Summary
Candidemia and other forms of invasive candidiasis are important causes of morbidity and mortality. The evolving challenge of antimicrobial resistance among fungal pathogens continues to highlight the need for potent, new antifungal agents. MEDLINE, EMBASE, Scopus, and Web of Science searches (up to January 2014) of the English-language literature were performed with the keywords “Candida” or “Candidemia” or “Candidiasis” and terms describing investigational drugs with activity against Candida spp. Conference abstracts and the bibliographies of pertinent articles were also reviewed for relevant reports. ClinicalTrials.gov was searched for relevant clinical trials. Currently available antifungal agents for the treatment of candidemia are summarized. Investigational antifungal agents with potential activity against Candida bloodstream infections and other forms of invasive candidiasis and vaccines for prevention of Candida infections are also reviewed as are selected antifungal agents no longer in development. Antifungal agents currently in clinical trials include isavuconazole, albaconazole, SCY-078, VT-1161, and T-2307. Further data are needed to determine the role of these compounds in the treatment of candidemia and other forms of invasive candidiasis. The progressive reduction in antimicrobial drug development may result in a decline in antifungal drug discovery. Still there remains a critical need for new antifungal agents to treat and prevent invasive candidiasis and other life-threatening mycoses.
Keywords: antifungal agents, Candida, candidemia, investigational, vaccines
Introduction
Invasive fungal infections due to Candida species have been a growing challenge over the past decade, both because of a rise in the frequency of infections and an increasing resistance to standard antifungal therapy [1]. Candida species are the fourth leading cause of healthcare-associated infections, accounting for approximately 11% of all infections; Candida spp. are also responsible for nearly 12% of all central line-associated bloodstream infections, preceded only by Staphylococcus aureus and Enterococcus species [2]. In the National HealthCare Safety Network data from 2009 to 2010, non-albicans Candida spp. were the 4th and Candida albicans was the 7th cause of central line associated bloodstream infections [3].
Crude mortality associated with Candida infections ranges from 19% to approximately 60% with the wide variability likely due to differences in study populations [4,5]. Similarly, mortality rates attributable to candidemia range from 10% to 49% [6–8]. Candidemia has also been associated with an increase in length of hospital stay and subsequent cost [6,7,9].
Independent risk factors for the development of candidemia include use of broad-spectrum antimicrobial agents, neutropenia, presence of central venous catheters, administration of total parenteral nutrition (TPN), gastrointestinal surgery, chemotherapy, hemodialysis, previous colonization with Candida spp., and gastric acid suppression [9].
Critically ill patients are therefore particularly susceptible to Candida bloodstream infections, as they present with many of these risk factors. The incidence of candidemia in the intensive care unit (ICU) is variable depending on the studies queried. Recently, the EPIC II investigators surveyed 14000 patients in 1265 ICUs in 76 countries in diverse geographic locations and reported a one-day point prevalence of candidemia of 6.9 per 1000 patients in 2007 [10]. C. albicans was the most common organism, and fluconazole was the most common antifungal agent used. The presence of candidemia correlated with increased ICU length of stay and mortality [10].
Although more than one hundred species of Candida have been described, the vast majority of cases of candidemia are caused by only five species: C. albicans, Candida glabrata, Candida parapsilosis, Candida tropicalis, and Candida krusei [11]. While other Candida species account for only a very small fraction of infections, recognition and understanding of resistance patterns in these scarcer species may have therapeutic implications. Traditionally, treatment of candidemia has relied mainly on antifungal azoles, polyenes, and echinocandins. In general, C. albicans, C. parapsilosis, C. tropicalis, and Candida lusitaniae are susceptible to antifungal azoles and echinocandins, with C. parapsilosis and Candida guilliermondii displaying higher MICs for echinocandins [11]. Echinocandin use has recently been shown to correlate with improved outcomes and a decrease in mortality in a quantitative review of randomized trials for the treatment of invasive candidiasis [12]. However, infections due to C. glabrata have increased in frequency over the past decades paralleled by an increase in resistance to fluconazole as well as echinocandins [13,14]. Therefore, given the increasing incidence of multidrug resistant Candida species and high mortality with existing treatment, there is a need to develop new antifungal agents. The objective of this review is to summarize the safety and efficacy of investigational antifungal agents that may potentially be effective treatment for candidemia and other forms of invasive candidiasis.
Magnitude and impact of candidemia
Candida spp. are a common cause of bloodstream infections with a high attributable cost. Early onset candidemia is also occurring with more frequency and is associated with increased mortality, longer hospital stays and subsequently higher hospital costs when compared to bacterial bloodstream infections [15]. Using an Agency for Healthcare Research and Quality dataset from the Nationwide Inpatient Survey 2000 (NIS 2000), Zaoutis and colleagues compared crude data to propensity-score matched data and demonstrated in adults with candidemia a 14.5% increase in mortality and a mean increase in length of stay of 10.1 days resulting in almost $40,000 in additional costs [6]. In pediatric patients (> 30 days and < 18 years old), they compared data using the 2000 Kids’ Inpatient Database (KID 2000) and found with propensity-matched analysis, that pediatric candidemia was associated with an absolute 10% increase in mortality and an approximate 21-day increase in length of stay with an additional $92,000+ in hospital costs.
In neonates, Candida spp. are the third most common cause of bloodstream infection and the primary cause of invasive fungal infection [16]. In data extracted from the 2003 Kids’ Inpatient Database from the Healthcare Cost and Utilization Project [16], the highest risk neonates were extremely low birth weight (ELBW)(<1000g). The ELBW infants experienced propensity score-adjusted mortality rates of 11.9% without comparable increases in the non- ELBW infants. ELBW infants with candidiasis had an increased hospital cost of over $39,000. Non-ELBW neonates had longer hospital stays (16 days) and increased hospital costs ($122,302).
In the United States for the year 2000, there were 30 adult and 43 pediatric cases of candidemia per 100,000 hospital admissions [6]. These numbers suggest that more preventive action may be necessary.
Existing treatment
There has been a great expansion of antifungal agents against Candida spp. bloodstream infections over the past two decades, which now include three major classes of drugs: 1) Triazoles (fluconazole, voriconazole), 2) echinocandins (caspofungin, micafungin, anidulafungin) and 3) polyenes (amphotericin B deoxycholate [AmB-d], liposomal amphotericin B [L-AmB], amphotericin B lipid complex [ABLC], and amphotericin B colloidal dispersion [ABCD]) (Table 1). The Clinical and Laboratory Standards Institute (CLSI) has developed methodology for susceptibility testing of Candida species to fluconazole, itraconazole, voriconazole, flucytosine, and the echinocandins, which are increasingly used to manage the treatment of candidemia [17,18].
Table 1.
Currently available antifungal agents for the treatment of candidemia.
| Name | Class | Mechanism of action | Company | FDA approved indications |
|---|---|---|---|---|
| Fluconazole | Antifungal triazole | Inhibitor of lanosterol 14α-demethylase, leading to inhibition of ergosterol biosynthesis | Pfizer / various |
|
| Voriconazole | Antifungal triazole | Pfizer / various |
|
|
| Anidulafungin | Echinocandin | Inhibitor of (1→3)-β-D-glucan synthase | Pfizer |
|
| Caspofungin | Echinocandin | Merck |
|
|
| Micafungin | Echinocandin | Astellas Pharma US Inc. |
|
|
| Amphotericin B deoxycholate | Polyene | Binds ergosterol in the cell membrane leading to changes in membrane permeability | X-GEN Pharmaceuticals Inc. |
|
| Amphotericin B lipid complex | Polyene | Sigma-Tau Pharmaceuticals Inc. |
|
|
| Liposomal amphotericin B | Polyene | Astellas Pharma US Inc. |
|
Factors to consider when choosing an antifungal agent for the treatment of candidemia include prior antifungal exposure, intolerance to an antifungal agent, current epidemiology and susceptibility patterns, severity of illness, comorbidities, and involvement of the central nervous system, heart valves, or other organ systems [19].
For the treatment of candidemia in adult nonneutropenic patients, the Infectious Diseases Society of America (IDSA) Clinical Practice Guidelines suggest initial therapy with fluconazole 800 mg [12 mg/kg] IV x 1, then 400 mg [6 mg/kg] IV daily or an echinocandin including caspofungin 70 mg IV x 1, then 50 mg IV daily, micafungin 100 mg IV daily, or anidulafungin 200 mg IV x 1 then 100 mg IV daily for two weeks after clearance of Candida spp. from the blood [19]. For patients who are not critically ill and have had no recent azole exposure, fluconazole is recommended as initial therapy. In contrast, an echinocandin is recommended in patients with moderately severe to severe illness or with recent azole exposure.
Because of varying susceptibilities of clinical isolates, special attention must be paid to the species of Candida being treated. For candidemia due to C. glabrata or C. krusei, an echinocandin is preferred for initial therapy; for C. albicans or C. parapsilosis, fluconazole is recommended. AmB-d 0.5–1 mg/kg IV daily or a lipid formulation of amphotericin B 3–5 mg/kg IV daily are alternative antifungal agents if a patient is intolerant to-or there is limited availability of other drugs. Current IDSA guidelines recommend two weeks of therapy for candidemia without persistent fungemia or metastatic complication after clearance of Candida from the blood and resolution of symptoms of candidemia.
In neutropenic patients, candidemia may be life-threatening and was historically treated with AmB [20]. Fluconazole is often used for prophylaxis to prevent candidemia in neutropenic patients, which has led to a decreased role for treatment of candidemia in these patients [21]. The availability of echinocandins and antifungal triazoles such as voriconazole have resulted in increased use in neutropenic patients with candidemia and an echinocandin is now recommended for initial therapy [19]. It should be noted, however, that this recommendation comes from information from single-arm studies or from subset data from randomized controlled trials [19].
Scientific rationale: targets of antifungal agents
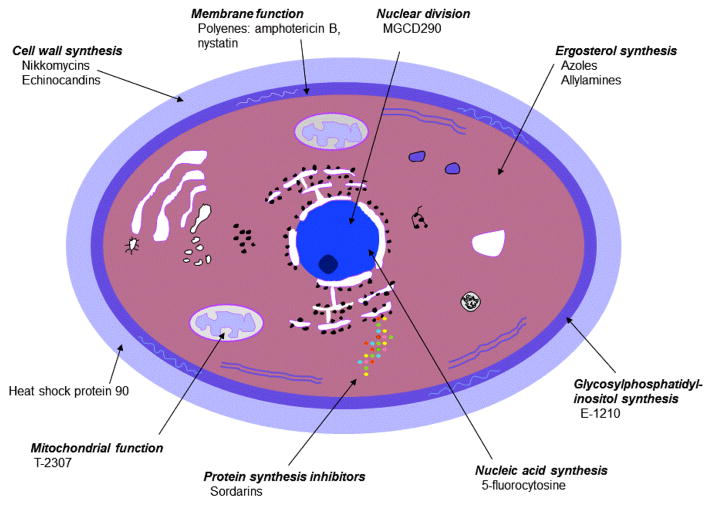
The cell of Candida spp. is composed of a protective outer cell wall surrounding an inner cell membrane. Components of the cell wall include (1→6)-β-D-glucan-branched (1→3)-β-D-glucan cross linked to chitin, branched (1→6)-β-D-glucan, and mannoproteins [22,23]. The cell wall functions as a barrier to protect the cell from the outside environment, is involved in adhesion to surfaces, and biofilm formation [23]. The main component of the cell membrane is ergosterol, which is involved in regulation of membrane fluidity, asymmetry, and integrity [22,24].
Figure 1 illustrates the site of action of selected antifungal agents [25]. The majority of available antifungal agents target the cell wall by inhibiting (1→3)-β-D-glucan synthase (echinocandins) or target the cell membrane by binding to ergosterol (amphotericin) or inhibiting ergosterol biosynthesis (antifungal azoles).
Figure 1.
Site of action in Candida spp. of selected antifungal agents.1
1Modified from figure by Georgopapadakou NH and Walsh TJ [25]
Novel targets of investigational drugs include glycosylphosphatidylinositol (GPI) biosynthesis, histone deacetylases, and heat shock proteins. Cell wall proteins may contain a GPI anchor, which anchors the protein to the plasma membrane [23]. Inhibiting GPI biosynthesis results in inhibition of fungal cell growth, hyphal elongation, and adhesion [26]. Histone deacetylases are enzymes involved in the regulation of chromatin structure and transcription through lysine deacetylation of histones [27,28]. They are involved in gene regulation, cell proliferation, cell death, and motility [28]. Heat shock protein 90 (HSP 90) is located on the cell wall, aiding the pathogen’s response to stressful stimuli, such as antifungal agents, by promoting formation of resistance mechanisms [29–31]. It also stimulates the release of endothelial nitric oxide and bradykinin, potentially contributing to symptoms associated with sepsis [32–35].
Investigational antifungal agents
Emerging antifungal agents currently in clinical trials for Candida spp. infections (Table 2) as well as selected antifungal agents no longer in development are reviewed below. While many compounds are not currently being evaluated in the treatment of candidemia or are not in active clinical development, they are included in this review as a proof of concept with regard to mechanism of action, and with the hope that investigation of promising compounds will be renewed in the future.
Table 2.
Investigational antifungal agents currently in clinical trials for Candida spp. infections.
| Compound | Company | Structure | Indication | Stage of development | Mechanism of action |
|---|---|---|---|---|---|
| Isavuconazole | Originator: Basilea Pharmaceutica Licensee: Astellas |
Antifungal triazole | Candidemia, other invasive Candida spp. infections, Aspergillus infection, Mucormycosis infection | Phase 3 | Inhibitor of lanosterol 14α-demethylase |
| Albaconazole | Originator: Palau Pharma Licensee: Actavis |
Antifungal triazole | Vulvovaginal candidiasis, tinea pedis, onychomycosis | Phase 2 | Inhibitor of lanosterol 14α-demethylase |
| MK-3118/ SCY-078 | Originator :Scynexis | Derivative of enfumafungin (hemiacetal triterpene glycoside) | Phase 1 | Inhibitor of (1→3)-β-D- glucan synthase | |
| VT-1161 | Viamet | Not in public domain | Onychomycosis, candidiasis | Phase 2 | Inhibitor of lanosterol demethylase (CYP51) |
| T-2307 | Toyama | Arylamidine | Phase 1 | Impairment of mitochondrial function |
Isavuconazole
Isavuconazonium (BAL-8557) is a water soluble prodrug of the antifungal triazole isavuconazole (BAL-4815) [36], which is being developed by Astellas Pharma in collaboration with Basilea Pharmaceutica for the treatment of invasive fungal infections including candidiasis and aspergillosis. Isavuconazonium has been granted an application under orphan drug designation by the FDA for treatment of invasive aspergillosis and mucormycosis. It inhibits ergosterol biosynthesis by inhibiting lanosterol 14α-demethylase [36]. BAL-8557 is converted by plasma esterases to isavuconazole and BAL-8728 (prodrug cleavage product) [36,37]; the conversion is rapid and complete with >98% converted to isavuconazole [37]. Advantages of BAL-8557 are that a cyclodextrin solubilizer is not required due to its water solubility [38], an oral dosage form is available, unlike the echinocandins, and it may be better tolerated than voriconazole.
Several in vitro and in vivo studies indicate that isavuconazole is active against Candida spp. [39–44]. Seifert and colleagues reported that isavuconazole was active in vitro against C. albicans, C. glabrata, C. krusei, C. parapsilosis, and C. tropicalis (Table 3) [44]. Isavuconazole was also active against C. guilliermondii and C. lusitaniae [42].
Table 3.
Summary of in vitro activity of investigational agents against Candida spp.
| Candida spp. | N1 | Antifungal agent | MIC50 (mg/L) | MIC90 (mg/L) | MIC range (mg/L) | Reference |
|---|---|---|---|---|---|---|
| C. albicans | 166 | isavuconazole | 0.002 | 0.004 | 0.0005 to 0.016 | [44] |
| 70 | ≤0.015 | 0.03 | ≤0.015 to 2 | [43] | ||
| 33 | 0.0007 | 0.0013 | <0.0004 to 0.0026 | [42] | ||
| 42 | 0.015 | 0.015 | ≤0.008 to 0.03 | [39] | ||
| 131 | albaconazole | ≤0.0002 | ≤0.0002 | ≤0.0002 to 0.03 | [51] | |
| 26 | 0.125 | 1 | 0.015 to 2 | [50] | ||
| 11 | MGCD290 | 4 | 16 | 0.5 to 16 | [28] | |
| 29 | MK-3118/ SCY-078 | 0.12 | 1 | 0.06 to 2 | [60] | |
| 25 | VT-1161 | ≤ 0.03 | ≤ 0.03 | ≤ 0.03 to >16 | [67] | |
| 40 | T-2307 | 0.0005 | 0.002 | 0.00025 to 0.0039 | [73] | |
| 291 | aminocandin | 0.25 | 0.25 | 0.06 to 0.5 | [92] | |
| 21 | E-1210 | 0.015 | 0.06 | 0.008 to 0.12 | [26] | |
| 52 | ≤0.008 | ≤0.008 | ≤0.008 to 0.016 | [101] | ||
| C. glabrata | 46 | isavuconazole | 0.25 | 0.5 | 0.063 to 4 | [44] |
| 37 | 0.25 | 1 | ≤0.015 to 2 | [43] | ||
| 25 | 0.0039 | 0.02 | 0.0011 to 0.64 | [42] | ||
| 25 | 0.5 | 1 | 0.12 to 8 | [39] | ||
| 34 | albaconazole | 0.06 | 0.12 | ≤0.0002 to 1 | [51] | |
| 17 | 0.25 | 1 | 0.25 to 8 | [50] | ||
| 14 | MGCD290 | 2 | 4 | 0.5 to 4 | [28] | |
| 29 | MK-3118/ SCY-078 | 0.5 | 2 | 0.5 to 2 | [60] | |
| 25 | VT-1161 | 0.06 | 0.25 | ≤0.03 to 2 | [67] | |
| 25 | T-2307 | 0.0039 | 0.0078 | 0.0039 to 0.0078 | [73] | |
| 67 | aminocandin | 0.25 | 0.25 | 0.12 to 0.5 | [92] | |
| 20 | E-1210 | 0.03 | 0.06 | ≤0.008 to 0.25 | [26] | |
| 44 | 0.06 | 0.06 | ≤0.008 to 0.06 | [101] | ||
| C. krusei | 11 | isavuconazole | 0.5 | 0.5 | 0.25 to 0.5 | [44] |
| 10 | 0.0041 | 0.011 | [42] | |||
| 8 | 0.25 | 0.25 to 0.5 | [39] | |||
| 12 | albaconazole | 0.015 | 0.06 | ≤0.0002 to 0.06 | [51] | |
| 5 | 0.25 | 0.25 | 0.06 to 0.25 | [50] | ||
| 5 | MGCD290 | 4 | 2 to 8 | [28] | ||
| 19 | MK-3118/ SCY-078 | 0.5 | 2 | 0.5 to 2 | [60] | |
| 16 | T-2307 | 0.001 | 0.002 | 0.0005 to 0.002 | [73] | |
| 25 | aminocandin | 0.12 | 0.5 | 0.03 to 4 | [93] | |
| 4 | E-1210 | 2 to > 32 | [101] | |||
| C. parapsilosis | 23 | isavuconazole | 0.016 | 0.031 | 0.004 to 0.25 | [44] |
| 84 | ≤0.015 | 0.03 | ≤0.015 to 0.125 | [43] | ||
| 17 | 0.0015 | 0.011 | <0.0004 to 0.017 | [42] | ||
| 22 | 0.03 | 0.06 | ≤0.008 to 0.06 | [39] | ||
| 62 | albaconazole | ≤ 0.0002 | ≤ 0.0002 | ≤ 0.0002 to 0.25 | [51] | |
| 15 | MK-3118/ SCY-078 | 0.25 | 0.5 | 0.25 to 1 | [60] | |
| 10 | VT-1161 | <0.03 | <0.03 | [67] | ||
| 20 | T-2307 | 0.0005 | 0.0005 | 0.00025 to 0.002 | [73] | |
| 14 | aminocandin | 1 | 2 | 1 to 2 | [92] | |
| 25 | 1 | 2 | 0.03 to 2 | [93] | ||
| 25 | E-1210 | 0.03 | 0.06 | ≤0.008 to 0.5 | [26] | |
| 26 | ≤0.008 | 0.016 | ≤0.008 to 0.016 | [101] | ||
| C. tropicalis | 25 | isavuconazole | 0.031 | 0.063 | 0.004 to 0.25 | [44] |
| 15 | ≤0.015 | 0.06 | ≤0.015 to 0.125 | [43] | ||
| 24 | 0.0046 | 0.008 | <0.0004 to 0.0094 | [42] | ||
| 14 | 0.06 | 0.5 | 0.03 to 0.5 | [39] | ||
| 36 | albaconazole | ≤ 0.0002 | 0.03 | ≤ 0.0002 to 64 | [51] | |
| 21 | MK-3118/ SCY-078 | 0.25 | 1 | 0.06 to 2 | [60] | |
| 20 | T-2307 | 0.0005 | 0.0005 | 0.00025 to 0.0005 | [73] | |
| 22 | aminocandin | 0.25 | 0.25 | 0.06 to 0.5 | [92] | |
| 25 | 0.25 | 1 | 0.06 to 2 | [93] | ||
| 24 | E-1210 | 0.03 | 0.06 | 0.008 to 0.06 | [26] | |
| 23 | 0.016 | 0.03 | ≤0.008 to 0.03 | [101] | ||
| C. guilliermondii | 15 | isavuconazole | 0.051 | 0.11 | 0.0069 to 0.18 | [42] |
| 8 | albaconazole | ≤ 0.0002 | ≤ 0.0002 | ≤ 0.0002 | [51] | |
| 17 | T-2307 | 0.002 | 0.0039 | 0.001 to 0.0039 | [73] | |
| 25 | aminocandin | 0.5 | 1 | 0.12 to 1 | [93] | |
| C. lusitaniae | 17 | isavuconazole | 0.00059 | 0.0024 | <0.0004 to 0.0025 | [42] |
Number of isolates.
Limited available data suggest that isavuconazole increased survival and decreased tissue burden in a C. albicans mouse model of disseminated candidiasis [41]. Isavuconazole was active against C. tropicalis but less active against C. krusei in a disseminated murine model of candidiasis [40]. Four days post infection in temporarily neutropenic mice infected with C. tropicalis, isavuconazole 60 mg/kg PO BID and 90 mg/kg PO BID reduced kidney fungal burden by 2.29 log10 CFU/g and 2.23 log10 CFU/g, respectively, compared to controls (p<0.001). Furthermore, in persistently neutropenic mice infected with C. tropicalis, isavuconazole 120 mg/kg PO BID reduced kidney fungal burden by 2.15 log10 CFU/g and 3.01 log10 CFU/g compared to controls 4 and 7 days post infection (p<0.001). Additionally, in persistently neutropenic mice infected with C. krusei, isavuconazole 90 mg/kg PO BID and 150 mg/kg PO BID decreased kidney fungal burden by 1.53 log10 CFU/g and 1.69 log10 CFU/g compared to controls (p<0.0001). Brain fungal burden of C. krusei in mice treated with isavuconazole 120 mg/kg PO BID (temporarily neutropenic) and 150 mg/kg PO BID (persistently neutropenic) significantly decreased by 1.21 log10 CFU/g and 0.84 log10 CFU/g, respectively, compared to controls.
The pharmacodynamic parameter associated with isavuconazole efficacy in a C. albicans murine model of disseminated candidiasis was the AUC/MIC ratio [45]. The postantifungal effect in vitro in a C. albicans strain exposed to isavuconazole for three hours was 2 hours at concentrations 2 X MIC, 5 hours at concentrations 5 to 100 X MIC, but not seen at concentrations below the MIC [45].
Two phase 1 clinical trials (single and multiple dose studies) of IV and oral BAL-8557 in healthy volunteers and one phase 1 clinical trial in patients with mild to moderate liver disease have been published [37,46,47]. The pharmacokinetic parameters of IV isavuconazole in healthy volunteers included a half-life of 76.2 to 104 hours [37], a dose proportional AUC, a clearance of 3.19 to 4.06 L/hour, and a Vd of 470 to 542 L [46]. The isavuconazole half-life was increased and clearance was decreased in patients with mild to moderate liver disease due to alcoholic cirrhosis [47]. After a single IV dose of BAL-8557 180.5 mg, the isavuconazole half-life and clearance were 302 hours and 1.43 L/hour, respectively, in patients with moderate liver disease compared to 123 hours and 2.73 L/hour, respectively, in healthy volunteers.
Adverse events observed in the multiple dose phase 1 trial were dose dependent [46]. In the isavuconazole 50 mg/day group there were 18 adverse events and in the isavuconazole 100 mg/day group there were 21 adverse events. One serious adverse event, rhinitis, was reported in the high dose group but was not thought to be related to isavuconazole. Mild to moderate adverse events in the high dose group included headache (5), rhinitis (3), nasopharyngitis (3), abnormal liver function test (1), back pain (1), pharyngolaryngeal pain (1), abdominal pain (1), gingivitis (1), and fatigue (1). Mild to moderate adverse events in the low dose group included nasopharyngitis (4), headache (2), rhinitis (2), back pain (1), pain in extremity (1), dizziness (1), cough (1), abdominal pain (1), diarrhea (1), and fatigue (1).
Isavuconazole is currently being studied in a Phase 3 clinical trial for the treatment of candidemia and other invasive Candida infections (NCT00413218).
Albaconazole
Albaconazole (UR-9825) is an orally and topically administered antifungal triazole being developed by Actavis. As with other antifungal triazoles, albaconazole inhibits ergosterol biosynthesis by inhibiting lanosterol 14α-demethylase [48]. Phase 2 clinical trials have been conducted with albaconazole for vulvovaginal candidiasis, tinea pedis and toenail onychomycosis [49].
While albaconazole is being targeted for dermatophyte infections, it is active in vitro and in vivo against Candida spp. [50–52]. Ramos and colleagues reported that albaconazole was active in vitro against Candida spp., including C. albicans, C. parapsilosis, C. tropicalis, C. glabrata, C. krusei, and C. guilliermondii (Table 3) [51]. However, albaconazole MICs were increased in isolates with a fluconazole MIC ≥ 16 mg/L, including C. albicans (MIC50 ≤ 0.0002 mg/L, MIC90 0.03 mg/L), C. tropicalis (MIC50 ≥ 8 mg/L, MIC90 64 mg/L), C. glabrata (MIC50 0.06 mg/L, MIC90 1 mg/L) and C. krusei (MIC50 ≤ 0.0002 mg/L, MIC90 0.06 mg/L).
In a rabbit model of disseminated candidiasis, albaconazole (0.5 mg/kg/day PO) significantly decreased C. albicans CFU in kidneys and lungs compared to controls [52]. In the albaconazole (0.5 mg/kg/day PO) and control groups, CFU in kidneys and lungs were 0.68 log CFU/g and 0.39 log CFU/g and 4.48 log CFU/g and 3.04 log CFU/g, respectively.
MGCD290
Shared targets for histone deacetylase inhibitors are similar for cancer and Candida spp. therapy. The histone deacetylase inhibitor 4-sodium phenylbutyrate was being evaluated by Lunamed for cervical intraepithelial neoplasia and chronic candidiasis in a terminated Phase 2 clinical trial. MGCD290 was a Hos2 fungal histone deacetylase inhibitor being developed by Mirati Therapeutics [28]. While it was targeted for the treatment of vulvovaginal candidiasis, a recent Phase 2 clinical trial failed to show a significant benefit of oral MGCD290 combined with fluconazole compared to fluconazole alone [53].
MGCD290 was only moderately active in vitro against Candida spp., including C. albicans, C. glabrata, and C. krusei [28]. However, MGCD290 potentiates the effect of antifungal triazoles [28]. With Candida spp. that were not susceptible to fluconazole, the combination of MGCD290 and fluconazole resulted in a reduction of fluconazole MIC. Furthermore, the combination of MGCD290 and fluconazole was synergistic in 26 out of 30 Candida spp. isolates. Limited available in vivo data also indicated that the combination of fluconazole and MGCD290 increased survival and decreased kidney fungal load compared to fluconazole alone in a mouse model of disseminated candidiasis [54,55].
Four Phase 1 single and multiple ascending dose studies of oral MGCD290 with or without fluconazole have been presented as conference abstracts [56–58]. Limited available data suggest a mean half-life of 10 to 11 hours and an AUC that is dose proportional in humans. No significant adverse effects were observed in these studies.
MK-3118/SCY-078
MK-3118 is an oral and parenteral semi-synthetic enfumafungin derivative being developed by Scynexis Inc. [59–60]. It acts as a specific inhibitor of (1→3)-β-D-glucan synthase, preventing appropriate cell wall formation [59,61,62]. In an in vitro study of 113 clinical isolates of Candida spp. tested by CLSI broth microdilution, MK-3118 and caspofungin showed similar MICs against C. parapsilosis (MIC90 0.5 mg/L, for both), C. tropicalis (MIC90 1 mg/L, for both), C. albicans (MIC90 1 mg/L versus 2 mg/L, respectively), and C. krusei (MIC90 2 mg/L versus 1 mg/L, respectively); it showed lower MICs than caspofungin against C. glabrata (MIC90 2 mg/L versus 16 mg/L, respectively) [60]. MK-3118 was shown to be active (with an MIC of ≤ 1 mg/L) against 32 out of 34 fluconazole-resistant isolates and was also active (with an MIC of ≤ 1 mg/L) against 22 out of 31 isolates with mutations associated with echinocandin resistance; in contrast, caspofungin was only active against 4 out of 31 such isolates.
MK-3118 was evaluated in a complement-deficient murine model [63]. Mice infected with a strain of C. albicans were treated with MK-3118 12.5 mg/kg PO BID for 7 days, resulting in a 4.7-log reduction in CFU/g of kidney at the end of treatment compared with control mice. In mice infected with C. tropicalis, MK-3118 25 mg/kg PO BID resulted in a 2.6 to 3.8 log reduction in CFU/g of kidney compared to control.
Two Phase 1 randomized, double-blinded, placebo-controlled ascending dose escalation studies in healthy volunteers have also been conducted, in which subjects received single or multiple doses of MK-3118 from 10 mg to 1600 mg or 300 mg to 800 mg once daily for 28 days, respectively [64,65]. Limited available data suggest a harmonic mean half-life of approximately 20 hours and an AUC that is dose-proportional in humans [65]. MK-3118 was generally well tolerated [64,65]. The most common adverse effects were diarrhea/loose stools, abdominal pain/cramps, and headache [64,65]. Gastrointestinal complaints were most notable at higher doses (≥600 mg daily) and on the first day of therapy [64].
VT-1161
Viamet Pharmaceuticals is developing several metalloenzyme inhibitors of lanosterol demethylase (CYP51) including VT-1598, VT-1129, and VT-1161 [66]. While oral VT-1129 is in preclinical development targeted for cryptococcal meningitis, it is also active in vitro and in vivo against Candida spp. [67–69]. Oral VT-1161 is currently in Phase 2 clinical trials targeted for onychomycosis and candidiasis.
VT-1161 is active in vitro and in vivo against Candida spp. [67–71]. It was active in vitro against C. albicans, C. glabrata, and C. parapsilosis (Table 3) [67]. In C. albicans isolates with reduced susceptibility to fluconazole (MIC 32 to > 64 mg/L), the VT-1161 MIC ranged from ≤ 0.03 to 0.5 mg/L [69]. However, in C. albicans isolates that were resistant to fluconazole (MIC > 64 mg/L), the VT-1161 MIC ranged from 0.25 to > 16 mg/L [67].
VT-1161 has demonstrated activity in several murine models of candidiasis [68,70,71]. Hoekstra and colleagues reported that VT-1161 significantly increased survival in mice infected with a strain of C. albicans that was susceptible to fluconazole (MIC50 2 mg/L) [68]. The percent survival with VT-1161 5, 10, and 20 mg/kg/day PO ranged from 90 to 100% compared to 0% in controls (p=0.0001). Kidney fungal burden with VT-1161 10 and 20 mg/kg/day PO ranged from 1.7 to 2.1 log CFU, compared with 5.7 log CFU with fluconazole 10 mg/kg/day. In mice infected with C. albicans with decreased susceptibility to fluconazole (MIC 32 mg/L), the percent survival with VT-1161 at doses ≥ 2.5 mg/kg/day PO was ≥90% compared to 10% (p ≤ 0.01) survival in control mice who received vehicle [70]. Kidney fungal burden with VT-1161 dosed at ≥ 2.5 mg/kg/day PO was 3.45 to 4.03 log10 CFU/g and with controls was 6.2 log10 CFU/g (p<0.001). VT-1161 also increased survival and decreased kidney fungal burden in a C. glabrata murine infection model [71].
T-2307
T-2307 is an injectable arylamidine being developed by Toyama currently in Phase 1 clinical trials. The mechanism of action in yeast involves impairment of mitochrondrial function [72]. T-2307 is active in vitro against Candida spp. including C. albicans, C. glabrata, C. krusei, C. parapsilosis, C. tropicalis, and C. guilliermondii (Table 3) [73]. It was also active against C. albicans strains that were not susceptible to fluconazole (MIC 16 to > 64 mg/L), with T-2307 MIC ranging from 0.0005 to 0.001 mg/L. In addition, in a C. albicans mouse model of disseminated candidiasis, T-2307 0.01 mg/kg/day and 0.02 mg/kg/day subcutaneously significantly increased survival compared to controls [73]. In a C. glabrata murine model of disseminated candidiasis, kidney fungal burden in the T-2307 0.05 mg/kg/day, 0.1 mg/kg/day, and control groups were 4 log CFU/ kidneys, 3.86 log CFU/ kidneys, and 5.26 log CFU/ kidneys, respectively (p<0.05) [74].
Investigational antifungal vaccines
A Candida vaccine may be a useful preventive strategy to avoid the morbidity, mortality, and cost associated with Candida spp. infections. Possible indications include prevention of vaginal candidiasis, denture stomatitis, oral candidiasis, and candidiasis in immunocompromised patients [75]. The rationale and strategies in Candida vaccine development has been recently reviewed [75,76]. Currently, there are no commercial vaccines for the prevention of Candida spp. infections. Emerging Candida vaccines in development are briefly reviewed below.
NDV-3 (rAls3p-N)
NDV-3 is a prophylactic recombinant vaccine with an Alhydrogel® adjuvant against the N-terminal region of the agglutinin-like sequence 3 protein (Als3p) being developed by NovaDigm Therapeutics [77,78]. Als3p is an adhesin and invasin protein, with a broad array of substrate affinity, allowing Candida spp. to bind to each other, as well as host epithelial and endothelial surfaces [77–83]. Because of colonization and/or previous infection, the majority of healthy individuals have detectable anti-Als3p antibodies [77,84]. This priming facilitates strong B-cell and T-cell responses upon vaccination, which promote opsonophagocytic killing and block fungal adherence [77,78,83,85]. Aside from its potential use in Candida infections, it has also shown activity against S. aureus [77,78].
In a murine model of disseminated candidiasis, mice were immunized with a subcutaneous injection of rAls3p-N with Freund’s adjuvant at day 0, and a booster at day 2 [78]. Mice were subsequently infected with C. albicans two weeks following the final dose. Serum antibody titers and animal survival were evaluated. Antibody titers in vaccinated mice were significantly higher compared to titers in unvaccinated mice. Survival at day 28 was also improved with vaccination. However, antibody titers in vaccinated mice did not significantly correlate with survival.
rAls3p-N with an Alhydrogel® adjuvant also increased survival and antibody titers compared to control mice in a model of disseminated candidiasis [85]. In IFN-γ deficient mice, vaccination only marginally improved survival. However, IFN-γ deficient mice who received CD4+ lymphocytes from vaccinated wild type mice had improved survival, suggesting that vaccine efficacy is dependent upon Type 1 immunity [83,85]. Additionally, gp91phox-/- deficient mice (which have defective phagocytes) and IL-17A deficient mice (Th17 cells are known to act by recruiting phagocytes) also were not protected by vaccination, indicating that functional phagocytes are also necessary to mediate vaccine efficacy [83]. Nonetheless, an IgG titer cut-off of ≥1:6400 was found to have sensitivity of 84% and specificity of 86% for protection from infection, suggesting that despite cell-mediated immunity, antibody titer thresholds may serve as a potential surrogate marker [85].
A Phase 1 double-blinded, placebo-controlled ascending dose escalation study in healthy volunteers (n = 40) has also been conducted, in which subjects received a single dose of NDV-3 30 mcg or 300 mcg on day 0; approximately 60% of subjects received a second dose at day 180 [77]. Plasma antibody titers (IgG and IgA1) significantly rose following vaccination, compared with placebo (p < 0.001). Based on seroconversion, defined as ≥ 4-fold rise in antibody titer over baseline, a single dose of 30 mcg or 300 mcg resulted in seroconversion rates of 13% and 53%, respectively, by day 7, and 100% seroconversion in both groups by day 14. Revaccination resulted in a further increase in antibody titers. Maximal response rates with IFN-γ and IL-17A producing PBMCs were seen at day 28 in the 30 mcg group, and seen earlier, at day 7, in the 300 mcg group. Revaccination increased these response rates further.
rAls3p-N was generally well-tolerated [77]. The most common adverse events was mild injection site pain, which resolved within 2–3 days following vaccination. Common systemic adverse events (not dose-dependent) included fatigue and headache, were usually mild or moderate, and all resolved within a few days [77].
rHyr1p-N
rHyr1p-N is a prophylactic recombinant vaccine against the N-terminal region of Hyr1p being developed by NovaDigm Therapeutics [86,87]. Hyr1 encodes glycosylphosphatidylinositol, a protein expressed on the cell surface of C. albicans and non-albicans Candida spp., mediating resistance to opsonophagocytic killing [86–89]. Direct vaccination results in the development of neutralizing antibodies, allowing for protection via development of active immunity; these neutralizing antibodies have also been shown to provide protection via passive immunity [87].
In a disseminated candidiasis murine model, mice were vaccinated with 2 subcutaneous injections of rHyr1p-N at day 0 and day 21 [86]. Mice were subsequently infected with C. albicans at day 35. Survival was significantly improved with vaccination with rHyr1p-N (Freunds or Alhydrogel® adjuvant) compared to controls at 35 days post-infection (60–100% versus 0%).
Furthermore, in a C. albicans mouse model of disseminated candidiasis vaccination with rHyr1p-N with an Alhydrogel® adjuvant resulted in increased survival and an approximately 1 log10 decrease in CFU/g of kidney 3 days post-infection compared to control mice [87]. Vaccinated mice infected with non-albicans Candida spp. also demonstrated a 0.65 to 1.69 log10 reduction in CFU/g of kidney compared to controls. Passive immunization with rabbit anti-Hyr1p antibodies (1 mg or 3 mg) given 2 hours prior to C. albicans infection was protective in mice up to 28 days post-infection (survival was 30 – 40% in vaccinated mice versus 0% in the control group, p = 0.001). rHyr1p-N was also evaluated in a neutropenic murine model. Following vaccination, mice were made neutropenic with cyclophosphamide and subsequently infected with C. albicans. Vaccination resulted in a 1.5 log10 reduction in CFU/g of kidney 10 days post-infection compared with control mice (p = 0.002). The survival rate at 28 days post-infection also significantly improved. All mice in the control group died before day 14; however, 20% of vaccinated mice were alive 28 days post-infection (p = 0.007).
Investigational antifungal agents and vaccines no longer in development
Aminocandin
Aminocandin was an echinocandin antifungal agent being developed by Novexel/AstraZeneca. It was a noncompetitive inhibitor of (1→3)-β-D glucan synthase [90]. Aminocandin was a promising antifungal agent, with its long half-life of 48 to 58 hours [91] it had a potentially useful role as long term antifungal therapy in the ambulatory setting. As with other echinocandins, aminocandin was active in vitro against C. albicans, C. glabrata, C. tropicalis, and C. krusei (Table 3) [92,93]. However, aminocandin MICs were increased with C. parapsilosis and C. guilliermondii [92,93]. Aminocandin was also active in vivo against C. albicans, C. tropicalis, and C. glabrata in murine infection models [90,94–96]. The pharmacodynamic parameter that correlated with aminocandin efficacy in a C. albicans murine model of disseminated candidiasis was the peak/MIC ratio [97]. Fungistatic activity in vivo occurred at a peak/MIC ratio of 3.72 with maximum killing at a peak/MIC ratio of 10.
E-1210
E-1210 was a novel, broad spectrum antifungal agent being developed by Eisai. It had a unique mechanism of action as an inhibitor of GPI biosynthesis [98]. Only preclinical information has been presented and published. Chemical, in vivo pharmacokinetic, and microbiological studies of E-1211, a water soluble prodrug of E-1210, have also been presented as conference abstracts [99,100]. E-1210 was active in vitro against several Candida spp., including C. albicans, C. glabrata, C. parapsilosis, and C. tropicalis (Table 3) [26]. However, it had poor activity in vitro against C. krusei with MIC values of 2 to > 32 mg/L [26,101]. E-1210 was also active in vitro against Candida spp. that were resistant to fluconazole and caspofungin [26]. In mouse models of disseminated candidiasis, E-1210 significantly increased survival of mice infected with C. albicans and C. tropicalis [102]. Pharmacodynamic parameters that correlated with the efficacy of E-1210 in a murine model of disseminated candidiasis were AUC/MIC and T>MIC [103]. In a time kill assay E-1210 was fungistatic with maximum activity at 8 X MIC against a strain of C. albicans [101].
Efungumab
Efungumab was a recombinant monoclonal antibody being developed by Novartis Pharmaceuticals [29,32,104,105]. It was derived from anti-HSP 90 antibody cDNA of patients who recovered from deep-seated, invasive candidiasis. Efungumab binds to the middle region of HSP 90, preventing communication between the terminal regions, hence preventing necessary conformational changes [29]. In addition to promising data from preclinical investigations and patient case reports with efungumab, a randomized, double-blinded trial in patients with culture-confirmed invasive candidiasis, compared liposomal amphotericin B or amphotericin B lipid complex alone with liposomal amphotericin B or amphotericin B lipid complex in combination with efungumab [32–34,105–108]. The primary outcome, the composite of complete clinical response and mycological clearance on day 10, favored combination therapy (84% vs. 48%, p <0.001). The rate of mycological clearance was more than twice as fast with efungumab and mycological resolution was achieved in 89% of the combination therapy group compared with 54% in the amphotericin group at day 10 (p < 0.001).
Efungumab was generally well tolerated. Safety concerns centered on a cytokine release syndrome characterized by hypertension, nausea, and vomiting [29,33,34,104]. Unfortunately, additional concerns regarding structural inconsistencies between manufactured batches, possibly due to an unpaired cysteine at position 28, also remained [104]. Despite these promising clinical results, development of this product was abandoned in 2010. Nonetheless, the concept of HSP-90 inhibition holds promise as a novel strategy to combat invasive candidiasis.
Sordarins
Sordarin is a natural product isolated from Sordaria araneosa [109,110]. Since its discovery, several structurally related compounds have been isolated and/or developed [110–113]. Sordarin, and its derivatives, bind to elongation factor 2 (eEF2), stabilizing the eEF2/ribosome complex via a likely additional interaction with ribosomal subunit protein (rpP0), and preventing the final step of translocation in protein synthesis [109–114]. Despite eEF2 being highly conserved among eukaryotes, sordarin and its derivatives only interact with a very specific region of eEF2, which is unique to fungal species, particularly Candida spp. [109–111,113–115]. Sordarin derivatives have been evaluated in murine models of disseminated candidiasis [112,113,116]. While these agents have not been tested in humans to date, low cytotoxicity has been reported for some compounds [111].
MAb B6.1
MAb B6.1 was a prophylactic and therapeutic IgM-monoclonal antibody that specifically targeted (1→2)-β-mannotriose; it was formerly under development by LigoCyte Pharmaceuticals [117–122]. (1→2)-β-mannotriose is an acid-labile component of the phospholipomannan complex, expressed uniformly on the surface of C. albicans and other pathogenic non-albicans Candida species, which displays adhesion [117,118,120,121,123,124]. The vaccine facilitates opsonophagocytic killing via the classical pathway of the complement system [117–123,125]. By using B6.1 in combination with conventional antifungal agents, which damage cell wall structure, B6.1 may be able to more efficiently bind its target [117,123]. Simultaneously, B6.1 interferes with the binding ability of adhesin, and may allow conventional antifungal agents to exert their full effects [123].
In a disseminated candidiasis murine model, mice were infected with C. albicans and subsequently treated with a single dose of B6.1 intraperitoneally one hour later, resulting in a 28% reduction in CFU/g of kidney at 48 hours after infection compared with control mice [123]. Mean survival time was significantly increased from 9.2 +/− 3.4 days in control mice to 30.2±16.9 days with amphotericin B 0.5 mg/kg/B6.1 combination therapy (p < 0.001). In addition, in a mouse model of disseminated candidiasis, B6.1 was given in combination with fluconazole 0.8 mg/kg, which resulted in a 70% reduction in CFU/g at 48 hours after infection compared with control mice (p < 0.05) [117]. Mean survival time was 9.2 ± 3.3 days in control mice and 33.0±16.6 days with fluconazole 0.8 mg/kg/B6.1 combination therapy (p < 0.05). B6.1 was also evaluated in a C. albicans neutropenic murine model, in which vaccination resulted in a significant reduction in CFU/g of kidney at 48 hours post-infection compared with control mice (p < 0.01) [121]. Furthermore, mean survival time in mice with prolonged neutropenia was also significantly increased from 5.2 days to 12.3 days if mice continued to receive B6.1 every other day following infection.
Potential development challenges
There has been an alarming decline in research, development, and FDA approval of new antimicrobial agents. A study by Spellberg and colleagues indicated that from 1983 to 1987 compared to 1998 to 2002, there was a 56% decrease in new antibacterial agents approved by the FDA [126]. A recent update on the progress of the IDSA 10 x ′20 initiative suggests a continued decline of antibacterial drugs approved by the FDA, with five drugs approved from 2003 to 2007 and two drugs approved from 2008 to 2012 [127]. The etiology of the decline in antimicrobial drug development includes high cost with a low rate of return compared to drugs used to treat chronic conditions, limitations on the use of newly approved antibacterial agents, and trial design and regulatory issues [128].
As large pharmaceutical companies continued to withdraw from antimicrobial drug development, antifungal drug discovery suffers as an innocent bystander. In the last 13 years, only five antifungal agents have been approved by the FDA including caspofungin (2001), voriconazole (2002), micafungin (2005), posaconazole (2006), and anidulafungin (2006) [129]. This is especially alarming with the emergence of resistant Candida spp. and the paucity of oral antifungal agents currently available to treat infections caused by these organisms. However, despite the current outlook with regard to the development of new antifungal agents, we retain hope that ongoing regulatory reform will rejuvenate the development of these important antimicrobial compounds in the future [130].
Future Directions/Conclusion
Candida spp. are a common cause of bloodstream infections with high attributable mortality and cost. Rapid diagnostics and new antifungal agents are needed for the treatment of candidemia, as the mortality rate with existing antifungal agents is high and resistance is emerging. Antifungal agents in clinical trials include isavuconazole, albaconazole, SCY-078, VT-1161, and T-2307. Currently, only isavuconazole is being studied in a Phase 3 clinical trial for the treatment of candidemia and other invasive Candida infections. Further data is needed to determine the role of these antifungal agents in the treatment of candidemia and other forms of invasive candidiasis. Unfortunately, the progressive decline in antimicrobial drug development by large pharmaceutical companies may result in a dearth of antifungal drug discovery and development. Nonetheless, there still remains interest and recognition of the critical need for developing new antifungal agents for treatment and prevention of invasive candidiasis, as well as other life-threatening mycoses. Indeed, compounds that are beyond the scope of this manuscript are undergoing early development that may lead to important new advances in patient care.
Acknowledgments
This work was supported in part by the intramural research program of the National Institutes of Health. The opinions expressed in this paper are the authors’ and do not reflect those of the National Institutes of Health (NIH) Clinical Center, NIH, Department of Health and Human Services, or the Federal government. Dr. Walsh is a Scholar of the Henry Schueler Foundation and a Scholar of Pediatric Infectious Diseases of the Sharpe Family Foundation. The assistance of Judith Welsh for the library research is gratefully acknowledged. We thank Dr. Anthony Suffredini and Dr. Parizad Torabi-Parizi, NIH Clinical Center, Department of Critical Care Medicine for the thoughtful review of the manuscript.
Footnotes
Financial Disclosures
Dr. Moriyama: Stock: Merck
Dr. Walsh:
Research Grants: Novartis, Astellas, Merck, ContraFect, Pfizer
Consultancies: Vestagen, ICo Therapeutics, Inc, Trius, Sigma Tau, Astellas, and Drais Pharmaceuticals, ContraFect, Novartis, Pfizer, Methylgene
References
- 1.Pfaller MA. Antifungal drug resistance: mechanisms, epidemiology, and consequences for treatment. Am J Med. 2012;125:S3–13. doi: 10.1016/j.amjmed.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 2.Hidron AI, Edwards JR, Patel J, et al. NHSN annual update: antimicrobial-resistant pathogens associated with healthcare-associated infections: annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006–2007. Infect Control Hosp Epidemiol. 2008;29:996–1011. doi: 10.1086/591861. [DOI] [PubMed] [Google Scholar]
- 3.Sievert DM, Ricks P, Edwards JR, et al. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2009–2010. Infect Control Hosp Epidemiol. 2013;34:1–14. doi: 10.1086/668770. [DOI] [PubMed] [Google Scholar]
- 4.Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis. 2004;39:309–17. doi: 10.1086/421946. [DOI] [PubMed] [Google Scholar]
- 5.Eggimann P, Garbino J, Pittet D. Epidemiology of Candida species infections in critically ill non-immunosuppressed patients. Lancet Infect Dis. 2003;3:685–702. doi: 10.1016/s1473-3099(03)00801-6. [DOI] [PubMed] [Google Scholar]
- 6.Zaoutis TE, Argon J, Chu J, Berlin JA, Walsh TJ, Feudtner C. The epidemiology and attributable outcomes of candidemia in adults and children hospitalized in the United States: a propensity analysis. Clin Infect Dis. 2005;41:1232–9. doi: 10.1086/496922. [DOI] [PubMed] [Google Scholar]
- 7.Wey SB, Mori M, Pfaller MA, Woolson RF, Wenzel RP. Hospital-acquired candidemia. The attributable mortality and excess length of stay. Arch Intern Med. 1988;148:2642–5. doi: 10.1001/archinte.148.12.2642. [DOI] [PubMed] [Google Scholar]
- 8.Gudlaugsson O, Gillespie S, Lee K, et al. Attributable mortality of nosocomial candidemia, revisited. Clin Infect Dis. 2003;37:1172–7. doi: 10.1086/378745. [DOI] [PubMed] [Google Scholar]
- 9.Pfaller MA, Diekema DJ. Epidemiology of invasive candidiasis: a persistent public health problem. Clin Microbiol Rev. 2007;20:133–63. doi: 10.1128/CMR.00029-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kett DH, Azoulay E, Echeverria PM, Vincent JL Extended Prevalence of Infection in ICU Study (EPIC II) Group of Investigators. Candida bloodstream infections in intensive care units: analysis of the extended prevalence of infection in intensive care unit study. Crit Care Med. 2011;39:665–70. doi: 10.1097/CCM.0b013e318206c1ca. [DOI] [PubMed] [Google Scholar]
- 11.Pfaller MA, Diekema DJ. Epidemiology of invasive mycoses in North America. Crit Rev Microbiol. 2010;36:1–53. doi: 10.3109/10408410903241444. [DOI] [PubMed] [Google Scholar]
- 12.Andes DR, Safdar N, Baddley JW, et al. Impact of treatment strategy on outcomes in patients with candidemia and other forms of invasive candidiasis: a patient-level quantitative review of randomized trials. Clin Infect Dis. 2012;54:1110–22. doi: 10.1093/cid/cis021. [DOI] [PubMed] [Google Scholar]
- 13.Pfaller MA, Messer SA, Hollis RJ, et al. Variation in susceptibility of bloodstream isolates of Candida glabrata to fluconazole according to patient age and geographic location in the United States in 2001 to 2007. J Clin Microbiol. 2009;47:3185–90. doi: 10.1128/JCM.00946-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pfaller MA, Castanheira M, Messer SA, Moet GJ, Jones RN. Variation in Candida spp. distribution and antifungal resistance rates among bloodstream infection isolates by patient age: report from the SENTRY Antimicrobial Surveillance Program (2008–2009) Diagn Microbiol Infect Dis. 2010;68:278–83. doi: 10.1016/j.diagmicrobio.2010.06.015. [DOI] [PubMed] [Google Scholar]
- 15.Shorr AF, Gupta V, Sun X, Johannes RS, Spalding J, Tabak YP. Burden of early-onset candidemia: analysis of culture-positive bloodstream infections from a large U.S. database. Crit Care Med. 2009;37:2519–26. doi: 10.1097/CCM.0b013e3181a0f95d. [DOI] [PubMed] [Google Scholar]
- 16.Zaoutis TE, Heydon K, Localio R, Walsh TJ, Feudtner C. Outcomes attributable to neonatal candidiasis. Clin Infect Dis. 2007;44:1187–93. doi: 10.1086/513196. [DOI] [PubMed] [Google Scholar]
- 17.Clinical and Laboratory Standards Institute. CLSI document M27-A3. Wayne, PA: CLSI; 2008. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved Standard–Third Edition. [Google Scholar]
- 18.Clinical and Laboratory Standards Institute. CLSI document M27-S4. Wayne, PA: CLSI; 2012. Reference method for broth dilution antifungal susceptibility testing of yeasts; fourth informational supplement. [Google Scholar]
- 19.Pappas PG, Kauffman CA, Andes D, et al. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;48:503–35. doi: 10.1086/596757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Linden P, Lee L, Walsh TJ. Retrospective analysis of the dosage of amphotericin B lipid complex for the treatment of invasive fungal infections. Pharmacotherapy. 1999;19:1261–8. doi: 10.1592/phco.19.16.1261.30870. [DOI] [PubMed] [Google Scholar]
- 21.Ng TB, Cheung RC, Ye Xi, et al. Pharmacotherapy approaches to antifungal prophylaxis. Expert Opin Pharmacother. 2012;13:1695–705. doi: 10.1517/14656566.2012.698263. [DOI] [PubMed] [Google Scholar]
- 22.Tada R, Latgé JP, Aimanianda V. Undressing the fungal cell wall/cell membrane - the antifungal drug targets. Curr Pharm Des. 2013;19:3738–47. doi: 10.2174/1381612811319200012. [DOI] [PubMed] [Google Scholar]
- 23.Free SJ. Fungal cell wall organization and biosynthesis. Adv Genet. 2013;81:33–82. doi: 10.1016/B978-0-12-407677-8.00002-6. [DOI] [PubMed] [Google Scholar]
- 24.Ghannoum MA, Rice LB. Antifungal agents: mode of action, mechanisms of resistance, and correlation of these mechanisms with bacterial resistance. Clin Microbiol Rev. 1999;12:501–17. doi: 10.1128/cmr.12.4.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Georgopapadakou NH, Walsh TJ. Human mycoses: drugs and targets for emerging pathogens. Science. 1994;264:371–3. doi: 10.1126/science.8153622. [DOI] [PubMed] [Google Scholar]
- 26.Pfaller MA, Hata K, Jones RN, Messer SA, Moet GJ, Castanheira M. In vitro activity of a novel broad-spectrum antifungal, E1210, tested against Candida spp. as determined by CLSI broth microdilution method. Diagn Microbiol Infect Dis. 2011;71:167–70. doi: 10.1016/j.diagmicrobio.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 27.Mai A. Small-molecule chromatin-modifying agents: therapeutic applications. Epigenomics. 2010;2:307–24. doi: 10.2217/epi.10.7. [DOI] [PubMed] [Google Scholar]
- 28.Pfaller MA, Messer SA, Georgopapadakou N, Martell LA, Besterman JM, Diekema DJ. Activity of MGCD290, a Hos2 histone deacetylase inhibitor, in combination with azole antifungals against opportunistic fungal pathogens. J Clin Microbiol. 2009;47:3797–804. doi: 10.1128/JCM.00618-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wirk B. Heat shock protein inhibitors for the treatment of fungal infections. Recent Pat Antiinfect Drug Discov. 2011;6:38–44. doi: 10.2174/157489111794407840. [DOI] [PubMed] [Google Scholar]
- 30.Burnie J, Matthews R. Genetically recombinant antibodies: New therapeutics against candidiasis. Expert Opin Biol Ther. 2004;4:233–41. doi: 10.1517/14712598.4.2.233. [DOI] [PubMed] [Google Scholar]
- 31.Cowen LE, Lindquist S. Hsp90 potentiates the rapid evolution of new traits: drug resistance in diverse fungi. Science. 2005;309:2185–9. doi: 10.1126/science.1118370. [DOI] [PubMed] [Google Scholar]
- 32.Matthews RC, Rigg G, Hodgetts S, et al. Preclinical assessment of the efficacy of mycograb, a human recombinant antibody against fungal HSP90. Antimicrob Agents Chemother. 2003;47:2208–16. doi: 10.1128/AAC.47.7.2208-2216.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sutherland A, Ellis D. Treatment of a critically ill child with disseminated Candida glabrata with a recombinant human antibody specific for fungal heat shock protein 90 and liposomal amphotericin B, caspofungin, and voriconazole. Pediatr Crit Care Med. 2008;9:e23–5. doi: 10.1097/PCC.0b013e31817286e8. [DOI] [PubMed] [Google Scholar]
- 34.Pachl J, Svoboda P, Jacobs F, et al. A randomized, blinded, multicenter trial of lipid-associated amphotericin B alone versus in combination with an antibody-based inhibitor of heat shock protein 90 in patients with invasive candidiasis. Clin Infect Dis. 2006;42:1404–13. doi: 10.1086/503428. [DOI] [PubMed] [Google Scholar]
- 35.Garcia-Cardena G, Fan R, Shah V, et al. Dynamic activation of endothelial nitric oxide synthase by Hsp90. Nature. 1998;392:821–4. doi: 10.1038/33934. [DOI] [PubMed] [Google Scholar]
- 36.Odds FC. Drug evaluation: BAL-8557--a novel broad-spectrum triazole antifungal. Curr Opin Investig Drugs. 2006;7:766–72. [PubMed] [Google Scholar]
- 37.Schmitt-Hoffmann A, Roos B, Heep M, et al. Single-ascending-dose pharmacokinetics and safety of the novel broad-spectrum antifungal triazole BAL4815 after intravenous infusions (50, 100, and 200 milligrams) and oral administrations (100, 200, and 400 milligrams) of its prodrug, BAL8557, in healthy volunteers. Antimicrob Agents Chemother. 2006;50:279–85. doi: 10.1128/AAC.50.1.279-285.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Livermore J, Hope W. Evaluation of the pharmacokinetics and clinical utility of isavuconazole for treatment of invasive fungal infections. Expert Opin Drug Metab Toxicol. 2012;8:759–65. doi: 10.1517/17425255.2012.683859. [DOI] [PubMed] [Google Scholar]
- 39.Pfaller MA, Messer SA, Rhomberg PR, Jones RN, Castanheira M. In vitro activities of isavuconazole and comparator antifungal agents tested against a global collection of opportunistic yeasts and molds. J Clin Microbiol. 2013;51:2608–16. doi: 10.1128/JCM.00863-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Majithiya J, Sharp A, Parmar A, Denning DW, Warn PA. Efficacy of isavuconazole, voriconazole and fluconazole in temporarily neutropenic murine models of disseminated Candida tropicalis and Candida krusei. J Antimicrob Chemother. 2009;63:161–6. doi: 10.1093/jac/dkn431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Warn PA, Parmar A, Sharp A, Heep M, Spickermann J, Denning DW. In vivo efficacy of the triazole BAL8557 against disseminated Candida albicans in mice assessed by survival and tissue burden in temporarily and persistently neutropenic mice treated with 1–7 doses of drug over a dose range of 20–80% of Emax. Int J Antimicrob Agents. 2007;29:S560, Abstract P1950. [Google Scholar]
- 42.Yamazaki T, Inagaki Y, Fujii T, et al. In vitro activity of isavuconazole against 140 reference fungal strains and 165 clinically isolated yeasts from Japan. Int J Antimicrob Agents. 2010;36:324–31. doi: 10.1016/j.ijantimicag.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 43.Guinea J, Peláez T, Recio S, Torres-Narbona M, Bouza E. In vitro antifungal activities of isavuconazole (BAL4815), voriconazole, and fluconazole against 1,007 isolates of zygomycete, Candida, Aspergillus, Fusarium, and Scedosporium species. Antimicrob Agents Chemother. 2008;52:1396–400. doi: 10.1128/AAC.01512-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seifert H, Aurbach U, Stefanik D, Cornely O. In vitro activities of isavuconazole and other antifungal agents against Candida bloodstream isolates. Antimicrob Agents Chemother. 2007;51:1818–21. doi: 10.1128/AAC.01217-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Warn PA, Sharp A, Parmar A, Majithiya J, Denning DW, Hope WW. Pharmacokinetics and pharmacodynamics of a novel triazole, isavuconazole: mathematical modeling, importance of tissue concentrations, and impact of immune status on antifungal effect. Antimicrob Agents Chemother. 2009;53:3453–61. doi: 10.1128/AAC.01601-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schmitt-Hoffmann A, Roos B, Maares J, et al. Multiple-dose pharmacokinetics and safety of the new antifungal triazole BAL4815 after intravenous infusion and oral administration of its prodrug, BAL8557, in healthy volunteers. Antimicrob Agents Chemother. 2006;50:286–93. doi: 10.1128/AAC.50.1.286-293.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schmitt-Hoffmann A, Roos B, Spickermann J, et al. Effect of mild and moderate liver disease on the pharmacokinetics of isavuconazole after intravenous and oral administration of a single dose of the prodrug BAL8557. Antimicrob Agents Chemother. 2009;53:4885–90. doi: 10.1128/AAC.00319-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aperis G, Mylonakis E. Newer triazole antifungal agents: pharmacology, spectrum, clinical efficacy and limitations. Expert Opin Investig Drugs. 2006;15:579–602. doi: 10.1517/13543784.15.6.579. [DOI] [PubMed] [Google Scholar]
- 49.Bartroli J, Merlos M. Overview of albaconazole. European Infectious Disease. 2011;5:88–91. [Google Scholar]
- 50.Alves SH, Da Matta DA, Azevedo AC, et al. In vitro activities of new and conventional antimycotics against fluconazole-susceptible and non-susceptible Brazilian Candida spp. isolates. Mycoses. 2006;49:220–5. doi: 10.1111/j.1439-0507.2006.01226.x. [DOI] [PubMed] [Google Scholar]
- 51.Ramos G, Cuenca-Estrella M, Monzón A, Rodríguez-Tudela JL. In-vitro comparative activity of UR-9825, itraconazole and fluconazole against clinical isolates of Candida spp. J Antimicrob Chemother. 1999;44:283–6. doi: 10.1093/jac/44.2.283. [DOI] [PubMed] [Google Scholar]
- 52.Bartroli J, Turmo E, Algueró M, et al. New azole antifungals. 3. Synthesis and antifungal activity of 3-substituted-4(3H)-quinazolinones. J Med Chem. 1998;41:1869–82. doi: 10.1021/jm9707277. [DOI] [PubMed] [Google Scholar]
- 53.Augenbraun M, Livingston J, Parker R, et al. Fluconazole and MGCD290 in vulvo vaginal candidiasis (VVC): Results from a randomized phase II study. Presented at ID Week; 2–6 October 2013; San Francisco CA. 2013. p. Abstract 1330. [Google Scholar]
- 54.Besterman J, Nguyen DT, Ste-Croix H. MGCD290, an oral fungal Hos2 inhibitor, enhances the antifungal properties of fluconazole following multiple or single oral dose administration in pre- and post-infection settings. Presented at the 52nd ICAAC meeting; 9–12 September 2012; San Francisco CA. p. Abstract M-1711. [Google Scholar]
- 55.Besterman JM, Nguyen D, Akache B, et al. In vivo properties of MGCD290, an antifungal histone deactylase (HDAC) inhibitor. Presented at the 48th ICAAC meeting; 25–28 October 2008; Washington DC. p. Abstract M-2133. [Google Scholar]
- 56.Martell LA, Reid GK, Martell RE, Drouin M, Wilhelm J, Besterman J. Multiple phase 1 studies in healthy subjects demonstrates safety and pharmacokinetics of MGCD290, an oral fungal Hos2 inhibitor +/− fluconazole. Presented at the 49th ICAAC meeting; 12–15 September 2009; San Francisco CA. p. Abstract M-1029. [Google Scholar]
- 57.Besterman J, Reid GK, Drouin M. Multiple ascending dose phase 1 studies in healthy subjects demonstrates safety and pharmacokinetics of MGCD290, an oral fungal Hos2 inhibitor +/− fluconazole. Presented at the 50th ICAAC meeting; 12–15 September 2010; Boston MA. p. Abstract F1-862. [Google Scholar]
- 58.Reid G, Drouin M, Besterman J. SAD and MAD studies in healthy subjects demonstrate safety and pharmacokinetics of MGCD290, an oral fungal Hos2 inhibitor +/− fluconazole. Presented at ID Week; 17–21 October 2012; San Diego CA. 2012. p. Abstract 1619. [Google Scholar]
- 59.Hector RF, Bierer DE. New β-glucan inhibitors as antifungal drugs. Expert Opin Ther Pat. 2011;21:1597–610. doi: 10.1517/13543776.2011.603899. [DOI] [PubMed] [Google Scholar]
- 60.Pfaller MA, Messer SA, Motyl MR, Jones RN, Castanheira M. Activity of MK-3118, a new oral glucan synthase inhibitor, tested against Candida spp. by two international methods (CLSI and EUCAST) J Antimicrob Chemother. 2013;68:858–63. doi: 10.1093/jac/dks466. [DOI] [PubMed] [Google Scholar]
- 61.Onishi J, Meinz M, Thompson J, et al. Discovery of novel antifungal (1,3)-beta-D-glucan synthase inhibitors. Antimicrob Agents Chemother. 2000;44:368–77. doi: 10.1128/aac.44.2.368-377.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Martins IM, Cortes JC, Munoz J, et al. Differential activities of three families of specific beta(1,3)glucan synthase inhibitors in wild-type and resistant strains of fission yeast. J Biol Chem. 2011;286:3484–96. doi: 10.1074/jbc.M110.174300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Flattery A, Abruzzo G, Gill C, et al. Evaluation of orally active enfumafungin derivative MK-3118 in mouse models of disseminated candidiasis. Presented at the 50th ICAAC meeting; 12–15 September 2010; Boston MA. p. Abstract F1-848. [Google Scholar]
- 64.Trucksis M, Garrett G, Bautmans A, et al. A phase I multiple-rising dose study evaluating safety, tolerability, and pharmacokinetics of MK-3118, oral glucan synthase inhibitor in healthy volunteers. Presented at the 51st ICAAC meeting; 17–20 September 2011; Chicago IL. p. Abstract F1-1390. [Google Scholar]
- 65.Trucksis M, Garrett G, Heirman I, et al. A phase I single rising dose study evaluating the safety, tolerability and pharmacokinetics of an oral glucan synthase inhibitor in healthy male volunteers. Presented at the 50th ICAAC meeting; 12–15 September 2010; Boston MA. p. Abstract F1-1975. [Google Scholar]
- 66.Viamet Pharmaceuticals: Product Pipeline. Viamet; [Accessed May 23, 2014]. Available from http://www.viamet.com/pipeline.asp. [Google Scholar]
- 67.Fothergill AW, Rinaldi MG, Hoekstra WJ, et al. In vitro activity of two metalloenzyme inhibitors compared to caspofungin and fluconazole against a panel of 74 Candida spp. Presented at the 50th ICAAC meeting; 12–15 September 2010; Boston MA. p. Abstract F1-851. [Google Scholar]
- 68.Hoekstra WJ, O’Leary AL, Moore WR, Schotzinger RJ. Novel metalloenzyme inhibitors, VT-1161 and VT-1129, exhibit efficacy and survival benefit in a murine systemic candidiasis model. Presented at the 50th ICAAC meeting; 12–15 September 2010; Boston MA. p. Abstract F1-852. [Google Scholar]
- 69.Fothergill AW, Wiederhold NP, Hoekstra WJ, et al. The fungal Cyp51 inhibitors VT-1129 and VT-1161 maintain in vitro activity against Candida albicans isolates with reduced antifungal susceptibility. Presented at the 51st ICAAC meeting; 17–20 September 2011; Chicago IL. p. Abstract F1-1381. [Google Scholar]
- 70.Najvar LK, Wiederhold NP, Garvey EP, et al. Efficacy of the novel fungal Cyp51 inhibitor VT-1161 against invasive candidiasis caused by resistant Candida albicans. Mycoses. 2012;55(Supp 4):106, Abstract P036. [Google Scholar]
- 71.Garvey EP, O’Leary AL, Hoekstra WJ, Moore RW, Schotzinger RJ. Single or repeat oral doses of the novel CYP51 inhibitor VT-1161 suppress fungal growth and provide a survival benefit in a murine Candida glabrata infection model. Presented at the 51st ICAAC meeting; 17–20 September 2011; Chicago IL. p. Abstract F1-1387. [Google Scholar]
- 72.Shibata T, Takahashi T, Yamada E, et al. T-2307 causes collapse of mitochondrial membrane potential in yeast. Antimicrob Agents Chemother. 2012;56:5892–7. doi: 10.1128/AAC.05954-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mitsuyama J, Nomura N, Hashimoto K, et al. In vitro and in vivo antifungal activities of T-2307, a novel arylamidine. Antimicrob Agents Chemother. 2008;52:1318–24. doi: 10.1128/AAC.01159-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yamada E, Nishikawa H, Nomura N, Mitsuyama J. T-2307 shows efficacy in a murine model of Candida glabrata infection despite in vitro trailing growth phenomena. Antimicrob Agents Chemother. 2010;54:3630–4. doi: 10.1128/AAC.00355-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Moragues MD, Rementeria A, Sevilla MJ, Eraso E, Quindos G. Candida antigens and immune responses: implications for a vaccine. Expert Rev Vaccines. 2014;13:1001–12. doi: 10.1586/14760584.2014.932253. [DOI] [PubMed] [Google Scholar]
- 76.Mochon AB, Cutler JE. Is a vaccine needed against Candida albicans? Med Mycol. 2005;43:97–115. doi: 10.1080/13693780500035979. [DOI] [PubMed] [Google Scholar]
- 77.Schmidt CS, White CJ, Ibrahim AS, et al. NDV-3, a recombinant alum-adjuvanted vaccine for Candida and Staphylococcus aureus is safe and immunogenic in healthy adults. Vaccine. 2012;30:7594–600. doi: 10.1016/j.vaccine.2012.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Spellberg BJ, Ibrahim AS, Avanesian V, et al. Efficacy of the anti-Candida rAls3p-N or rAls1p-N vaccines against disseminated and mucosal candidiasis. J Infect Dis. 2006;194:256–60. doi: 10.1086/504691. [DOI] [PubMed] [Google Scholar]
- 79.Phan QT, Myers CL, Fu Y, et al. Als3 is a Candida albicans invasin that binds to cadherins and induces endocytosis by host cells. PLoS Biol. 2007;5:e64. doi: 10.1371/journal.pbio.0050064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Otoo HN, Lee KG, Qiu W, Lipke PN. Candida albicans Als adhesins have conserved amyloid-forming sequences. Eukaryot Cell. 2008;7:776–82. doi: 10.1128/EC.00309-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Laforce-Nesbitt SS, Sullivan MA, Hoyer LL, Bliss JM. Inhibition of Candida albicans adhesion by recombinant human antibody single-chain variable fragment specific for Als3p. FEMS Immunol Med Microbiol. 2008;54:195–202. doi: 10.1111/j.1574-695X.2008.00465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cleary IA, Reinhard SM, Miller CL, et al. Candida albicans adhesin Als3p is dispensable for virulence in the mouse model of disseminated candidiasis. Microbiology. 2011;157:1806–15. doi: 10.1099/mic.0.046326-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lin L, Ibrahim AS, Xu X, et al. Th1-Th17 cells mediate protective adaptive immunity against Staphylococcus aureus and Candida albicans infection in mice. PLoS Pathog. 2009;5:e1000703. doi: 10.1371/journal.ppat.1000703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Baquir B, Lin L, Ibrahim AS, et al. Immunological reactivity of blood from healthy humans to the rAls3p-N vaccine protein. J Infect Dis. 2010;201:473–7. doi: 10.1086/649901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Spellberg B, Ibrahim AS, Lin L, et al. Antibody titer threshold predicts anti-candidal vaccine efficacy even though the mechanism of protection is induction of cell-mediated immunity. J Infect Dis. 2008;197:967–71. doi: 10.1086/529204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Luo G, Ibrahim AS, Spellberg B, Nobile CJ, Mitchell AP, Fu Y. Candida albicans Hyr1p confers resistance to neutrophil killing and is a potential vaccine target. J Infect Dis. 2010;201:1718–28. doi: 10.1086/652407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Luo G, Ibrahim AS, French SW, Edwards JE, Jr, Fu Y. Active and passive immunization with rHyr1p-N protects mice against hematogenously disseminated candidiasis. PloS One. 2011;6:e25909. doi: 10.1371/journal.pone.0025909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bailey DA, Feldmann PJF, Bovey M, Gow NA, Brown AJ. The Candida albicans HYR1 gene, which is activated in response to hyphal development, belongs to a gene family encoding yeast cell wall proteins. J Bacteriol. 1996;178:5353–60. doi: 10.1128/jb.178.18.5353-5360.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Luo G, Ibrahim A, Spellberg B, et al. Neutrophils inhibit Candidal expression of HYR1, which mediates resistance to neutrophil killing. Presented at the 48th ICAAC meeting; 25–28 October 2008; Washington DC. p. Abstract M-1583. [Google Scholar]
- 90.Brzankalski GE, Najvar LK, Wiederhold NP, et al. Evaluation of aminocandin and caspofungin against Candida glabrata including isolates with reduced caspofungin susceptibility. J Antimicrob Chemother. 2008;62:1094–100. doi: 10.1093/jac/dkn304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sandage B, Cooper G, Najarian N, Lowther J. Pharmacokinetics and fungicidal activity of aminocandin (HMR3270), a novel echinocandin in healthy volunteers. Clin Microbiol Infect. 2005;11(Supp 2):350, Abstract P1115. [Google Scholar]
- 92.Laverdiere M, Labbé AC, Restieri C, et al. Susceptibility patterns of Candida species recovered from Canadian intensive care units. J Crit Care. 2007;22:245–50. doi: 10.1016/j.jcrc.2006.10.038. [DOI] [PubMed] [Google Scholar]
- 93.Isham N, Ghannoum MA. Determination of MICs of aminocandin for Candida spp. and filamentous fungi. J Clin Microbiol. 2006;44:4342–4. doi: 10.1128/JCM.01550-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Najvar LK, Bocanegra R, Wiederhold NP, et al. Therapeutic and prophylactic efficacy of aminocandin (IP960) against disseminated candidiasis in mice. Clin Microbiol Infect. 2008;14:595–600. doi: 10.1111/j.1469-0691.2008.01994.x. [DOI] [PubMed] [Google Scholar]
- 95.Ghannoum MA, Kim HG, Long L. Efficacy of aminocandin in the treatment of immunocompetent mice with haematogenously disseminated fluconazole-resistant candidiasis. J Antimicrob Chemother. 2007;59:556–9. doi: 10.1093/jac/dkl525. [DOI] [PubMed] [Google Scholar]
- 96.Warn PA, Sharp A, Morrissey G, Denning DW. Activity of aminocandin (IP960) compared with amphotericin B and fluconazole in a neutropenic murine model of disseminated infection caused by a fluconazole-resistant strain of Candida tropicalis. J Antimicrob Chemother. 2005;56:590–3. doi: 10.1093/jac/dki268. [DOI] [PubMed] [Google Scholar]
- 97.Andes D, Marchillo K, Lowther J, Bryskier A, Stamstad T, Conklin R. In vivo pharmacodynamics of HMR 3270, a glucan synthase inhibitor, in a murine candidiasis model. Antimicrob Agents Chemother. 2003;47:1187–92. doi: 10.1128/AAC.47.4.1187-1192.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Watanabe NA, Miyazaki M, Horii T, Sagane K, Tsukahara K, Hata K. E1210, a new broad-spectrum antifungal, suppresses Candida albicans hyphal growth through inhibition of glycosylphosphatidylinositol biosynthesis. Antimicrob Agents Chemother. 2012;56:960–71. doi: 10.1128/AAC.00731-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hata K, Yamamoto E, Okubo M. Physicochemical properties and nonclinical pharmacokinetics of E1211: a water-soluble prodrug of E1210. Presented at the 51st ICAAC meeting; 17–20 September 2011; Chicago IL. p. Abstract F1-1376. [Google Scholar]
- 100.Hata K, Miyazaki M, Horii T, Watanabe N. In vitro and in vivo antifungal activities of E1211: a water-soluble prodrug of E1210. Presented at the 51st ICAAC meeting; 17–20 September 2011; Chicago IL. p. Abstract F1-1377. [Google Scholar]
- 101.Miyazaki M, Horii T, Hata K, et al. In vitro activity of E1210, a novel antifungal, against clinically important yeasts and molds. Antimicrob Agents Chemother. 2011;55:4652–8. doi: 10.1128/AAC.00291-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hata K, Horii T, Miyazaki M, et al. Efficacy of oral E1210, a new broad-spectrum antifungal with a novel mechanism of action, in murine models of candidiasis, aspergillosis, and fusariosis. Antimicrob Agents Chemother. 2011;55:4543–51. doi: 10.1128/AAC.00366-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Horii T, Okubo M, Miyazaki M, Hata K, Watanabe N. In vivo pharmacodynamic correlates of success for E1210 treatment of disseminated candidiasis. Presented at the 50th ICAAC meeting; 12–15 September 2010; Boston MA. p. Abstract F1-843. [Google Scholar]
- 104.Richie DL, Ghannoum MA, Isham N, Thompson KV, Ryder NS. Nonspecific effect of Mycograb on amphotericin B MIC. Antimicrob Agents Chemother. 2012;56:3963–4. doi: 10.1128/AAC.00435-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hodgetts S, Nooney L, Al-Akeel R, et al. Efungumab and caspofungin: pre-clinical data supporting synergy. J Antimicrob Chemother. 2008;61:1132–9. doi: 10.1093/jac/dkn075. [DOI] [PubMed] [Google Scholar]
- 106.Matthews R, Hodgetts S, Burnie J. Preliminary assessment of a human recombinant antibody fragment to hsp90 in murine invasive candidiasis. J Infect Dis. 1995;171:1668–71. doi: 10.1093/infdis/171.6.1668. [DOI] [PubMed] [Google Scholar]
- 107.Rowlands HE, Morris K, Graham C. Human recombinant antibody against Candida. Pediatr Infect Dis J. 2006;25:959–60. doi: 10.1097/01.inf.0000237922.28863.ab. [DOI] [PubMed] [Google Scholar]
- 108.Krenova Z, Pavelka Z, Lokaj P, et al. Successful treatment of life-threatening Candida peritonitis in a child with abdominal non-Hodgkin lymphoma using Efungumab and amphotericin B colloid dispersion. J Pediatr Hematol Oncology. 2010;32:128–30. doi: 10.1097/MPH.0b013e3181cb49a8. [DOI] [PubMed] [Google Scholar]
- 109.Dominguez JM, Gomez-Lorenzo MG, Martin JJ. Sordarin inhibits fungal protein synthesis by blocking translocation differently to fusidic acid. J Biol Chem. 1999;274:22423–7. doi: 10.1074/jbc.274.32.22423. [DOI] [PubMed] [Google Scholar]
- 110.Soe R, Mosley RT, Justice M, et al. Sordarin derivatives induce a novel conformation of the yeast ribosome translocation factor eEF2. J Biol Chem. 2007;282:657–66. doi: 10.1074/jbc.M607830200. [DOI] [PubMed] [Google Scholar]
- 111.Herreros E, Almela MJ, Lozano S, Gomez de las Heras F, Gargallo-Viola D. Antifungal activities and cytotoxicity studies of six new azasordarins. Antimicrob Agents Chemother. 2001;45:3132–9. doi: 10.1128/AAC.45.11.3132-3139.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Aviles P, Falcoz C, San Roman R, Gargallo-Viola D. Pharmacokinetics-pharmacodynamics of a sordarin derivative (GM 237354) in a murine model of lethal candidiasis. Antimicrob Agents Chemother. 2000;44:2333–40. doi: 10.1128/aac.44.9.2333-2340.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kamai Y, Kakuta M, Shibayama T, Fukuoka T, Kuwahara S. Antifungal activities of R-135853, a sordarin derivative, in experimental candidiasis in mice. Antimicrob Agents Chemother. 2005;49:52–6. doi: 10.1128/AAC.49.1.52-56.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chakraborty B, Mukherjee R, Sengupta J. Structural insights into the mechanism of translational inhibition by the fungicide sordarin. J Comput Aided Mol Des. 2013;27:173–84. doi: 10.1007/s10822-013-9636-8. [DOI] [PubMed] [Google Scholar]
- 115.Dominguez JM, Martin JJ. Identification of elongation factor 2 as the essential protein targeted by sordarins in Candida albicans. Antimicrob Agents Chemother. 1998;42:2279–83. doi: 10.1128/aac.42.9.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Martinez A, Aviles P, Jimenez E, Caballero J, Gargallo-Viola D. Activities of sordarins in experimental models of candidiasis, aspergillosis, and pneumocystosis. Antimicrob Agents Chemother. 2000;44:3389–94. doi: 10.1128/aac.44.12.3389-3394.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lee JH, Jang EC, Han Y. Combination immunotherapy of MAb B6. 1 with fluconazole augments therapeutic effect to disseminated candidiasis. Arch Pharm Res. 2011;34:399–405. doi: 10.1007/s12272-011-0307-9. [DOI] [PubMed] [Google Scholar]
- 118.Han Y, Kanbe T, Cherniak R, Cutler JE. Biochemical characterization of Candida albicans epitopes that can elicit protective and nonprotective antibodies. Infect Immun. 1997;65:4100–7. doi: 10.1128/iai.65.10.4100-4107.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhang MX, Cutler JE, Han Y, Kozel TR. Contrasting roles of mannan-specific monoclonal immunoglobulin M antibodies in the activation of classical and alternative pathways by Candida albicans. Infect Immun. 1998;66:6027–9. doi: 10.1128/iai.66.12.6027-6029.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Han Y, Morrison RP, Cutler JE. A vaccine and monoclonal antibodies that enhance mouse resistance to Candida albicans vaginal infection. Infect Immun. 1998;66:5771–6. doi: 10.1128/iai.66.12.5771-5776.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Han Y, Cutler JE. Assessment of a mouse model of neutropenia and the effect of an anti-candidiasis monoclonal antibody in these animals. J Infect Dis. 1997;175:1169–75. doi: 10.1086/516455. [DOI] [PubMed] [Google Scholar]
- 122.Han Y, Kozel TR, Zhang MX, MacGill RS, Carroll MC, Cutler JE. Complement is essential for protection by an IgM and an IgG3 monoclonal antibody against experimental, hematogenously disseminated candidiasis. J Immunol. 2001;167:1550–7. doi: 10.4049/jimmunol.167.3.1550. [DOI] [PubMed] [Google Scholar]
- 123.Han Y. Efficacy of combination immunotherapy of IgM MAb B6. 1 and amphotericin B against disseminated candidiasis. Int Immunopharmacol. 2010;10:1526–31. doi: 10.1016/j.intimp.2010.08.027. [DOI] [PubMed] [Google Scholar]
- 124.Trinel PA, Delplace F, Maes E, et al. Candida albicans serotype B strains synthesize a serotype-specific phospholipomannan overexpressing a beta-1,2-linked mannotriose. Mol Microbiol. 2005;58:984–98. doi: 10.1111/j.1365-2958.2005.04890.x. [DOI] [PubMed] [Google Scholar]
- 125.Caesar-TonThat TC, Cutler JE. A monoclonal antibody to Candida albicans enhances mouse neutrophil candidacidal activity. Infect Immun. 1997;65:5354–7. doi: 10.1128/iai.65.12.5354-5357.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Spellberg B, Powers JH, Brass EP, Miller LG, Edwards JE., Jr Trends in antimicrobial drug development: implications for the future. Clin Infect Dis. 2004;38:1279–86. doi: 10.1086/420937. [DOI] [PubMed] [Google Scholar]
- 127.Boucher HW, Talbot GH, Benjamin DK, Jr, et al. 10 x ′20 Progress--development of new drugs active against gram-negative bacilli: an update from the Infectious Diseases Society of America. Clin Infect Dis. 2013;56:1685–94. doi: 10.1093/cid/cit152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Spellberg B, Guidos R, Gilbert D, et al. The epidemic of antibiotic-resistant infections: a call to action for the medical community from the Infectious Diseases Society of America. Clin Infect Dis. 2008;46:155–64. doi: 10.1086/524891. [DOI] [PubMed] [Google Scholar]
- 129.Ostrosky-Zeichner L, Casadevall A, Galgiani JN, Odds FC, Rex JH. An insight into the antifungal pipeline: selected new molecules and beyond. Nat Rev Drug Discov. 2010;9:719–27. doi: 10.1038/nrd3074. [DOI] [PubMed] [Google Scholar]
- 130.Shlaes DM, Sahm D, Opiela C, Spellberg B. The FDA reboot of antibiotic development. Antimicrob Agents Chemother. 2013;57:4605–7. doi: 10.1128/AAC.01277-13. [DOI] [PMC free article] [PubMed] [Google Scholar]