Accumulating evidence supports an effect of aspirin in reducing cancer incidence and mortality. Our analyses show that prophylactic aspirin use for a minimum of 5 years at doses between 75 and 325 mg/day appears to have favourable benefit–harm profile; longer use is likely to have greater benefits. Further research is needed to determine the optimum dose and duration of use.
Keywords: aspirin, prevention, benefit-harm, cancer, cardiovascular disease, gastrointestinal bleeding
Abstract
Background
Accumulating evidence supports an effect of aspirin in reducing overall cancer incidence and mortality in the general population. We reviewed current data and assessed the benefits and harms of prophylactic use of aspirin in the general population.
Methods
The effect of aspirin for site-specific cancer incidence and mortality, cardiovascular events was collated from the most recent systematic reviews. Studies identified through systematic Medline search provided data regarding harmful effects of aspirin and baseline rates of harms like gastrointestinal bleeding and peptic ulcer.
Results
The effects of aspirin on cancer are not apparent until at least 3 years after the start of use, and some benefits are sustained for several years after cessation in long-term users. No differences between low and standard doses of aspirin are observed, but there were no direct comparisons. Higher doses do not appear to confer additional benefit but increase toxicities. Excess bleeding is the most important harm associated with aspirin use, and its risk and fatality rate increases with age. For average-risk individuals aged 50–65 years taking aspirin for 10 years, there would be a relative reduction of between 7% (women) and 9% (men) in the number of cancer, myocardial infarction or stroke events over a 15-year period and an overall 4% relative reduction in all deaths over a 20-year period.
Conclusions
Prophylactic aspirin use for a minimum of 5 years at doses between 75 and 325 mg/day appears to have favourable benefit–harm profile; longer use is likely to have greater benefits. Further research is needed to determine the optimum dose and duration of use, to identify individuals at increased risk of bleeding, and to test effectiveness of Helicobacter pylori screening–eradication before starting aspirin prophylaxis.
introduction
Aspirin reduces the incidence of cardiovascular events by 12%, both in the general population and in high-risk groups [1]. However, with increasing awareness of risk factors and the wide range of effective agents available for high-risk individuals, use of aspirin in the general population does not have a major impact on cardiovascular mortality. An increasing body of evidence supports the role of aspirin for cancer prevention [2–7]. Aspirin use is associated with an age-dependent increased risk of bleeding [8], especially gastrointestinal (GI) bleeding, and peptic ulcer; and benefits need to be balanced against harms.
The US Preventive Services Task Force (USPSTF) have reported on benefits and harms of aspirin use for prevention of specific diseases like colorectal cancer (CRC) [9] and cardiovascular disease (CVD) [10]. However, they have not investigated overall benefits and harms based on all major diseases. A recent economic model incorporating aspirin's effect on cancer has suggested that prophylactic aspirin use can be cost-effective [11].
Previously, we reviewed the role of aspirin for cancer prevention [12]. Although there was strong evidence for protection against colorectal and other cancers [13], we concluded that it was premature to recommend routine use in the general population and recommended further long-term follow-up of existing aspirin trials. Since our publication, several such extended follow-up results have become available from initiatives underway at the time. As a result, an augmented group reconvened on 6 May 2011 to review current data and assess the benefits and harms of prophylactic use of aspirin in the general population. A substantial amount of the new data we considered and used for the benefit-harm analyses were unpublished at that time [3, 5–7, 14], and the group concluded that it should be publicly available before we report our review. Most of these data are now published. Here, we summarise current evidence regarding the effect of aspirin on cancer and estimate overall benefits and harms of prophylactic aspirin use.
methods
materials: evidence and data collation
The evidence for the effect of aspirin for incidence and death by cancer site was collated from the most recent systematic reviews [2–7] and some individual studies reporting on specific sites or long-term aspirin use [15–18]. Systematic reviews were undertaken by the members of the group and these data, although only published subsequently, were available for discussion at the evidence review meeting (Table 1). Cancer incidence and mortality rates in the UK for year 2008 [19] were used for baseline rates.
Table 1.
Relative risks of aspirin use on the incidence and mortality of major cancers from recent overviews and major studies
| Cancer incidence |
Cancer mortality |
|||||
|---|---|---|---|---|---|---|
| No. of studies and source | No. of cases | Relative risk (95% CI) | No. of studies and source | No. of cases | Relative risk (95% CI) | |
| Colorectal cancer | ||||||
| Case–control | 15a | 21 414 | 0.63 (0.56–0.70) | 1b | 433 | 0.72 (0.56–0.92) |
| 22c | 17 231 | 0.61 (0.55–0.67) | ||||
| Cohort | 15a | 16 105 | 0.82 (0.75–0.89) | 2d | 1124 | 0.68 (0.56–0.83) |
| 8c | 2955 | 0.78 (0.71–0.84) | 1*e | 149 | 0.64 (0.42–0.98) | |
| RCT | 3f | 196 | 0.75 (0.56–0.97) | 3f | 130 | 0.61 (0.43–0.87) |
| 3*f | 135 | 0.62 (0.43–0.94) | 3*f | 91 | 0.48 (0.30–0.77) | |
| Oesophageal cancer | ||||||
| Case–control | 7a | 1075 | 0.54 (0.44–0.67) | – | – | – |
| 9c | 2307 | 0.58 (0.44–0.76) | ||||
| Cohort | 4a | 1118 | 0.73 (0.51–1.07) | 2d | 194 | 0.56 (0.35–0.91) |
| 1c | 102 | 0.78 (0.42–1.44) | 1*e | 45 | 0.61 (0.30–1.23) | |
| RCT | – | – | – | 3g | 62 | 0.42 (0.25–0.71) |
| Stomach cancer | ||||||
| Case–control | 7a | 2411 | 0.60 (0.44–0.82) | – | – | – |
| 8c | 3000 | 0.61 (0.40–0.93) | ||||
| Cohort | 6a | 2108 | 0.77 (0.58–1.04) | 2d | 314 | 0.59 (0.40–0.86) |
| 1c | 184 | 0.49 (0.22–1.12) | 1*e | 39 | 0.36 (0.15–0.88) | |
| RCT | – | – | – | 3g | 71 | 0.69 (0.43–1.10) |
| Pancreatic cancer | ||||||
| Case–control | 3a | 1406 | 0.82 (0.68–1.00) | – | – | – |
| 5c | 1619 | 1.02 (0.83–1.26) | ||||
| Cohort | 7a | 6471 | 0.95 (0.85–1.05) | 2h | 4655 | 0.97 (0.86–1.09) |
| 3c | 2415 | 1.00 (0.79–1.27) | 1*e | 186 | 1.03 (0.73–1.46) | |
| RCT | – | – | – | 3g | 77 | 0.81 (0.51–1.26) |
| Lung cancer | ||||||
| Case–control | 5a | 4863 | 0.73 (0.55–0.98) | 1b | 979 | 0.88 (0.73–1.05) |
| 12c | 11 683 | 0.84 (0.66–1.08) | ||||
| Cohort | 15a | 11 356 | 0.98 (0.92–1.05) | 2i | 410j | 0.97 (0.83–1.14) |
| 5c | 1856 | 1.07 (0.96–1.19) | 1*e | 462 | 1.04 (0.84–1.29) | |
| RCT | – | – | – | 3g | 326 | 0.71 (0.58–0.89) |
| Prostate cancer | ||||||
| Case–control | 9a | 5795 | 0.87 (0.74–1.02) | – | – | – |
| 8c | 7857 | 0.86 (0.69–1.08) | ||||
| Cohort | 15a | 31 657 | 0.91 (0.85–0.97) | 1k | 580m | 0.84 (0.69–1.02) |
| 5c | 3865 | 0.93 (0.86–1.01) | 1*e | 43 | 0.57 (0.28–1.15) | |
| RCT | – | – | – | 3g | 210 | 0.81 (0.61–1.06) |
| Breast cancer | ||||||
| Case–control | 10a | 25 835 | 0.83 (0.76–0.91) | 1b | 864 | 0.95 (0.80–1.13) |
| 12c | 22 046 | 0.81 (0.72–0.93) | ||||
| Cohort | 22a | 27 091 | 0.93 (0.87–1.00) | 2d | 131j | 0.86 (0.65–1.15) |
| 9c | 7713 | 0.88 (0.82–0.93) | 1*e | 32 | 0.28 (0.06–1.20) | |
| RCT | 1n | 1230 | 0.98 (0.87–1.09) | –c | 23 | 1.17 (0.50–2.71) |
Several studies appear in more than one overview.
A number of cases are the number of events, either cancer diagnoses or cancer deaths.
Relative risks for >5 years daily use are also given where available.
From Bosetti et al. [7].
From Chan et al. [16] (Women only Nested Case–control study, current users versus never users).
From Algra et al. [6] (based on maximum aspirin use data).
From Jacobs et al. [15].
From Rothwell et al. [4].
From Rothwell et al. [2].
Pooled risk ratios from Ratnasinghe et al. [25] and Thun et al. [26]; Thun et al. [26] reported all respiratory cancer deaths as one group, which have been approximated as lung cancer deaths.
Number of deaths (lung cancer or breast cancer) not reported in Cancer Prevention Study II, Thun et al. [26].
From Dhillon et al. [18].
Number of lethal prostate cancers, i.e. any metastatic prostate cancer or prostate cancer death.
From Women's Health Study, Cook et al. [17].
The evidence for the effect of aspirin on cardiovascular events was based on the Antithrombotic Trialists' (ATT) Collaboration meta-analysis [1]. Baseline CVD incidence and mortality rates are based on a downward adjustment of the rates observed in the UK in 1998 [20], to reflect a 25% reduction in incidence [21] and a 30% reduction in mortality as seen in the USA [22] (UK shows similar trends) between 1998 and 2008 to project the rates forward.
A detailed analysis of the harmful effects of aspirin has been reported elsewhere [23] and is summarised briefly in the supplementary Material, available at Annals of Oncology online.
statistical analysis: benefit–harm analysis
We considered the overall benefits and harms for taking aspirin for 10 years starting from age 50, 55, 60 and 65 years separately for men and women. We assumed: (i) that the cardiovascular benefit and adverse-effects (Table 2) only occur during active treatment, i.e. 10 years; (ii) the protection against cancer begins 3 years after initiating aspirin [3] and continues for an additional 5 years after stopping aspirin [24]; (iii) the protection against cancer mortality begins 5 years after the commencement of aspirin use [2] and lasts for an additional 10 years after treatment cessation and (iv) the protective effects are seen only in colorectal, oesophageal, gastric, breast, prostate and lung cancers (Table 3) [or only colorectal, oesophageal and gastric cancers for sensitivity analyses]. Details of derivation of effect sizes (Table 3) used for benefit–harm analyses are given in the supplementary Material, available at Annals of Oncology online.
Table 2:
Age and Sex specific baseline major extracranial bleeding [1], any GI Bleeding [51, 52], peptic ulcer [51] and any GI complication (GI bleed or peptic ulcer) event rates estimated in the UK general population (per 1000 person years) not using NSAID.
| Age-group (y) | Major extracranial Bleeding |
Any GI bleeding |
Uncomplicated Peptic ulcer |
Any GI complication |
||||
|---|---|---|---|---|---|---|---|---|
| Men | Women | Men | Women | Men | Women | Men | Women | |
| 50–54 | 0.44 | 0.22 | 1.31 | 0.76 | 0.60 | 0.52 | 1.91 | 1.28 |
| 55–59 | 0.78 | 0.39 | 1.04 | 0.86 | 0.77 | 0.64 | 1.81 | 1.50 |
| 60–64 | 1.12 | 0.56 | 2.41 | 1.28 | 0.95 | 0.78 | 3.36 | 2.06 |
| 65–69 | 1.46 | 0.74 | 3.19 | 2.27 | 1.17 | 0.95 | 4.36 | 3.22 |
| 70–74 | 1.81 | 0.92 | 4.38 | 2.66 | 1.27 | 1.04 | 5.65 | 3.70 |
| 75–79 | 2.27 | 1.21 | 7.00 | 4.46 | 1.30 | 1.09 | 8.30 | 5.55 |
| 80–84 | 2.95 | 1.75 | 8.21 | 6.41 | 1.30 | 1.09 | 9.51 | 7.50 |
GI Bleeding and peptic ulcer rates are adjusted for baseline NSAID use (18-25%) in the population. Details of estimation of these rates are reported elsewhere [23].
Uncomplicated peptic ulcers refer to ulcers that are neither bleeding nor perforated.
Any GI bleeding comprises of both upper and lower GI bleeding, including bleeding from peptic ulcer. Any GI complication comprises of any GI bleeding and uncomplicated peptic ulcers.
Table 3:
Risk ratios for incidence and mortality of different events due to aspirin use; used in benefit-harm calculations.
| Event | Incidence |
Mortality |
||
|---|---|---|---|---|
| Best estimate | Conservative | Best estimate | Conservative | |
| Colorectal cancer | 0.65 | 0.70 | 0.60 | 0.65 |
| Oesophageal cancer | 0.70 | 0.75 | 0.50 | 0.55 |
| Gastric cancer | 0.70 | 0.75 | 0.65 | 0.70 |
| Lung cancer | 0.95 | 1.00 | 0.85 | 0.90 |
| Prostate cancer | 0.90 | 0.95 | 0.85 | 0.90 |
| Breast cancer | 0.90 | 0.95 | 0.95 | 1.00 |
| Myocardial infarction | 0.82 | 0.82 | 0.95 | 0.95 |
| Stroke | 0.95 | 0.95 | 1.21 | 1.21 |
| Major extracranial bleeding | 1.54 | 1.70 | - | - |
| GI bleeding | - | - | 1.60 | 1.70 |
| Peptic Ulcer | - | - | 1.60 | 1.70 |
A qualitative estimation of site-specific relative risks for various cancers is done based on data in Table 1 as described in the supplementary material, available at Annals of Oncology online, the relative risks for cardiovascular events are based on the ATT Collaboration meta-analysis [1] and those for adverse gastrointestinal events are estimated as described elsewhere [23].
The inter-current mortality (England and Wales, 2008) adjusted rates were used to compute the probabilities for different events by computing 1 − exp(−inter-current mortality adjusted cumulative hazard) for incidence (not mortality) calculations. The calculations for the incidence of major events (cancer, myocardial infarction, stroke, major bleeding) excluded uncomplicated peptic ulcers or other more minor bleeding events since they are not comparable in severity.
findings
summary of evidence for a reduction in cancer incidence and mortality
The main results are summarised in Table 1.
colorectal cancer
There is now overwhelming evidence for a reduction in CRC incidence and mortality from regular aspirin use. A 20-year follow-up of two high-dose aspirin trials showed an overall 37% reduction in CRC incidence in participants who had scheduled treatment of 5 years or more, but the effect was seen only 10 years after randomisation [13]. Subsequent long-term follow-up of three trials of low-dose aspirin (75–300 mg/day) use found a smaller (25%) but significant reduction in CRC incidence [4]. The effects were not apparent immediately and showed larger benefit with increasing duration of aspirin use. Two trials of alternate day use, the Women's Health Study (WHS) [17] and the Physicians' Health Study (PHS) [28], have not shown any reduction within 10 years of follow-up; although a 43% reduction after 10 years has been observed in the WHS [14]. Evidence for mortality reduction is based on a greater number of studies [2] and the effect size appears to be larger than for incidence—a 40% overall reduction or 52% reduction with at least 5 years of scheduled treatment [2, 4]. Rothwell et al. [5] suggest that the greater effect on mortality is due to a reduction in metastatic spread, possibly through a platelet-mediated mechanism with benefits both before and after the diagnosis of cancer [29, 30].
The effects from observational studies are based on a much larger number of cases and are largely consistent with those from RCTs (Table 1)—a 27% overall reduction in CRC incidence (38% in case–control studies, 19% in cohort studies) [6, 7]. Although not clearly observed for other cancers, observational studies show larger reductions for standard or high-dose aspirin compared with low-dose aspirin for CRC [7].
Aspirin shows similar effects in individuals at high-risk of CRC [31]. Colorectal Adenoma/Carcinoma Prevention Programme 2 (CAPP2), a randomised trial of 600 mg aspirin daily in carriers of Lynch syndrome, showed a 63% reduction (P = 0.008) in incidence among those completing 2 years of treatment, although results with shorter follow-up [32] or for first events in all enrolled patients were not significant [31].
oesophageal cancer
Although data are less extensive, consistent reductions in mortality have also been seen for oesophageal cancer, with a 58% reduction after 5 years of follow-up in randomised trials, and a 44% reduction in cohort studies [25, 26]. A 43% reduction in incidence of oesophageal cancer was seen in case–control studies, whereas cohort studies reported a 27% reduction. Although the meta-analyses of RCTs have suggested the effect of aspirin is primarily on adenocarcinomas (all sites), the observational studies [7] have found similar reductions in squamous cell (39%) and adenocarcinomas (36%) including gastric cardia.
other gastrointestinal cancers
Stomach cancer also emerges as a site for which aspirin may provide substantial protection, although the extent of the effect appears to be smaller, and the data are less extensive and more variable. In the RCTs, an overall 31% reduction in deaths was reported (P = 0.11), based mostly on a 58% reduction (P = 0.007) after 10 years of use [2]. A 41% reduction in mortality was also observed in two cohort studies [25, 26]. Case–control studies found a 39% reduction in gastric cancer incidence while cohort studies reported a 25% reduction. Pancreatic cancer appears to be little affected with a non-significant 4% reduction in incidence and 3% reduction in mortality [25, 27] in cohort studies and a non-significant 19% reduction in mortality in the RCTs. Case–control studies showed a non-significant 7% reduction in incidence.
other sites
At most small effects are seen at other cancer sites. Case–control studies indicate an 18% reduction in breast cancer incidence, and an 8% reduction was seen in cohort studies. A similar but non-significant reduction in mortality [16, 25, 26] was seen; 5% in case–control studies and 14% in cohort studies. However, no effect on incidence was seen in the WHS [17]. A non-significant increase in breast cancer mortality was seen in the overview of RCTs [6], although this may be unreliable in view of the small number of events.
Some effect has also been seen for prostate cancer with a non-significant 19% reduction in mortality in the RCTs, and a non-significant 16% reduction in lethal prostate cancers (metastatic or fatal) in the Health Professionals Follow-up study (HPFS) [18]. A significant 9% reduction in incidence in cohort studies has been observed. Case–control studies show a significant 13% reduction when analyses are restricted to aggressive (high-grade) tumours, with a non-significant 14% reduction overall [7].
More variable but generally favourable evidence was seen for lung cancer, with a 29% reduction in mortality in the RCTs, which became apparent only after 5 years of follow-up, and a non-significant 12% reduction in mortality in one case–control study [16] and a 19% reduction in one cohort study [25]. The effect on incidence in observational studies was confined to case–control studies (19% reduction) with no effect seen in cohort studies.
Although a large reduction in endometrial cancer was seen in one study of patients with mismatch repair defects [31], its relevance to the general population is unknown and a significant preventive effect of aspirin has not been seen for any other cancer site, either for incidence or mortality.
dose and duration
There is consistent evidence that long-term use of aspirin is necessary to achieve a cancer prevention benefit. This is most clearly seen in the RCTs where no benefit was seen in years 0–3, but incidence was reduced after 3 years of treatment [3], and mortality was reduced only after 5 years [2, 3], but continued for as long as follow-up was available. This is supported by observational studies, especially for CRC where the reduced incidence is much clearer in long-term users [6, 7, 24]. Reduced incidence and mortality have been seen for all daily doses above 75 mg, and there is no clear indication of a greater reduction with increasing dose [4] in average-risk individuals. Some observational studies have suggested that doses <300 mg/day are not effective [24, 33] and an RCT [31] in high-risk individuals has shown efficacy at 600 mg daily dose. However, Baron et al. [34] observed greater reductions in all or advanced colorectal adenomas with an 81 mg daily aspirin compared with a 325 mg daily dose. In a meta-analysis of colorectal adenoma prevention trials, similar reductions were observed with low- (81 or 160 mg daily) versus standard-dose (300 or 325 mg daily) aspirin, but reductions in advanced adenomas were greater with the higher dose [35]. Of the alternate daily dosing trials WHS [17] and PHS [28, 36]; PHS [28, 36] has not shown clear benefits, whereas WHS has shown a delayed post-treatment benefit in CRC incidence [14].
age and sex
In an overview of six RCTs of daily low-dose aspirin involving over 35 000 individuals and 1632 incident cancers, no difference has been seen between men and women, or between those aged <60 years of age at randomisation versus older ages [3]. These results appear robust, as ∼40% of these cancers occurred in women and 30% in individuals younger than 60 years of age at randomisation [3]. Observational studies generally support this finding, but data are less complete. A possible exception to these findings is the smaller and late effect seen in the WHS, which investigated 100 mg aspirin on alternate days in women only [17].
cardiovascular disease
When used in primary prevention settings, aspirin has been shown to reduce serious vascular events among individuals at average/low risk [1] by 12% (0.51% versus 0.57%/year, P = 0.0001). This was primarily due to a 21% reduction in non-fatal myocardial infarction (MI), with little overall effect on strokes. Overall effects on serious vascular events were similar in men and women, although the effect of aspirin on coronary heart disease was larger in men and the effect on stroke was larger in women. Despite the effect on incidence, no reduction has been seen in cardiovascular mortality in the primary prevention trials [relative risk (RR) = 0.97, 95% confidence interval (CI) = 0.87–1.09 ATT Collaboration [1]; odds ratio (OR) = 0.99, 95% CI = 0.87–1.12, Rothwell et al. [3]], although some reduction has been seen in the high-risk individuals in the secondary prevention trials (RR = 0.91, 95% CI 0.82–1.00, P = 0.06) [1].
bleeding and other side-effects
Without a doubt increased bleeding is the most important side-effect of aspirin. The side-effects are discussed briefly in the supplementary Material, available at Annals of Oncology online and more fully elsewhere [23] (Table 2). Haemorrhagic stroke, although rare, is the most serious and potentially fatal side-effect. Estimates suggest a relative increase of 32%–36% in haemorrhagic strokes in aspirin users from a baseline rate of 0.03% per year [1]. Much commoner are the extracranial (predominantly gastrointestinal) bleeds, where the risk for major events is increased by about 30%–70% from an overall baseline risk of 0.7 per 1000 per year with low or standard-dose aspirin treatment [23]. Overall, the rates of gastrointestinal complications increase steeply beyond age 70 years and fatality rates show a similar trend, but the rates and fatality ratios are low below 70 years of age [23].
overall benefits and harms of aspirin prophylaxis in the general population
Using our ‘best estimates’ for individuals taking aspirin for 10 years, there would be a ‘relative’ reduction of ∼9% in the number of men and 7% in the number of women with a cancer, myocardial infarction or stroke event over a 15-year period (Table 4). ‘Absolute’ reductions are age and sex dependent. There would be between 0.95% (women starting at age 50 years) and 3.84% (men starting at age 65 years) fewer individuals with cancer, myocardial infarction or stroke (Table 4). Reductions in cancer incidence would account for 61%–80% of the overall benefit, and reductions in CRC alone would account for 30%–36% of it. Our conservative estimate gives absolute reductions ranging from 0.68% to 3.09% (Table 4). Depending on age and sex, major bleeding events would increase (absolute) by between 0.16% (0.21%) and 0.81% (1.05%) over their baseline rates of 0.57% to 2.37% over a 15-year period (conservative estimates in parentheses). Thus, the net relative benefit on these serious events is about 6% (4% conservative) in both men and women, but absolute benefits are greater in men and at older ages, due to higher baseline event rates and range from 0.79% (0.47%) to 3.03% (2.03%). The number needed to treat (NNT) for 10 years ranges from 33 to 127 to prevent one major event.
Table 4.
Benefits and harms of 10 years of aspirin use on the incidence of major events by age and sex
| Age at starting | 50 years |
55 years |
60 years |
65 years |
||||
|---|---|---|---|---|---|---|---|---|
| Incidence | Baseline | Reduction | Baseline | Reduction | Baseline | Reduction | Baseline | Reduction |
| Cancer | ||||||||
| Men | 9.70 | 0.92 (0.65) | 15.20 | 1.52 (1.07) | 20.75 | 2.09 (1.45) | 25.39 | 2.51 (1.75) |
| Women | 10.41 | 0.76 (0.48) | 13.19 | 1.03 (0.67) | 15.78 | 1.26 (0.85) | 18.08 | 1.48 (1.03) |
| MI | ||||||||
| Men | 5.13 | 0.52 | 6.75 | 0.68 | 8.72 | 0.89 | 10.92 | 1.15 |
| Women | 1.62 | 0.15 | 2.59 | 0.23 | 4.22 | 0.37 | 6.69 | 0.61 |
| Stroke | ||||||||
| Men | 2.14 | 0.06 | 3.16 | 0.08 | 4.66 | 0.12 | 6.66 | 0.18 |
| Women | 1.71 | 0.05 | 2.54 | 0.07 | 3.84 | 0.10 | 5.75 | 0.15 |
| Total | ||||||||
| Men | 16.97 | 1.50 (1.22) | 25.11 | 2.29 (1.83) | 34.13 | 3.10 (2.47) | 42.97 | 3.84 (3.09) |
| Women | 13.74 | 0.95 (0.68) | 18.32 | 1.32 (0.97) | 23.83 | 1.73 (1.32) | 30.53 | 2.24 (1.79) |
| Adverse events | Baseline | Excess | Baseline | Excess | Baseline | Excess | Baseline | Excess |
| Major extracranial bleeding | ||||||||
| Men | 1.12 | 0.32 (0.42) | 1.58 | 0.49 (0.64) | 2.00 | 0.66 (0.85) | 2.37 | 0.81 (1.05) |
| Women | 0.57 | 0.16 (0.21) | 0.81 | 0.25 (0.32) | 1.05 | 0.34 (0.44) | 1.30 | 0.43 (0.55) |
| Net benefit | Baseline | Reduction | Baseline | Reduction | Baseline | Reduction | Baseline | Reduction |
| Men | 18.09 | 1.18 (0.81) | 26.70 | 1.80 (1.19) | 36.13 | 2.44 (1.62) | 45.34 | 3.03 (2.03) |
| Women | 14.31 | 0.79 (0.47) | 19.13 | 1.07 (0.65) | 24.88 | 1.39 (0.88) | 31.83 | 1.82 (1.24) |
Baseline probabilities of an event and aspirin-related reductions (per 100 individuals in 15 years) using best (and conservative) estimates for prophylactic use of aspirin for 10 years on the incidence of major events namely cancer, myocardial infarction, stroke and major bleeding according to sex and age at starting use. All estimates are adjusted for inter-current mortality.
Effects on cardiovascular and bleeding events are assumed to occur only during active treatment (10 years) and those for cancer do not start until after 3 years of use but persist for an additional 5 years after treatment completion. Baseline rates are for the entire 15-year period. Figures in parentheses are conservative estimates.
The figures in bold represent overall benefits, overall harms and net balance of benefit and harm.
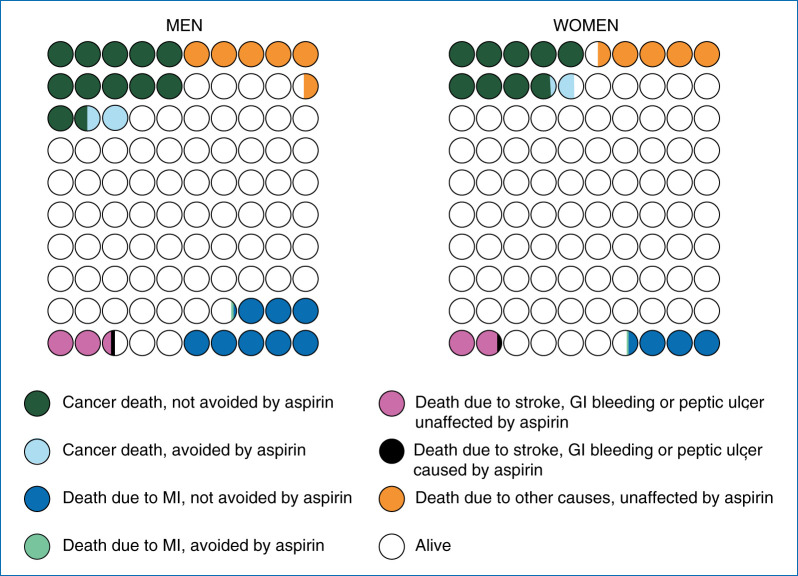
The magnitude of the relative reduction in cancer deaths is somewhat larger than that for incidence (13% in men and 9% in women) leading to a 4% (3% conservative estimate) relative reduction in all deaths, since there is no net reduction in cardiovascular or other deaths (Table 5). The net absolute benefits are slightly smaller than for incidence due to lower baseline rates. There would be between 0.47% (0.31%) (women starting at age 50 years) and 2.18% (1.64%) (men starting at age 65 years) fewer deaths (net benefit) over a 20-year period, with NNTs to save one life ranging from 46 to 213 (conservative estimates in parentheses). This is almost entirely (89%–96%) due to a reduction in deaths from cancer. The benefits of aspirin are at least equivalent in magnitude to those from statins [37], and as they mostly relate to cancer, are complimentary to statins. Although the relative benefit is similar, the absolute magnitude of benefit is smaller for women than for men as they have a lower baseline death rate from these major diseases (Figure 1). The net absolute benefit is 2% or more (incidence or deaths) in men starting aspirin at age 60 years or above. Calculations for 5 years of aspirin use show similar trends (supplementary Tables W1 and W2, available at Annals of Oncology online), but net benefits are ∼50% of those for 10 years of use for major event incidence and 60% for deaths.
Table 5.
Benefits and harms of 10 years of aspirin on mortality by age and sex
| Age at starting | 50 years |
55 years |
60 years |
65 years |
||||
|---|---|---|---|---|---|---|---|---|
| Mortality | Baseline | Reduction | Baseline | Reduction | Baseline | Reduction | Baseline | Reduction |
| Cancer | ||||||||
| Men | 7.45 | 0.99 (0.80) | 11.59 | 1.48 (1.19) | 16.40 | 2.04 (1.62) | 20.53 | 2.41 (1.91) |
| Women | 6.12 | 0.53 (0.39) | 8.80 | 0.78 (0.58) | 12.04 | 1.09 (0.82) | 15.26 | 1.39 (1.06) |
| MI | ||||||||
| Men | 5.08 | 0.07 | 8.05 | 0.12 | 11.80 | 0.20 | 15.13 | 0.31 |
| Women | 1.80 | 0.02 | 3.44 | 0.04 | 6.02 | 0.08 | 9.33 | 0.14 |
| Total | ||||||||
| Men | 12.53 | 1.05 (0.86) | 19.64 | 1.60 (1.30) | 28.19 | 2.24 (1.82) | 35.66 | 2.72 (2.22) |
| Women | 7.92 | 0.55 (0.40) | 12.24 | 0.82 (0.61) | 18.06 | 1.16 (0.89) | 24.60 | 1.53 (1.20) |
| Adverse events | Baseline | Excess | Baseline | Excess | Baseline | Excess | Baseline | Excess |
| Stroke | ||||||||
| Men | 1.03 | 0.06 | 1.85 | 0.09 | 3.21 | 0.17 | 4.83 | 0.32 |
| Women | 0.74 | 0.04 | 1.47 | 0.06 | 2.90 | 0.11 | 5.12 | 0.26 |
| GI bleeding | ||||||||
| Men | 0.19 | 0.04 (0.04) | 0.34 | 0.05 (0.06) | 0.57 | 0.08 (0.09) | 0.74 | 0.17 (0.19) |
| Women | 0.12 | 0.02 (0.03) | 0.22 | 0.03 (0.04) | 0.39 | 0.05 (0.06) | 0.59 | 0.11 (0.13) |
| Peptic ulcer | ||||||||
| Men | 0.08 | 0.02 (0.02) | 0.12 | 0.03 (0.03) | 0.15 | 0.03 (0.04) | 0.17 | 0.05 (0.06) |
| Women | 0.07 | 0.02 (0.02) | 0.10 | 0.02 (0.02) | 0.13 | 0.03 (0.03) | 0.16 | 0.04 (0.05) |
| Total | ||||||||
| Men | 1.29 | 0.11 (0.12) | 2.31 | 0.17 (0.18) | 3.93 | 0.28 (0.30) | 5.73 | 0.54 (0.58) |
| Women | 0.93 | 0.09 (0.09) | 1.79 | 0.11 (0.12) | 3.42 | 0.19 (0.21) | 5.86 | 0.41 (0.44) |
| All-cause deaths | Baseline | Reduction | Baseline | Reduction | Baseline | Reduction | Baseline | Reduction |
| Men | 18.02 | 0.94 (0.74) | 27.67 | 1.43 (1.12) | 41.99 | 1.96 (1.52) | 58.74 | 2.18 (1.64) |
| Women | 11.82 | 0.47 (0.31) | 18.55 | 0.70 (0.49) | 29.86 | 0.97 (0.69) | 47.45 | 1.12 (0.76) |
Baseline ‘20-year’ event-specific mortality probabilities and aspirin-related reductions (per 100 individuals) using best (and conservative) estimates for prophylactic use of aspirin for 10 years on mortality due to cancer, myocardial infarction, stroke and aspirin-related adverse events (peptic ulcer and gastrointestinal bleeding) according to sex and age at starting use.
Effects on cardiovascular and bleeding events are assumed to occur only during active treatment (10 years) and those for cancer do not start until after 5 years of use but persist for an additional 10 years after treatment completion. Baseline rates are for the entire 20-year period. Figures in parentheses are conservative estimates.
The figures in bold represent overall benefits, overall harms and net balance of benefit and harm.
Figure 1.
Cumulative effects of aspirin taken for 10 years starting at 55 years of age: on deaths over next 20 years in 100 average-risk men (A) and women (B).
discussion
When based solely on the primary prevention of CVD, the value of aspirin prophylaxis in the general population is uncertain, because even though a reduction in vascular events is achieved, it is accompanied by an increase in major bleeding and there is no significant reduction in vascular deaths [1]. Thus, analyses based only on effects on CVD benefits have suggested that aspirin is cost-effective only in individuals at high risk of CVD [38–40]. However, recent evidence suggests that aspirin's effect on overall mortality is mainly through a reduction in cancer deaths [2, 3, 41]. Other studies of incidence have also supported a role for aspirin in cancer prevention [3, 4, 6, 7, 14]. A simple economic model assuming a 22% reduction in all cancer mortality has suggested that aspirin could be cost-effective even in individuals without CVD risk factors, [11]. However, this model has used a very optimistic estimate for aspirin's impact on cancer mortality, and does not consider the impact of age and sex on benefits and harms. It also does not address the most appropriate duration of use and is likely to be simplistic and overly optimistic. Here, we have synthesised all available evidence of aspirin's effects on individual cancers, CVD and its harms. We modelled these effects using population data from the UK for both sexes across different age groups to analyse benefits and harms of prophylactic aspirin use in the average-risk general population in the developed world. Although cancer incidence and/or mortality vary to some degree across the developed world, with small overall adjustments for this, our results are likely to be generalisable to other countries in Western Europe and North America.
uncertainties in benefits
Three members of the group (EJJ, NRC and JAJ) felt that the evidence is still too limited to include reductions in breast, prostate and lung cancer in analyses of the benefits and harms of aspirin use, and favoured a more conservative analysis that included reductions in only colorectal, oesophageal and stomach cancer. However, the balance of benefits and harms in such analysis (supplementary Online Tables W3 and W4, available at Annals of Oncology online) still appears favourable, although fewer individuals would benefit (and the same number would be harmed). Analyses with cancer benefit restricted to CRC alone also show net benefit across all age groups and in both sexes (data not shown), although we claim that these are excessively conservative. Furthermore, uncertainties also exist due to the impact CRC screening may have on CRC prevention by aspirin. Complexities in the use of CRC screening methods, their variable uptake and interplay of aspirin use with sensitivity of screening methods make it almost impossible to predict the magnitude of impact of CRC screening on aspirin benefit in future. Uncomplicated peptic ulcers or other minor bleeding events have been excluded in these calculations since they are not comparable in severity. However, these have effects on morbidity, quality of life and the associated medical expense should also be considered.
It should also be recognised that our best estimates may be conservative, as bigger effects have been seen in several studies, and the overview of trials with long-term follow-up found a 20% relative mortality reduction in all cancers [2]. In addition, the results from RCTs are based on randomised allocation to aspirin and the effects of actual usage could be larger due to cross-over and non-compliance. Recent results using updated aspirin usage [15] from the CPS-II Nutrition Cohort subset show a 16% reduction in cancer mortality in both sexes combined. This is comparable with our main estimates.
These benefit–harm calculations are based on data from the developed world. Further research is needed to determine the benefits and harms in the developing world, where cancer incidence is lower and Helicobacter pylori prevalence is higher.
minimising harm
Although often not as serious as MI, stroke or cancer for the age groups considered here, major bleeding is the most important serious side-effect of aspirin. Efforts to identify high-risk individuals and either reduce their risk or not offer them prophylactic aspirin would greatly improve the benefit–harm ratio. Clear contraindications are those with peptic ulcer, recent bleeding episodes or bleeding tendencies. Other risk factors for bleeding in aspirin or non-steroidal anti-inflammatory drug (NSAID) users are: increasing age, male sex, diabetes, hypertension, being overweight or obese, smoking, alcohol consumption and H. pylori infection [1, 42]. Age is a key factor in weighing benefits and harms, with a roughly doubling of risk with each advancing decade of age. If aspirin has a long-term post-treatment carry-over benefit after more than 5 years of use, restricting prophylactic use to age <70 years in average-risk individuals may be prudent at this stage. However, since the cancer risk also increases steeply with age, use at older ages may be beneficial if the carry-over benefit of aspirin is limited. The increased risk of bleeding in men is not a useful factor in restricting use, because men also have greater benefits. Increased bleeding risk in smokers is a more serious issue, but clearly smoking cessation is a more important preventive action where possible. The risk of gastrointestinal bleeding increases with increasing alcohol consumption [42], and aspirin increases this risk at all levels of consumption. Caution is necessary for prophylactic use in those with high alcohol consumption.
In NSAID users, H. pylori infection is associated with a 2- to 3.5-fold higher risk of uncomplicated peptic ulcer, and with a 2- to 2.5-fold higher risk of gastrointestinal bleeding [23, 43, 44]. We estimate it to account for about 25%–30% of peptic ulcers [23] and upper gastrointestinal ulcer bleeding events in NSAID users. There is limited evidence [45, 46] on an H. pylori screen-and-treat strategy before starting aspirin. Studies investigating the cost-effectiveness of H. pylori screening to prevent gastric cancer [47, 48] support it in general but a trial will provide better quality evidence. HEAT trial in aspirin users (ClinicalTrials.gov Identifier: NCT01506986) is scheduled to start soon. Concomitant use of proton pump inhibitors (PPIs) reduced adverse GI events by 66% (OR 0.34; 95% CI 0.21–0.57) in a meta-analysis of 35 trials [49]. The role of routine prolonged use of PPIs in the general population is less clear. The ongoing AspECT trial [50] is addressing the question as to whether co-administration of PPIs with aspirin will be effective in reducing peptic ulcer disease and gastrointestinal bleeding, but in a population that will mostly not be infected with H. pylori.
research priorities
Several uncertainties exist in our estimates which would benefit from more data. Key among these is the extent of a carry-over effect after stopping aspirin. This is an important issue in determining the most appropriate duration of use, which could be longer than the 10 years used in our base case scenario. In randomised trials, the effects on cancer mortality persisted for several years after the end of the 5- to 9-year intervention period [2]. However, the extent to which the participants continued aspirin use after completing scheduled treatment is not clear. Observational studies suggest greater effect sizes with longer duration of use [6, 7, 24] especially for more than 10 years of aspirin use [26], but in the absence of long-term follow-up, these studies are unable to determine the duration of benefit after treatment cessation. Further research is needed to investigate the duration of cancer prevention effect after stopping the drug.
There is also uncertainty about whether there is an upper age at which the harms outweigh the benefits. For example the balance of benefit–harm in usage above the age of 70 may be different since bleeding events become more common and serious after this age, but the cancer rates also become higher. Ongoing ASPREE trial (ClinicalTrials.gov Identifier: NCT01038583) may help address the question of low-dose aspirin use in elderly. There is also more heterogeneity and consequent uncertainty in the results for women, with smaller effects seen in the WHS trial, than in other studies.
The optimum dose for cancer prevention is also uncertain. Indirect comparisons show little difference between low-dose (75–100 mg/day) and standard-dose (300–325 mg/day) aspirin, although there are no direct randomised comparisons. There is no clear indication that doses higher than 300–325 mg are more effective in general population, although they may be needed in the adjuvant setting or for high-risk populations.
Although at current H. pylori prevalence, screen and treat before starting prophylactic aspirin appears a reasonable strategy, it may not remain cost-effective with declining prevalence.
A 2 × 2 × 2 factorial trial could address all three of these questions—low versus standard dose, 5 versus 10 years duration of use and H. pylori screen-and-treat versus symptom-directed management. However, separate trials could be done if deemed logistically more attractive.
Further research is also needed to identify additional (e.g. genetic) factors associated with the risk of bleeding. Reliable data on minor bleeding episodes in general population are sparse. These events have an important influence on acceptability and adherence, and research to gather such data are needed. Much still remains to be learned in special populations at high risk, such as those with Barrett's oesophagus, where placebo-controlled trials are ongoing. It is important that these are continued and completed.
In summary, analysis of benefits and harms in the general population in the developed world suggests a net benefit for a minimum 5 years of aspirin prophylaxis starting between ages 50 and 65, for both men and women, with larger benefits for 10 years of use. Continuing aspirin use for a longer duration also appears to be beneficial; however, there is uncertainty about the age at which it should be stopped.
funding
This evidence review meeting was sponsored by International Society of Cancer Prevention (ISCaP), Cancer Research UK (CRUK), British Heart Foundation (BHF) and American Cancer Society (ACS) and received funding from CRUK, BHF and ACS. Sponsors and funding sources had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
disclosure
The findings and conclusions in this report are those of the authors and do not represent the official position of the authors' respective institutions. JC: Member of the Bayer advisory board. JB: Consultancy for Bayer Pharma. Research funding from Bayer Pharma. A stockholder and medical director in QuantuMDx, a new medical devices company which will develop point of care pharmacogenetic testing. Aspirin sensitivity is one of company's targets. JAJ: Consultant to Astra-Zeneca, Dr Falk Pharmaceuticals, Chief investigator of AspECT trial and ChoPIN trial. PMR: Has received honoraria for talks, advisory boards and clinical trial committees from several pharmaceutical companies with an interest in antiplatelet agents including Astra-Zeneca, Bayer, Boehringer Ingelheim, Sanofi-BMS, Biotronic, Johnson & Johnson and Servier, and is on the executive committee of the ARRIVE trial. Research funding from Boehringer Ingelheim. All remaining authors have declared no conflicts of interest.
Supplementary Material
references
- 1.Baigent C, Blackwell L, Collins R, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet. 2009;373:1849–1860. doi: 10.1016/S0140-6736(09)60503-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rothwell PM, Fowkes FG, Belch JF, et al. Effect of daily aspirin on long-term risk of death due to cancer: analysis of individual patient data from randomised trials. Lancet. 2011;377:31–41. doi: 10.1016/S0140-6736(10)62110-1. [DOI] [PubMed] [Google Scholar]
- 3.Rothwell PM, Price JF, Fowkes FG, et al. Short-term effects of daily aspirin on cancer incidence, mortality, and non-vascular death: analysis of the time course of risks and benefits in 51 randomised controlled trials. Lancet. 2012;379:1602–1612. doi: 10.1016/S0140-6736(11)61720-0. [DOI] [PubMed] [Google Scholar]
- 4.Rothwell PM, Wilson M, Elwin CE, et al. Long-term effect of aspirin on colorectal cancer incidence and mortality: 20-year follow-up of five randomised trials. Lancet. 2010;376:1741–1750. doi: 10.1016/S0140-6736(10)61543-7. [DOI] [PubMed] [Google Scholar]
- 5.Rothwell PM, Wilson M, Price JF, et al. Effect of daily aspirin on risk of cancer metastasis: a study of incident cancers during randomised controlled trials. Lancet. 2012;379:1591–1601. doi: 10.1016/S0140-6736(12)60209-8. [DOI] [PubMed] [Google Scholar]
- 6.Algra AM, Rothwell PM. Effects of regular aspirin on long-term cancer incidence and metastasis: a systematic comparison of evidence from observational studies versus randomised trials. Lancet Oncol. 2012;13:518–527. doi: 10.1016/S1470-2045(12)70112-2. [DOI] [PubMed] [Google Scholar]
- 7.Bosetti C, Rosato V, Gallus S, et al. Aspirin and cancer risk: a quantitative review to 2011. Ann Oncol. 2012;23:1403–1415. doi: 10.1093/annonc/mds113. [DOI] [PubMed] [Google Scholar]
- 8.Patrono C, Garcia Rodriguez LA, Landolfi R, Baigent C. Low-dose aspirin for the prevention of atherothrombosis. N Engl J Med. 2005;353:2373–2383. doi: 10.1056/NEJMra052717. [DOI] [PubMed] [Google Scholar]
- 9.USPSTF. Routine aspirin or nonsteroidal anti-inflammatory drugs for the primary prevention of colorectal cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2007;146:361–364. [PubMed] [Google Scholar]
- 10.USPSTF. Aspirin for the prevention of cardiovascular disease: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;150:396–404. doi: 10.7326/0003-4819-150-6-200903170-00008. [DOI] [PubMed] [Google Scholar]
- 11.Pignone M, Earnshaw S, McDade C, Pletcher MJ. Effect of including cancer mortality on the cost-effectiveness of aspirin for primary prevention in men. J Gen Intern Med. 2013;28:1483–1491. doi: 10.1007/s11606-013-2465-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cuzick J, Otto F, Baron JA, et al. Aspirin and non-steroidal anti-inflammatory drugs for cancer prevention: an international consensus statement. Lancet Oncol. 2009;10:501–507. doi: 10.1016/S1470-2045(09)70035-X. [DOI] [PubMed] [Google Scholar]
- 13.Flossmann E, Rothwell PM. Effect of aspirin on long-term risk of colorectal cancer: consistent evidence from randomised and observational studies. Lancet. 2007;369:1603–1613. doi: 10.1016/S0140-6736(07)60747-8. [DOI] [PubMed] [Google Scholar]
- 14.Cook NR, Lee IM, Zhang SM, et al. Alternate-day, low-dose aspirin and cancer risk: long-term observational follow-up of a randomized trial. Ann Intern Med. 2013;159:77–85. doi: 10.7326/0003-4819-159-2-201307160-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacobs EJ, Newton CC, Gapstur SM, Thun MJ. Daily aspirin use and cancer mortality in a large US cohort. J Natl Cancer Inst. 2012;104:1208–1217. doi: 10.1093/jnci/djs318. [DOI] [PubMed] [Google Scholar]
- 16.Chan AT, Manson JE, Feskanich D, et al. Long-term aspirin use and mortality in women. Arch Intern Med. 2007;167:562–572. doi: 10.1001/archinte.167.6.562. [DOI] [PubMed] [Google Scholar]
- 17.Cook NR, Lee IM, Gaziano JM, et al. Low-dose aspirin in the primary prevention of cancer: the Women's Health Study: a randomized controlled trial. JAMA. 2005;294:47–55. doi: 10.1001/jama.294.1.47. [DOI] [PubMed] [Google Scholar]
- 18.Dhillon PK, Kenfield SA, Stampfer MJ, Giovannucci EL. Long-term aspirin use and the risk of total, high-grade, regionally advanced and lethal prostate cancer in a prospective cohort of health professionals, 1988–2006. Int J Cancer. 2011;128:2444–2452. doi: 10.1002/ijc.25811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cancer Research UK. CancerStats—Cancer Statistics for the UK. London: Cancer Research UK; 2011. http://www.cancerresearchuk.org/cancer-info/cancerstats/ (April 2011, date last accessed) [Google Scholar]
- 20.Law M, Wald N, Morris J. Lowering blood pressure to prevent myocardial infarction and stroke: a new preventive strategy. Health Technol Assess. 2003;7:1–94. doi: 10.3310/hta7310. [DOI] [PubMed] [Google Scholar]
- 21.Yeh RW, Sidney S, Chandra M, et al. Population trends in the incidence and outcomes of acute myocardial infarction. N Engl J Med. 2010;362:2155–2165. doi: 10.1056/NEJMoa0908610. [DOI] [PubMed] [Google Scholar]
- 22.Roger VL, Go AS, Lloyd-Jones DM, et al. Executive summary: heart disease and stroke statistics–2012 update: a report from the American Heart Association. Circulation. 2012;125:188–197. doi: 10.1161/CIR.0b013e3182456d46. [DOI] [PubMed] [Google Scholar]
- 23.Thorat MA, Cuzick J. Prophylactic use of aspirin: systematic review of harms and approaches to mitigation in the general population. (Manuscript submitted) [DOI] [PubMed]
- 24.Chan AT, Giovannucci EL, Meyerhardt JA, et al. Aspirin dose and duration of use and risk of colorectal cancer in men. Gastroenterology. 2008;134:21–28. doi: 10.1053/j.gastro.2007.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ratnasinghe LD, Graubard BI, Kahle L, et al. Aspirin use and mortality from cancer in a prospective cohort study. Anticancer Res. 2004;24:3177–3184. [PubMed] [Google Scholar]
- 26.Thun MJ, Namboodiri MM, Calle EE, et al. Aspirin use and risk of fatal cancer. Cancer Res. 1993;53:1322–1327. [PubMed] [Google Scholar]
- 27.Jacobs EJ, Connell CJ, Rodriguez C, et al. Aspirin use and pancreatic cancer mortality in a large United States cohort. J Natl Cancer Inst. 2004;96:524–528. doi: 10.1093/jnci/djh084. [DOI] [PubMed] [Google Scholar]
- 28.Gann PH, Manson JE, Glynn RJ, et al. Low-dose aspirin and incidence of colorectal tumors in a randomized trial. J Natl Cancer Inst. 1993;85:1220–1224. doi: 10.1093/jnci/85.15.1220. [DOI] [PubMed] [Google Scholar]
- 29.Chan AT, Ogino S, Fuchs CS. Aspirin use and survival after diagnosis of colorectal cancer. JAMA. 2009;302:649–658. doi: 10.1001/jama.2009.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holmes MD, Chen WY, Li L, et al. Aspirin intake and survival after breast cancer. J Clin Oncol. 2010;28:1467–1472. doi: 10.1200/JCO.2009.22.7918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burn J, Gerdes A-M, Macrae F, et al. Long-term effect of aspirin on cancer risk in carriers of hereditary colorectal cancer: an analysis from the CAPP2 randomised controlled trial. Lancet. 2011;378:2081–2087. doi: 10.1016/S0140-6736(11)61049-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burn J, Bishop DT, Mecklin JP, et al. Effect of aspirin or resistant starch on colorectal neoplasia in the Lynch syndrome. N Engl J Med. 2008;359:2567–2578. doi: 10.1056/NEJMoa0801297. [DOI] [PubMed] [Google Scholar]
- 33.Friis S, Sorensen HT, McLaughlin JK, et al. A population-based cohort study of the risk of colorectal and other cancers among users of low-dose aspirin. Br J Cancer. 2003;88:684–688. doi: 10.1038/sj.bjc.6600760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baron JA, Cole BF, Sandler RS, et al. A randomized trial of aspirin to prevent colorectal adenomas. N Engl J Med. 2003;348:891–899. doi: 10.1056/NEJMoa021735. [DOI] [PubMed] [Google Scholar]
- 35.Cole BF, Logan RF, Halabi S, et al. Aspirin for the chemoprevention of colorectal adenomas: meta-analysis of the randomized trials. J Natl Cancer Inst. 2009;101:256–266. doi: 10.1093/jnci/djn485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Steering Committee of the Physicians' Health Study Research Group. Final report on the aspirin component of the ongoing Physicians' Health Study. N Engl J Med. 1989;321:129–135. doi: 10.1056/NEJM198907203210301. [DOI] [PubMed] [Google Scholar]
- 37.Mihaylova B, Emberson J, Blackwell L, et al. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet. 2012;380:581–590. doi: 10.1016/S0140-6736(12)60367-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Greving JP, Buskens E, Koffijberg H, Algra A. Cost-effectiveness of aspirin treatment in the primary prevention of cardiovascular disease events in subgroups based on age, gender, and varying cardiovascular risk. Circulation. 2008;117:2875–2883. doi: 10.1161/CIRCULATIONAHA.107.735340. [DOI] [PubMed] [Google Scholar]
- 39.Earnshaw SR, Scheiman J, Fendrick AM, et al. Cost-utility of aspirin and proton pump inhibitors for primary prevention. Arch Intern Med. 2011;171:218–225. doi: 10.1001/archinternmed.2010.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pignone M, Earnshaw S, Tice JA, Pletcher MJ. Aspirin, statins, or both drugs for the primary prevention of coronary heart disease events in men: a cost-utility analysis. Ann Intern Med. 2006;144:326–336. doi: 10.7326/0003-4819-144-5-200603070-00007. [DOI] [PubMed] [Google Scholar]
- 41.Mills EJ, Wu P, Alberton M, et al. Low-dose aspirin and cancer mortality: a meta-analysis of randomized trials. Am J Med. 2012;125:560–567. doi: 10.1016/j.amjmed.2012.01.017. [DOI] [PubMed] [Google Scholar]
- 42.Kaufman DW, Kelly JP, Wiholm BE, et al. The risk of acute major upper gastrointestinal bleeding among users of aspirin and ibuprofen at various levels of alcohol consumption. Am J Gastroenterol. 1999;94:3189–3196. doi: 10.1111/j.1572-0241.1999.01517.x. [DOI] [PubMed] [Google Scholar]
- 43.Huang JQ, Sridhar S, Hunt RH. Role of Helicobacter pylori infection and non-steroidal anti-inflammatory drugs in peptic-ulcer disease: a meta-analysis. Lancet. 2002;359:14–22. doi: 10.1016/S0140-6736(02)07273-2. [DOI] [PubMed] [Google Scholar]
- 44.Papatheodoridis GV, Sougioultzis S, Archimandritis AJ. Effects of Helicobacter pylori and nonsteroidal anti-inflammatory drugs on peptic ulcer disease: a systematic review. Clin Gastroenterol Hepatol. 2006;4:130–142. doi: 10.1016/j.cgh.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 45.Chan FK, Sung JJ, Chung SC, et al. Randomised trial of eradication of Helicobacter pylori before non-steroidal anti-inflammatory drug therapy to prevent peptic ulcers. Lancet. 1997;350:975–979. doi: 10.1016/s0140-6736(97)04523-6. [DOI] [PubMed] [Google Scholar]
- 46.Chan FK, To KF, Wu JC, et al. Eradication of Helicobacter pylori and risk of peptic ulcers in patients starting long-term treatment with non-steroidal anti-inflammatory drugs: a randomised trial. Lancet. 2002;359:9–13. doi: 10.1016/s0140-6736(02)07272-0. [DOI] [PubMed] [Google Scholar]
- 47.Parsonnet J, Harris RA, Hack HM, Owens DK. Modelling cost-effectiveness of Helicobacter pylori screening to prevent gastric cancer: a mandate for clinical trials. Lancet. 1996;348:150–154. doi: 10.1016/s0140-6736(96)01501-2. [DOI] [PubMed] [Google Scholar]
- 48.Roderick P, Davies R, Raftery J, et al. The cost-effectiveness of screening for Helicobacter pylori to reduce mortality and morbidity from gastric cancer and peptic ulcer disease: a discrete-event simulation model. Health Technol Assess. 2003;7:1–86. doi: 10.3310/hta7060. [DOI] [PubMed] [Google Scholar]
- 49.Lanas A, Wu P, Medin J, Mills EJ. Low doses of acetylsalicylic acid increase risk of gastrointestinal bleeding in a meta-analysis. Clin Gastroenterol Hepatol. 2011;9:762–768 e766. doi: 10.1016/j.cgh.2011.05.020. [DOI] [PubMed] [Google Scholar]
- 50.Jankowski J, Moayyedi P. Re: cost-effectiveness of aspirin chemoprevention for Barrett's esophagus. J Natl Cancer Inst. 2004;96:885–887. doi: 10.1093/jnci/djh171. author reply 887. [DOI] [PubMed] [Google Scholar]
- 51. doi: 10.1111/j.1365-2036.2009.04131.x. Cai S, Garcia Rodriguez LA, Masso-Gonzalez EL, Hernandez-Diaz S. Uncomplicated peptic ulcer in the UK: trends from 1997 to 2005. Aliment Pharmacol Ther 2009; 30: 1039–1048. [DOI] [PubMed] [Google Scholar]
- 52. doi: 10.1136/bmj.331.7528.1310. Hippisley-Cox J, Coupland C, Logan R. Risk of adverse gastrointestinal outcomes in patients taking cyclo-oxygenase-2 inhibitors or conventional non-steroidal anti-inflammatory drugs: population based nested case-control analysis. BMJ 2005; 331: 1310–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.