In neutropenic patients with lung infiltrates, Aspergillus spp., P. jirovecii, multi-resistant gram-negative pathogens, mycobacteria, viruses and toxic effects from cytotoxic drugs, radiation, or pulmonary involvement by the underlying malignancy should be included into differential diagnosis. Pre-emptive treatment with mould-active systemic antifungal agents improves clinical outcome.
Keywords: diagnosis, fever, lung infiltrates, neutropenia, pneumonia, treatment
Abstract
Up to 25% of patients with profound neutropenia lasting for >10 days develop lung infiltrates, which frequently do not respond to broad-spectrum antibacterial therapy. While a causative pathogen remains undetected in the majority of cases, Aspergillus spp., Pneumocystis jirovecii, multi-resistant Gram-negative pathogens, mycobacteria or respiratory viruses may be involved. In at-risk patients who have received trimethoprim–sulfamethoxazole (TMP/SMX) prophylaxis, filamentous fungal pathogens appear to be predominant, yet commonly not proven at the time of treatment initiation. Pathogens isolated from blood cultures, bronchoalveolar lavage (BAL) or respiratory secretions are not always relevant for the etiology of pulmonary infiltrates and should therefore be interpreted critically. Laboratory tests for detecting Aspergillus galactomannan, β-d-glucan or DNA from blood, BAL or tissue samples may facilitate the diagnosis; however, most polymerase chain reaction assays are not yet standardized and validated. Apart from infectious agents, pulmonary side-effects from cytotoxic drugs, radiotherapy or pulmonary involvement by the underlying malignancy should be included into differential diagnosis and eventually be clarified by invasive diagnostic procedures. Pre-emptive treatment with mold-active systemic antifungal agents improves clinical outcome, while other microorganisms are preferably treated only when microbiologically documented. High-dose TMP/SMX is first choice for treatment of Pneumocystis pneumonia, while cytomegalovirus pneumonia is treated primarily with ganciclovir or foscarnet in most patients. In a considerable number of patients, clinical outcome may be favorable despite respiratory failure, so that intensive care should be unrestrictedly provided in patients whose prognosis is not desperate due to other reasons.
introduction consensus process
See supplementary Material, available at Annals of Oncology online (Table 7).
clinical baseline
See supplementary Material, available at Annals of Oncology online.
diagnostic procedures
With respect to the critical prognosis of lung infiltrates (LI) in febrile neutropenic patients, diagnostic procedures are of major importance, but should not cause a substantial delay in the start of adequate antimicrobial therapy.
imaging
Conventional chest radiographs show abnormalities in <2% of febrile neutropenic patients without clinical findings indicating lower respiratory tract infection [2–4]. It is undetermined how many of these patients would have abnormalities on computed tomography (CT) scans, because no randomized head-to-head comparisons have been published so far. In patients persistently febrile after >48 h of broad-spectrum antibacterial therapy, ∼10% of chest radiographs are abnormal, whereas high-resolution CT scans at this time reveal pathological findings in ∼50% of patients [5, 6]. Early detection of lesions indicating invasive mold infection or Pneumocystis pneumonia (PcP) is of utmost importance, facilitating targeted bronchoscopy and bronchoalveolar lavage (BAL) and a prompt institution of pre-emptive antimicrobial treatment [7–9], enabling better survival of these patients. CT findings such as consolidation, ‘halo sign’ and ‘air-crescent sign’, obtained by high-resolution or multislice CT scans, may be important signs of filamentous fungal disease [8, 10]. While the ‘halo sign’ has been described typically in neutropenic patients, other CT findings indicative of IPA are comparable in neutropenic and in non-neutropenic patients [11]. A ‘reversed halo sign’, showing a focal rounded area of ground-glass opacity surrounded by a crescent or complete ring of consolidation, has been reported as relatively specific for fungal pneumonia due to zygomycetes/mucorales [8]; however, it may represent a broad spectrum of other differential diagnoses including tuberculosis, sarcoidosis or cryptogenic organizing pneumonia [12].
Beyond early identification of LI, CT findings may allow for distinguishing fungal from nonfungal LI [13–18]. Diffuse bilateral perihilar infiltrates, patchy areas of ground-glass attenuation (peripheral sparing), cysts and septal thickening, consolidation and centrilobular nodules may indicate PcP [19–21]. Nodular or cavitary lesions are suggestive of invasive filamentous fungal infection; however, differential diagnoses include pneumonia due to other microorganisms including mycobacteria [22] (which may be relevant in regions with high prevalence), Nocardia, Pneumocystis or Pseudomonas aeruginosa (P. aeruginosa) as well as lung involvement by underlying malignancies [23], so that comparison to previous CT scans in an individual patient is essential. Combination of CT scan with angiography has been found to increase the diagnostic specificity in some patients with pulmonary mold infections [24, 25]; however, this more labor intensive method has not yet become widely applied and is therefore not included into current clinical practice guidelines. In selected patients where pulmonary CT scan is not wanted or feasible, magnetic resonance tomography (MRI) is a valid alternative (B-II) [26, 27]. As yet, consensus definitions of invasive fungal diseases [10] have not included thoracic MRI findings. In selected patients with unexplained fever during neutropenia, [18F]2-fluoro-2-deoxy-D-glucose–positron emission tomography combined with computed tomography (PET-CT) may be helpful, particularly to rule out undetected infection [28].
Follow-up thoracic CT scans should in general not be ordered <7 days after start of treatment (A-II). In patients with IPA may show increasing volume of pulmonary infiltrates during the first week despite effective antifungal therapy [29]. This finding alone should not give reason to assess the treatment course as refractory (A-II). Reduction of the ‘halo’ and the development of an ‘air-crescent’ sign, however, typically indicate favorable response [30].
microbiology and histopathology
In the majority of febrile neutropenic patients with LI, no proving microbiological finding is available, so that the therapeutic management is based upon clinical and imaging findings (see below). In microbiologically documented cases, pathogens typically are isolated from blood cultures, bronchial secretions or BAL fluid. It often means a challenge to assess the diagnostic relevance of culture results [31–34], because unselected bronchial samples from these patients grow colonizing and contaminating microorganisms with no etiological significance [35], or blood cultures may show isolates not etiologically related to pneumonias. At the same time, if autopsies show invasive fungal infections, 75% of them have not been detected ante mortem [36, 37]. Therefore, in contrast to the majority of other microbiological findings, isolation of Aspergillus spp. or other filamentous fungi from upper respiratory tract specimens of severely immunocompromised patients typically indicates a respiratory tract mycosis [38].
The diagnostic yield and the outcome of clinical management in critically ill, febrile cancer patients with severe pulmonary infiltrates have not been improved by invasive diagnostic procedures including BAL [39]. The detection rate of potential pathogens from BAL samples has been described to be 25%–50% or even higher [11, 40–42], depending on the risk profile of patients included. A retrospective analysis of microbiological findings from BAL samples in cancer patients with LI showed 34% bacteria, 22% cytomegalovirus (CMV), 15% Pneumocystis jirovecii (P. jirovecii) and 2% Aspergillus spp. [43], and another report of 246 bronchoscopies in 199 febrile patients with hematological malignancies described pathogens with possible etiological significance in 48% of samples, of which 70 samples grew only bacteria, 13 showed both fungi and bacteria, 15 samples Aspergillus spp., 16 samples Candida species and 2 samples both Aspergillus and Candida spp. [33]. Many LI in severely immunocompromised patients may also have polymicrobial etiology [34], with molds (predominantly Aspergillus spp.) plus bacteria in 12% and multiple fungal species in 22% of samples. Although the etiological relevance of BAL findings may be questionable in many cases, the results trigger the change of antimicrobial treatment in up to 50% of patients [33, 44, 45]. As a diagnostic ‘gold standard’ is lacking, the number of false-positive and false-negative findings are unknown, and the rates of success or failure of ‘pathogen-directed’ antimicrobial treatment therefore remain undetermined. A proposal for the assessment of the etiological significance of microbiological findings in febrile neutropenic patients with LI is given in antimicrobial treatment in patients with documented pathogens section.
While for the proven diagnosis of IPA, cultural isolation of fungi and histological proof from lung tissue are regarded as diagnostic ‘gold standard’ [10], quality standards for diagnostic procedures are not available and patients undergoing biopsy are highly selected. Histological proof alone has an accuracy for Aspergillus of around 78%, so that histology should always be combined with fungal culture and with a culture independent method, e.g. nucleic acid based [46]. Polymerase chain reaction (PCR) may be helpful especially in patients who already receive antifungal treatment and for difficult-to-culture pathogens such as Mucorales.
Transbronchial biopsy is not recommended in severely thrombocytopenic patients with lung infiltrates [45]. Open-lung biopsy (OLB), mini-thoracotomy or video-assisted thoracoscopic surgery may be safely carried out in patients with treatment-refractory LI not cleared-up by other diagnostic approaches, primarily in order to rule out noninfectious origin [42, 47–50]. OLB is a relatively safe procedure with a complication rate of ∼6% [51], including the risk of hemorrhage [49, 52] even in thrombocytopenic patients [51, 53]. Histologically, no infection or malignancy, but nonspecific inflammation is detected in the majority of patients [31, 51, 53]. Notably, findings from OLB and BAL obtained simultaneously may show different microbiological results [31].
CT-guided percutaneous side-cut core needle biopsy may provide informative results in ∼80% of cases, allowing for species identification using molecular methods for tissue workup [54–58]. Percutaneous biopsy requires platelet counts >50 000/µl plus sufficient coagulation indices, e.g. an aPTT ratio of ≤1.4 [59], and should be limited to patients without an obvious risk of respiratory failure in case of complications such as a pneumothorax. As yet, there are no reports from prospective studies comparing different methods for invasive approaches to identify the causes of LI in febrile neutropenic patients.
nonculture-based diagnostic methods
cytomegalovirus and respiratory viruses
In patients with profound cellular immunosuppression, respiratory viruses may be the cause of LI, so that diagnostic programs used for workup of BAL or oro-/nasopharyngeal swabs should include CMV as well as Influenza, Parainfluenza, Respiratory Syncytial Virus, Coronavirus, Rhinovirus and Human Metapneumovirus [60–63]. In febrile neutropenic patients with LI, CMV PCR applied on BAL samples has a high negative, but low positive predictive value [64], while positive rapid culture, immediate early antigen, direct fluorescent antibody tests, DNA hybridization or cytology from BAL cultures are required to confirm the diagnosis of CMV pneumonia [65, 66].
Pneumocystis jirovecii
Besides microscopic identification, which has been the classical reference method for detecting P. jirovecii, PCR has been introduced in the 1990s for early detection of this pathogen with a high sensitivity [67]. It is essential to distinguish between infection and colonization, which may be present in >50% of individuals without signs or symptoms of PcP [68]. A meta-analysis showed a very high sensitivity of 99% and a specificity of 90% [69], so that a negative Pneumocystis-PCR from a BAL sample at the time of diagnosis allows to put aside anti-Pneumocystis therapy [70]. More recently developed quantitative PCR assays appear to increase the specificity [71, 72]. A report on 71 non-HIV patients with proven PcP showed a positive predictive value of 98% when >1450 pathogens per ml were detected in BAL samples [73]. Determining β-d-glucan in serum may add to the differential diagnosis [74, 75], because a negative result of this test makes PcP highly unlikely [76].
filamentous fungi
Numerous methods have been developed for detecting fungal cell antigens such as Aspergillus galactomannan (GM), 1,3-β-d-glucan or nuclear amplification assays to identify fungal DNA for early noninvasive detection of filamentous fungi in febrile neutropenic patients with LI of undetermined etiology [77–81]. A positive (i.e. >0.5 OD) GM test from blood or from BAL samples, where a cutoff of ≥1.0 might be more appropriate [82], has been accepted as a significant finding indicating a probable invasive fungal infection in severely immunocompromised patients [83, 84]. It is questionable if Aspergillus GM in blood will become positive earlier than a chest CT scan [85]. Notably, the GM test may give false-positive results in patients treated with semisynthetic β-lactam antibiotics such as amoxicillin–clavulanate, piperacillin–tazobactam, carbapenems, ceftriaxone or cefepime [86, 87] as well as in those given enteral nutrition [88], those with other fungal infections such as fusariosis [89] and in BAL samples obtained using specific lavage solutions such as Plasmalyte™ [90]. False-positive Aspergillus antigenemia may also be due to blood product conditioning fluids [91]. A significant decline in the galactomannan Aspergillus antigen signal was described during storage of serum samples, in contrast to BAL samples, so that the time from taking a blood sample to testing should be minimized [92].
Details on antigen testing for fungal infection, including those other than aspergillosis [93], have been reviewed in a separate evidence-based guideline of our group [94].
Studies on panfungal or Aspergillus-specific PCR assays indicate that the use of these techniques on BAL samples seems superior when compared with blood samples, particularly in patients undergoing systemic antifungal therapy [81, 84, 95–97]. On lung biopsy specimens, PCR added to histopathology and culture may improve specification of pathogens [58]. Since there is no widely accepted international standardization of these assays available as yet for blood and BAL samples [98], PCR results have not become part of definition criteria for invasive fungal infections by now [10, 94, 99]. PCR presumably will become part of a diagnostic program for LI, including thoracic CT scans, serology and conventional microbiology from blood and BAL samples [100]. The combination of Aspergillus PCR and GM in BAL samples enhances diagnostics since positive results for both GM and PCR make a pulmonary aspergillosis highly likely [100], as confirmed recently by meta-analyses [101, 102].
Previous exposure to antifungal therapy may reduce the sensitivity of the galactomannan as well as quantitative PCR assays [103, 104].
Legionella pneumophila serogroup 1 antigen
While nosocomial outbreaks of legionellosis among cancer and leukemia patients have become very rare, a single-center report from 2007 has indicated that this differential diagnosis should not be ignored [105]. Testing of Legionella pneumophila serogroup 1 in urine helps to detect this diagnosis rapidly. Controlled clinical studies on the usefulness of routine testing for this antigen among febrile neutropenic patients with lung infiltrates are not available.
biomarkers
Nonspecific proinflammatory laboratory parameters like C-reactive protein, interleukin-6 [106], interleukin-8, tumor necrosis factor-α or procalcitonin plasma levels [107] are frequently used to assess the severity of infections and the response to antimicrobial therapy. In febrile neutropenic patients with LI, the predictive value of these parameters has not been investigated in prospective studies as yet. In clinical practice, the repeated measurement of these parameters typically parallels clinical observation and should be used for therapeutic decisions only in the context with clinical and imaging findings. Persisting fever, progressive or newly emerged LI and rising proinflammatory parameters typically indicate the need for a change in the antimicrobial treatment regimen [108].
algorithms for the clinical management of febrile neutropenic patients with LI
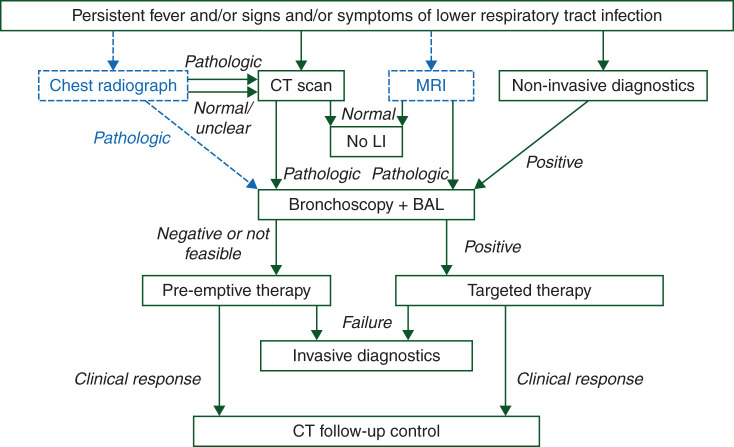
An algorithm for the clinical management of febrile neutropenic patients with LI is proposed in Figure 1. Synopses of recommendations for diagnostic measures are given in Tables 1–4. Recommendations for antimicrobial treatment and clinical management are summarized in Tables 5 and 6.
Figure 1.
Diagnostic procedures and treatment of neutropenic patients with fever and suspected or proven lung infiltrates.
Table 1.
Recommendations for imaging diagnostic procedures
| Recommendation | Strength |
|---|---|
| In febrile neutropenic patients with signs or symptoms of lower respiratory tract infection, multislice or high-resolution computed tomography (CT) scan of the lungs is the diagnostic method of choice | A-II |
| Conventional chest radiographs are not recommended for the diagnosis of lung infiltrates in febrile neutropenic patients | E-II |
| If a pulmonary CT scan is not feasible, MRI of the lungs is recommended | B-II |
| In most cases, thoracic CT scan can be done without contrast media | B-II |
| Multislice or high-resolution CT scan must be available at a maximum of 24 h after clinical indication has been established | A-II |
| If infiltrates are detected on pulmonary CT scans, bronchoalveolar lavage should be carried out at a segmental bronchus supplying an area of radiographic abnormalities | B-III |
| Whenever possible, thoracic CT showing abnormalities scans should be compared with previous scans | A-II |
| CT or magnetic resonance angiography may be considered if feeding vessel sign, reversed halo sign or hemoptysis are observed in suspected fungal pneumonia | B-III |
Table 4.
Clinical assessment of microbiological findings in febrile neutropenic patients with lung infiltrates
|
Table 5.
Recommendations for antimicrobial treatment and clinical management—I
| Recommendation | Strength |
|---|---|
| Febrile neutropenic patients with LI not typical for Pneumocystis pneumonia (PcP) or lobar bacterial pneumonia should receive mold-active systemic antifungal therapy | A-II |
| Preferred first-line therapy in this setting is voriconazole or liposomal amphotericin B | A-II |
| -Patients under current oral posa- or voriconazole prophylaxis should be switched to liposomal amphotericin B | C-III |
| The dosage of antifungal drugs in this setting is equal to the dosage used for proven mold infection | B-III |
| In severely neutropenic, hospitalized patients addressed here, antiviral agents, macrolide antibiotics, aminoglycosides or fluoroquinolones should only be given based on a conclusive microbiological finding | D-II |
| If PcP is suspected because of the pattern of lung infiltrates and new LDH elevation, treatment should be initiated also before bronchoscopy and BAL | B-II |
| Positive quantitative PCR (>1450 copies/ml) for P. jirovecii from BAL should trigger the start of systemic Pneumocystis treatment | B-II |
| First choice for treatment of PcP is high-dose trimethoprim–sulfamethoxazole (TMP/SMX) | A-II |
| In PcP patients intolerant of or refractory to high-dose TMP/SMX, a combination of clindamycin plus primaquine is the preferred alternative | B-II |
| In (non-HIV) patients with critical respiratory insufficiency due to PcP, adjunctive administration of glucocorticosteroids is not generally recommended and should only be considered in individual patients | C-II |
| Patients who have been successfully treated for PcP should receive secondary oral prophylaxis to prevent PcP recurrence | A-II |
| Drugs of choice for secondary PcP prophylaxis are intermittent TMP/SMX or monthly aerosolized pentamidine | B-II |
Table 6.
Recommendations for antimicrobial treatment and clinical management—II
| Recommendation | Strength |
|---|---|
| In patients with documented P. aeruginosa pneumonia, treatment with an antipseudomonal β-lactam plus an aminoglycoside is preferred when local in vitro resistance patterns indicate suboptimal activity of antipseudomonal β-lactam antibiotics | B-II |
| Antipseudomonal β-lactams suitable for treatment of P. aeruginosa pneumonia are piperacillin (±tazobactam), ceftazidime, imipenem/cilstatin, meropenem or cefepime | A-I |
| In patients who cannot be treated with an aminoglycoside, the antipseudomonal β-lactam should be combined with ciprofloxacin | B-II |
| The preferred regimen for documented S. maltophilia pneumonia is TMP/SMX | A-II |
| The dose of TMP/SMX for treatment of S. maltophilia pneumonia is similar to the treatment of P. jirovecii pneumonia | B-III |
| Preferred treatment regimens for CMV pneumonia are i.v. ganciclovir or foscarnet | A-II |
| The selection between ganciclovir and foscarnet should be based on the known toxicity profiles of these compounds and, if present, known resistance patterns | A-II |
| Response to antimicrobial treatment should be clinically assessed on a daily basis | A-II |
| Imaging studies to re-assess treatment response should generally not be ordered earlier than after 7 days of antimicrobial treatment | B-II |
| In patients with lack of clinical improvement, CT scan should be repeated after 7 days of treatment | B-II |
| Persisting fever, progressive or newly emerged LI and rising proinflammatory parameters after 7 days of treatment typically indicate the need for repeated microbiological diagnostics and a change in the antimicrobial treatment regimen | A-III |
| Intensive care should unrestrictedly be provided to patients with respiratory failure unless their prognosis is desperate due to other reasons | A-II |
| Multidisciplinary professionals should be involved in intensive care of cancer patients with respiratory failure caused by lung infiltrates | A-II |
diagnostic procedures
In patients with acute myeloid leukemia or myelodysplastic syndrome undergoing aggressive myelosuppressive chemotherapy expecting severe neutropenia lasting ≥10 days, serial monitoring of Aspergillus galactomannan from blood samples is recommended (B-II). The place for 1,3-β-d-glucan is not yet clearly defined, and PCR should be studied in the frame of clinical trials only. Serial panfungal PCR monitoring in patients with acute leukemia undergoing intensive myelosuppressive chemotherapy has failed to identify patients with a particularly high risk of developing invasive fungal disease [109]. Importantly, diagnostic procedures aim at obtaining microbiological results that confirm or help to modify the antimicrobial therapy, which should be initiated without awaiting results from diagnostic procedures (A-II).
Patients with fever of unknown origin not responding to an appropriate first-line therapy after 72–96 h should undergo thorough physical re-examination, imaging (Table 1) and microbiological diagnostics including a native thoracic CT scan and a CT scan of paranasal sinuses if symptoms or signs of sinusitis are present (A-II). A high-resolution or multislice thoracic CT scan must be available at a maximum of 24 h after clinical indication has been established (A-II). When LI are documented, noninvasive diagnostic tests should be repeated and bronchoscopy and BAL (Table 2) should be arranged within a maximum of 24 h (B-III). BAL samples must be sent immediately to the microbiological laboratory for workup, to be started within 4 h after sampling (A-III). Recommended microbiological procedures are listed in Table 3. A standardized procedure for bronchoscopy and BAL is recommended (A-II) [111].
Table 2.
Recommendations for bronchoscopy and bronchoalveolar lavage (BAL)
| Recommendation | Strength |
|---|---|
| Bronchoscopy and bronchoalveolar lavage should be carried out using a standardized protocol | A-II |
| Transbronchial biopsies are not recommended in febrile neutropenic (and thrombocytopenic) patients | D-II |
| If a tissue sample for histological, microbiological and molecular workup is required, CT-guided side-cut percutaneous biopsy, video-assisted thoracoscopy or open-lung biopsy should be used | B-II |
| Microbiological workup of BAL samples should follow a standardized protocol. | B-II |
| Bronchoscopy and BAL should be available within 24 h after clinical indication has been established | B-III |
| Urgent need to start or modify antimicrobial therapy should not be postponed by bronchoscopy and BAL | A-II |
| Bronchoscopy and BAL should only be carried out in patients without critical hypoxemia | B-II |
Table 3.
Diagnostic workup of bronchoalveolar lavage (BAL) samples from febrile neutropenic patients with lung infiltrates
| Recommended diagnostic program | Evidence level |
|---|---|
| Cytospin preparations for distinguishing intracellular from extracellular pathogens and identifying infiltration by underlying malignancy | B |
| Gram stain | B |
| Giemsa or May-Grünwald-Giemsa stain (assessment of macrophages, ciliated epithelium, leukocytes) | B |
| Mycobacterium tuberculosis (M. tuberculosis) polymerase chain reaction (PCR) | A |
| PCR for Pneumocystis jirovecii (P. jirovecii); quantitative if possible | A |
| Calcofluor white or equivalent (assessment of fungi and P. jirovecii) | A |
| Direct immunofluorescence test for P. jirovecii (confirmatory) | A |
| Aspergillus antigen (Galactomannan Sandwich ELISA) | A |
| Bacteriological cultures (Quantitative or semi-quantitative): dilutions of 10−2 and 10−4; culture media: blood agar, MacConkey/Endo, Levinthal/blood (bacterial culture), Legionella-BCYE or equivalent (Legionella spp.), for mycobacteria at least one solid and one liquid medium (e.g. Löwenstein–Jensen agar and Middlebrook 7H9 broth or equivalent), Sabouraud/Kimmig or equivalent (fungal culture) | A |
| Optional program | |
| Enrichment culture (Brain–Heart Infusion broth, dextrose broth) | C |
| Legionella PCR | B |
| PCR for cytomegalovirus (CMV), Respiratory Syncytial Virus, influenza A/B, parainfluenza 1-3, metapneumovirus and adenovirus | B |
| Quantitative PCR for Varicella Zoster Virus | B |
| Panfungal or Aspergillus PCR | B |
| Peripheral blood cultures 1 h after bronchoscopy to detect transient bacteremia | C |
| Throat swab to assess oral flora in comparison with BAL | C |
Invasive procedures such as open-lung or percutaneous core needle biopsy should be considered in patients with undetermined LI who urgently require histological identification while bronchoscopy and BAL have failed (B-II).
antimicrobial therapy in patients without documented causative pathogens
Considering the dismal prognosis of febrile neutropenic patients with LI not treated promptly with an appropriate antimicrobial regimen, it is recommended to start therapy on the basis of clinical, imaging and/or laboratory findings indicative of a particular infection in patients at risk for, but without proof of this infection. The type of underlying malignancy or immunosuppression has an instrumental impact on the selection of antimicrobial agents suitable for systemic therapy. In patients without a conclusive microbiological finding (Table 4) and a lack of response to antimicrobial treatment, re-assessment including thoracic CT scan and eventually also bronchoscopy and BAL should be arranged after 7 days (A-II).
patients with severe neutropenia due to chemotherapy for acute leukemia or other aggressive hematologic malignancy
This subgroup of febrile neutropenic patients with LI should be treated with a broad-spectrum β-lactam with antipseudomonal activity, as used for empirical treatment of fever of unknown origin (A-II). Streptococci including cephalosporin-resistant strains [112] must be included in the antimicrobial spectrum (B-II). Additionally, patients with LI not typical for PcP or lobar bacterial pneumonia should receive mold-active systemic antifungal therapy with voriconazole or liposomal amphotericin B (A-II) [113]. This high-risk subgroup of patients has a significant benefit from prompt when compared with delayed mold-active antifungal therapy [114]. It has been shown that patients with invasive aspergillosis treated with voriconazole or liposomal amphotericin B had superior response and survival rates when treated early versus later in the course of the disease (A-II) [115, 116]. In patients pretreated with voriconazole or posaconazole for systemic antifungal prophylaxis and in whom a breakthrough filamentous fungal pneumonia is suspected, measurement of antifungal drug levels and invasive diagnostic procedures should be taken into consideration (B-III) and treatment should be switched to liposomal amphotericin B (C-III). Particularly in patients in whom mucormycosis (zygomycosis) is suspected, liposomal amphotericin B is recommended (A-II). Independent from unequivocal documentation of pulmonary fungal infection, systemic antifungal treatment should be continued until hematopoietic recovery and regression of clinical and radiological signs of infection (B-III).
In patients without a microbiologically proven indication, the addition of an aminoglycoside or 5-flucytosine is not recommended due to a lack of benefit (E-I) [117]. In patients who had not received routine anti-Pneumocystis prophylaxis, have a thoracic CT scan suggesting PcP, and who have a rapid and otherwise unexplained rise of serum lactate dehydrogenase, prompt start of high-dose trimethoprim–sulfamethoxazole (TMP/SMX) therapy should be considered before bronchoscopy and BAL (B-II) [118]. In case of PcP, BAL will remain positive for this pathogen over several days despite appropriate antimicrobial therapy [119].
Except from selected patients who also have a severe cellular immunosuppression, antiviral agents such as ganciclovir are not recommended for early pre-emptive therapy in febrile neutropenic patients with LI (E-II). In general, glycopeptides, fluoroquinolones or macrolide antibiotics without a specific pathogen documented from clinically significant samples should not be used as well (D-III).
other subgroups of febrile patients with hematological malignancies
In individual patients undergoing high-dose chemotherapy and autologous hematopoietic stem-cell transplantation (AHSCT) with febrile neutropenia and LI of unknown origin, whose conditioning regimen included total body irradiation or who have been treated with alemtuzumab, antithymocyte globulin or fludarabine, bronchoscopy with BAL to check for CMV disease may be considered (B-III) [120]. A positive rapid culture or ‘immediate early antigen’ should prompt ganciclovir treatment (5 mg/kg every 12 h) (B-III), while foscarnet has not been investigated in this setting. Since patients after AHSCT have a very low risk of fungal pneumonia [121–123], pre-emptive antifungal therapy should not be given (D-II).
antimicrobial treatment in patients with documented pathogens
The interpretation of microbiological findings in neutropenic patients with LI is difficult (Table 4). Isolates typically originate from blood cultures or BAL samples. They may represent nonpathogenic contaminants, colonizers, co-pathogens or microorganisms causing a separate infection. If etiologically significant pathogens are detected, particularly multidrug-resistant bacteria, critical reappraisal of antimicrobial treatment to avoid fatal outcome due to delayed effective therapy is recommended (A-II) [124].
antimicrobial treatment of complicated bacterial pneumonias
In patients with a documented P. aeruginosa pneumonia, primary combination antibacterial therapy including an antipseudomonal β-lactam plus preferably an aminoglycoside or (if an aminoglycoside is contraindicated) ciprofloxacin is recommended by many authors [125–128]. However, meta-analyses have not unequivocally supported this recommendation [129–131], so that adequate β-lactam monotherapy may also be appropriate in this setting (B-II). Antipseudomonal β-lactams suitable for treatment of P. aeruginosa pneumonia are piperacillin (±tazobactam), ceftazidime, imipenem/cilastatin, meropenem and cefepime (A-I). Depending on their in vitro susceptibility pattern, multi-resistant Gram-negative aerobes such as extended-spectrum-β-lactamase-(ESBL-) producing E. coli, Enterobacter spp. or Klebsiella spp. as well as Acinetobacter spp. or P. aeruginosa require antimicrobial treatment selected appropriately according to this pattern (A-II). Pharmacokinetic aspects (penetration to lung tissue, possible inactivation by surfactant) must always be included in this selection (A-II). In individual patients with pneumonia caused by multi-resistant Gram-negative pathogens, aerosolized colistin has been successfully used as a part of the antimicrobial strategy [132]. Stenotrophomonas maltophilia (S. maltophilia) rarely causes pneumonia, while it is more frequently isolated from respiratory secretions representing selection of opportunistic microorganisms under broad-spectrum antibacterial treatment. In patients with suspected or documented S. maltophilia pneumonia, early antimicrobial intervention with high-dose TMP/SMX (15–20 mg/kg/day of trimethoprim) is recommended (B-II) [133, 134]. In individual patients, tigecycline-based treatment may be an appropriate alternative (C-II) [135]. It should be kept in mind that in vitro susceptibility may not predict clinical efficacy of antimicrobial agents in S. maltophilia infections [136].
While pneumonia caused by methicillin-susceptible Staphylococcus aureus (S. aureus) should be treated with oxacillin or flucloxacillin, methicillin-resistant S. aureus should preferably be treated with vancomycin, if no serious renal insufficiency is present (B-II). Linezolid is a possible alternative for first-line treatment (B-II) [137–139]; however, the risk of severe thrombocytopenia or even pancytopenia associated with linezolid must be taken into consideration [140]. Daptomycin should not be used for treatment of pneumonia, because it is inactivated by surfactant (E-I) [141].
treatment of CMV pneumonia
CMV pneumonia typically affects allogeneic stem-cell transplant recipients, but is also relevant in patients treated with lymphocyte-depleting agents like alemtuzumab or fludarabine. First-choice antiviral treatment options are foscarnet or ganciclovir (A-II). Foscarnet is associated with less myelosuppression, which is a serious adverse effect of ganciclovir [142]. On the other hand, reversible nephrotoxicity is one of the typical side-effects of foscarnet [143].
treatment of documented fungal pneumonia
Detailed recommendations for treatment of documented fungal pneumonia are provided in evidence-based guidelines [113, 144, 145]. Intravenous voriconazole (6 mg/kg every 12 h day 1, 4 mg/kg every 12 h thereafter) (A-I) or liposomal amphotericin B (3 mg/kg/day) (A-II) are recommended first-line choices for treatment of IPA. For mucormycosis (zygomycosis), liposomal amphotericin B is preferred (A-II), the recommended dose is ≥5 mg/kg/day (A-II). In patients with worsening LI and gas exchange within the first week of treatment, failure of antifungal therapy should only be considered if new LI emerge on control CT scans (B-III). At the same time, other causes such as a second infection, immune reconstitution syndrome, infiltrates caused by the underlying malignancy, toxicity from cancer treatment or yet insufficient duration of antifungal treatment should be ruled out (B-III) [146, 147]. Combination antifungal first-line treatment in patients with invasive mold infections is controversial. A prospective clinical study comparing voriconazole alone with the combination of voriconazole with anidulafungin in patients with proven and probable aspergillosis has not yet been published in detail [148]. For treatment of mucormycosis, a combination of liposomal amphotericin B and an echinocandin may be promising [149, 150]; however, randomized studies on this subject have not been conducted. A combination of liposomal amphotericin B and the iron chelator deferasirox for the treatment of mucormycoses has shown inferior clinical results for the combination when compared with the antifungal agent alone [151].
treatment of documented Pneumocystis pneumonia
If PcP is suspected, treatment with TMP/SMX (co-trimoxazole) at a dosage of TMP 15–20 mg/kg plus SMX 75–100 mg/kg daily (A-II) should be initiated immediately after asservation of representative samples (e.g. induced sputum or BAL) (B-II), since treatment delay may enhance mortality [152, 153]. In mild-to-moderate cases (oxygen partial pressure pO2 ≥70 mmHg or alveolar-arterial oxygen difference AaDO2 <45 mmHg) an oral therapy can be discussed, otherwise it should be administered i.v. In patients with proven PcP, treatment with TMP/SMX should be continued for at least 2 weeks (A-II). Clinical improvement should develop within 8 days, otherwise a second infection should be considered and diagnostic procedures repeated. In individual patients with persistent PcP, mutations in the genes for dihydropteroate synthase or dihydrofolate reductase may be taken into consideration [154–157]. In case of treatment failure or TMP/SMX intolerance, atovaquone oral suspension (750 mg twice times daily with meal), i.v. pentamidine (4 mg/kg daily) or clindamycin (600 mg four times daily or 900 mg three times daily i.v.) plus primaquine (30 mg daily p.o.) may represent treatment alternatives [158], with clindamycin + primaquine presumably being the most effective option (C-III) [159]. Glucose-6-phosphate dehydrogenase deficiency must be excluded before administration of dapsone or primaquine (A-I). Subsequently, patients should be given secondary prophylaxis (A-II) using oral TMP/SMX at a daily dosage of 160/800 mg given on 3 days per week (B-II) or with monthly pentamidine inhalation at a dose of 300 mg (B-II) [160, 161]. In patients with respiratory failure due to PcP, systemic corticosteroids may be beneficial in AIDS patients, but data are conflicting in non-HIV patients [162, 163]. Recent studies could not show a clinical benefit [164] and were even associated with increased mortality [165].
intensive care medicine
Reports on the outcome of cancer patients requiring intensive care have shown hospital survival rates of 60%–70% and higher [166–168]. Neutropenic patients with respiratory failure due to LI may have a favorable outcome under appropriate intensive care including mechanical ventilation [169–171]. Even if respiratory failure is due to IPA, survival can be achieved in around one third of patients [172]. It is therefore not justified to reject cancer patients from intensive care only because of their underlying malignancy [173]. Multidisciplinary care involving hematology-oncology professionals should be provided during intensive care treatment of these patients (A-II). Intensive care should unrestrictedly be provided to patients with respiratory failure (A-II), except from those whose prognosis is desperate due to other reasons or who have given a personal directive in order to abstain from it.
funding
None. Travel expenses and costs for group meetings were reimbursed by the German Society for Hematology and Medical Oncology.
disclosure
GM: Consultations: Gilead; Sponsored research: Pfizer; Honoraria: Astellas, Gilead, MSD, Pfizer. DB: Consultations: Gilead; Sponsored research: Gilead, Pfizer; Honoraria: Astellas, Gilead, Pfizer, MSD; Travel grants: Astellas, MSD, Pfizer. AH: Sponsored research: Pfizer; Honoraria: Astellas, MSD. CPH: Consultations: MSD, Basilea, Astellas, Gilead; Sponsored research: Pfizer; Honoraria: Gilead, MSD, Pfizer. SN: Honoraria: MSD, MR: Consultations: Gilead; Honoraria: Gilead, Pfizer. EA: Consultations: Gilead; Sponsored research: MSD, Pfizer. All other co-authors: nothing to disclose.
Supplementary Material
references
- 1.Einsele H, Bertz H, Beyer J, et al. Infectious complications after allogeneic stem cell transplantation: epidemiology and interventional therapy strategies—guidelines of the Infectious Diseases Working Party (AGIHO) of the German Society of Hematology and Oncology (DGHO) Ann Hematol. 2003;82(Suppl 2):S175–S185. doi: 10.1007/s00277-003-0772-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Korones DN. Is routine chest radiography necessary for the initial evaluation of fever in neutropenic children with cancer? Pediatr Blood Cancer. 2004;43:715–717. doi: 10.1002/pbc.20226. [DOI] [PubMed] [Google Scholar]
- 3.Navigante AH, Cerchietti LC, Costantini P, et al. Conventional chest radiography in the initial assessment of adult cancer patients with fever and neutropenia. Cancer Control. 2002;9:346–351. doi: 10.1177/107327480200900411. [DOI] [PubMed] [Google Scholar]
- 4.Oude Nijhuis CS, Gietema JA, Vellenga E, et al. Routine radiography does not have a role in the diagnostic evaluation of ambulatory adult febrile neutropenic cancer patients. Eur J Cancer. 2003;39:2495–2498. doi: 10.1016/j.ejca.2003.06.002. [DOI] [PubMed] [Google Scholar]
- 5.Heussel CP, Kauczor HU, Heussel G, et al. Early detection of pneumonia in febrile neutropenic patients: use of thin-section CT. Am J Roentgenol. 1997;169:1347–1353. doi: 10.2214/ajr.169.5.9353456. [DOI] [PubMed] [Google Scholar]
- 6.Heussel CP, Kauczor HU, Heussel GE, et al. Pneumonia in febrile neutropenic patients and in bone marrow and blood stem-cell transplant recipients: use of high-resolution computed tomography. J Clin Oncol. 1999;17:796–805. doi: 10.1200/JCO.1999.17.3.796. [DOI] [PubMed] [Google Scholar]
- 7.Caillot D, Mannone L, Cuisenier B, Couaillier JF. Role of early diagnosis and aggressive surgery in the management of invasive pulmonary aspergillosis in neutropenic patients. Clin Microbiol Infect. 2001;7(Suppl 2):54–61. doi: 10.1111/j.1469-0691.2001.tb00010.x. [DOI] [PubMed] [Google Scholar]
- 8.Georgiadou SP, Sipsas NV, Marom EM, Kontoyiannis DP. The diagnostic value of halo and reversed halo signs for invasive mold infections in compromised hosts. Clin Infect Dis. 2011;52:1144–1155. doi: 10.1093/cid/cir122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rámila E, Sureda A, Martino R, et al. Bronchoscopy guided by high-resolution computed tomography for the diagnosis of pulmonary infections in patients with hematologic malignancies and normal plain chest X-ray. Haematologica. 2000;85:961–966. [PubMed] [Google Scholar]
- 10.De Pauw B, Walsh TJ, Donnelly JP, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group Consensus Group. Clin Infect Dis. 2008;46:1813–1821. doi: 10.1086/588660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cornillet A, Camus C, Nimubona S, et al. Comparison of epidemiological, clinical, and biological features of invasive aspergillosis in neutropenic and nonneutropenic patients: a 6-year survey. Clin Infect Dis. 2006;43:577–584. doi: 10.1086/505870. [DOI] [PubMed] [Google Scholar]
- 12.Godoy MC, Viswanathan C, Marchiori E, et al. The reversed halo sign: update and differential diagnosis. Br J Radiol. 2012;85:1226–1235. doi: 10.1259/bjr/54532316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suzuki HI, Asai T, Tamaki Z, et al. Drug-induced hypersensitivity syndrome with rapid hematopoietic reconstitution during treatment for acute myeloid leukemia. Haematologica. 2008;93:469–470. doi: 10.3324/haematol.12029. [DOI] [PubMed] [Google Scholar]
- 14.Caillot D, Latrabe V, Thiébaut A, et al. Computer tomography in pulmonary invasive aspergillosis in hematological patients with neutropenia: a useful tool for diagnosis and assessment of outcome in clinical trials. Eur J Radiol. 2010;74:e172–e175. doi: 10.1016/j.ejrad.2009.05.058. [DOI] [PubMed] [Google Scholar]
- 15.Escuissato DL, Gasparetto EL, Marchiori E, et al. Pulmonary infections after bone marrow transplantation: high-resolution CT findings in 111 patients. Am J Roentgenol. 2005;185:608–615. doi: 10.2214/ajr.185.3.01850608. [DOI] [PubMed] [Google Scholar]
- 16.Gasparetto EL, Escuissato DL, Marchiori E, et al. High-resolution CT findings of respiratory syncytial virus pneumonia after bone marrow transplantation. Am J Roentgenol. 2004;182:1133–1137. doi: 10.2214/ajr.182.5.1821133. [DOI] [PubMed] [Google Scholar]
- 17.Hachem R, Sumoza D, Hanna H, et al. Clinical and radiologic predictors of invasive pulmonary aspergillosis in cancer patients: should the European Organization for Research and Treatment of Cancer/Mycosis Study Group criteria be revised? Cancer. 2006;106:1581–1586. doi: 10.1002/cncr.21755. [DOI] [PubMed] [Google Scholar]
- 18.Heussel CP, Kauczor HU, Ullmann AJ. Pneumonia in neutropenic patients. Eur Radiol. 2004;14:256–271. doi: 10.1007/s00330-003-1985-6. [DOI] [PubMed] [Google Scholar]
- 19.Kanne JP, Yandow DR, Meyer CA. Pneumocystis jiroveci pneumonia: high-resolution CT findings in patients with and without HIV infection. AJR Am J Roentgenol. 2012;198:W555–W561. doi: 10.2214/AJR.11.7329. [DOI] [PubMed] [Google Scholar]
- 20.Tasaka S, Tokuda H, Sakai F, et al. Comparison of clinical and radiological features of Pneumocystis pneumonia between malignancy cases and acquired immunodeficiency syndrome cases: a multicenter study. Intern Med. 2010;49:273–281. doi: 10.2169/internalmedicine.49.2871. [DOI] [PubMed] [Google Scholar]
- 21.Vogel MN, Vatlach M, Weissgerber P, et al. HRCT-features of Pneumocystis jiroveci pneumonia and their evolution before and after treatment in non-HIV immunocompromised patients. Eur J Radiol. 2012;81:1315–1320. doi: 10.1016/j.ejrad.2011.02.052. [DOI] [PubMed] [Google Scholar]
- 22.Georgiadou SP, Kontoyiannis DP. Concurrent lung infections in patients with hematological malignancies and invasive pulmonary aspergillosis: how firm is the Aspergillus diagnosis? J Infect. 2012;65:262–268. doi: 10.1016/j.jinf.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shorr AF, Susla GM, O'Grady NP. Pulmonary infiltrates in the non-HIV-infected immunocompromised patient: etiologies, diagnostic strategies, and outcomes. Chest. 2004;125:260–271. doi: 10.1378/chest.125.1.260. [DOI] [PubMed] [Google Scholar]
- 24.Sonnet S, Buitrago-Téllez CH, Tamm M, et al. Direct detection of angioinvasive pulmonary aspergillosis in immunosuppressed patients: preliminary results with high-resolution 16-MDCT angiography. AJR Am J Roentgenol. 2005;184:746–751. doi: 10.2214/ajr.184.3.01840746. [DOI] [PubMed] [Google Scholar]
- 25.Stanzani M, Battista G, Sassi C, et al. Computed tomographic pulmonary angiography for diagnosis of invasive mold diseases in patients with hematological malignancies. Clin Infect Dis. 2012;54:610–616. doi: 10.1093/cid/cir861. [DOI] [PubMed] [Google Scholar]
- 26.Attenberger UI, Morelli JN, Henzler T, et al. 3Tesla proton MRI for the diagnosis of pneumonia/lung infiltrates in neutropenic patients with acute myeloid leukemia: initial results in comparison to HRCT. Eur J Radiol. 2014;83:e61–e66. doi: 10.1016/j.ejrad.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 27.Eibel R, Herzog P, Dietrich O, et al. Pulmonary abnormalities in immunocompromised patients: comparative detection with parallel acquisition MR imaging and thin-section helical CT. Radiology. 2006;241:880–891. doi: 10.1148/radiol.2413042056. [DOI] [PubMed] [Google Scholar]
- 28.Vos FJ, Donnelly JP, Oyen WJ, et al. 18F-FDG PET/CT for diagnosing infectious complications in patients with severe neutropenia after intensive chemotherapy for haematological malignancy or stem cell transplantation. Eur J Nucl Med Mol Imaging. 2012;39:120–128. doi: 10.1007/s00259-011-1939-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Caillot D, Couaillier JF, Bernard A, et al. Increasing volume and changing characteristics of invasive pulmonary aspergillosis on sequential thoracic computed tomography scans in patients with neutropenia. J Clin Oncol. 2001;19:253–259. doi: 10.1200/JCO.2001.19.1.253. [DOI] [PubMed] [Google Scholar]
- 30.Brodoefel H, Vogel M, Hebart H, et al. Long-term CT follow-up in 40 non-HIV immunocompromised patients with invasive pulmonary aspergillosis. Am J Roentgenol. 2006;187:404–413. doi: 10.2214/AJR.05.0513. [DOI] [PubMed] [Google Scholar]
- 31.Ellis ME, Spence D, Bouchama A, et al. Open lung biopsy provides a higher and more specific diagnostic yield compared to bronchoalveolar lavage in immunocompromised patients. Scand J Infect Dis. 1995;27:157–162. doi: 10.3109/00365549509018998. [DOI] [PubMed] [Google Scholar]
- 32.Azoulay E, Mokart D, Rabbat A, et al. Diagnostic bronchoscopy in hematology and oncology patients with acute respiratory failure. Crit Care Med. 2008;36:100–107. doi: 10.1097/01.CCM.0000295590.33145.C4. [DOI] [PubMed] [Google Scholar]
- 33.Hummel M, Rudert S, Hof H, et al. Diagnostic yield of bronchoscopy with bronchoalveolar lavage in febrile patients with hematologic malignancies and pulmonary infiltrates. Ann Hematol. 2008;87:291–297. doi: 10.1007/s00277-007-0391-6. [DOI] [PubMed] [Google Scholar]
- 34.Rolston KV, Bodey GP, Safdar A. Polymicrobial infection in patients with cancer: an underappreciated and underreported entity. Clin Infect Dis. 2007;45:228–233. doi: 10.1086/518873. [DOI] [PubMed] [Google Scholar]
- 35.Dettenkofer M, Wenzler-Rottele S, Babikir R, et al. Surveillance of nosocomial sepsis and pneumonia in patients with a bone marrow or peripheral blood stem cell transplant. Clin Infect Dis. 2005;40:926–931. doi: 10.1086/428046. [DOI] [PubMed] [Google Scholar]
- 36.Chamilos G, Luna M, Lewis RE, et al. Invasive fungal infections in patients with hematologic malignancies in a tertiary care cancer center: an autopsy study over a 15-year period (1989–2003) Haematologica. 2006;91:986–989. [PubMed] [Google Scholar]
- 37.Sharma S, Nadrous HF, Peters SG, et al. Pulmonary complications in adult blood and marrow transplant recipients: autopsy findings. Chest. 2005;128:1385–1392. doi: 10.1378/chest.128.3.1385. [DOI] [PubMed] [Google Scholar]
- 38.Perfect JR, Cox GM, Lee JY, et al. The impact of culture isolation of Aspergillus species: a hospital-based survey of aspergillosis. Clin Infect Dis. 2001;33:1824–1833. doi: 10.1086/323900. [DOI] [PubMed] [Google Scholar]
- 39.Azoulay E, Mokart D, Lambert J, et al. Diagnostic strategy for hematology and oncology patients with acute respiratory failure: randomized controlled trial. Am J Respir Crit Care Med. 2010;182:1038–1046. doi: 10.1164/rccm.201001-0018OC. [DOI] [PubMed] [Google Scholar]
- 40.Boersma WG, Erjavec Z, van der Werf TS, et al. Bronchoscopic diagnosis of pulmonary infiltrates in granulocytopenic patients with hematologic malignancies: BAL versus PSB and PBAL. Respir Med. 2007;101:317–325. doi: 10.1016/j.rmed.2006.04.021. [DOI] [PubMed] [Google Scholar]
- 41.Jain P, Sandur S, Meli Y, et al. Role of flexible bronchoscopy in immunocompromised patients with lung infiltrates. Chest. 2004;125:712–722. doi: 10.1378/chest.125.2.712. [DOI] [PubMed] [Google Scholar]
- 42.Zihlif M, Khanchandani G, Ahmed HP, Soubani AO. Surgical lung biopsy in patients with hematological malignancy or hematopoietic stem cell transplantation and unexplained pulmonary infiltrates: improved outcome with specific diagnosis. Am J Hematol. 2005;78:94–99. doi: 10.1002/ajh.20258. [DOI] [PubMed] [Google Scholar]
- 43.Mulabecirovic A, Gaulhofer P, Auner HW, et al. Pulmonary infiltrates in patients with haematologic malignancies: transbronchial lung biopsy increases the diagnostic yield with respect to neoplastic infiltrates and toxic pneumonitis. Ann Hematol. 2004;83:420–422. doi: 10.1007/s00277-004-0876-5. [DOI] [PubMed] [Google Scholar]
- 44.Patel NR, Lee PS, Kim JH, et al. The influence of diagnostic bronchoscopy on clinical outcomes comparing adult autologous and allogeneic bone marrow transplant patients. Chest. 2005;127:1388–1396. doi: 10.1378/chest.127.4.1388. [DOI] [PubMed] [Google Scholar]
- 45.Peikert T, Rana S, Edell ES. Safety, diagnostic yield, and therapeutic implications of flexible bronchoscopy in patients with febrile neutropenia and pulmonary infiltrates. Mayo Clin Proc. 2005;80:1414–1420. doi: 10.4065/80.11.1414. [DOI] [PubMed] [Google Scholar]
- 46.Shah AA, Hazen KC. Diagnostic accuracy of histopathologic and cytopathologic examination of Aspergillus species. Am J Clin Pathol. 2013;139:55–61. doi: 10.1309/AJCPO8VTSK3HRNUT. [DOI] [PubMed] [Google Scholar]
- 47.Daniels CE, Myers JL, Utz JP, et al. Organizing pneumonia in patients with hematologic malignancies: a steroid-responsive lesion. Respir Med. 2007;101:162–168. doi: 10.1016/j.rmed.2006.03.035. [DOI] [PubMed] [Google Scholar]
- 48.Camus P, Costabel U. Drug-induced respiratory disease in patients with hematological diseases. Semin Respir Crit Care Med. 2005;26:458–481. doi: 10.1055/s-2005-922030. [DOI] [PubMed] [Google Scholar]
- 49.Armenian SH, La Via WV, Siegel SE, Mascarenhas L. Evaluation of persistent pulmonary infiltrates in pediatric oncology patients. Pediatr Blood Cancer. 2007;48:165–172. doi: 10.1002/pbc.20747. [DOI] [PubMed] [Google Scholar]
- 50.Theodore S, Liava'a M, Antippa P, et al. Surgical management of invasive pulmonary fungal infection in hematology patients. Ann Thorac Surg. 2009;87:1532–1538. doi: 10.1016/j.athoracsur.2009.02.069. [DOI] [PubMed] [Google Scholar]
- 51.Georgiadou SP, Sampsonas FL, Rice D, et al. Open-lung biopsy in patients with undiagnosed lung lesions referred at a tertiary cancer center is safe and reveals noncancerous, noninfectious entities as the most common diagnoses. Eur J Clin Microbiol Infect Dis. 2013;32:101–105. doi: 10.1007/s10096-012-1720-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.White DA, Wong PW, Downey R. The utility of open lung biopsy in patients with hematologic malignancies. Am J Respir Crit Care Med. 2000;161:723–729. doi: 10.1164/ajrccm.161.3.9904016. [DOI] [PubMed] [Google Scholar]
- 53.Hoffer FA, Gow K, Flynn PM, Davidoff A. Accuracy of percutaneous lung biopsy for invasive pulmonary aspergillosis. Pediatr Radiol. 2001;31:144–152. doi: 10.1007/s002470000402. [DOI] [PubMed] [Google Scholar]
- 54.Carrafiello G, Lagana D, Nosari AM, et al. Utility of computed tomography and of fine needle aspiration biopsy in early diagnosis of fungal pulmonary infections. Radiol Med. 2006;111:33–41. doi: 10.1007/s11547-006-0004-9. [DOI] [PubMed] [Google Scholar]
- 55.Gupta S, Sultenfuss M, Romaguera JE, et al. CT-guided percutaneous lung biopsies in patients with haematologic malignancies and undiagnosed pulmonary lesions. Hematol Oncol. 2010;28:75–81. doi: 10.1002/hon.923. [DOI] [PubMed] [Google Scholar]
- 56.Lass-Flörl C, Resch G, Nachbaur D, et al. The value of computed tomography-guided percutaneous lung biopsy for diagnosis of invasive fungal infection in immunocompromised patients. Clin Infect Dis. 2007;45:e101–e104. doi: 10.1086/521245. [DOI] [PubMed] [Google Scholar]
- 57.Reinwald M, Spiess B, Heinz WJ, et al. Aspergillus PCR-based investigation of fresh tissue and effusion samples in patients with suspected invasive Aspergillosis enhances diagnostic capabilities. J Clin Microbiol. 2013;51:4178–4185. doi: 10.1128/JCM.02387-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rickerts V, Mousset S, Lambrecht E, et al. Comparison of histopathological analysis, culture, and polymerase chain reaction assays to detect invasive mold infections from biopsy specimens. Clin Infect Dis. 2007;44:1078–1083. doi: 10.1086/512812. [DOI] [PubMed] [Google Scholar]
- 59.Manhire A, Charig M, Clelland C, et al. Guidelines for radiologically guided lung biopsy. Thorax. 2003;58:920–936. doi: 10.1136/thorax.58.11.920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chemaly RF, Hanmod SS, Rathod DB, et al. The characteristics and outcomes of parainfluenza virus infections in 200 patients with leukemia or recipients of hematopoietic stem cell transplantation. Blood. 2012;119:2738–2745. doi: 10.1182/blood-2011-08-371112. [DOI] [PubMed] [Google Scholar]
- 61.Hirsch HH, Martino R, Ward KN, et al. Fourth European Conference on Infections in Leukaemia (ECIL-4): guidelines for diagnosis and treatment of human respiratory syncytial virus, parainfluenza virus, metapneumovirus, rhinovirus, and coronavirus. Clin Infect Dis. 2013;56:258–266. doi: 10.1093/cid/cis844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Debur MC, Vidal LR, Stroparo E, et al. Human metapneumovirus infection in hematopoietic stem cell transplant recipients. Transpl Infect Dis. 2010;12:173–179. doi: 10.1111/j.1399-3062.2009.00465.x. [DOI] [PubMed] [Google Scholar]
- 63.Williams JV, Martino R, Rabella N, et al. A prospective study comparing human metapneumovirus with other respiratory viruses in adults with hematologic malignancies and respiratory tract infections. J Infect Dis. 2005;192:1061–1065. doi: 10.1086/432732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hohenthal U, Itälä M, Salonen J, et al. Bronchoalveolar lavage in immunocompromised patients with haematological malignancy—value of new microbiological methods. Eur J Haematol. 2005;74:203–211. doi: 10.1111/j.1600-0609.2004.00373.x. [DOI] [PubMed] [Google Scholar]
- 65.Boeckh M. Complications, diagnosis, management, and prevention of CMV infections: current and future. Hematology Am Soc Hematol Educ Program. 2011;2011:305–309. doi: 10.1182/asheducation-2011.1.305. [DOI] [PubMed] [Google Scholar]
- 66.Ljungman P, Griffiths P, Paya C. Definitions of cytomegalovirus infection and disease in transplant recipients. Clin Infect Dis. 2002;34:1094–1097. doi: 10.1086/339329. [DOI] [PubMed] [Google Scholar]
- 67.Sing A, Trebesius K, Roggenkamp A, et al. Evaluation of diagnostic value and epidemiological implications of PCR for Pneumocystis carinii in different immunosuppressed and immunocompetent patient groups. J Clin Microbiol. 2000;38:1461–1467. doi: 10.1128/jcm.38.4.1461-1467.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ponce CA, Gallo M, Bustamante R, Vargas SL. Pneumocystis colonization is highly prevalent in the autopsied lungs of the general population. Clin Infect Dis. 2010;50:347–353. doi: 10.1086/649868. [DOI] [PubMed] [Google Scholar]
- 69.Lu Y, Ling G, Qiang C, et al. PCR diagnosis of Pneumocystis pneumonia: a bivariate meta-analysis. J Clin Microbiol. 2011;49:4361–4363. doi: 10.1128/JCM.06066-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Azoulay E, Bergeron A, Chevret S, et al. Polymerase chain reaction for diagnosing Pneumocystis pneumonia in non-HIV immunocompromised patients with pulmonary infiltrates. Chest. 2009;135:655–661. doi: 10.1378/chest.08-1309. [DOI] [PubMed] [Google Scholar]
- 71.Alanio A, Desoubeaux G, Sarfati C, et al. Real-time PCR assay-based strategy for differentiation between active Pneumocystis jirovecii pneumonia and colonization in immunocompromised patients. Clin Microbiol Infect. 2011;17:1531–1537. doi: 10.1111/j.1469-0691.2010.03400.x. [DOI] [PubMed] [Google Scholar]
- 72.Hardak E, Neuberger A, Yigla M, et al. Outcome of Pneumocystis jirovecii pneumonia diagnosed by polymerase chain reaction in patients without human immunodeficiency virus infection. Respirology. 2012;17:681–686. doi: 10.1111/j.1440-1843.2012.02158.x. [DOI] [PubMed] [Google Scholar]
- 73.Mühlethaler K, Bögli-Stuber K, Wasmer S, et al. Quantitative PCR to diagnose Pneumocystis pneumonia in immunocompromised non-HIV patients. Eur Respir J. 2012;39:971–978. doi: 10.1183/09031936.00095811. [DOI] [PubMed] [Google Scholar]
- 74.Onishi A, Sugiyama D, Kogata Y, et al. Diagnostic accuracy of serum 1,3-β-D-glucan for Pneumocystis jiroveci pneumonia, invasive candidiasis, and invasive aspergillosis: systematic review and meta-analysis. J Clin Microbiol. 2012;50:7–15. doi: 10.1128/JCM.05267-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Theel ES, Jespersen DJ, Iqbal S, et al. Detection of (1, 3)-β-D-glucan in bronchoalveolar lavage and serum samples collected from immunocompromised hosts. Mycopathologia. 2013;175:33–41. doi: 10.1007/s11046-012-9579-y. [DOI] [PubMed] [Google Scholar]
- 76.Karageorgopoulos DE, Qu JM, Korbila IP, et al. Accuracy of β-D-glucan for the diagnosis of Pneumocystis jirovecii pneumonia: a meta-analysis. Clin Microbiol Infect. 2013;19:39–49. doi: 10.1111/j.1469-0691.2011.03760.x. [DOI] [PubMed] [Google Scholar]
- 77.Busca A, Locatelli F, Barbui A, et al. Usefulness of sequential Aspergillus galactomannan antigen detection combined with early radiologic evaluation for diagnosis of invasive pulmonary aspergillosis in patients undergoing allogeneic stem cell transplantation. Transplant Proc. 2006;38:1610–1613. doi: 10.1016/j.transproceed.2006.02.072. [DOI] [PubMed] [Google Scholar]
- 78.Einsele H, Hebart H, Roller G, et al. Detection and identification of fungal pathogens in blood by using molecular probes. J Clin Microbiol. 1997;35:1353–1360. doi: 10.1128/jcm.35.6.1353-1360.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Maertens J, Verhaegen J, Demuynck H, et al. Autopsy-controlled prospective evaluation of serial screening for circulating galactomannan by a sandwich enzyme-linked immunosorbent assay for hematological patients at risk for invasive aspergillosis. J Clin Microbiol. 1999;37:3223–3228. doi: 10.1128/jcm.37.10.3223-3228.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Maertens JA, Klont R, Masson C, et al. Optimization of the cutoff value for the Aspergillus double-sandwich enzyme immunoassay. Clin Infect Dis. 2007;44:1329–1336. doi: 10.1086/514349. [DOI] [PubMed] [Google Scholar]
- 81.Spiess B, Buchheidt D, Baust C, et al. Development of a LightCycler PCR assay for detection and quantification of Aspergillus fumigatus DNA in clinical samples from neutropenic patients. J Clin Microbiol. 2003;41:1811–1818. doi: 10.1128/JCM.41.5.1811-1818.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zou M, Tang L, Zhao S, et al. Systematic review and meta-analysis of detecting galactomannan in bronchoalveolar lavage fluid for diagnosing invasive aspergillosis. PLoS One. 2012;7:e43347. doi: 10.1371/journal.pone.0043347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Becker MJ, Lugtenburg EJ, Cornelissen JJ, et al. Galactomannan detection in computerized tomography-based broncho-alveolar lavage fluid and serum in haematological patients at risk for invasive pulmonary aspergillosis. Br J Haematol. 2003;121:448–457. doi: 10.1046/j.1365-2141.2003.04308.x. [DOI] [PubMed] [Google Scholar]
- 84.Buchheidt D, Baust C, Skladny H, et al. Detection of Aspergillus species in blood and bronchoalveolar lavage samples from immunocompromised patients by means of 2-step polymerase chain reaction: clinical results. Clin Infect Dis. 2001;33:428–435. doi: 10.1086/321887. [DOI] [PubMed] [Google Scholar]
- 85.Weisser M, Rausch C, Droll A, et al. Galactomannan does not precede major signs on a pulmonary computerized tomographic scan suggestive of invasive aspergillosis in patients with hematological malignancies. Clin Infect Dis. 2005;41:1143–1149. doi: 10.1086/444462. [DOI] [PubMed] [Google Scholar]
- 86.Aubry A, Porcher R, Bottero J, et al. Occurrence and kinetics of false-positive Aspergillus galactomannan test results following treatment with beta-lactam antibiotics in patients with hematological disorders. J Clin Microbiol. 2006;44:389–394. doi: 10.1128/JCM.44.2.389-394.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Boonsarngsuk V, Niyompattama A, Teosirimongkol C, Sriwanichrak K. False-positive serum and bronchoalveolar lavage Aspergillus galactomannan assays caused by different antibiotics. Scand J Infect Dis. 2010;42:461–468. doi: 10.3109/00365541003602064. [DOI] [PubMed] [Google Scholar]
- 88.Girmenia C, Santilli S, Ballarò D, et al. Enteral nutrition may cause false-positive results of Aspergillus galactomannan assay in absence of gastrointestinal diseases. Mycoses. 2011;54:e883–e884. doi: 10.1111/j.1439-0507.2011.02022.x. [DOI] [PubMed] [Google Scholar]
- 89.Tortorano AM, Esposto MC, Prigitano A, et al. Cross-reactivity of Fusarium spp. in the Aspergillus galactomannan enzyme-linked immunosorbent assay. J Clin Microbiol. 2012;50:1051–1053. doi: 10.1128/JCM.05946-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hage CA, Reynolds JM, Durkin M, et al. Plasmalyte as a cause of false-positive results for Aspergillus galactomannan in bronchoalveolar lavage fluid. J Clin Microbiol. 2007;45:676–677. doi: 10.1128/JCM.01940-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Martín-Rabadán P, Gijón P, Alonso Fernández R, et al. False-positive Aspergillus antigenemia due to blood product conditioning fluids. Clin Infect Dis. 2012;55:e22–e27. doi: 10.1093/cid/cis493. [DOI] [PubMed] [Google Scholar]
- 92.Johnson GL, Sarker SJ, Hill K, et al. Significant decline in galactomannan signal during storage of clinical serum samples. Int J Mol Sci. 2013;14:12970–7. doi: 10.3390/ijms140712970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ostrosky-Zeichner L, Alexander BD, Kett DH, et al. Multicenter clinical evaluation of the (1,3) beta-D-glucan assay as an aid to diagnosis of fungal infections in humans. Clin Infect Dis. 2005;41:654–659. doi: 10.1086/432470. [DOI] [PubMed] [Google Scholar]
- 94.Ruhnke M, Böhme A, Buchheidt D, et al. Diagnosis of invasive fungal infections in hematology and oncology guidelines from the Infectious Diseases Working Party in Haematology and Oncology of the German Society for Haematology and Oncology (AGIHO) Ann Oncol. 2012;23:823–833. doi: 10.1093/annonc/mdr407. [DOI] [PubMed] [Google Scholar]
- 95.Lass-Flörl C, Gunsilius E, Gastl G, et al. Diagnosing invasive aspergillosis during antifungal therapy by PCR analysis of blood samples. J Clin Microbiol. 2004;42:4154–4157. doi: 10.1128/JCM.42.9.4154-4157.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ljungman P, von Dobeln L, Ringholm L, et al. The value of CMV and fungal PCR for monitoring for acute leukaemia and autologous stem cell transplant patients. Scand J Infect Dis. 2005;37:121–127. [PubMed] [Google Scholar]
- 97.Musher B, Fredricks D, Leisenring W, et al. Aspergillus galactomannan enzyme immunoassay and quantitative PCR for diagnosis of invasive aspergillosis with bronchoalveolar lavage fluid. J Clin Microbiol. 2004;42:5517–5522. doi: 10.1128/JCM.42.12.5517-5522.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.White PL, Bretagne S, Klingspor L, et al. Aspergillus PCR: one step closer to standardization. J Clin Microbiol. 2010;48:1231–1240. doi: 10.1128/JCM.01767-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mengoli C, Cruciani M, Barnes RA, et al. Use of PCR for diagnosis of invasive aspergillosis: systematic review and meta-analysis. Lancet Infect Dis. 2009;9:89–96. doi: 10.1016/S1473-3099(09)70019-2. [DOI] [PubMed] [Google Scholar]
- 100.White PL, Linton CJ, Perry MD, et al. The evolution and evaluation of a whole blood polymerase chain reaction assay for the detection of invasive aspergillosis in hematology patients in a routine clinical setting. Clin Infect Dis. 2006;42:479–486. doi: 10.1086/499949. [DOI] [PubMed] [Google Scholar]
- 101.Avni T, Levy I, Sprecher H, et al. Diagnostic accuracy of PCR alone compared to galactomannan in bronchoalveolar lavage fluid for diagnosis of invasive pulmonary aspergillosis: a systematic review. J Clin Microbiol. 2012;50:3652–3658. doi: 10.1128/JCM.00942-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Heng SC, Morrissey O, Chen SC, et al. Utility of bronchoalveolar lavage fluid galactomannan alone or in combination with PCR for the diagnosis of invasive aspergillosis in adult hematology patients: a systematic review and meta-analysis. Crit Rev Microbiol. 2013 doi: 10.3109/1040841X.2013.804033. June 25 [epub ahead of print], doi: 10.3109/1040841X.2013.804033. [DOI] [PubMed] [Google Scholar]
- 103.Marr KA, Laverdiere M, Gugel A, Leisenring W. Antifungal therapy decreases sensitivity of the Aspergillus galactomannan enzyme immunoassay. Clin Infect Dis. 2005;40:1762–1769. doi: 10.1086/429921. [DOI] [PubMed] [Google Scholar]
- 104.McCulloch E, Ramage G, Rajendran R, et al. Antifungal treatment affects the laboratory diagnosis of invasive aspergillosis. J Clin Pathol. 2012;65:83–86. doi: 10.1136/jcp.2011.090464. [DOI] [PubMed] [Google Scholar]
- 105.Gudiol C, Verdaguer R, Angeles Domínguez M, et al. Outbreak of Legionnaires' disease in immunosuppressed patients at a cancer centre: usefulness of universal urine antigen testing and early levofloxacin therapy. Clin Microbiol Infect. 2007;13:1125–1128. doi: 10.1111/j.1469-0691.2007.01805.x. [DOI] [PubMed] [Google Scholar]
- 106.von Lilienfeld-Toal M, Dietrich MP, Glasmacher A, et al. Markers of bacteremia in febrile neutropenic patients with haematological malignancies: procalcitonin and IL-6 are more reliable than C-reactive protein. Eur J Clin Microbiol Infect Dis. 2004;23:539–544. doi: 10.1007/s10096-004-1156-y. [DOI] [PubMed] [Google Scholar]
- 107.Monneret G, Doche C, Durand DV, et al. Procalcitonin as a specific marker of bacterial infection in adults. Clin Chem Lab Med. 1998;36:67–68. doi: 10.1515/CCLM.1998.012. [DOI] [PubMed] [Google Scholar]
- 108.Robinson JO, Lamoth F, Bally F, et al. Monitoring procalcitonin in febrile neutropenia: what is its utility for initial diagnosis of infection and reassessment in persistent fever? PLoS One. 2011;6:e18886. doi: 10.1371/journal.pone.0018886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Maschmeyer G, Heinz WJ, Hertenstein B, et al. Immediate versus deferred empirical antifungal (IDEA) therapy in high-risk patients with febrile neutropenia: a randomized, double-blind, placebo-controlled, multicenter study. Eur J Clin Microbiol Infect Dis. 2013;32:679–689. doi: 10.1007/s10096-012-1794-4. [DOI] [PubMed] [Google Scholar]
- 110.Kish MA Infectious Diseases Society of America . Guide to development of practice guidelines. Clin Infect Dis. 2001;32:851–854. doi: 10.1086/319366. [DOI] [PubMed] [Google Scholar]
- 111.Sampsonas F, Kontoyiannis DP, Dickey BF, Evans SE. Performance of a standardized bronchoalveolar lavage protocol in a comprehensive cancer center: a prospective 2-year study. Cancer. 2011;117:3424–3433. doi: 10.1002/cncr.25905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Carratalà J, Rosón B, Fernández-Sevilla A, et al. Bacteremic pneumonia in neutropenic patients with cancer: causes, empirical antibiotic therapy, and outcome. Arch Intern Med. 1998;158:868–872. doi: 10.1001/archinte.158.8.868. [DOI] [PubMed] [Google Scholar]
- 113.Walsh TJ, Anaissie EJ, Denning DW, et al. Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis. 2008;46:327–360. doi: 10.1086/525258. [DOI] [PubMed] [Google Scholar]
- 114.Schiel X, Link H, Maschmeyer G, et al. A prospective, randomized multicenter trial of the empirical addition of antifungal therapy for febrile neutropenic cancer patients. Infection. 2006;34:118–126. doi: 10.1007/s15010-006-5113-9. [DOI] [PubMed] [Google Scholar]
- 115.Cornely OA, Maertens J, Bresnik M, et al. Efficacy outcomes in a randomised trial of liposomal amphotericin B based on revised EORTC/MSG 2008 definitions of invasive mould disease. Mycoses. 2011;54:e449–e455. doi: 10.1111/j.1439-0507.2010.01947.x. [DOI] [PubMed] [Google Scholar]
- 116.Greene RE, Schlamm HT, Oestmann JW, et al. Imaging findings in acute invasive pulmonary aspergillosis: clinical significance of the halo sign. Clin Infect Dis. 2007;44:373–379. doi: 10.1086/509917. [DOI] [PubMed] [Google Scholar]
- 117.Paul M, Soares-Weiser K, Leibovici L. Beta lactam monotherapy versus beta lactam-aminoglycoside combination therapy for fever with neutropenia: systematic review and meta-analysis. BMJ. 2003;326:1111–1119. doi: 10.1136/bmj.326.7399.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ainoda Y, Hirai Y, Fujita T, et al. Analysis of clinical features of non-HIV Pneumocystis jirovecii pneumonia. J Infect Chemother. 2012;18:722–728. doi: 10.1007/s10156-012-0408-5. [DOI] [PubMed] [Google Scholar]
- 119.Roger PM, Vandenbos F, Pugliese P, et al. Persistence of Pneumocystis carinii after effective treatment of P. carinii pneumonia is not related to relapse or survival among patients infected with human immunodeficiency virus. Clin Infect Dis. 1998;26:509–510. doi: 10.1086/517099. [DOI] [PubMed] [Google Scholar]
- 120.Fassas AB, Bolaños-Meade J, Buddharaju LN, et al. Cytomegalovirus infection and non-neutropenic fever after autologous stem cell transplantation: high rates of reactivation in patients with multiple myeloma and lymphoma. Br J Haematol. 2001;112:237–241. doi: 10.1046/j.1365-2141.2001.02487.x. [DOI] [PubMed] [Google Scholar]
- 121.Pagano L, Caira M, Nosari A, et al. Fungal infections in recipients of hematopoietic stem cell transplants: results of the SEIFEM B-2004 study. Clin Infect Dis. 2007;45:1161–1170. doi: 10.1086/522189. [DOI] [PubMed] [Google Scholar]
- 122.Post MJ, Lass-Floerl C, Gastl G, Nachbaur D. Invasive fungal infections in allogeneic and autologous stem cell transplant recipients. Transpl Infect Dis. 2007;9:189–195. doi: 10.1111/j.1399-3062.2007.00219.x. [DOI] [PubMed] [Google Scholar]
- 123.Reich G, Mapara MY, Reichardt P, et al. Infectious complications after high-dose chemotherapy and autologous stem cell transplantation. Bone Marrow Transplant. 2001;27:525–529. doi: 10.1038/sj.bmt.1702822. [DOI] [PubMed] [Google Scholar]
- 124.Hyle EP, Lipworth AD, Zaoutis TE, et al. Impact of inadequate initial antimicrobial therapy on mortality in infections due to extended-spectrum beta-lactamase-producing enterobacteriaceae: variability by site of infection. Arch Intern Med. 2005;165:1375–1380. doi: 10.1001/archinte.165.12.1375. [DOI] [PubMed] [Google Scholar]
- 125.Garnacho-Montero J, Sa-Borges M, Sole-Violan J, et al. Optimal management therapy for Pseudomonas aeruginosa ventilator-associated pneumonia: an observational, multicenter study comparing monotherapy with combination antibiotic therapy. Crit Care Med. 2007;35:1888–1895. doi: 10.1097/01.CCM.0000275389.31974.22. [DOI] [PubMed] [Google Scholar]
- 126.Park SY, Park HJ, Moon SM, et al. Impact of adequate empirical combination therapy on mortality from bacteremic Pseudomonas aeruginosa pneumonia. BMC Infect Dis. 2012;12:308. doi: 10.1186/1471-2334-12-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Safdar N, Handelsman J, Maki DG. Does combination antimicrobial therapy reduce mortality in Gram-negative bacteraemia? A meta-analysis. Lancet Infect Dis. 2004;4:519–527. doi: 10.1016/S1473-3099(04)01108-9. [DOI] [PubMed] [Google Scholar]
- 128.Vidaur L, Sirgo G, Rodríguez AH, Rello J. Clinical approach to the patient with suspected ventilator-associated pneumonia. Respir Care. 2005;50:965–974. [PubMed] [Google Scholar]
- 129.Chatzinikolaou I, Abi-Said D, Bodey GP, et al. Recent experience with Pseudomonas aeruginosa bacteremia in patients with cancer: retrospective analysis of 245 episodes. Arch Intern Med. 2000;160:501–509. doi: 10.1001/archinte.160.4.501. [DOI] [PubMed] [Google Scholar]
- 130.Peña C, Suarez C, Ocampo-Sosa A, et al. Effect of adequate single-drug vs combination antimicrobial therapy on mortality in Pseudomonas aeruginosa bloodstream infections: a post hoc analysis of a prospective cohort. Clin Infect Dis. 2013;57:208–216. doi: 10.1093/cid/cit223. [DOI] [PubMed] [Google Scholar]
- 131.Vardakas KZ, Tansarli GS, Bliziotis IA, Falagas ME. β-Lactam plus aminoglycoside or fluoroquinolone combination versus β-lactam monotherapy for Pseudomonas aeruginosa infections: a meta-analysis. Int J Antimicrob Agents. 2013;41:301–310. doi: 10.1016/j.ijantimicag.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 132.Michalopoulos A, Kasiakou SK, Mastora Z, et al. Aerosolized colistin for the treatment of nosocomial pneumonia due to multidrug-resistant Gram-negative bacteria in patients without cystic fibrosis. Crit Care. 2005;9:R53–R59. doi: 10.1186/cc3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Aisenberg G, Rolston KV, Dickey BF, et al. Stenotrophomonas maltophilia pneumonia in cancer patients without traditional risk factors for infection, 1997–2004. Eur J Clin Microbiol Infect Dis. 2007;26:13–20. doi: 10.1007/s10096-006-0243-7. [DOI] [PubMed] [Google Scholar]
- 134.Tada K, Kurosawa S, Hiramoto N, et al. Stenotrophomonas maltophilia infection in hematopoietic SCT recipients: high mortality due to pulmonary hemorrhage. Bone Marrow Transplant. 2013;48:74–79. doi: 10.1038/bmt.2012.87. [DOI] [PubMed] [Google Scholar]
- 135.Tekçe YT, Erbay A, Cabadak H, Sen S. Tigecycline as a therapeutic option in Stenotrophomonas maltophilia infections. J Chemother. 2012;24:150–154. doi: 10.1179/1120009X12Z.00000000022. [DOI] [PubMed] [Google Scholar]
- 136.Carroll KC, Cohen S, Nelson R, et al. Comparison of various in vitro susceptibility methods for testing Stenotrophomonas maltophilia. Diagn Microbiol Infect Dis. 1998;32:229–235. doi: 10.1016/s0732-8893(98)00089-3. [DOI] [PubMed] [Google Scholar]
- 137.Kalil AC, Klompas M, Haynatzki G, Rupp ME. Treatment of hospital-acquired pneumonia with linezolid or vancomycin: a systematic review and meta-analysis. BMJ Open. 2013;3:e003912. doi: 10.1136/bmjopen-2013-003912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Walkey AJ, O'Donnell MR, Wiener RS. Linezolid vs glycopeptide antibiotics for the treatment of suspected methicillin-resistant Staphylococcus aureus nosocomial pneumonia: a meta-analysis of randomized controlled trials. Chest. 2011;139:1148–1155. doi: 10.1378/chest.10-1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wunderink RG, Rello J, Cammarata SK, et al. Linezolid vs vancomycin: analysis of two double-blind studies of patients with methicillin- resistant Staphylococcus aureus nosocomial pneumonia. Chest. 2003;124:1789–1797. [PubMed] [Google Scholar]
- 140.Faguer S, Kamar N, Fillola G, et al. Linezolid-related pancytopenia in organ-transplant patients: report of two cases. Infection. 2007;35:275–277. doi: 10.1007/s15010-007-6197-6. [DOI] [PubMed] [Google Scholar]
- 141.Silverman JA, Mortin LI, Vanpraagh AD, et al. Inhibition of daptomycin by pulmonary surfactant: in vitro modeling and clinical impact. J Infect Dis. 2005;191:2149–2152. doi: 10.1086/430352. [DOI] [PubMed] [Google Scholar]
- 142.Mori T, Kato J. Cytomegalovirus infection/disease after hematopoietic stem cell transplantation. Int J Hematol. 2010;91:588–595. doi: 10.1007/s12185-010-0569-x. [DOI] [PubMed] [Google Scholar]
- 143.Wagstaff AJ, Bryson HM. Foscarnet. A reappraisal of its antiviral activity, pharmacokinetic properties and therapeutic use in immunocompromised patients with viral infections. Drugs. 1994;48:199–226. doi: 10.2165/00003495-199448020-00007. [DOI] [PubMed] [Google Scholar]
- 144.Limper AH, Knox KS, Sarosi GA, et al. American Thoracic Society Fungal Working Group. An official American Thoracic Society statement: treatment of fungal infections in adult pulmonary and critical care patients. Am J Respir Crit Care Med. 2011;183:96–128. doi: 10.1164/rccm.2008-740ST. [DOI] [PubMed] [Google Scholar]
- 145.Mousset S, Buchheidt D, Heinz W, et al. Treatment of invasive fungal infections in cancer patients-updated recommendations of the Infectious Diseases Working Party (AGIHO) of the German Society of Hematology and Oncology (DGHO) Ann Hematol. 2014;93:13–32. doi: 10.1007/s00277-013-1867-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Maschmeyer G, Haas A. Defining clinical failure for salvage studies. Med Mycol. 2006;44:S315–S318. doi: 10.1080/13693780600835690. [DOI] [PubMed] [Google Scholar]
- 147.Segal BH, Herbrecht R, Stevens DA, et al. Defining responses to therapy and study outcomes in clinical trials of invasive fungal diseases: Mycoses Study Group and European Organization for Research and Treatment of Cancer consensus criteria. Clin Infect Dis. 2008;47:674–683. doi: 10.1086/590566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Marr KA, Schlamm H, Rottinghaus ST, et al. A Randomised, Double-Blind Study of Combination Antifungal Therapy with Voriconazole and Anidulafungin Versus Voriconazole Monotherapy for Primary Treatment of Invasive Aspergillosis. 22nd European Congress of Clinical Microbiology and Infectious Diseases; London, UK: 2012. 31 March–3 April, abstr #LB2812. [Google Scholar]
- 149.Reed C, Bryant R, Ibrahim AS, et al. Combination polyene-caspofungin treatment of rhino-orbital-cerebral mucormycosis. Clin Infect Dis. 2008;47:364–371. doi: 10.1086/589857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Spellberg B, Ibrahim A, Roilides E, et al. Combination therapy for mucormycosis: why, what, and how? Clin Infect Dis. 2012;54(Suppl 1):S73–S78. doi: 10.1093/cid/cir885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Spellberg B, Ibrahim AS, Chin-Hong PV, et al. The Deferasirox-AmBisome Therapy for Mucormycosis (DEFEAT Mucor) study: a randomized, double-blinded, placebo-controlled trial. J Antimicrob Chemother. 2012;67:715–722. doi: 10.1093/jac/dkr375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Asai N, Motojima S, Ohkuni Y, et al. Early diagnosis and treatment are crucial for the survival of Pneumocystis pneumonia patients without human immunodeficiency virus infection. J Infect Chemother. 2012;18:898–905. doi: 10.1007/s10156-012-0441-4. [DOI] [PubMed] [Google Scholar]
- 153.Li MC, Lee NY, Lee CC, et al. Pneumocystis jiroveci pneumonia in immunocompromised patients: delayed diagnosis and poor outcomes in non-HIV-infected individuals. J Microbiol Immunol Infect. 2014;47:42–47. doi: 10.1016/j.jmii.2012.08.024. [DOI] [PubMed] [Google Scholar]
- 154.Dini L, du Plessis M, Frean J, Fernandez V. High prevalence of dihydropteroate synthase mutations in Pneumocystis jirovecii isolated from patients with Pneumocystis pneumonia in South Africa. J Clin Microbiol. 2010;48:2016–2021. doi: 10.1128/JCM.02004-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Le Gal S, Damiani C, Perrot M, et al. Circulation of Pneumocystis dihydropteroate synthase mutants in France. Diagn Microbiol Infect Dis. 2012;74:119–124. doi: 10.1016/j.diagmicrobio.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 156.Nahimana A, Rabodonirina M, Zanetti G, et al. Association between a specific Pneumocystis jiroveci dihydropteroate synthase mutation and failure of pyrimethamine/sulfadoxine prophylaxis in human immunodeficiency virus-positive and -negative patients. J Infect Dis. 2003;188:1017–1023. doi: 10.1086/378239. [DOI] [PubMed] [Google Scholar]
- 157.Queener SF, Cody V, Pace J, et al. Trimethoprim resistance of dihydrofolate reductase (DHFR) variants from clinical isolates of Pneumocystis jirovecii. Antimicrob Agents Chemother. 2013;57:4990–4998. doi: 10.1128/AAC.01161-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Thomas CF, Jr, Limper AH. Pneumocystis pneumonia. N Engl J Med. 2004;350:2487–2498. doi: 10.1056/NEJMra032588. [DOI] [PubMed] [Google Scholar]
- 159.Smego RA, Jr, Nagar S, Maloba B, Popara M. A meta-analysis of salvage therapy for Pneumocystis carinii pneumonia. Arch Intern Med. 2001;161:1529–1533. doi: 10.1001/archinte.161.12.1529. [DOI] [PubMed] [Google Scholar]
- 160.Hughes WT, Rivera GK, Schell MJ, et al. Successful intermittent chemoprophylaxis for Pneumocystis carinii pneumonitis. N Engl J Med. 1987;316:1627–1632. doi: 10.1056/NEJM198706253162604. [DOI] [PubMed] [Google Scholar]
- 161.Schneider MM, Hoepelman AI, Eeftinck Schattenkerk JK, et al. A controlled trial of aerosolized pentamidine or trimethoprim-sulfamethoxazole as primary prophylaxis against Pneumocystis carinii pneumonia in patients with human immunodeficiency virus infection. The Dutch AIDS Treatment Group. N Engl J Med. 1992;327:1836–1841. doi: 10.1056/NEJM199212243272603. [DOI] [PubMed] [Google Scholar]
- 162.Delclaux C, Zahar JR, Amraoui G, et al. Corticosteroids as adjunctive therapy for severe Pneumocystis carinii pneumonia in non-human immunodeficiency virus-infected patients. Clin Infect Dis. 1999;29:670–672. doi: 10.1086/598651. [DOI] [PubMed] [Google Scholar]
- 163.Pareja JG, Garland R, Koziel H. Use of adjunctive corticosteroids in severe adult non-HIV Pneumocystis carinii pneumonia. Chest. 1998;113:1215–1224. doi: 10.1378/chest.113.5.1215. [DOI] [PubMed] [Google Scholar]
- 164.Moon SM, Kim T, Sung H, et al. Outcomes of moderate-to-severe Pneumocystis pneumonia treated with adjunctive steroid in non-HIV-infected patients. Antimicrob Agents Chemother. 2011;55:4613–4618. doi: 10.1128/AAC.00669-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Lemiale V, Debrumetz A, Delannoy A, et al. Adjunctive steroid in HIV-negative patients with severe Pneumocystis pneumonia. Respir Res. 2013;14:87. doi: 10.1186/1465-9921-14-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Azoulay E, Mokart D, Pène F, et al. Outcomes of critically ill patients with hematologic malignancies: prospective multicenter data from France and Belgium—a groupe de recherche respiratoire en reanimation onco-hematologique study. J Clin Oncol. 2013;31:2810–2818. doi: 10.1200/JCO.2012.47.2365. [DOI] [PubMed] [Google Scholar]
- 167.Bird GT, Farquhar-Smith P, Wigmore T, et al. Outcomes and prognostic factors in patients with haematological malignancy admitted to a specialist cancer intensive care unit: a 5 yr study. Br J Anaesth. 2012;108:452–459. doi: 10.1093/bja/aer449. [DOI] [PubMed] [Google Scholar]
- 168.Taccone FS, Artigas AA, Sprung CL, et al. Characteristics and outcomes of cancer patients in European ICUs. Crit Care. 2009;13:R15. doi: 10.1186/cc7713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Cherif H, Martling CR, Hansen J, et al. Predictors of short and long-term outcome in patients with hematological disorders admitted to the intensive care unit for a life-threatening complication. Support Care Cancer. 2007;15:1393–1398. doi: 10.1007/s00520-007-0268-1. [DOI] [PubMed] [Google Scholar]
- 170.Maschmeyer G, Bertschat FL, Moesta KT, et al. Outcome analysis of 189 consecutive cancer patients referred to the intensive care unit as emergencies during a 2-year period. Eur J Cancer. 2003;39:783–792. doi: 10.1016/s0959-8049(03)00004-2. [DOI] [PubMed] [Google Scholar]
- 171.Owczuk R, Wujtewicz MA, Sawicka W, et al. Patients with haematological malignancies requiring invasive mechanical ventilation: differences between survivors and non-survivors in intensive care unit. Support Care Cancer. 2005;13:332–338. doi: 10.1007/s00520-004-0750-y. [DOI] [PubMed] [Google Scholar]
- 172.Burghi G, Lemiale V, Seguin A, et al. Outcomes of mechanically ventilated hematology patients with invasive pulmonary aspergillosis. Intensive Care Med. 2011;37:1605–1612. doi: 10.1007/s00134-011-2344-8. [DOI] [PubMed] [Google Scholar]
- 173.Sculier JP. Intensive care and oncology. Support Care Cancer. 1995;3:93–105. doi: 10.1007/BF00365848. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.