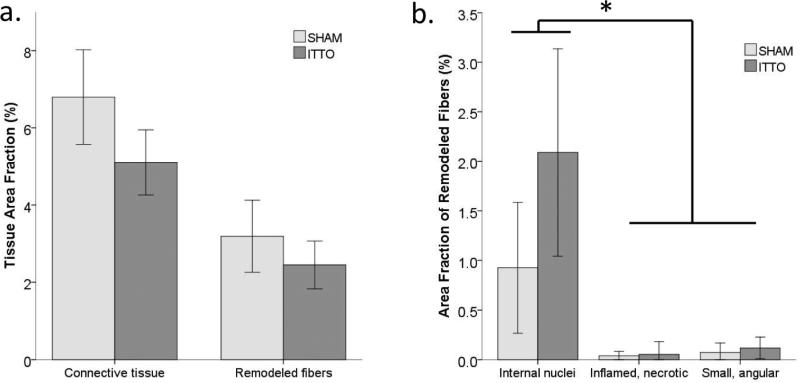
The morphological AA did not differ according to group. The majority of the parasternal intercostal muscle fibers were normal fibers that occupied a significantly larger AA than connective tissue or remodeling fibers (p<0.001). The AA of connective tissue exceeded the AA of remodeled muscle fibers (p<0.001). Only minute quantities of remodeling tissue were identified. Internally-nucleated cells occupied the largest AA (pooled AA: 1.5 ± 1.0 %), suggesting a small yet active muscle regeneration process. Figure 3 depicts the remodeling features and AA of remodeling tissue in the parasternal intercostal muscles. Neither group contained areas of widespread cellular membrane disruption or inflammatory cell abundance indicative of an acute muscular injury.
Figure 3. Morphological assessment of the third parasternal intercostal muscles, in animals that received ITTO or SHAM conditioning.
(a) Area fraction (AA) of remodeling fibers and connective tissue in ITTO animals and SHAM animals. The AA was not different based on group assignment. (b.) Classifications of remodeling fibers. In each category, there were no AA differences between the training groups (*p<0.001 AA of internal nuclei, compared to inflamed necrotic and small angular AA categories).
ITTO and Pax7-positive nuclei in the respiratory muscles
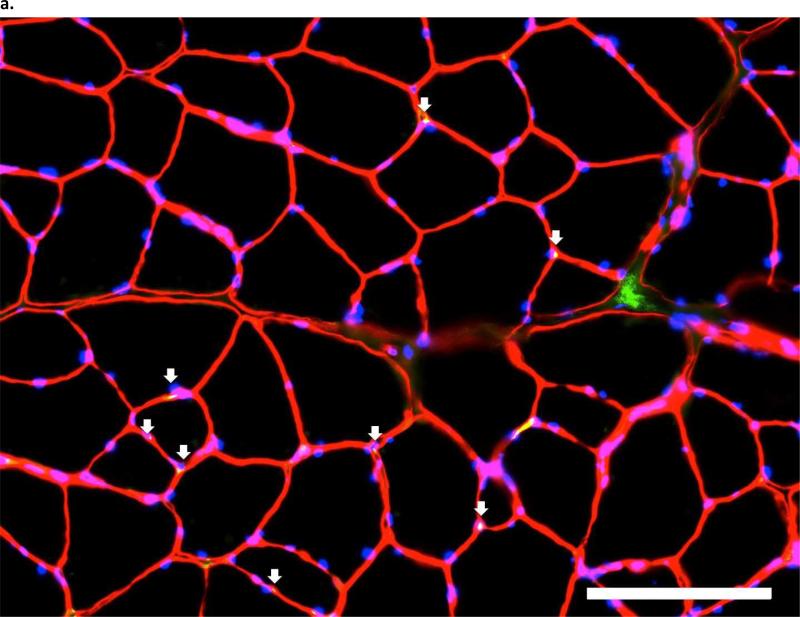
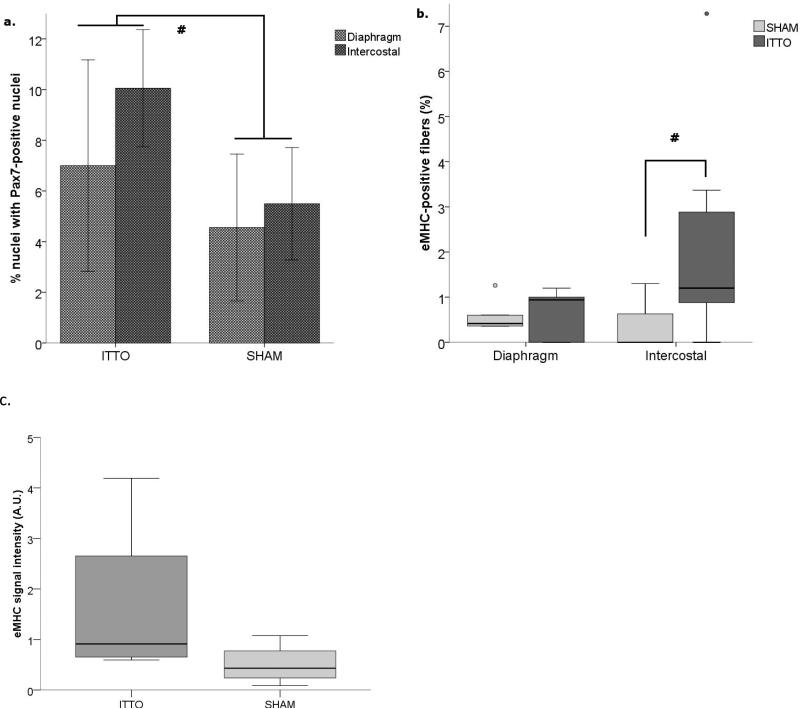
Pax7-positive nuclei were identified by green fluorescent nuclei located between the basal lamina and the extracellular membrane, and overlaid by DAPI-positive tissue. Figure 4 illustrates Pax7 microscopy from an ITTO and a SHAM animal. Of the sample used to compare myogenic activity, diaphragm tissue was unavailable from two animals (ITTO=1, SHAM=1). There was a significant main effect for training on the number of fibers containing Pax7-positive nuclei (ANOVA, F = 6.205, p<0.05). Animals exposed to ITTO training contained more Pax7-positive nuclei in the diaphragm and parasternal muscles, compared to SHAM-trained animals (Diaphragm: ITTO: 7.0±4.2%, SHAM: 4.6±2.9%, Parasternal: ITTO: 10.1±2.3%, SHAM: 5.5±2.2%, Figure 5A).
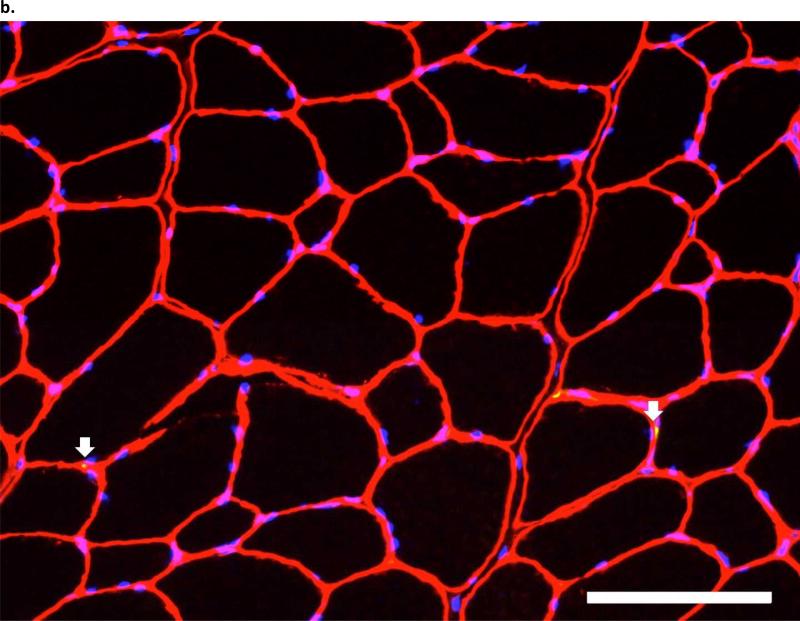
Figure 4. pbhImmunofluorescent images of Pax7 in the third parasternal intercostals, from (a.) an ITTO animal and (b.).
a SHAM animal. Arrows show Pax7-positive nuclei, which were defined as areas that fluoresced with Pax7-AlexaFluor-488, were contained within the sarcolemma and basal membrane, and co-localized with DAPI staining. Each image layer was acquired with exposure times of 150-220 msec and gain of 2.0. At least 250 fibers were analyzed with 10X eyepiece and 20X magnification. Scale bar = 100 μm.
Figure 5. Expression of myogenic biomarkers in the respiratory muscles.
(a.) The proportion of Pax7-positive nuclei (mean ±SD) was greater in ITTO diaphragm and parasternal intercostal tissue, when compared to SHAM-trained animals (#, p<0.05). (b.) The presence of embryonic myosin (median, IQR) was increased only in the parasternal intercostal muscles of the ITTO animals (# p<0.05). (c.) With Western blot, the signal intensity of eMHC protein (median, IQR) in a subset (n=8) of parasternal intercostal muscles was variable in ITTO animals and did not differ significantly between the groups (p=0.12). Protein measures were normalized to a standard dilution curve.
ITTO and eMHC-positive fibers in the respiratory muscles
Intensely-fluorescing areas within the exact borders of the sarcolemma were interpreted as eMHC-positive fibers (Figure S1A-D). Fibers with a faint or incomplete area of fluorescence were not counted. The eMHC-positive fiber CSA was small (465±78 μm2). Data for eMHC were power-transformed in order to meet normality assumptions for two-way ANOVA. There was a significant interaction between the type of training and muscle (F=3.764, p<0.05). That is, eMHC-positive fibers were prevalent in ITTO parasternal tissue (ITTO: 1.2 (3.4-0.6)%, SHAM: 0 (0.6-0)%, Figure 5B), but not in the diaphragm. Western blot analysis revealed small yet distinct bands at ~200kDa. The signal intensity tended to be larger in the ITTO animals, but individual readings were variable and did not reach statistical significance (Mann-Whitney U, p=0.12, Figure 5C).
Discussion
The current study measured the CSA and myogenic remodeling responses of parasternal inspiratory intercostal muscles following brief, intense pressure overload training. There are two advantageous aspects of this investigation. First, we present novel analyses of the hypertrophic and myogenic responses to training in the parasternal intercostals, and compare these findings to the diaphragm. Additionally, fiber remodeling was elicited from the innovative ITTO model of inspiratory muscle strength training, which provided an occlusive stimulus needed to recruit fast-fatigable fibers [30] while avoiding the inflammatory responses incurred by chronic tracheal banding or tracheostomy. The results support the hypothesis that ITTO training was associated with fiber remodeling in the rodent parasternal intercostal muscles. However, we reject our hypothesis that remodeling of the parasternals would mirror those of the diaphragm. The distinct patterns of myogenic marker prevalence in these respiratory muscles may offer insights into their motor recruitment and remodeling capabilities with clinical IMST.
In patients with respiratory insufficiency, IMST has been found to increase strength [12], reduce dyspnea [31], and improve weaning from mechanical ventilation [9]. Functional gains in patients have been observed in conjunction with fiber hypertrophy and regeneration of the lower intercostals [12,32]. However, exercise-induced cellular remodeling is not well understood in the human diaphragm or parasternal intercostals. The ITTO model could offer potential insights on the rate, extent, and location of neuromuscular plasticity induced by IMST, and enable a more direct investigation of optimal exercise doses to yield changes in muscular endurance and power. Research on intercostal remodeling is scant but could be especially crucial for managing diseases where the diaphragm is at a mechanical disadvantage (i.e. COPD) [31] or paralyzed (i.e. cervical spinal cord injury, multiple sclerosis) [33,34].
After only 10 training sessions, type IIx/b fibers were significantly (+20%) larger in the parasternals, which was consistent with our recent report of rapid, fast-fiber hypertrophy in the mid-costal diaphragms of ITTO-trained animals [16]. Acute anabolic responses can be detected in humans and rodents within hours of the first strength-training bout [35-37]. Fast-fiber hypertrophy may occur after ITTO training [38,39], because airway occlusion appears necessary to appreciably recruit fast-fatigable rodent diaphragm fibers [30]. Type IIx/b fibers are prevalent in parasternal tissue, but the time and extent to which they became recruited during ITTO is not well understood. However, human patients reach peak volitional inspiratory pressure within 15-20 seconds of occlusion [40,41]. We did not measure pressure generated by the animals during ITTO, but consistently observed costal retractions reflecting robust respiratory efforts with occlusions.
Fiber hypertrophy occurred together with a greater prevalence of Pax7-positive nuclei in both the ITTO diaphragm and parasternals, an indication of satellite cell activity [21]. Both in rodents and humans, resistance training increases satellite cell content, with concomitant gains in fiber CSA and muscle force [42-44]. The pattern of satellite cell accretion may retain an isoform specificity [45], and recent work found strength training of humans promoted fast-fiber hypertrophy, accompanied by a greater satellite cell content in type II, but not type I fibers [42]. Pax7 is downregulated during terminal differentiation of myotubes, leaving the possibility that some cells were not counted [46]. Although it has been noted previously that the fiber-type distribution of satellite cells may differ between slow and fast limb muscles [47], this was not a primary aim of this study. The naturally occurring proportions of Pax7, Pax3, and other regenerative transcription factors also appear species- and muscle-dependent [32,48], and require further investigation.
While similar hypertrophic responses were detected in both the diaphragm and parasternal intercostal muscles, only the parasternals contained an increased prevalence of slow fibers and eMHC fiber expression. These distinctions suggest ITTO could have elicited different patterns of loading and remodeling in the diaphragm and parasternals. Clinically, a chronically elevated work of breathing is associated with a fast-to-slow MHC shift in the parasternals but not the diaphragms of patients with COPD [49]. Similarly, early and regular muscular activity appears to elicit a greater expression of slow MHC in the fast muscles of rodents, but only when the muscle is exposed to damage-regeneration phenomena [50]. Recruitment of inspiratory synergistic muscles enhances maximal respiratory pressure and reduces diaphragmatic muscular tension [51], at the cost of high intramuscular tension within individual intercostal segments [23]. Consequently, progressive or sustained inspiratory loading may render the intercostals susceptible to fatigue or injury [52,53]. In contrast, diaphragmatic activation becomes attenuated by sustained loaded breathing [54], which could minimize the risk of diaphragmatic fatigue, even in the presence of task failure. While the recruitment patterns of the lower intercostals are best understood, it is reasonable to speculate that ITTO training could recruit the parasternal segments and subsequently promote myogenic remodeling [55]. While it has been previously reported that ITTO facilitated diaphragm EMG [14], interpretation of the current findings in the context of parasternal muscle loading must be made cautiously without parasternal EMG data during ITTO.
Although ITTO appeared to promote modest Pax7 and eMHC expression, we did not observe widespread regenerative activity akin to large induced mechanical or chemical injuries [56-58]. In conjunction, H&E staining did not reveal large quantities of small or centrally nucleated fibers. Using different methodologies, McCarthy et al. [59] and Wang and McPherron [60] tested the necessity of satellite cells for fiber hypertrophy. Both studies noted that satellite cell activation was not required to elicit short-term fiber hypertrophy, yet McCarthy et al. observed that hyperplasia and fiber regeneration appear to be satellite cell dependent [59]. The separate and synergistic roles of fiber hypertrophy and fiber regeneration remain topics of great interest and offer potential avenues for promoting muscle rehabilitation without inducing injury [61]. We note that the number of Pax7-positive fibers observed in this study is relatively small, when compared to the numbers reported in typical slow-twitch muscles. Interestingly, the proportions of eMHC-positive fibers in the ITTO parasternals were similar to levels reported in humans, following strength training [62].
We acknowledge that there are limitations to the study design. Analysis of single muscle fibers permits more precise measurements at the optimal length for force generation (L0) [63]. We elected to analyze fiber bundles at resting length, in order to capture greater quantities of muscle fibers using standardized cryopreservation techniques [25-27]. In a subset of randomly selected animals (n=3 from each group), electron microscopy confirmed the resting diaphragm and parasternal sarcomere lengths were equivalent between the groups (p=0.96). Further, the study did not prospectively address whether ITTO training modified minute ventilation, EMG or muscle force mechanics. Additionally, single occlusions in anesthetized animals robustly facilitate the lower external intercostals to a greater extent than the diaphragm or parasternals [13] and thus may undergo an even greater relative muscular remodeling with ITTO. Additional study is needed regarding in vivo activation of the diaphragm and parasternals using EMG during ITTO, in order to further elucidate mechanisms of remodeling. We recognize all of these considerations could have important implications regarding the exercise doses recommended for patient use, and they require further study.
Conclusions
Ten sessions of ITTO was associated with increased CSA of type IIx/b muscle fibers and increased prevalence of Pax7-positive nuclei identified in the diaphragm and parasternal intercostal muscles. Further, the additional fast to slow fiber shift and eMHC-positive fibers suggested differences in the loading and remodeling of the parasternal intercostals. It is recommended that future studies address the functional transcriptional and translational pathways that accompany respiratory muscle training and examine the force adaptations of the respiratory muscles to provide us with an understanding of the mechanisms underlying muscle adaptation for clinical rehabilitation.
Supplementary Material
At a glance.
This research has clinical implications for patients with respiratory muscle weakness and dyspnea. A rodent experimental model was used to identify fiber hypertrophy and regeneration in the parasternal intercostals after two weeks of respiratory muscle training. Compared to the diaphragm, the exercises increased parasternal fiber size equally and regeneration to a greater extent. This work begins to clarify our understanding of the cellular mechanisms by which respiratory muscle training improves muscle function. The investigation and application of these findings to patients may help providers to understand the optimal type, frequency, and duration of exercises needed to improve inspiratory muscle function.
Acknowledgements
Training support to Smith was provided by NIH T32 HD043730 and K12HD055929-02. Some disposable supplies were provided by NIH HD059751-01A1 to Vandenborne and by a Howard Hughes Medical Institute-sponsored mini-grant to Attia.
The A4.840, SC-71, Pax, and F1.652 antibodies generated by Helen M. Blau were obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by The University of Iowa, Department of Biology, Iowa City, IA 52242. No real or potential conflict of interest exists for any of the authors in this study.
The authors express gratitude to Mark Hotchkiss, Katherine Pate, and Vipa Bernhardt for their assistance with animal care and tissue collection. We thank the University of Florida Electron Microscopy Core Laboratory for assistance with ultrastructural assessments.
Abbreviations
- AA
Area fraction
- CSA
Cross-sectional area
- eMHC
Embryonic myosin heavy chain
- IMST
Inspiratory muscle strength training
- ITTO
Intermittent transient tracheal occlusion
- L0
Optimal length for muscle force generation
- MHC
Myosin heavy chain
- SHAM
Sham training group
Footnotes
- Conception and design of the experiments: Davenport, Mathur, Martin, Smith, Vandenborne
- Collection, analysis and interpretation of data: Smith, Mathur, Ye, Attia, Davenport
- Drafting the article or revising it critically for important intellectual content: Davenport, Martin, Smith, Mathur, Ye, Attia, Vandenborne All authors approved the final version of the manuscript.
References
- 1.Saboisky JP, Gorman RB, De Troyer A, Gandevia SC, Butler JE. Differential activation among five human inspiratory motoneuron pools during tidal breathing. J Appl Physiol. 2007;102:772–780. doi: 10.1152/japplphysiol.00683.2006. [DOI] [PubMed] [Google Scholar]
- 2.Farkas GA, Cerny FJ, Rochester DF. Contractility of the ventilatory pump muscles. Med Sci Sports Exerc. 1996;28:1106–1114. doi: 10.1097/00005768-199609000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Butler JE, Gandevia SC. The output from human inspiratory motoneurone pools. J Physiol. 2008;586:1257–1264. doi: 10.1113/jphysiol.2007.145789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Troyer A, Kirkwood PA, Wilson TA. Respiratory action of the intercostal muscles. Physiol Rev. 2005;85:717–756. doi: 10.1152/physrev.00007.2004. [DOI] [PubMed] [Google Scholar]
- 5.De Troyer A. The electro-mechanical response of canine inspiratory intercostal muscles to increased resistance: the cranial rib-cage. J Physiol. 1992;451:445–461. doi: 10.1113/jphysiol.1992.sp019172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Troyer A, Estenne M. Coordination between rib cage muscles and diaphragm during quiet breathing in humans. J Appl Physiol. 1984;57:899–906. doi: 10.1152/jappl.1984.57.3.899. [DOI] [PubMed] [Google Scholar]
- 7.Axen K, Bishop M, Haas F. Respiratory load compensation in neuromuscular disorders. J Appl Physiol. 1988;64:2659–2666. doi: 10.1152/jappl.1988.64.6.2659. [DOI] [PubMed] [Google Scholar]
- 8.Cader SA, Vale RG, Castro JC, Bacelar SC, Biehl C, et al. Inspiratory muscle training improves maximal inspiratory pressure and may assist weaning in older intubated patients: a randomised trial. J Physiother. 2010;56:171–177. doi: 10.1016/s1836-9553(10)70022-9. [DOI] [PubMed] [Google Scholar]
- 9.Martin AD, Smith BK, Davenport P, Harman E, Gonzalez-Rothi RJ, et al. Inspiratory muscle strength training improves weaning outcome in failure to wean patients: a randomized trial. Crit Care. 2011;15:R84. doi: 10.1186/cc10081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kellerman BA, Martin AD, Davenport PW. Inspiratory strengthening effect on resistive load detection and magnitude estimation. Med Sci Sports Exerc. 2000;32:1859–1867. doi: 10.1097/00005768-200011000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Capdevila X, Lopez S, Bernard N, Rabischong E, Ramonatxo M, et al. Effects of controlled mechanical ventilation on respiratory muscle contractile properties in rabbits. Intensive Care Med. 2003;29:103–110. doi: 10.1007/s00134-002-1548-3. [DOI] [PubMed] [Google Scholar]
- 12.Ramirez-Sarmiento A, Orozco-Levi M, Guell R, Barreiro E, Hernandez N, et al. Inspiratory muscle training in patients with chronic obstructive pulmonary disease: structural adaptation and physiologic outcomes. Am J Respir Crit Care Med. 2002;166:1491–1497. doi: 10.1164/rccm.200202-075OC. [DOI] [PubMed] [Google Scholar]
- 13.De Troyer A. Differential control of the inspiratory intercostal muscles during airway occlusion in the dog. J Physiol. 1991;439:73–88. doi: 10.1113/jphysiol.1991.sp018657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pate KM, Davenport PW. Tracheal occlusions evoke respiratory load compensation and neural activation in anesthetized rats. J Appl Physiol. 2012;112:435–442. doi: 10.1152/japplphysiol.01321.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bernhardt V, Hotchkiss MT, Garcia-Reyero N, Escalon BL, Denslow N, et al. Tracheal occlusion conditioning in conscious rats modulates gene expression profile of medial thalamus. Front Physiol. 2011;2:24. doi: 10.3389/fphys.2011.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith BK, Martin AD, Vandenborne K, Darragh BD, Davenport PW. Chronic intrinsic transient tracheal occlusion elicits diaphragmatic muscle fiber remodeling in conscious rodents. PLoS One. 2012;7:e49264. doi: 10.1371/journal.pone.0049264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bernhardt V, Garcia-Reyero N, Vovk A, Denslow N, Davenport PW. Tracheal occlusion modulates the gene expression profile of the medial thalamus in anesthetized rats. J Appl Physiol. 2011;111:117–124. doi: 10.1152/japplphysiol.01317.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gasier HG, Riechman SE, Wiggs MP, Buentello A, Previs SF, et al. Cumulative responses of muscle protein synthesis are augmented with chronic resistance exercise training. Acta Physiol (Oxf) 2010;201:381–389. doi: 10.1111/j.1748-1716.2010.02183.x. [DOI] [PubMed] [Google Scholar]
- 19.Mauro A. Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol. 1961;9:493–495. doi: 10.1083/jcb.9.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jayaraman A, Liu M, Ye F, Walter GA, Vandenborne K. Regenerative responses in slow-and fast-twitch muscles following moderate contusion spinal cord injury and locomotor training. European journal of applied physiology. 2012:1–10. doi: 10.1007/s00421-012-2429-2. [DOI] [PubMed] [Google Scholar]
- 21.Zammit PS, Partridge TA, Yablonka-Reuveni Z. The skeletal muscle satellite cell: the stem cell that came in from the cold. J Histochem Cytochem. 2006;54:1177–1191. doi: 10.1369/jhc.6R6995.2006. [DOI] [PubMed] [Google Scholar]
- 22.Schiaffino S, Reggiani C. Fiber types in mammalian skeletal muscles. Physiol Rev. 2011;91:1447–1531. doi: 10.1152/physrev.00031.2010. [DOI] [PubMed] [Google Scholar]
- 23.Leenaerts P, Decramer M. Respiratory changes in parasternal intercostal intramuscular pressure. J Appl Physiol. 1990;68:868–875. doi: 10.1152/jappl.1990.68.3.868. [DOI] [PubMed] [Google Scholar]
- 24.Pate KM, Davenport PW. Neural activation associated with chronic tracheal obstructions (TO) in conscious rats. FASEB J. 2010;24:1064.1067. [Google Scholar]
- 25.Larsson L, Skogsberg C. Effects of the interval between removal and freezing of muscle biopsies on muscle fibre size. J Neurol Sci. 1988;85:27–38. doi: 10.1016/0022-510x(88)90033-0. [DOI] [PubMed] [Google Scholar]
- 26.Levine S, Nguyen T, Taylor N, Friscia ME, Budak MT, et al. Rapid disuse atrophy of diaphragm fibers in mechanically ventilated humans. N Engl J Med. 2008;358:1327–1335. doi: 10.1056/NEJMoa070447. [DOI] [PubMed] [Google Scholar]
- 27.Roy RR, Pierotti DJ, Edgerton VR. Skeletal muscle fiber cross-sectional area: effects of freezing procedures. Acta Anat (Basel) 1996;155:131–135. doi: 10.1159/000147798. [DOI] [PubMed] [Google Scholar]
- 28.Reid WD, Huang J, Bryson S, Walker DC, Belcastro AN. Diaphragm injury and myofibrillar structure induced by resistive loading. J Appl Physiol. 1994;76:176–184. doi: 10.1152/jappl.1994.76.1.176. [DOI] [PubMed] [Google Scholar]
- 29.Scott A, Wang X, Road JD, Reid WD. Increased injury and intramuscular collagen of the diaphragm in COPD: autopsy observations. Eur Respir J. 2006;27:51–59. doi: 10.1183/09031936.06.00143004. [DOI] [PubMed] [Google Scholar]
- 30.Mantilla CB, Seven YB, Zhan WZ, Sieck GC. Diaphragm motor unit recruitment in rats. Respir Physiol Neurobiol. 2010;173:101–106. doi: 10.1016/j.resp.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang CH, Yang GG, Wu YT, Lee CW. Comparison of inspiratory muscle strength training effects between older subjects with and without chronic obstructive pulmonary disease. J Formos Med Assoc. 2011;110:518–526. doi: 10.1016/S0929-6646(11)60078-8. [DOI] [PubMed] [Google Scholar]
- 32.Martinez-Llorens J, Casadevall C, Lloreta J, Orozco-Levi M, Barreiro E, et al. [Activation of satellite cells in the intercostal muscles of patients with chronic obstructive pulmonary disease]. Arch Bronconeumol. 2008;44:239–244. [PubMed] [Google Scholar]
- 33.Fry DK, Pfalzer LA, Chokshi AR, Wagner MT, Jackson ES. Randomized control trial of effects of a 10-week inspiratory muscle training program on measures of pulmonary function in persons with multiple sclerosis. J Neurol Phys Ther. 2007;31:162–172. doi: 10.1097/NPT.0b013e31815ce136. [DOI] [PubMed] [Google Scholar]
- 34.Sheel AW, Reid WD, Townson AF, Ayas NT, Konnyu KJ. Effects of exercise training and inspiratory muscle training in spinal cord injury: a systematic review. J Spinal Cord Med. 2008;31:500–508. doi: 10.1080/10790268.2008.11753645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koopman R, Gleeson BG, Gijsen AP, Groen B, Senden JM, et al. Post-exercise protein synthesis rates are only marginally higher in type I compared with type II muscle fibres following resistance-type exercise. Eur J Appl Physiol. 2011;111:1871–1878. doi: 10.1007/s00421-010-1808-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tannerstedt J, Apro W, Blomstrand E. Maximal lengthening contractions induce different signaling responses in the type I and type II fibers of human skeletal muscle. J Appl Physiol. 2009;106:1412–1418. doi: 10.1152/japplphysiol.91243.2008. [DOI] [PubMed] [Google Scholar]
- 37.Garma T, Kobayashi C, Haddad F, Adams GR, Bodell PW, et al. Similar acute molecular responses to equivalent volumes of isometric, lengthening, or shortening mode resistance exercise. J Appl Physiol. 2007;102:135–143. doi: 10.1152/japplphysiol.00776.2006. [DOI] [PubMed] [Google Scholar]
- 38.Prezant DJ, Aldrich TK, Richner B, Gentry EI, Valentine DE, et al. Effects of long-term continuous respiratory resistive loading on rat diaphragm function and structure. J Appl Physiol. 1993;74:1212–1219. doi: 10.1152/jappl.1993.74.3.1212. [DOI] [PubMed] [Google Scholar]
- 39.Rollier H, Bisschop A, Gayan-Ramirez G, Gosselink R, Decramer M. Low load inspiratory muscle training increases diaphragmatic fiber dimensions in rats. Am J Respir Crit Care Med. 1998;157:833–839. doi: 10.1164/ajrccm.157.3.9512103. [DOI] [PubMed] [Google Scholar]
- 40.Truwit JD, Marini JJ. Validation of a technique to assess maximal inspiratory pressure in poorly cooperative patients. Chest. 1992;102:1216–1219. doi: 10.1378/chest.102.4.1216. [DOI] [PubMed] [Google Scholar]
- 41.Harikumar G, Moxham J, Greenough A, Rafferty GF. Measurement of maximal inspiratory pressure in ventilated children. Pediatr Pulmonol. 2008;43:1085–1091. doi: 10.1002/ppul.20905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Verdijk LB, Gleeson BG, Jonkers RA, Meijer K, Savelberg HH, et al. Skeletal muscle hypertrophy following resistance training is accompanied by a fiber type-specific increase in satellite cell content in elderly men. J Gerontol A Biol Sci Med Sci. 2009;64:332–339. doi: 10.1093/gerona/gln050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Petrella JK, Kim JS, Mayhew DL, Cross JM, Bamman MM. Potent myofiber hypertrophy during resistance training in humans is associated with satellite cell-mediated myonuclear addition: a cluster analysis. J Appl Physiol. 2008;104:1736–1742. doi: 10.1152/japplphysiol.01215.2007. [DOI] [PubMed] [Google Scholar]
- 44.Smith HK, Merry TL. Voluntary resistance wheel exercise during post-natal growth in rats enhances skeletal muscle satellite cell and myonuclear content at adulthood. Acta Physiol (Oxf) 2012;204:393–402. doi: 10.1111/j.1748-1716.2011.02350.x. [DOI] [PubMed] [Google Scholar]
- 45.Kalhovde JM, Jerkovic R, Sefland I, Cordonnier C, Calabria E, et al. “Fast” and “slow” muscle fibres in hindlimb muscles of adult rats regenerate from intrinsically different satellite cells. J Physiol. 2005;562:847–857. doi: 10.1113/jphysiol.2004.073684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zammit PS, Relaix F, Nagata Y, Ruiz AP, Collins CA, et al. Pax7 and myogenic progression in skeletal muscle satellite cells. J Cell Sci. 2006;119:1824–1832. doi: 10.1242/jcs.02908. [DOI] [PubMed] [Google Scholar]
- 47.Gibson MC, Schultz E. The distribution of satellite cells and their relationship to specific fiber types in soleus and extensor digitorum longus muscles. Anat Rec. 1982;202:329–337. doi: 10.1002/ar.1092020305. [DOI] [PubMed] [Google Scholar]
- 48.Relaix F, Montarras D, Zaffran S, Gayraud-Morel B, Rocancourt D, et al. Pax3 and Pax7 have distinct and overlapping functions in adult muscle progenitor cells. J Cell Biol. 2006;172:91–102. doi: 10.1083/jcb.200508044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Levine S, Nguyen T, Friscia M, Zhu J, Szeto W, et al. Parasternal intercostal muscle remodeling in severe chronic obstructive pulmonary disease. J Appl Physiol. 2006;101:1297–1302. doi: 10.1152/japplphysiol.01607.2005. [DOI] [PubMed] [Google Scholar]
- 50.Pette D, Sketelj J, Skorjanc D, Leisner E, Traub I, et al. Partial fast-to-slow conversion of regenerating rat fast-twitch muscle by chronic low-frequency stimulation. J Muscle Res Cell Motil. 2002;23:215–221. doi: 10.1023/a:1020974710389. [DOI] [PubMed] [Google Scholar]
- 51.Decramer M, Jiang TX, Reid MB. Respiratory changes in diaphragmatic intramuscular pressure. J Appl Physiol. 1990;68:35–43. doi: 10.1152/jappl.1990.68.1.35. [DOI] [PubMed] [Google Scholar]
- 52.Altose MD, Stanley NN, Cherniack NS, Fishman AP. Effects of mechanical loading and hypercapnia on inspiratory muscle EMG. J Appl Physiol. 1975;38:467–473. doi: 10.1152/jappl.1975.38.3.467. [DOI] [PubMed] [Google Scholar]
- 53.Hershenson MB, Kikuchi Y, Tzelepis GE, McCool FD. Preferential fatigue of the rib cage muscles during inspiratory resistive loaded ventilation. J Appl Physiol. 1989;66:750–754. doi: 10.1152/jappl.1989.66.2.750. [DOI] [PubMed] [Google Scholar]
- 54.Ross EZ, Nowicky AV, McConnell AK. Influence of acute inspiratory loading upon diaphragm motor-evoked potentials in healthy humans. J Appl Physiol. 2007;102:1883–1890. doi: 10.1152/japplphysiol.00694.2006. [DOI] [PubMed] [Google Scholar]
- 55.Gea J, Hamid Q, Czaika G, Zhu E, Mohan-Ram V, et al. Expression of myosin heavy-chain isoforms in the respiratory muscles following inspiratory resistive breathing. Am J Respir Crit Care Med. 2000;161:1274–1278. doi: 10.1164/ajrccm.161.4.99040103. [DOI] [PubMed] [Google Scholar]
- 56.Pizza FX, Peterson JM, Baas JH, Koh TJ. Neutrophils contribute to muscle injury and impair its resolution after lengthening contractions in mice. J Physiol. 2005;562:899–913. doi: 10.1113/jphysiol.2004.073965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Esposito A, Germinario E, Zanin M, Palade PT, Betto R, et al. Isoform switching in myofibrillar and excitation-contraction coupling proteins contributes to diminished contractile function in regenerating rat soleus muscle. J Appl Physiol. 2007;102:1640–1648. doi: 10.1152/japplphysiol.01397.2006. [DOI] [PubMed] [Google Scholar]
- 58.Liu M, Stevens-Lapsley JE, Jayaraman A, Ye F, Conover C, et al. Impact of treadmill locomotor training on skeletal muscle IGF1 and myogenic regulatory factors in spinal cord injured rats. Eur J Appl Physiol. 2010 doi: 10.1007/s00421-010-1392-z. [DOI] [PubMed] [Google Scholar]
- 59.McCarthy JJ, Mula J, Miyazaki M, Erfani R, Garrison K, et al. Effective fiber hypertrophy in satellite cell-depleted skeletal muscle. Development. 2011;138:3657–3666. doi: 10.1242/dev.068858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang Q, McPherron AC. Myostatin inhibition induces muscle fibre hypertrophy prior to satellite cell activation. J Physiol. 2012;590:2151–2165. doi: 10.1113/jphysiol.2011.226001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee SJ, Huynh TV, Lee YS, Sebald SM, Wilcox-Adelman SA, et al. Role of satellite cells versus myofibers in muscle hypertrophy induced by inhibition of the myostatin/activin signaling pathway. Proc Natl Acad Sci U S A. 2012;109:E2353–2360. doi: 10.1073/pnas.1206410109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kadi F, Thornell LE. Training affects myosin heavy chain phenotype in the trapezius muscle of women. Histochem Cell Biol. 1999;112:73–78. doi: 10.1007/s004180050393. [DOI] [PubMed] [Google Scholar]
- 63.Geiger PC, Cody MJ, Macken RL, Sieck GC. Maximum specific force depends on myosin heavy chain content in rat diaphragm muscle fibers. J Appl Physiol. 2000;89:695–703. doi: 10.1152/jappl.2000.89.2.695. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.