Abstract
Prototheca zopfii has been developed as an experimental system for studying the control of cytokinesis and daughter-cell number variation in multiple fission. Although mean daughter-cell number increases linearly with growth rate, and this dependency is genetically controlled, pedigree analysis shows that daughter-cell number variation is not under direct genetic control. In a population growing in steady-state balanced growth, each cell has a given probability of dividing into 2, 4, 8 16, or 32 daughter-cells. These probabilities are independent of the division number of the cell in the preceding generation, and can be altered by changes in the culture medium.
Keywords: cell division, growth rate effects, colorless alga, Chlorella
Full text
PDF

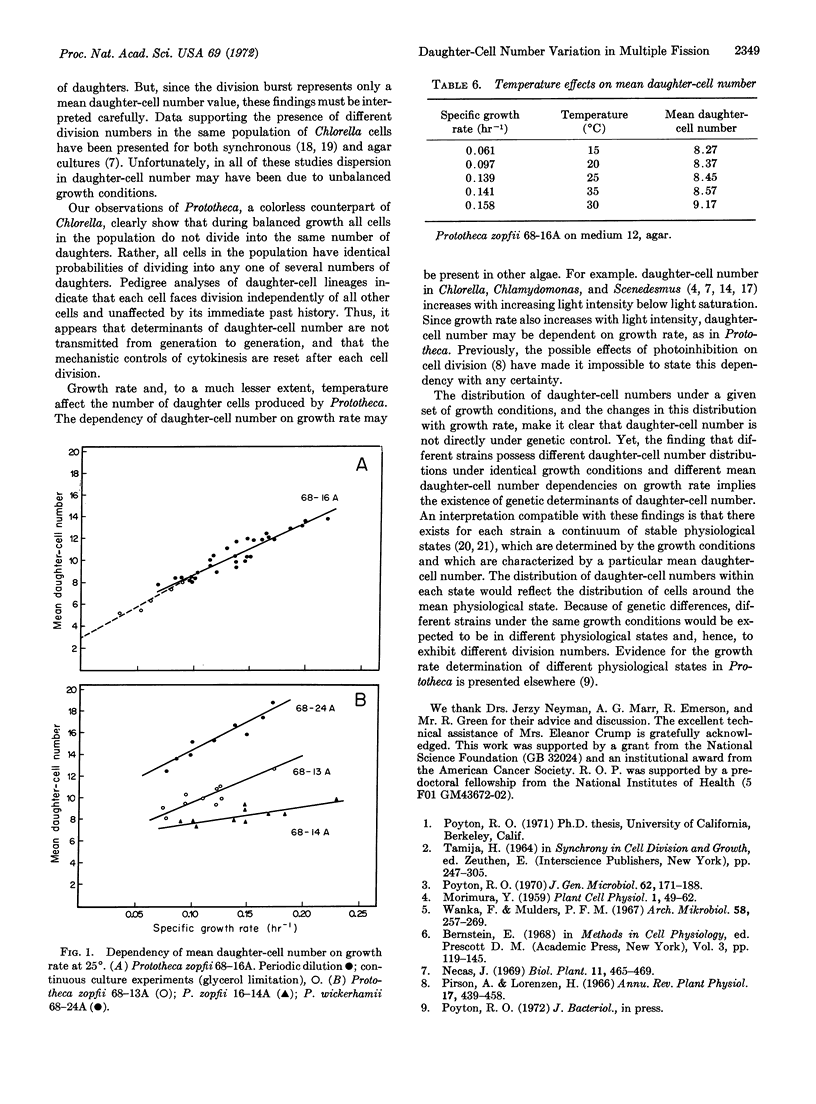

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CAMPBELL A. Synchronization of cell division. Bacteriol Rev. 1957 Dec;21(4):263–272. doi: 10.1128/br.21.4.263-272.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey R. J., Marr A. G., Painter P. R. Kinetics of growth of individual cells of Escherichia coli and Azotobacter agilis. J Bacteriol. 1967 Feb;93(2):605–617. doi: 10.1128/jb.93.2.605-617.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kates J. R., Chiang K. S., Jones R. F. Studies on DNA replication during synchronized vegetative growth and gametic differentiation in Chlamydomonas reinhardtii. Exp Cell Res. 1968 Jan;49(1):121–135. doi: 10.1016/0014-4827(68)90525-9. [DOI] [PubMed] [Google Scholar]
- Molloy G. R., Schmidt R. R. Studies on the regulation of ribulose-1,5-diphosphate carboxylase synthesis during the cell cycle ofthe eucaryote chlorella. Biochem Biophys Res Commun. 1970 Sep 10;40(5):1125–1133. doi: 10.1016/0006-291x(70)90911-3. [DOI] [PubMed] [Google Scholar]
- Poyton R. O., Branton D. A multipurpose microperfusion chamber. Exp Cell Res. 1970 Apr;60(1):109–114. doi: 10.1016/0014-4827(70)90494-5. [DOI] [PubMed] [Google Scholar]
- SCHAECHTER M., MAALOE O., KJELDGAARD N. O. Dependency on medium and temperature of cell size and chemical composition during balanced grown of Salmonella typhimurium. J Gen Microbiol. 1958 Dec;19(3):592–606. doi: 10.1099/00221287-19-3-592. [DOI] [PubMed] [Google Scholar]
- SOEDER C. J., RIED A. [On the course of sporulation and protoplast division in synchronous cultures of Chlorella pyrenoidosa]. Arch Mikrobiol. 1962;42:176–189. [PubMed] [Google Scholar]
- Schor S., Siekevitz P., Palade G. E. Cyclic Changes in Thylakoid Membranes of Synchronized Chlamydomonas reinhardi. Proc Natl Acad Sci U S A. 1970 May;66(1):174–180. doi: 10.1073/pnas.66.1.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitz T. O., Kent A. B., Hopkins H. A., Schmidt R. R. Equilibrium density-gradient procedure for selection of synchronous cells from asynchronous cultures. Science. 1970 Jun 5;168(3936):1231–1232. doi: 10.1126/science.168.3936.1231. [DOI] [PubMed] [Google Scholar]
- Wanka F., Mulders P. F. The effect of light on DNA synthesis and related processes in synchronous cultures of chlorella. Arch Mikrobiol. 1967;58(3):257–269. doi: 10.1007/BF00408808. [DOI] [PubMed] [Google Scholar]


