Abstract
Serotonin signaling suppresses generation of amyloid-β (Aβ) in vitro and in animal models of Alzheimer’s disease (AD). We show that in an aged transgenic AD mouse model (APP/PS1 plaque-bearing mice), the antidepressant citalopram, a selective serotonin reuptake inhibitor (SSRI), decreased Aβ in brain interstitial fluid (ISF) in a dose-dependent manner. Growth of individual amyloid plaques was assessed in plaque-bearing mice that were chronically administered citalopram. Citalopram arrested the growth of pre-existing plaques and reduced the appearance of new plaques by 78%. In healthy human volunteers, citalopram’s effects on Aβ production and Aβ concentrations in cerebrospinal fluid (CSF) were measured prospectively using stable-isotope labeling kinetics (SILK), with CSF sampling during acute dosing of citalopram. Aβ production in CSF was slowed by 37% in the citalopram group compared to placebo. This change was associated with a 38% decrease in total CSF Aβ concentrations in the drug-treated group. The ability to safely decrease Aβ concentrations is potentially important as a preventive strategy for AD. This study demonstrates key target engagement for future AD prevention trials.
Introduction
Alzheimer’s disease (AD) is the most common cause of dementia, and is characterized pathologically by amyloid plaques and neurofibrillary tangles. A devastating illness, AD typically leads to death within 7–8 years of diagnosis and currently affects 5 million patients in the United States (1). AD incidence doubles every 5 years after 65 years of age and the prevalence is projected to increase dramatically in the next decades to between 13.2 to 16 million patients in the US by mid-century, unless preventive measures are developed (1). Because asymptomatic participants in a large prospective prevention trial might be exposed to an experimental compound for many years, the compound should have a proven safety record, as is the case for selective serotonin reuptake inhibitor (SSRI) drugs. To date, there have not been drugs with both an established safety profile and a mechanism-based rationale that have been tested in primary or secondary prevention trials of AD.
The accumulation of amyloid-β (Aβ) peptide in the brain and its aggregation into amyloid plaques is currently considered the pathological trigger for AD (2). In humans, CSF Aβ concentrations begin changing before tau levels; amyloid deposition occurs in the brain decades before the onset of clinical symptoms (3–5). Given that aggregation of the Aβ peptide into plaques appears to be concentration dependent (6,7), methods for reducing Aβ concentrations are important targets for therapy.
In addition to its effect as an antidepressant, the neurotransmitter serotonin is a candidate for reducing Aβ concentrations, by reducing Aβ production. In depression, the antidepressant drug class known as selective serotonin reuptake inhibitors (SSRI) are thought to have their effect by blocking the reuptake of serotonin into the presynaptic terminal and thus increasing the availability of serotonin. A distinct effect has been demonstrated in many studies showing the link between serotonin, AD, and Aβ. Serotonin receptor levels are reduced in human AD brains (8,9). Activation of serotonin receptors has been shown to reduce Aβ production in vitro; treatment with serotonin or receptor agonists activates intracellular signaling cascades and increases levels of the α-secretase product sAPP-α (10–12). Modulating serotonin levels in vivo shows consistent effects: single-dose treatment of SSRIs in young APP/PS1 mice reduced Aβ levels in the brain interstitial fluid (ISF) by 25% (13). In those studies, serotonin signaling did not alter the rate of Aβ clearance, suggesting that reduced Aβ production was responsible for lower Aβ concentrations. Chronic SSRI treatment over the course of four months reduced Aβ plaque load by 50% in mice (13). SSRI treatment in the 3xTg AD mouse model showed similar reductions in Aβ (14). The beneficial effects of serotonin appear to carry into humans as well. Previously depressed patients who had undergone SSRI treatment in the five years preceding their enrollment in a positron emission tomography (PET) study to quantify amyloid binding, had less evidence of Aβ deposits than those who had not been exposed to SSRI treatment (13).
In this study, we first examined the dose-response effects of citalopram, one of the most selective SSRIs for serotonin, on lowering brain Aβ concentrations in mice. We also prospectively examined the effect of citalopram on individual plaque growth in the same mouse model of AD. Based on these studies we tested the effects of an approximately equivalent dose of citalopram on human Aβ concentrations and production. We used the stable-isotope labeling kinetics (SILK) method (15) that quantifies the amount of newly-generated Aβ, as well as the rate of production and clearance of Aβ, within the CSF in humans. We then examined the hypothesis that, similar to results in the AD transgenic mice, citalopram would prospectively lower Aβ production in the human CNS.
Results
Citalopram dose-response in aged APP/PS1 mice
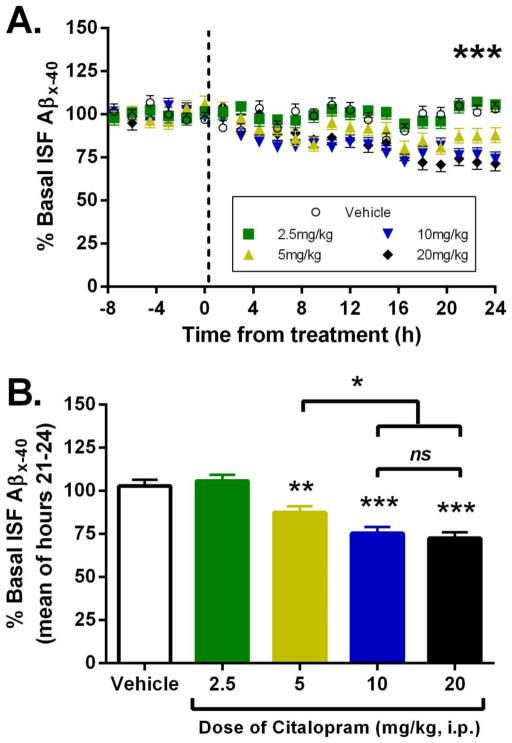
Previous studies demonstrated in 3 month old APP/PS1+/− mice, an age prior to Aβ pathology, that 10mg/kg citalopram reduces brain ISF Aβ concentrations by 25% (13). Here, we determined the acute effect of several doses of citalopram on brain ISF Aβ concentrations in 12 month old, plaque-bearing APP/PS1 mice. Mice were implanted with microdialysis probes into the hippocampus in order to sample brain ISF over time in awake, behaving animals. ISF was sampled every 90 minutes for up to 32 hours. Baseline ISF Aβ concentrations were established in each mouse over 8 hours, followed by intraperitoneal administration of citalopram or vehicle, then another 24 hours of ISF sampling (Fig. 1A). Mice administered citalopram at 2.5 mg/kg had no significant change in ISF Aβ concentrations compared to vehicle-treated mice at 24 hours after dosing (Fig. 1B). At 24 hours after administration, however, there was a significant dose-dependent reduction in ISF Aβ at both 5 and 10 mg/kg citalopram (mean reduction of 12.4% and 24.5%, respectively, compared to baseline concentrations). The maximum reduction in ISF Aβ occurred at 10 mg/kg, as a higher dose of 20 mg/kg citalopram had no additional effect (27.5% reduction). Plaque-load within the hippocampus was assessed histologically in all mice at the 24 hour time point and did not differ between groups (Fig. S1). Importantly, a calculation to determine roughly equivalent doses of citalopram between mice and humans estimated that a 10 mg/kg dose for a mouse is comparable to 50 mg/day in humans (16), a common though high dose of citalopram prescribed to treat depression (17). Citalopram is one of the most selective SSRIs for serotonin re-uptake and has among the most favorable side effect profiles compared with other antidepressants (17).
Fig. 1. Citalopram reduces ISF Aβ concentrations in aged APP/PS1 mice.
(A) 12 month old APP/PS1 mice were implanted with microdialysis probes in the hippocampus. Baseline ISF Aβ was established over 8 hours followed by administration of vehicle (PBS) or citalopram (2.5, 5, 10, 20 mg/kg ip). ISF Aβ was sampled every 90 minutes. A repeated measures ANOVA demonstrated a treatment effect of ***p <0.0001. N=5 per group. (B) The mean concentration of ISF Aβx-40 at hours 21–24 after drug administration was 103%, 105.8%, 87.6%, 75.5%, and 72.5% (mean % ± SE 3.56) for vehicle and ascending doses of citalopram, respectively, compared to baseline in each mouse. There was a significant reduction in ISF Aβ at 5, 10 and 20 mg/kg citalopram compared to vehicle-treated mice (p=0.003, p<0.0001, and p<0.0001, respectively). Aβ concentrations after 10 mg/kg citalopram were significantly lower than for 5 mg/kg (p=0.02) but did not differ significantly from the 20 mg/kg dose.
Chronic citalopram treatment blocks plaque-growth in APP/PS1 mice
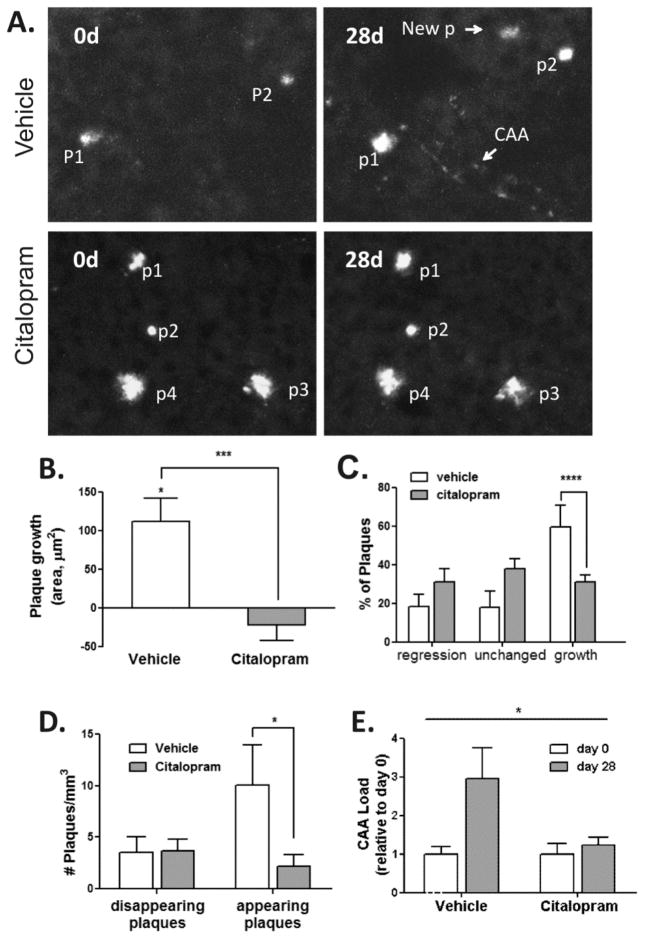
Using APP/PS1 mice, we examined the effect of chronic citalopram administration on amyloid plaque appearance and growth. Six month-old APP/PS1 mice were treated with citalopram, 10 mg/kg (n=8), or vehicle (n=6), i.p daily for 28 days. Brains from mice were non-invasively imaged using 2-photon microscopy on the day prior to the initial injection and on the day after the final injection (28 days), focusing on identical high-power fields so that individual plaques could be compared over time. During this 28-day interval, plaques in mice treated with vehicle grew on average 111.4 μm2, a growth rate significantly different from 0 (p = 0.0003), whereas plaques in citalopram-treated mice did not grow (−22.4 μm2, a growth rate not significantly different from 0, p = 0.26) (Fig 2A,B). The rate of plaque growth in the vehicle group was significantly higher relative to the citalopram group (p=0.0003). Plaques were classified as regressing in size (<0.75-fold growth), unchanged (0.75- to 1.25-fold growth), or growing (>1.25-fold growth). Citalopram reduced the likelihood of plaque growth [Odds Ratio, OR (95% CI)], 0.298 (0.148, 0.600), p=0.0008; but did not increase the likelihood of plaque regression, 2.1 (0.96, 4.7), p= 0.063 (Fig 2C). Furthermore, citalopram treatment reduced the number of newly appearing plaques [2.2 (1.1) vs. 10 (3.9), p= 0.01], but had no effect on the number of disappearing plaques [3.7 (1.1) vs. 3.6 (1.5), p = 0.93] (Fig 2D). A similar effect on amyloid angiopathy progression was observed (1.2 (0.2) versus 2.9 (0.8) p= 0.03) during 28 days of treatment (Fig 2E). Taken together, these results suggest that citalopram reduces plaque appearance and growth, but has no effect on plaque regression. These effects are consistent with citalopram’s inhibition of Aβ production without enhancing Aβ clearance mechanisms.
Fig. 2. Citalopram reduces amyloid plaque formation and growth in APP/PS1 mice.
Six month-old APP/PS1 mice were treated with Citalopram 10mg/kg per day or vehicle i.p. for 28d (Vehicle: n=6 mice, 49 plaques; Citalopram: n=8 mice, 116 plaques) and imaged using 2 photon microscopy. (A) Representative multiphoton micrographs of individual amyloid plaques in the cortex of APP/PS1 mice before (0d) and 28 days after treatment. A repeated measures ANOVA indicated that Citalopram attenuated the growth of pre-existing plaques. P: plaque; CAA: cerebral amyloid angiopathy; New P: newly appearing plaque. (B) Analysis using a cumulative logit model demonstrated that Citalopram reduced the likelihood of plaque growth, but did not induce plaque regression (C) Citalopram also reduced the appearance of new plaques based on comparison using a Wilcoxon rank-sum test (D), and inhibited CAA progression, based on a comparison using a t-test (E) during the 28 days of treatment compared to vehicle control. Scale bar, 50 μM; *P < 0.05; ** P < 0.01, ***P<0.001, **** P < 0.001 for Odds Ratio. Values represent mean ± SE.
Citalopram reduces CSF Aβ production and concentrations in humans
Healthy subjects age 18–50, without medical disease, electrocardiogram (ECG) or laboratory abnormalities and with no prior history of antidepressant treatment, were enrolled in a double-blind study. 40 volunteers were screened for inclusion and exclusion criteria; 12 were screen failures. Among the 28 enrolled subjects, 4 had no obtainable CSF and one subject was not included in the analysis because the citalopram dose occurred later than other subjects; diurnal variation in Aβ concentrations precluded adjusting the “start” time for measurement (18).
Three subjects had post-lumbar puncture headaches and their participation had to be halted during the study. Adverse events included 8 (33%) post-lumbar puncture headaches requiring epidural blood patch (a treatment for LP-induced headache), 2 episodes (8%) of back and leg pain and 4 episodes (16%) of nausea and vomiting. One subject received Compazine due to nausea. All events resolved and likely were related to lumbar CSF sampling or the high dose of citalopram used in this study. Of these side effects, the nausea and vomiting were most likely due to the SSRI. 23 subjects (11 females, 12 males) had measurements taken of CSF Aβ concentrations and 13C-labeled (newly produced) and unlabeled (residual) Aβ (see methods). The placebo versus citalopram treated volunteers included in the analysis did not differ in demographic characteristics, including age, gender or race. Placebo and citalopram treated volunteers were first matched on gender and then as closely as possible in age, always within 5 years. In addition, since the groups were not explicitly matched on race, at study mid-point the groups were checked by an unblinded research assistant to ensure that they were balanced in racial composition.
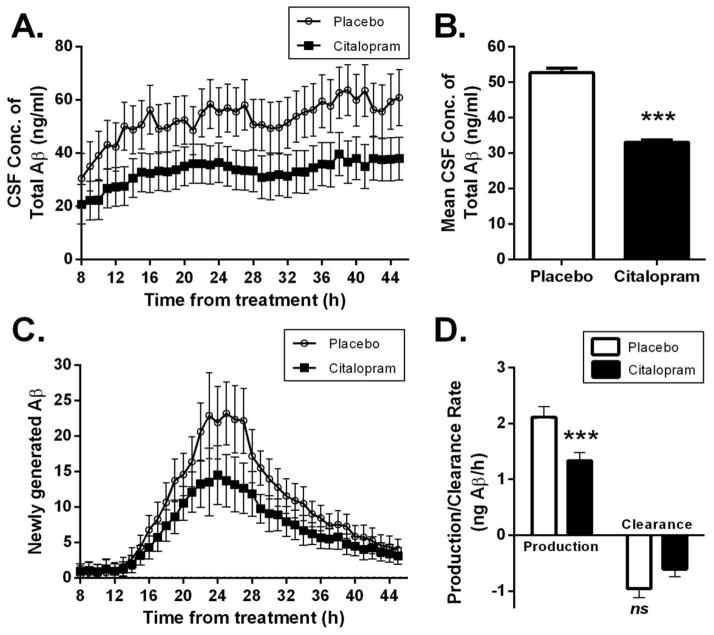
Hourly CSF sampling began 8 hours after initial treatment with citalopram or placebo. 13 subjects had CSF sampling for 37 hours and 10 subjects had CSF sampling for 18 hours, which includes the 12-hour production phase (see Bateman et al for description of the incorporation of 13C-labeled leucine as a measure of “newly generated” e.g., newly produced amyloid (19–20)). Citalopram-treated subjects had significantly different changes in Aβ concentrations over time compared to placebo (repeated measures ANOVA: time by treatment group p = 0.002), along with a significant main effect of the treatment group (p=0.001) (Fig. 3A). Post-hoc approximate t-tests revealed significant differences (p < 0.05) between the treatment and placebo groups in terms of change in the mean Aβ concentrations from baseline, at hours 10, 13–16, 19, 22–27, 29, 32–41, 44–45 from treatment. Citalopram treatment was associated with a 38% lower average Aβ concentration over the 37 hour sampling period compared to placebo (p=0.001) (Fig. 3B). The longitudinal change in concentrations of newly-produced Aβ, as assessed by plasma 13C6-Leucine normalized labeled Aβ, also differed significantly between citalopram-treated subjects relative to placebo (time by treatment group, p = 0.0004), along with a significant main effect of treatment group (p=0.008) (Fig. 3C). As with Aβ concentrations, newly-produced Aβ was reduced in citalopram-treated individuals, as post-hoc approximate t-tests revealed that the change in average concentration of newly-generated Aβ from baseline was significantly lower in the citalopram-treated group relative to placebo at hours 13–41 and 44–45 (p<0.05). The mean production rate of Aβ within CSF calculated over hours 13–22 was significantly lower in citalopram-treated individuals (mean, SE) [1.33 pg/hr (0.15)] versus placebo [2.11 pg/hr (0.20] (p=0.005), a reduction of 37%. However the clearance rate of Aβ was unchanged (−0.61 (0.14) versus −0.96 (0.16) per hour mean & 95% CI; p=0.115). These data suggest that citalopram reduced CSF Aβ concentrations by suppressing Aβ production while having a negligible effect on Aβ clearance mechanisms. Interestingly, the tracer/tracee ratio (labeled Aβ/unlabeled Aβ) (TTR) over the 37 hours of CSF sampling was overlapping for both groups (Fig. S2 suggesting that the fractional turnover of Aβ was unchanged at the time of the SILK study and that Aβ metabolism had reached a new steady state. The longitudinal course of TTR was not significantly different between the two groups (time by treatment group p = 0.99, main effect of treatment group p=0.91).
Fig. 3. Citalopram reduces CSF Aβ concentrations and production rate in healthy human volunteers.
(A) Comparison of hourly CSF Aβ concentrations over 37 hours of sampling in healthy human volunteers treated with citalopram or placebo. (B) Using repeated measures ANOVA, subjects who received citalopram had lower mean CSF concentrations of Aβ (31.4 +/− 0.64) ng/mL over hours 8–45 compared with placebo (50.3+/− 0.99) ng/mL (p=0.001). (C) Newly generated Aβ is decreased following administration of citalopram compared with placebo, determined from incorporation of 13C-Leucine; 13C-Leucine is not radioactive but the different isotope can be detected by mass spectrometry. (D) The mean production rate of Aβ over hours 13–22 was significantly slower in citalopram-treated individuals [1.33 (0.16) versus 2.11 (0.20) ng/ml/hr, p=0.005], but the clearance rate of Aβ was unchanged [−0.61 (0.14) versus −0.96 (0.16) ng/ml/hr, p=0.115]. Values represent mean and 95% CI (A, C) and mean ± SE (B, D) and p-values are based on repeated measures ANOVA derived approximate t-tests.
Discussion
In a prospective study of CSF measurements we show that an acute dose of citalopram administration was associated with a decreased amount of newly generated Aβ in young healthy humans, and decreased CSF Aβ concentrations compared with placebo. Importantly, this effect was seen in the production phase of Aβ with no significant group differences initially, but with subsequent differences that remained throughout the production phase and with no effect on clearance. In complementary rodent studies, we used serial two-photon imaging and found that administration of citalopram prospectively blocked the growth of existing amyloid plaques and significantly reduced the appearance of new plaques, compared to vehicle-treated animals, over a 4 week period. We have previously shown in mice that SSRI antidepressants lower the production of Aβ by 25% in the brain ISF, as demonstrated by in vivo microdialysis studies (13). To date, this effect has been determined for 4 different SSRI antidepressants. We have now demonstrated a dose-response curve for the effects of citalopram on lowering Aβ concentrations in plaque-bearing mice. Importantly, the maximal effective dose of citalopram that lowered brain Aβ concentrations and plaques in mice (10 mg/kg) is approximately comparable to a dose of citalopram (50 mg/day) that has been administered to humans as an antidepressant, and similar to the dose we used in this study, 60 mg. Further, we previously showed in retrospective studies in humans that SSRI exposure within the 5 years prior to amyloid PET scan was associated with lower amyloid binding as determined by PIB-PET imaging (13). A recently discovered protective mutation in APP (A673T) has approximately the same magnitude of Aβ lowering effect as the citalopram effect in the current study, strengthening the argument for SSRIs as a potential AD prevention strategy (21).
There is an interesting discrepancy in the timing of the response to SSRI treatment for depression compared with the current study. Depression therapy generally takes several weeks of SSRI treatment before amelioration of symptoms occurs. However, we see reductions in CSF Aβ within a matter of hours. The mechanisms by which SSRIs mediate these two effects are likely to be very different. Current theories for SSRI- mediated treatment of depression include increased production of BDNF (22), increased hippocampal neurogenesis (23–25) and reduced expression of serotonin autoreceptors (26). All of these processes require weeks to take full effect. In contrast, the mechanisms that regulate Aβ production use direct signaling pathways, which exert their effects much faster. Stimulation of serotonin receptors activates the ERK signaling cascade in as little as 5 minutes (27–29). Our previous studies demonstrated that ERK activation was required for serotonin-dependent suppression of Aβ (13). After citalopram treatment in mice, we also found increased α-secretase activity, which appears to be downstream of ERK signaling (30). ERK activity can also reduce γ-secretase activity, which would lower Aβ levels. Adding activated ERK to an in vitro γ-secretase cleavage assay reduced activity in a dose-dependent manner, and the γ-secretase subunit nicastrin is an ERK substrate (31). Phosphorylation of either of these secretase enzymes by activated ERK could occur within minutes of SSRI treatment, thus accounting for the rapid changes in Aβ levels. Future experiments are still necessary to further define the entire intracellular signaling pathway responsible for the reduced Aβ production following SSRI treatment.
Maintaining low Aβ concentrations with any agent, including secretase inhibitors or SSRIs, should limit the rate at which toxic species of Aβ are formed. Early in disease pathogenesis, soluble Aβ monomers aggregate into soluble Aβ oligomers and insoluble Aβ plaques, both of which can alter synaptic transmission and be toxic to neurons under certain conditions. Conversion of Aβ from its normal soluble form into these toxic conformations is concentration-dependent; high concentrations of Aβ are much more prone to aggregate than lower concentrations of Aβ (7). Small changes in CSF Aβ concentration have dramatic effects on Aβ accumulation as plaques. In animal models, a 12–25% reduction in Aβ concentrations was sufficient to dramatically decrease plaque formation (32). In studies using BACE knock-out mice, a 12% decrease in Aβ concentrations, assessed in total brain tissue, resulted in a marked blocking of plaque formation (33). Here, a decrease of 25% in Aβ concentration produced a 78% reduction in new plaque formation. Individuals with autosomal dominant AD or Down’s syndrome have only 20–50% higher CSF Aβ42 concentrations than normal but manifest disease 20–30 years before AD patients with the sporadic form of the disease. Therefore, development of safe and effective therapeutic approaches that can reduce CNS Aβ concentrations even modestly may prevent pathological amyloid accumulation and a subsequent cascade of neuronal damage, which would have an important impact on preventing or slowing progression to symptomatic AD. The ability to decrease CNS Aβ concentrations is thus potentially important as a preventive strategy for AD. A caveat is that there have been many clinical trials using Aβ lowering drugs in AD and MCI that were unsuccessful. However, these trials were conducted after the onset of clinical symptoms and thus may have been too late to prevent further disease progression. Nonetheless, the efficacy of the current proposed prevention strategy depends critically on the validity of the amyloid hypothesis of Alzheimer’s disease.
A limitation of the study is that data were not collected during the eight hours following drug/placebo administration. Thus an important unanswered question is the timing of when CSF concentration differences arose. The concentrations over the time course were such that steady-state kinetics applied reasonably well after the first 12 hours and the rate of change of concentration appeared similar in both groups. Tracer kinetics established that the fractional turnover rate in the two groups was very similar suggesting that Aβ metabolism had reached a new steady state at the time when the SILK study was carried out. In future studies, it will still be important to establish that the change in concentrations occurred as a result of drug administration.
Another limitation is that our human studies were conducted in young individuals, presumably prior to plaque formation. It is not known whether the same effect would be observed in older individuals with plaque deposition. However, the current dose-response studies and prospective plaque visualization studies in older mice (with plaques) suggest that the Aβ lowering effects of citalopram likely will be effective in older amyloid-plaque positive humans. The next step will be prospective trials in older cognitively normal individuals to test the ability of SSRI exposure to prevent the development or slow the growth of plaques and thus the subsequent cascade to dementia.
Materials and Methods
Study Design
The overall objective of the study was to determine in mouse AD models and in young healthy human volunteers whether citalopram had an amyloid protein lowering effect in brain ISF/CSF, respectively. In mice, this was assessed by dose-response curve microdialysis studies on amyloid concentrations and by multiphoton microscopy determination of individual plaque growth. In humans, this was assessed by determining the amyloid production rate and CSF concentration in double blind randomized studies of citalopram vs placebo. Based on the power analysis of the data, for Aβ concentration with alpha=0.05, the power of the time by treatment group interaction was > 99.99% and the power of the main effect of treatment was 95.03%. For newly generated Aβ, the power of time by treatment group interaction was also > 99.99% while the power of the main effect of treatment was 66.18%.
Mouse studies
All experimental procedures involving animals were performed in accordance with guidelines established by the Animal Studies Committee at Washington University. We bred APP/PS1+/− hemizygous mice (34) to wildtype C3H/B6 mice (Jackson Labs, Bar Harbor, Maine), then used the APP/PS1+/− offspring for experiments at 12 months of age, when this model contains ample Aβ deposits throughout the hippocampus and cortex. These mice contain a PS1ΔE9 mutation and human APP Swedish mutation that were inserted into a single locus. Animals were screened for the PS1 and APP transgenes by PCR from tail DNA. Male and female littermate mice were distributed between all experimental groups. For multiphoton studies, 6 month-old mice were injected i.p. daily with either citalopram (10 mg/kg) or saline for 28 days. Multiphoton imaging was performed on the day before the first injection, and after the last injection (see below).
In vivo Aβ Microdialysis
In vivo microdialysis to assess brain ISF Aβ in the hippocampus of awake, freely moving APP/PS1+/− mice was performed similar to previously described (35,13). This technique samples soluble molecules within the extracellular fluid that are smaller than 38-kilodaltons, the molecular-weight cut off of the probe membrane. Aβ capable of entering the probe has been dubbed “exchangeable Aβ or eAβ”.
Under isoflurane volatile anesthetic, guide cannulae (BR-style, Bioanalytical Systems, Indianapolis, IN) were cemented into the left hippocampus (3.1mm behind bregma, 2.5 mm lateral to midline, and 1.2 mm below dura at a 12° angle). Two millimeter microdialysis probes were inserted through the guides so the membrane was contained entirely within the hippocampus (BR-2, 30-kilodalton MWCO membrane, Bioanalytical Systems). Microdialysis perfusion buffer was artificial CSF (aCSF) (perfusion buffer in mM: 1.3 CaCl2, 1.2 MgSO4, 3 KCl, 0.4 KH2PO4, 25 NaHCO3, and 122 NaCl, pH 7.35) containing 4% bovine serum albumin (Sigma, St. Louis, MO) that was filtered through a 0.1μM membrane. Flow rate was a constant 1.0μl/min. Samples were collected every 90 minutes with a refrigerated fraction collector into polypropylene tubes and assessed for Aβx-40 by ELISA at the completion of each experiment. Basal concentrations of ISF Aβ were defined as the mean concentration of Aβ over the 8 hours preceding drug treatment. Once basal ISF Aβ concentrations were established, APP/PS1 mice were administered either vehicle (PBS) or citalopram at one of 4 doses intraperitoneally (2.5, 5, 10, 20mg/kg). After drug treatment, ISF Aβ concentrations were sampled every 90 minutes for an additional 24 hours then all samples were assayed for Aβ concentration by sandwich ELISA. All ISF Aβ concentrations were normalized to the basal Aβ concentration in each mouse.
Aβ Sandwich ELISA
ISF Aβ concentrations were assessed using sandwich ELISAs as described (13). Briefly, a mouse-anti-Aβ40 antibody (mHJ2) was used to capture and a biotinylated central domain antibody (mHJ5.1) was used to detect, followed by streptavidin-poly-HRP-40 (Fitzgerald Industries, Concord, MA). All ELISA assays were developed using Super Slow ELISA TMB (Sigma, St. Louis, MO) and absorbance read on a Bio-Tek Epoch plate reader (Winooski, Vermont) at 650nm. The standard curve for each assay utilized synthetic human Aβ1-40 peptide (American Peptide, Sunnyvale, CA).
Thinned-skull cranial window surgery
Thinned-skull cranial windows were prepared on the day of the first multiphoton imaging session as described previously (32). Briefly, mice were anesthetized under volatile isoflurane and the skin and periosteum were removed to expose the skull. A high-speed drill and microsurgical blade (Surgistar) were used to thin the skull until the skull was transparent and displayed flexibility. Two thinned-skull windows (each 0.8–1.0 mm in diameter) were prepared on each animal.
In vivo multiphoton microscopy
Longitudinal multiphoton microscopy to monitor amyloid plaque growth was performed as described previously (3). Briefly, mice were injected intraperitoneally with the fluorescent amyloid-binding compound methoxy-X04 (5 mg ml−1) 24 hours prior to each multiphoton imaging session (36). Animals were mounted on a custom-built stereotaxic apparatus and a small ring of molten bone wax was applied to the skull surrounding the perimeter of the window to create a water immersion chamber. The cranial window was centered under the objective lens on a two-photon microscope [LSM 510 META NLO system (Carl Zeiss Inc.) with a Cameleon Ti:Sapphire laser (Coherent Inc.)]. Two-photon fluorescence was generated with 750 nm excitation and fluorescence emission was detected at 435–485 nm. A 10X water-immersion objective [numerical aperture (NA) = 0.33, Zeiss] was used to create a site map during initial imaging and a 40X water-immersion objective (NA = 0.75, Zeiss) was used for high-resolution quantification of individual amyloid plaques. Incremental z-stack image series (step distance = 10 and 5 μm under 10X and 40X objectives, respectively) were acquired from the skull surface to approximately 200 μm into cortex.
To determine the effect of citalopram on amyloid plaque formation and growth, the same sites for each animal were imaged on day 0 and day 28. Collapsed z-stack images for each individual plaque were measured by radii of plaque and intensity using SigmaScan Pro Image Analysis Software (Systat Software) with a preset threshold (threshold = mean + 4 * SD). Plaques were excluded from analysis if they were located at the edge of the window, exhibited fluorescence intensity less than the mean intensity of an adjacent background region or if the image acquisition was affected by motion artifacts from heartbeat or respiration.
Human Studies
A randomized double-blind placebo controlled study was performed to determine the central nervous system effect of 60 mg of the SSRI antidepressant citalopram, given in divided oral doses (2 doses of 30 mg each). This study was approved by the Washington University Human Studies Committee and all participants gave written consent. Healthy 21–50 year old male and female volunteers were invited to participate, screened, and enrolled. Exclusion criteria included any serious or unstable medical illness including neurologic disease and prior use of antidepressants within the past 5 years. Each participant received either placebo or a dose of 30 mg of citalopram 8 hours and 6 hours before the start of the labeling study (total = 60 mg). A central nervous system stable isotope labeling kinetics study of Aβ was conducted, as previously described (19). Briefly, following an overnight fast, a lumbar subarachnoid catheter was placed at the L3-4 interspace, intra-venous catheters were placed in both arms, and hourly sampling of blood and cerebral-spinal fluid was conducted during and after administration of a stable-isotope labeled amino acid (13C6 -leucine) (19–20). Serial samples of 12 mLs of blood and 6 mLs of CSF were taken at one hour time intervals for up to 37 hours. CSF has a production rate of 20 mLs per hour and replenishes itself throughout the procedure. We administered 13C6-leucine with an initial bolus of 2 mg/kg over 10 min, followed by 9 hours of continuous intravenous infusion at a rate of 2 mg/kg/h. Participants were provided with 3 low-leucine diet meals during the leucine infusion to decrease the amount of ingested unlabeled leucine. Participants rested overnight following the study with discharge at 48 hours.
Labeled and unlabeled Aβ analysis by mass spectrometer
Incorporation of 13C6 leucine into Aβ was measured using the stable isotope labeling kinetics (SILK-Aβ) assay similar to Bateman et al 2006, with the addition of stable isotope spike absolute quantitation (SISAQ™) to allow for absolute quantitation of Aβ in the samples (C2N Diagnostics). A quantitation peptide (Aβ40) was added to all samples at a final concentration of 10 ng/mL prior to immunoprecipitation of Aβ from the sample. Aβ was immunoprecipitated using the Aβ mid domain specific antibody HJ5.1 (37) and the 3 isotopically different versions of the Aβ 17–28 tryptic peptide (unlabeled, 13C6 labeled, and the quantitation peptide) were monitored on the mass spectrometer (Thermo LTQ XL). Concentrations of Aβ total was calculated based on SISAQ™ standard curves (containing various concentrations of unlabeled and 13C6 labeled Aβ 40) and the tracer to tracee ratio (labeled Aβ/unlabeled Aβ) (TTR) for metabolically labeled Aβ was calculated based on SILK™ standard curves (containing various ratios of unlabeled and 13C6 labeled Aβ). The percentage of CSF Aβ that is labeled during its production is a function of the amount of 13C6-leucine present in the precursor pool. In prior SILK studies (19), plasma 13C6-leucine enrichment was found to adequately represent 13C6 labeling in the precursor pool for Aβ synthesis. Plasma 13C6-leucine enrichment was quantified using capillary gas chromatography-quadrupole-mass spectrometry (GC-MS) as described (20). Plasma leucine was converted to its heptafluorobutyric propyl ester, the 13C6-/12C6-leucine abundance ratio was quantified using GC-MS in negative chemical ionization mode by monitoring m/z 355 and 349 (Agilent 6890N gas chromatograph and Agilent 5973N mass selective detector, Palo Alto, CA, USA). The amount of newly-produced Aβ at each time point was calculated by dividing the labeled Aβ concentration by the average plasma 13C6-leucine enrichment during the 13C6-leucine infusion (hours 1–9). Citalopram has a long half-life, meaning there was likely a sustained effect on Aβ throughout CSF sampling despite the single administration at the beginning of the study.
Statistics
Repeated measures ANOVA (38) with fixed effects of treatment group, time and a treatment group by time interaction, was utilized to analyze the patterns of change in basal ISF Aβ, plaque growth, newly-generated Aβ, Aβ concentrations and TTR over time. In order to account for the within-subject dependencies in the outcome variables, these models incorporated a random intercept at the subject level and, for plaque growth, also a random intercept at the plaque level. The models also incorporated a Toeplitz covariance structure for the model errors in basal ISF Aβ, first-order autoregressive process heterogeneous variance covariance structure for the model errors in newly-generated Aβ and the same covariance structure but with homogeneous variance for CSF concentrations of total Aβ and TTR, as these provided the best measures on Akaike’s information criterion (39). Subsequent model derived post-hoc approximate t-tests were used to assess differences between treatment groups, at specific time points, in change in the mean concentrations of newly generated Aβ and Aβ concentrations from baseline while, for basal ISF Aβ, mean concentrations averaged over hours 21–24 were compared.
The production and clearance rates were estimated as the difference in mean newly generated Aβ at hours 14 and 5 and 30 and 20, respectively, divided by the number of hours between the each pair of measurements. Differences between the production and clearance rates between the treatment groups were tested with model-derived approximate t-tests. Admittedly, this model is limited by presuming that production and clearance rates are independent phenomena in the analysis, while practically production and clearance occur simultaneously.
The general linear mixed models were estimated using restricted maximum likelihood estimation, with the approximate F-test denominator degrees of freedom based on the method of Kenward and Roger (40). The likelihood of plaques exhibiting regression, growth or remaining unchanged was compared between the treatment groups with a non-proportional odds cumulative logit model. The number of disappearing/appearing plaques was compared between treatment groups using an exact Wilcoxon rank-sum test, with CAA load compared using a t-test. All analyses were conducted using SAS version 9.3 (SAS Institute, Inc, Cary, NC, USA).
Power Analysis
Based on a repeated measures ANOVA, with a compound symmetry covariance structure and alpha=0.05, for Aβ concentration the power of the time by treatment group interaction was > 99.99% and the power of the main effect of treatment was 95.03%. For newly generated Aβ, the power of time by treatment group interaction was also > 99.99% while the power of the main effect of treatment was 66.18%.
Supplementary Material
Supplemental Figure 1: Plaque load in aged APP/PS1 mice dose response.
Supplemental Figure 2: CSF Aβ Tracer to Tracee ratio did not differ between citalopram and placebo.
Accessible Summary.
Evidence in cognitively normal individuals implicates increased brain amyloid burden for subsequent higher rate of cognitive decline; increased amyloid burden can occur decades before the onset of cognitive symptoms. In mouse models of Alzheimer’s disease (AD) and healthy young humans this study tested for evidence of a drug lowering effect on brain amyloid burden. The drug used in this study, citalopram, belongs to the selective serotonin reuptake inhibitor (SSRI) class of antidepressant drugs. Here it was not used as an antidepressant but rather because serotonin signaling suppresses generation of amyloid-β (Aβ) in animal models of AD. We show that in an aged transgenic AD mouse model citalopram decreased brain Aβ concentrations in a dose-dependent manner. Further, citalopram halted the growth of pre-existing brain amyloid plaques and reduced the appearance of new plaques by 78%. In healthy human volunteers, citalopram’s effects on Aβ production and Aβ concentrations in cerebrospinal fluid (CSF) were measured prospectively. Aβ production in CSF was slowed by 37% in the citalopram group compared to placebo and there was a 38% decrease in total CSF Aβ concentrations. The ability to safely decrease Aβ concentrations is potentially important as a preventive strategy for AD.
Acknowledgments
The authors thank Wendy Sigurdson, R.N., for her expert assistance with the human catheter adjustments.
Funding. This study was funded by: R21 AG03969002 (YIS), R01 AG04150202 (YIS), NIH R01 AG042513 (JRC); R21 NS082529,(JML) R01 NS067905 (JML), Washington University Hope Center for Neurological Diseases (YIS, JML) and the Washington University Biomedical Mass Spectrometry Resource (P41 GM103422, P60 DK020579, and P30 DK056341).
Footnotes
Author contributions: YIS, JML and JRC designed the experiments, were responsible for carrying out the experiments and data analysis and wrote the manuscript; for microdialysis studies JRF, MG, WDF helped design experiments, performed experiments, and approved the final manuscript; for multiphoton studies PY helped design experiments, performed experiments, and approved the final manuscript; for human studies KEY, CRF, RC, and RS helped design experiments, performed experiments, and approved the final manuscript. TW and RB contributed to the design of the human stable isotope labeling study as well as the analysis and interpretation of this data and review of the manuscript; MJ and CX implemented statistical methods, performed data analysis, and helped write the manuscript; JCM and MAM contributed to experimental design and approved the final manuscript.
Competing interests: TW is an employee and has stock options in C2N Diagnostics LLC. MAM is an employee of Avid Radiopharmaceuticals. JCM has participated in or is participating in clinical trials of antidementia drugs sponsored by the companies Janssen Alzheimer Immunotherapy Program, Pfizer, and Eli Lilly/Avid Radiopharmaceuticals. JCM has served or is serving as a consultant or has received speaking honoraria for the company Lilly USA. Neither JCM nor his family owns stock or has equity interest (outside of mutual funds or other externally directed accounts) in any pharmaceutical or biotechnology company. The other authors declare no competing interests. TW is listed as an inventor on a patent related to this work: Title: Methods for measuring concentrations of biomolecules Publication #: WO2013082307 Application #: PCT/US2012/067110.
References
- 1.Hebert LE, Scherr PA, Bienias JL, Bennett DA, Evans DA. Alzheimer disease in the US population: prevalence estimates using the 2000 census. Arch Neurol. 2003 Aug;60:1119. doi: 10.1001/archneur.60.8.1119. [DOI] [PubMed] [Google Scholar]
- 2.Holtzman DM, Morris JC, Goate AM. Alzheimer’s disease: the challenge of the second century. Science translational medicine. 2011 Apr 6;3:77sr1. doi: 10.1126/scitranslmed.3002369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Price JL, Morris JC. Tangles and plaques in nondemented aging and “preclinical” Alzheimer’s disease. Ann Neurol. 1999 Mar;45:358. doi: 10.1002/1531-8249(199903)45:3<358::aid-ana12>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 4.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002 Jul 19;297:353. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 5.Bateman RJ, et al. Clinical and Biomarker Changes in Dominantly Inherited Alzheimer’s Disease. N Engl J Med. 2012 Jul 11; doi: 10.1056/NEJMoa1202753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bero AW, et al. Neuronal activity regulates the regional vulnerability to amyloid-beta deposition. Nat Neurosci. 2011 Jun;14:750. doi: 10.1038/nn.2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lomakin A, Teplow DB, Kirschner DA, Benedek GB. Kinetic theory of fibrillogenesis of amyloid beta-protein. Proc Natl Acad Sci U S A. 1997 Jul 22;94:7942. doi: 10.1073/pnas.94.15.7942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arai H, Kosaka K, Iizuka R. Changes of biogenic amines and their metabolites in postmortem brains from patients with Alzheimer-type dementia. J Neurochem. 1984 Aug;43:388. doi: 10.1111/j.1471-4159.1984.tb00913.x. [DOI] [PubMed] [Google Scholar]
- 9.Reynolds GP, et al. 5-Hydroxytryptamine (5-HT)4 receptors in post mortem human brain tissue: distribution, pharmacology and effects of neurodegenerative diseases. British journal of pharmacology. 1995 Mar;114:993. doi: 10.1111/j.1476-5381.1995.tb13303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arjona AA, Pooler AM, Lee RK, Wurtman RJ. Effect of a 5-HT(2C) serotonin agonist, dexnorfenfluramine, on amyloid precursor protein metabolism in guinea pigs. Brain Res. 2002 Sep 27;951:135. doi: 10.1016/s0006-8993(02)03153-0. [DOI] [PubMed] [Google Scholar]
- 11.Nitsch RM, Deng M, Growdon JH, Wurtman RJ. Serotonin 5-HT2a and 5-HT2c receptors stimulate amyloid precursor protein ectodomain secretion. J Biol Chem. 1996 Feb 23;271:4188. doi: 10.1074/jbc.271.8.4188. [DOI] [PubMed] [Google Scholar]
- 12.Robert SJ, Zugaza JL, Fischmeister R, Gardier AM, Lezoualc’h F. The human serotonin 5-HT4 receptor regulates secretion of non-amyloidogenic precursor protein. J Biol Chem. 2001 Nov 30;276:44881. doi: 10.1074/jbc.M109008200. [DOI] [PubMed] [Google Scholar]
- 13.Cirrito JR, et al. Serotonin signaling is associated with lower amyloid-beta levels and plaques in transgenic mice and humans. Proc Natl Acad Sci U S A. 2011 Sep 6;108:14968. doi: 10.1073/pnas.1107411108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nelson RL, et al. Prophylactic treatment with paroxetine ameliorates behavioral deficits and retards the development of amyloid and tau pathologies in 3xTgAD mice. Exp Neurol. 2007 May;205:166. doi: 10.1016/j.expneurol.2007.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bateman RJ, et al. A gamma-secretase inhibitor decreases amyloid-beta production in the central nervous system. Ann Neurol. 2009 Jul;66:48. doi: 10.1002/ana.21623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008 Mar;22:659. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- 17.Cipriani A, Furukawa T, Salanti G, Geddes J, Higgins J, Churchill R, Watanabe N, Nakagawa A, Omori I, McGuire H, Tansella M, Barbui C. Comparative efficacy and acceptability of 12 new-generation antidepressants: a multiple-treatments meta-analysis. Lancet. 2009;373:746–758. doi: 10.1016/S0140-6736(09)60046-5. [DOI] [PubMed] [Google Scholar]
- 18.Kang JE, et al. Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle. Science. 2009 Nov 13;326:1005. doi: 10.1126/science.1180962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bateman RJ, et al. Human amyloid-beta synthesis and clearance rates as measured in cerebrospinal fluid in vivo. Nat Med. 2006 Jul;12:856. doi: 10.1038/nm1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bateman RJ, Munsell LY, Chen X, Holtzman DM, Yarasheski KE. Stable isotope labeling tandem mass spectrometry (SILT) to quantify protein production and clearance rates. J Am Soc Mass Spectrom. 2007 Jun;18:997. doi: 10.1016/j.jasms.2007.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jonsson T, Atwal JK, Steinberg S, Snaedal J, Jonsson PV, Bjornsson S, Stefansson H, Sulem P, Gudbjartsson D, Maloney J, Hoyte K, Gustafson A, Liu Y, Lu Y, Bhangale T, Graham RR, Huttenlocher J, Bjornsdottir G, Andreassen OA, Jönsson EG, Palotie A, Behrens TW, Magnusson OT, Kong A, Thorsteinsdottir U, Watts RJ, Stefansson K. A mutation in APP protects against Alzheimer’s disease and age-related cognitive decline. Nature. 2012 Aug 2;488(7409):96–9. doi: 10.1038/nature11283. [DOI] [PubMed] [Google Scholar]
- 22.Sen S, Duman R, Sanacora G. Serum brain-derived neurotrophic factor, depression, and antidepressant medications: meta-analyses and implications. Biol Psychiatry. 2008 Sep 15;64(6):527–32. doi: 10.1016/j.biopsych.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malberg J, Eisch A, Nestler E, Duman R. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci. 2000 Dec 15;20(24):9104–10. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kempermann G, Kronenberg G. Depressed new neurons--adult hippocampal neurogenesis and a cellular plasticity hypothesis of major depression. Biol Psychiatry. 2003 Sep 1;54(5):499–503. doi: 10.1016/s0006-3223(03)00319-6. [DOI] [PubMed] [Google Scholar]
- 25.Eisch AJ, Petrik D. Depression and hippocampal neurogenesis: a road to remission? Science. 2012 Oct 5;338:72–5. doi: 10.1126/science.1222941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hamon M, Blier P. Monoamine neurocircuitry in depression and strategies for new treatments. Prog Neuropsychopharmacol Biol Psychiatry. 2013 Aug 1;45:54–63. doi: 10.1016/j.pnpbp.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 27.Lione A, Errico M, Lin S, Cowen D. Activation of extracellular signal-regulated kinase (ERK) and Akt by human serotonin 5-HT1B receptors in transfected BE(2)-C neuroblastoma cells is inhibited by RGS4. J Neurochem. 2000 Sep;75(3):934–8. doi: 10.1046/j.1471-4159.2000.0750934.x. [DOI] [PubMed] [Google Scholar]
- 28.Johnson-Farley N, Kertesy S, Dubyak G, Cowen D. Enhanced activation of Akt and extracellular-regulated kinase pathways by simultaneous occupancy of Gq-coupled 5-HT2A receptors and Gs-coupled 5-HT7A receptors in PC12 cells. J Neurochem. 2005 Jan;92(1):72–82. doi: 10.1111/j.1471-4159.2004.02832.x. [DOI] [PubMed] [Google Scholar]
- 29.Norum J, Hart K, Levy F. Ras-dependent ERK activation by the human Gs-coupled serotonin receptors 5-HT4(b) and 5-HT7(a) J Biol Chem. 2003 Jan 31;278(5):3098–104. doi: 10.1074/jbc.M206237200. [DOI] [PubMed] [Google Scholar]
- 30.Cisse M, Duplan E, Guillot-Sestier MV, Rumigny J, Bauer C, Pages G, Orzechowski HD, Slack B, Checler F, Vincent B. The extracellular regulated kinase-1 (ERK1) controls regulated alpha-secretase-mediated processing, promoter transactivation, and mRNA levels of the cellular prion protein. J Biol Chem. 2011 Aug 19;286(33):29192–206. doi: 10.1074/jbc.M110.208249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim SK, Park HJ, Hong HS, Baik EJ, Jung MW, Mook-Jung I. ERK1/2 is an endogenous negative regulator of the gamma-secretase activity. FASEB J. 2006 Jan;20(1):157–9. doi: 10.1096/fj.05-4055fje. [DOI] [PubMed] [Google Scholar]
- 32.Yan P, et al. Characterizing the appearance and growth of amyloid plaques in APP/PS1 mice. J Neurosci. 2009 Aug 26;29:10706. doi: 10.1523/JNEUROSCI.2637-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McConlogue L, et al. Partial reduction of BACE1 has dramatic effects on Alzheimer plaque and synaptic pathology in APP Transgenic Mice. J Biol Chem. 2007 Sep 7;282:26326. doi: 10.1074/jbc.M611687200. [DOI] [PubMed] [Google Scholar]
- 34.Savonenko A, et al. Episodic-like memory deficits in the APPswe/PS1dE9 mouse model of Alzheimer’s disease: relationships to beta-amyloid deposition and neurotransmitter abnormalities. Neurobiol Dis. 2005 Apr;18:602. doi: 10.1016/j.nbd.2004.10.022. [DOI] [PubMed] [Google Scholar]
- 35.Cirrito JR, et al. In vivo assessment of brain interstitial fluid with microdialysis reveals plaque-associated changes in amyloid-beta metabolism and half-life. J Neurosci. 2003 Oct 1;23:8844. doi: 10.1523/JNEUROSCI.23-26-08844.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Klunk WE, et al. Imaging Abeta plaques in living transgenic mice with multiphoton microscopy and methoxy-X04, a systemically administered Congo red derivative. J Neuropathol Exp Neurol. 2002 Sep;61:797. doi: 10.1093/jnen/61.9.797. [DOI] [PubMed] [Google Scholar]
- 37.Castellano JM, et al. Low-density lipoprotein receptor overexpression enhances the rate of brain-to-blood Abeta clearance in a mouse model of beta-amyloidosis. Proc Natl Acad Sci U S A. 2012 Sep 18;109:15502. doi: 10.1073/pnas.1206446109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Diggle P, Heagerty P, Liang K, Zeger S. Analysis of Longitudinal Data. 2. Oxofrd Universal Press; New York: 2002. [Google Scholar]
- 39.Akaike H. A new look at the statistical model identification. IEEE Transactions on Automatic Control. 1974;19:716. [Google Scholar]
- 40.Kenward MG, Roger JH. Small sample inference for fixed effects from restricted maximum likelihood. Biometrics. 1997 Sep;53:983. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Plaque load in aged APP/PS1 mice dose response.
Supplemental Figure 2: CSF Aβ Tracer to Tracee ratio did not differ between citalopram and placebo.