Abstract
Aims
Cresols are present in antiseptics, coal tar, some resins, pesticides, and industrial solvents. Cresol intoxication leads to hepatic injury due to coagulopathy as well as disturbance of hepatic circulation in fatal cases. Patients with uremia suffer from cardiovascular complications, such as atherosclerosis, thrombosis, hemolysis, and bleeding, which may be partly due to p-cresol toxicity and its effects on vascular endothelial and mononuclear cells. Given the role of reactive oxygen species (ROS) and inflammation in vascular thrombosis, the objective of this study was to evaluate the effect of p-cresol on endothelial and mononuclear cells.
Methods
EA.hy926 (EAHY) endothelial cells and U937 cells were exposed to different concentrations of p-cresol. Cytotoxicity was evaluated by 3-(4,5-Dimethylthiazol-2-yl)-2,5 -diphenyltetrazolium bromide (MTT) assay and trypan blue dye exclusion technique, respectively. Cell cycle distribution was analyzed by propidium iodide flow cytometry. Endothelial cell migration was studied by wound closure assay. ROS level was measured by 2′,7′-dichlorofluorescein diacetate (DCF) fluorescence flow cytometry. Prostaglandin F2α (PGF2α), plasminogen activator inhibitor-1 (PAI-1), soluble urokinase plasminogen activator receptor (suPAR), and uPA production were determined by Enzyme-linked immunosorbant assay (ELISA).
Results
Exposure to 100–500 µM p-cresol decreased EAHY cell number by 30–61%. P-cresol also decreased the viability of U937 mononuclear cells. The inhibition of EAHY and U937 cell growth by p-cresol was related to induction of S-phase cell cycle arrest. Closure of endothelial wounds was inhibited by p-cresol (>100 µM). P-cresol (>50 µM) also stimulated ROS production in U937 cells and EAHY cells but to a lesser extent. Moreover, p-cresol markedly stimulated PAI-1 and suPAR, but not PGF2α, and uPA production in EAHY cells.
Conclusions
p-Cresol may contribute to atherosclerosis and thrombosis in patients with uremia and cresol intoxication possibly due to induction of ROS, endothelial/mononuclear cell damage and production of inflammation/atherosclerosis-related molecules.
Introduction
Cresol is a widely used disinfectant. For example, formalin-cresol (FC) is often utilized for root canal procedures and as a dressing after pulpectomy [1]–[4]. P-cresol is also an end product of protein breakdown in healthy individuals and an amino acid metabolite of intestinal bacteria [5], [6]. O- and p-cresol are also present in coal tar, some resins, pesticides and industrial solvents [7] and are the metabolic products of toluene [8] and menthofuran [9], two environmental toxicants. Exposure to cresol via inhalation, cutaneous absorption or oral intake may result in intoxication, leading to hepatic injury possibly due to coagulopathy and disturbance of hepatic circulation in fatal cases [10].
Plasma p-cresol levels in uremia patients, which range from 100–250 µM [11], may be responsible for the cardiovascular diseases commonly observed in chronic kidney disease patients [12] and is considered a modifiable cardiovascular risk factor in uremic patients [13], [14]. The vascular changes induced by p-cresol include arterial calcification, atherosclerosis and arterial stiffness [15], [16], and are related to endothelial and vascular smooth cell dysfunction [17], [18], as well as platelet and leukocyte activation [19]. Thrombosis and atherosclerosis occur due to an imbalance between thrombogenic factors, including vessel wall damage, platelet aggregation, activation of blood coagulation and stasis, and anti-thrombotic factors [20]. Plasminogen activator inhibitor-1 (PAI-1) is elevated in obesity, diabetes and metabolic syndrome, and may inhibit the fibrinolysis and enhance vascular thrombosis [21]. Endothelial injury may also cause loss of barrier function, concomitant with smooth muscle cell proliferation and migration within the site of injury. Elevated serum soluble urokinase plasminogen activator receptor (suPAR) is also noted in patients with renal and peripheral vascular damage [22].
Uremia-related cardiovascular diseases are often associated with tissue inflammation and endothelial damage [23]. Complex cellular and inflammatory interactions are involved in the progression of vascular diseases [24]. Prostaglandin F2α (PGF2α) is a critical mediator of inflammatory diseases, such as rheumatic diseases, atherosclerosis, diabetes, septic shock, and ischemia reperfusion [25]. In addition, oxidative stress and endothelial cell injury are responsible for the acceleration of atherosclerosis in patients with chronic renal failure as well as the progression of renal damage [26]–[28]. However, it is not known if these vascular changes are due to the effects of uremic toxins, such as p-cresol, on endothelial cells.
P-cresol suppresses normal endothelial function, such as proliferation, wound repair and response to cytokines [29], [30]; it also inhibits the release of platelet-activating factor by rat peritoneal macrophages, which is crucial for platelet function [31]. P-cresol reduces ROS levels in monocytes, lymphocytes and granulocytes [32] and inhibits the leukocyte trans-endothelial migration [33]. In the presence of albumin, p-cresol alters the actin cytoskeleton and permeability to endothelial cells [34]. Oxidative stress and various inflammatory modulators, such as PGF2α, plasminogen activator inhibitor-1 (PAI-1) and uPAR, have roles in cardiovascular disease and chronic kidney disease progression [35]–[39]. However, the effects of p-cresol on inflammatory mediator levels as well as endothelial and mononuclear cell dysfunction remain unknown.
To know more about p-cresol intoxication on the vascular changes, we studied the effects of p-cresol on ROS production, cell proliferation, cell cycle progression and various inflammation/atherosclerosis-related mediators (e.g., PGF2α, PAI-1, uPA and suPAR) were determined using in vitro analyses.
Materials and Methods
Materials
EA.hy926 (EAHY) endothelial cells were kindly provided by Professor Cora-Jean S. Edgell (Pathology Department, University of North Carolina, USA) and were previously described by Tseng et al. [40]. Human U937 mononuclear cells were obtained from American Type Culture Collection [41]. EAHY and U937 cells were cultured in Dulbecco's modified Eagle's Medium (DMEM) and RPMI1640, respectively supplemented with 10% fetal bovine serum (FBS), antibiotics and glutamine (Sigma Chemical Company, USA). ELISA kits for PAI-1, uPA and suPAR were purchased from R&D Systems (Minneapolis, MN, USA). PGF2α ELISA kits were obtained from Cayman Chemical (Ann Arbor, MI, USA). P-cresol and 3-(4,5-Dimethylthiazol-2-yl)-2,5 -diphenyltetrazolium bromide (MTT) were purchased from Sigma (St. Louis, MO, USA). 2′,7′-dichlorofluorescein diacetate (DCFH-DA) was obtained from Molecular Probes (Invitrogen Detection Technologies, Grand Island, NY, USA). Flow cytometry reagents were from Becton Dickinson (Franklin Lakes, New Jersey, USA).
Effect of P-cresol on the Growth of EAHY Endothelial Cells and U937 Mononuclear Cells
We evaluated the cytotoxicity of p-cresol on EAHY cells and U937 cell growth. Briefly, 1×104 EAHY cells were seeded into 24-well culture plates. After 24 h, the medium was changed to that containing various concentrations of p-cresol (final 10, 50, 100, 250, 500 µM). After 72 h, fresh medium containing 0.5 mg/mL MTT was added for 3 h. Viable cell number was estimated using the MTT assay by dissolving the produced formazan with dimethylsulfoxide (DMSO); the optical density (OD) values were determined as described previously [42].
U937 cells (1×106 cells) were inoculated into 24-well culture wells and exposed to various concentrations of p-cresol. After 24 h, the number of viable U937 cells was determined by a trypan blue exclusion assay as previously described [43].
Cell Cycle Analysis of EAHY and U937 Cells after Exposure to P-cresol
EAHY (5×105 cells/6-well) and U937 (1×106 cells/24-well) cells were treated with p-cresol for 24 h. EAHY (both floating and attached cells) and U937 cells were collected for analysis of cell cycle distribution by staining with propidium iodide (PI) and flow cytometric analysis as described previously [43], [44]. In short, cells were washed with phosphate buffered saline (PBS), fixed in 70% ice-cold ethanol with 2 mg/mL RNase for 30 min, and stained with PI (40 µg/mL) for 10 min. The PI fluorescence was analyzed by single cell flow cytometric analysis (FACSCalibur, Becton Dickinson, Worldwide Inc., San-Jose, California) at an excitation wavelength at 488 nm with an emission wavelength >590 nm. The PI fluorescence of 20,000 cells was determined for both untreated cells (control) and p-cresol-treated cells. The percentage of cells in the sub-G0/G1, G0/G1, S, and G2/M phases was determined using the ModiFit software and CellQuest programs [44], [45]. Cytotoxicity was also evaluated using the MTT assay (for EAHY cells) and trypan blue dye exclusion technique (for U937 cells).
Effect of P-cresol on the Migration of Endothelial Cells
The effect of p-cresol on the wound closure by EAHY endothelial cells was performed similar to that described previously [41]. Briefly, 5×105 EAHY cells were seeded into 6-well plates. After 24 h, a wound was created by a pipet tip on the EAHY cell layer. Then, culture medium containing different concentrations of p-cresol was added, and wound closure was assessed after 6, 24, and 30 h using phase contrast microscopy.
Effect of P-cresol on Cellular ROS Levels in EAHY and U937 Cells
EAHY cells (5×105 cells/6-well) and U937 cells (1×106 cells/24-well) were cultured with their respective culture medium that contained different concentrations of p-cresol for 24 h. Cells were then stained with 10 µM DCFH-DA for 30 min at 37°C and collected. After washing with phosphate-buffered saline (PBS), cells were resuspended in PBS and subjected immediately for flow cytometry analysis of DCF fluorescence as previously described [45].
Effect of P-cresol on PGF2α, PAI-1, uPA and suPAR Secretion by EAHY and U937 Cells
EAHY cells (5×105 cells/6-well) and U937 cells (1×106 cells/24-well) were cultured in their respective culture medium that contained different concentrations of p-cresol for 24 or 72 h. Culture medium was collected (centrifugation was needed for U937 cells) for analysis of PGF2α, uPA, PAI-1 and suPAR secretion by ELISA following the manufacturer's instructions.
Statistical Analysis
At least five or more separate experiments were performed for each analysis. The data were analyzed with one-way ANOVA and post-hoc Tukey test. A P-value <0.05 was considered to be statistically significant between the groups.
Results
Effect of P-cresol on EAHY and U937 Cell growth
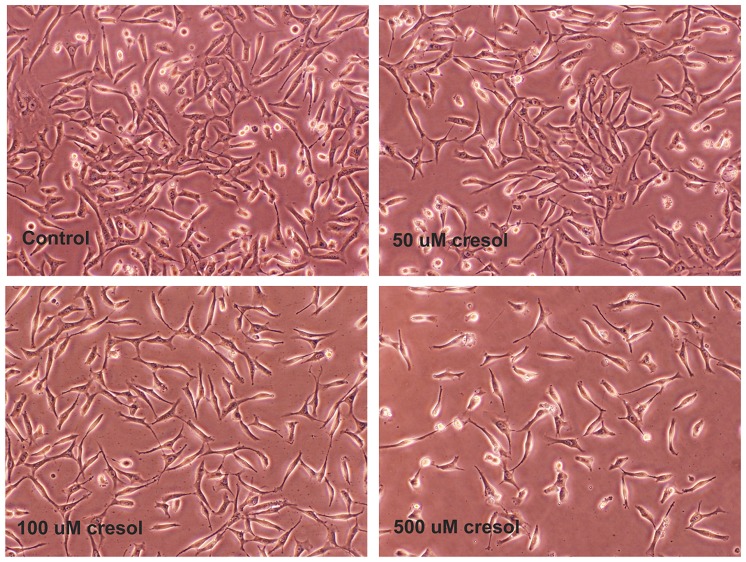
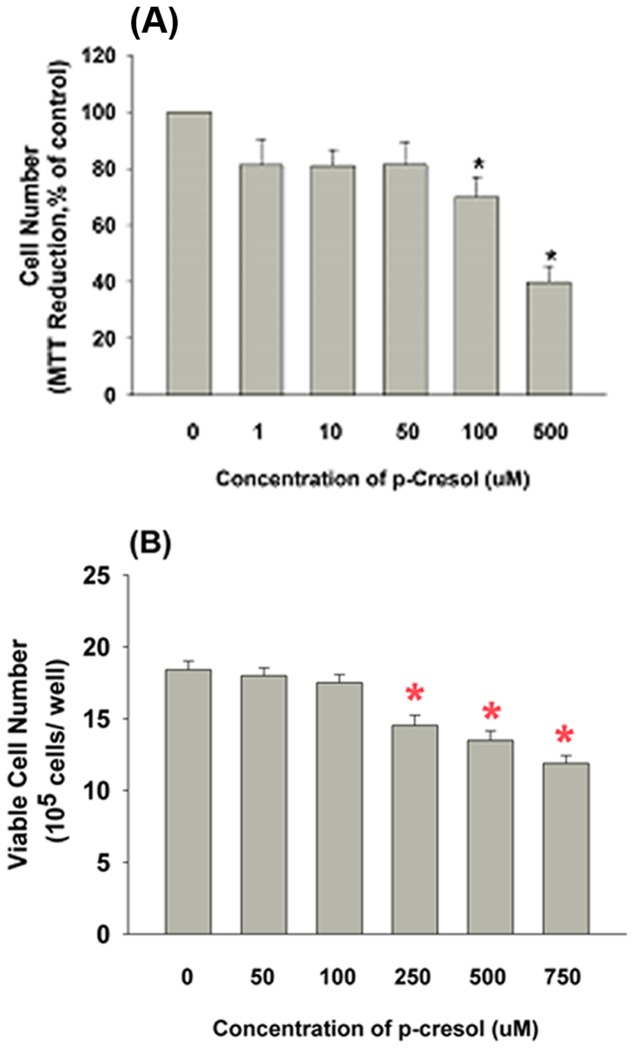
Exposure to p-cresol may inhibit cell growth. As shown in Fig. 1 , 50, 100 and 500 µM p-cresol decreased the EAHY cell density; however, no marked differences in the proportion of floating EAHY cells were observed. Specifically, 100 and 500 µM p-cresol decreased the EAHY cell number by 30 and 61%, respectively as analyzed by the MTT assay (P <0.05; Fig. 2A ). Similarly, exposure of U937 cells to p-cresol significantly decreased the number of viable cells from 1.84×106 cells/well (control) to 1.45×106 and 1.19×106 cells/well after treatment with 250 and 750 µM p-cresol, respectively (P <0.05; Fig. 2B ).
Figure 1. Morphology of EAHY endothelial cells (1×104 cells/well) after exposure to p-cresol for 3 days.
Density of EAHY cells markedly decreased. One representative picture of EAHY cells was shown.
Figure 2. Effect of p-cresol on cell growth of EAHY and U937 cells.
(A) P-cresol obviously suppressed the growth of endothelial cells as analyzed by MTT assay (as % of control) (n = 6) and (B) U937 cells (x 105 cells/well) as analyzed by trypan blue dye exclusion technique. *denotes significant difference when compared with control group (n = 13).
Effect of P-cresol on Cell Cycle Progression and Apoptosis of EAHY and U937 Cells
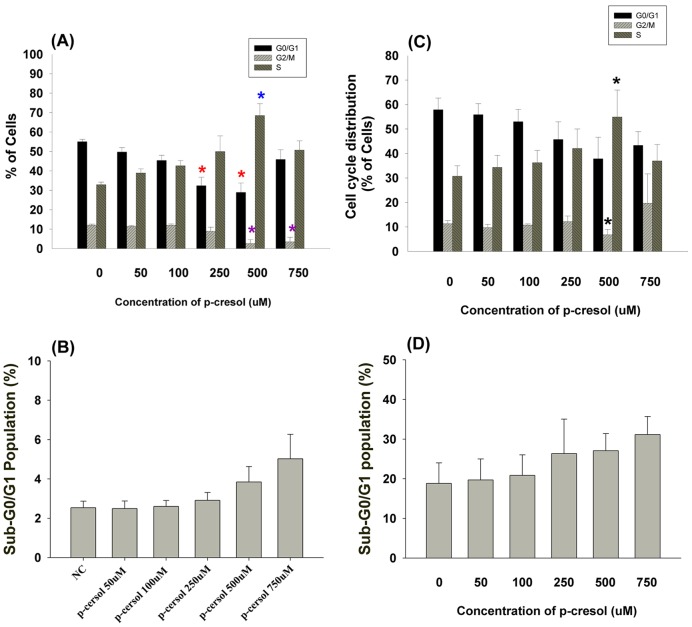
We next evaluated if the effects of p-cresol on EAHY and U937 cell growth were due to induction of cell cycle arrest and apoptosis. As shown in Fig. 3A , p-cresol (>500 µM) induced S-phase cell cycle arrest (P <0.05); the percentage of apoptotic EAHY endothelial cells showed slightly increased (P >0.05) ( Fig. 3B ). In these conditions, 250–500 µM p-cresol decreased the cell number by 21–32% as analyzed by the MTT assay (data not shown). Similarly 500 µM p-cresol also stimulated S-phase cell cycle arrest in U937 cells (P <0.05; Fig. 3C ). However, this effect was not evident at 750 µM, possibly due to slight induction of apoptosis of U937 mononuclear cells (P >0.05) ( Fig. 3D ).
Figure 3. Effect of p-cresol on cell cycle distribution of EAHY and U937 cells.
(A) Induction of S-phase cell cycle arrest of endothelial cells by p-cresol, (B) Induction of apoptosis of EAHY endothelial cells by p-cresol (C) Induction of S-phase cell cycle arrest of U937 mononuclear cells by cresol, (D) Induction of apoptosis of U937 cells by p-cresol (sub-G0/G1 population, %) (Mean±SE).
Effect of P-cresol on Wound Closure of EAHY Endothelial Cell Monolayer
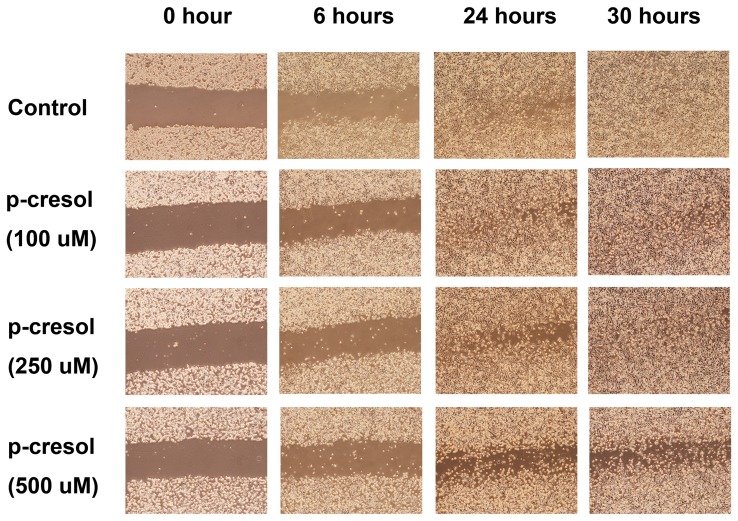
In order to evaluate the effects of p-cresol on the proliferation and migration of endothelial cells and thus wound closure, we created a wound on EAHY cell monolayers. Cells were then exposed to different concentrations of p-cresol. In untreated controls, EAHY cells migrated into the wound after 24 h ( Fig. 4 ). However, exposure to 250 and 500 µM p-cresol delayed the wound closure of endothelial cells as shown by the presence of gaps at the wound edges after 24 h ( Fig. 4 ).
Figure 4. Effect of p-cresol on closure of wounds in EAHY cell monolayer.
Briefly 5×105 EAHY cells were seeded into 6-well plates. After 24 hours, a wound was created by a pipet tip on EAHY cell layer. Then culture medium with different concentrations of p-cresol (final 100, 250 and 500 µM) was added and the closure of wound at 0 hour (immediate after wounding), 6 hours, 24 hours and 30 hours after wounding was recorded by taking pictures for comparison. One representative study pictures were shown.
Effect of P-cresol on ROS Production of EAHY and U937 Cells
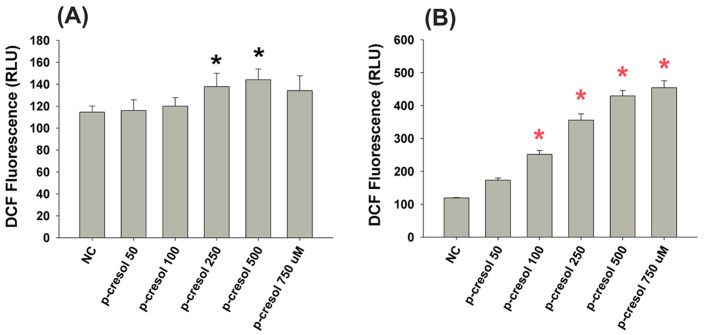
Chemical toxicity is often associated with oxidative stress. As shown in Fig. 5A , exposure to 250 and 500 µM p-cresol stimulated ROS production as indicated by increased DCF fluorescence in EAHY endothelial cells (P <0.05). Similarly, p-cresol (≥100 µM) stimulated ROS production in U937 mononuclear cells, but to a greater extent ( Fig. 5B ).
Figure 5. Effect of p-cresol on ROS production of EAHY and U937 cells.
(A) EAHY endothelial cells, (B) U937 mononuclear cells. Results were expressed as DCF fluorescence (RFU) (Mean ±SE).
Effect of P-cresol on PGF2α Production of EAHY and U937 Cells
As shown in Fig. 6 , p-cresol slightly induced PGF2α production in both EAHY endothelial cells and U937 mononuclear cells (p>0.05). In addition, Western blot analysis revealed that cresol induced COX-2 expression in EAHY endothelial cells (data not shown).
Figure 6. Effect of p-cresol on PGF2α production of EAHY and U937 cells.
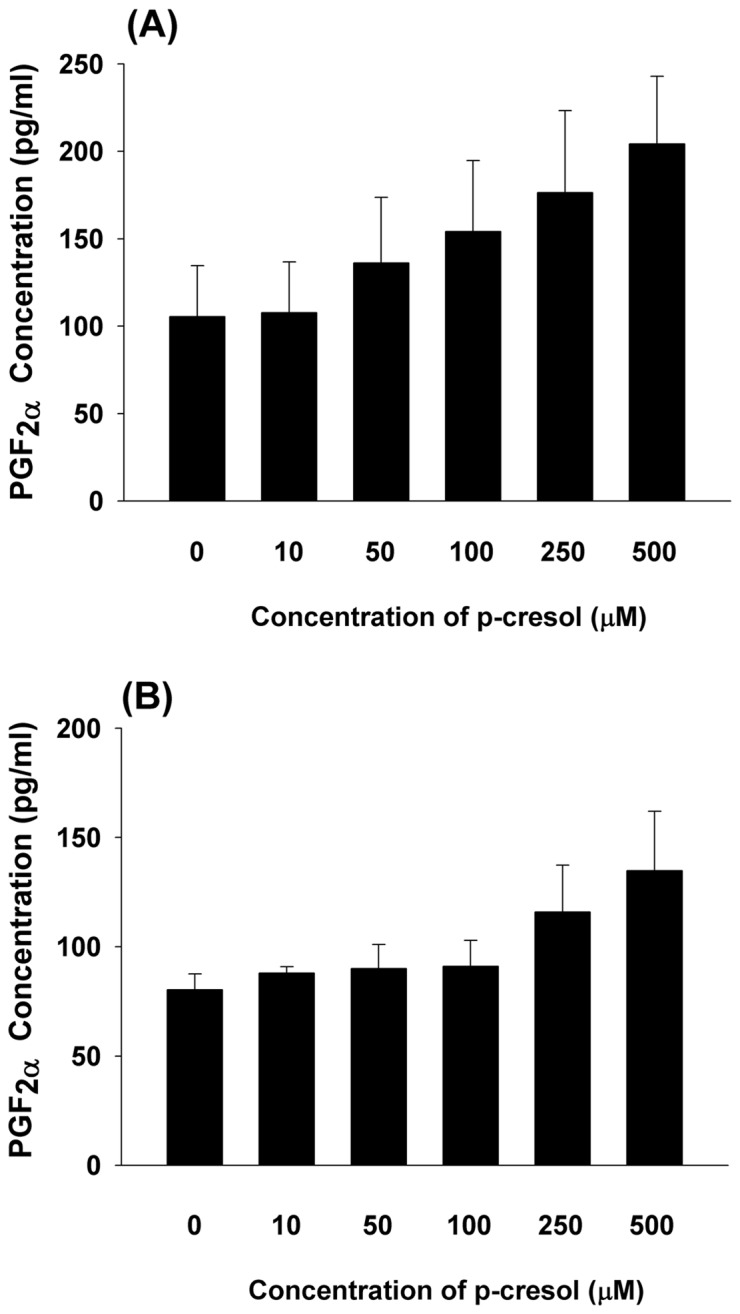
(A) EAHY endothelial cells, and (B) U937 mononuclear cells. Results were expressed as Mean ±SE. No statistically significant difference when compared with control was noted (P>0.05).
Effect of P-cresol on PAI-1, uPA and suPAR Production of EAHY and U937 Cells
As shown in Fig. 7A , p-cresol significantly increased PAI-1 production by EAHY endothelial cells (P <0.05). P-cresol also slightly decreased uPA production (P>0.05) ( Fig. 7B ). Furthermore, significantly increased suPAR levels were observed in endothelial cells exposed to 500 µM p-cresol (P <0.05; Fig. 7C ).
Figure 7. Effect of cresol on PAI-1, uPA and suPAR production of EAHY cells.
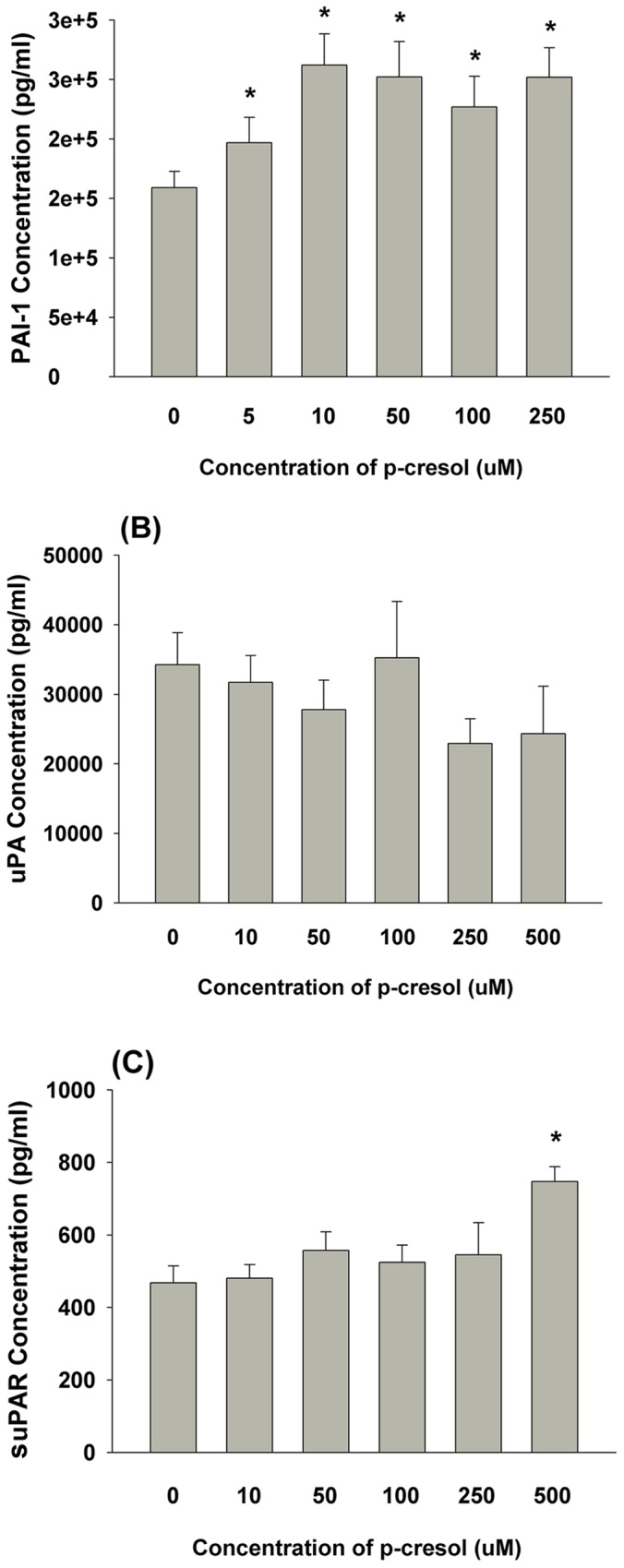
(A) PAI-1 level in medium, (B) uPA level in medium, (C) suPAR level in medium. Results were expressed as Mean ±SE *denotes statistically significant difference when compared with control (P<0.05).
Discussion
P-cresol, a metabolite of environmental toxins, including benzene, toluene and menthofuran, is also a toxic metabolite in uremic patients. Some cases of fatal cresol intoxication have also been reported [10]. However, the toxicity of p-cresol on the cardiovascular system has not been fully addressed. In this study, p-cresol slightly inhibited EAHY endothelial and U937 mononuclear cell growth at concentrations higher than 100 µM, which is in agreement with Ying et al. (2011) [46]. The growth inhibitory effect of p-cresol on EAHY cells is possibly due to a cytostatic effect but not cytotoxicity by p-cresol as evidenced by the lack of cell death or floating cells. Given that vascular damage and atherosclerosis may be due to endothelial dysfunction and resultant vascular smooth muscle cell proliferation and platelet aggregation [47], inhibition of endothelial cell wound closure by p-cresol was also observed as in a previous study [30]. This is possibly due to alteration ROS levels, actin cytoskeleton, ERK1/2, p38 and Akt signaling by p-cresol in different kind of cells, such as endothelial cells, bone marrow derived stem cells and platelets [34], [46], [48], [49]. These effects could further enhance the vascular damage response, thereby altering local and systemic circulation and vascular health. This may partly explain why cresol intoxication induces hepatic injury with coagulopathy and disturbance of hepatic circulation [30] as well as the presence of vascular disorders (e.g., atherosclerosis, thrombosis, arterial stiffness, hemolysis and bleeding) in uremic patients [15], [16].
P-cresol but not p-cresyl sulfate may induce G2/M cell cycle arrest of endothelial progenitor cells [50]. In addition, we observed that the cytostatic effect of p-cresol may be due to induction of late S-phase arrest and apoptosis of EAHY endothelial cells and U937 mononuclear cells. Immunosuppression may be important for the disease pathogenesis in uremic patients [33]. However, little is known regarding the effect of p-cresol on inflammatory cells. P-cresol decreased CD11b expression and phorbol-12 myristate-13 acetate (PMA)-induced ROS production, but not polymorphonuclear leukocyte (PMN) viability and apoptosis [51]. Furthermore, transendothelial migration by peripheral blood mononuclear cells and THP-1 mononuclear cells was inhibited by p-cresol [33]; it also inhibited Lactobacilli–induced IL-12 production, but showed little cytotoxicity in murine macrophages [52]. In this study, inhibition U937 mononuclear cell growth with concomitant apoptosis by p-cresol suggests that it may induce immune dysfunction in uremic patients.
Toxicity is often associated with the induction of cellular oxidative stress. Suppression of the respiratory burst activity of whole blood, granulocytes and monocytes [53] as well as arachidonic acid (AA)-induced ROS production in rabbit platelets by p-cresol was previously reported [49]. Consistently, p-cresol stimulated ROS production in EAHY endothelial cells and U937 mononuclear cells, suggesting the involvement of ROS in uremia-associated vascular changes and cresol intoxication. ROS contributed to vascular damage and induced vascular dysfunction, such as leukocyte activation, neointimal hyperplasia, endothelial cell dysregulation, arterial stiffness, abnormal vascular repair, vascular calcification, and atherosclerosis, in uremic patients [54]. Moreover, the antioxidant, N-acetyl-L-cysteine, attenuated endothelial dysfunction in uremic patients [55]. Taken together, the consumption or supplementation of antioxidants may be of clinical significance for the prevention and treatment of p-cresol-induced toxicity in uremic patients.
Local inflammation and expression of metalloproteinases may stimulate the proliferation and migration of vascular smooth muscle cells [54], thereby contributing to atherosclerosis. In uremic patients, this is possibly induced by toxins, such as p-cresol, indoxyl sulfate, and p-cresyl sulfate [54]. P-cresol inhibited Interleukin (IL)-1-induced monocyte chemoattractant protein-1 (MCP-1) and IL-8 expression by endothelial cells [33]; it also inhibited IL-1β- and TNF-α-induced cell adhesion molecule (intercellular cell adhesion molecule - ICAM-1, vascular cell adhesion molecule - VCAM-1) expression and the cytokine-stimulated adhesion of THP-1 cells to endothelial cells [29], suggesting that p-cresol is capable of impair immune function and modulating cell adhesion in uremic patients. Roles for PGF2α in inflammatory diseases have been previously reported [25]; it also induces tachycardia in systemic inflammation and vascular hypo-response in septic condition [56]. In this study, p-cresol slightly stimulated PGF2α production by endothelial and mononuclear cells (P>0.05), suggesting its possible involvement in uremia-related tissue inflammation and cardiovascular diseases.
In renal thrombotic angiopathy and uremic patients, immunohistochemical (IHC) staining revealed elevated PAI-1 and uPAR expression in the glomeruli and arterial walls, leading to fibrin deposition and tissue fibrosis [54], [57]. In atherosclerotic plaques, IHC staining also showed increased PAI-1 and uPAR expression in the vascular intima and media [36], suggesting their involvement in atherosclerosis. However, little was known regarding the effect of p-cresol on the plasminogen activation system. In the present study, p-cresol stimulated PAI-1 and suPAR secretion but slightly decreased uPA secretion by endothelial cells. Because elevated PAI-1 is important for tissue fibrosis and atherosclerosis, p-cresol-induced PAI-1 secretion may play a role in the induction of atherosclerosis in uremic patients.
The impact of suPAR in the cardiovascular system is relatively unknown. It may stimulate angiogenesis [58], thrombosis [59] and leukocyte chemotaxis [60], and it was elevated in patients with infection, inflammation, and cancer [22]. An elevated suPAR in serum and atherosclerosis plaques is associated with inflammation of the vulnerable and symptomatic human atherosclerotic plaques [61]. Furthermore, elevated suPAR is a marker of renal and peripheral vascular damage [22] and of poor prognosis in patients with cancer [58]. PAI-1 may also alter the interaction of suPAR with other co-receptors, such as vitronectin and high molecular weight kininogen [60], [62], and thus leukocyte chemotaxis. Stimulation of suPAR production by p-cresol in this study suggests its role in vascular inflammation, and the pathogenesis of vascular dysfunction in patients with cresol intoxication, atherosclerosis and uremia. Further studies will assess the prognostic value of determining plasma PAI-1 and suPAR levels in patients with uremia or cresol intoxication.
In conclusion, p-cresol suppressed cell growth by inducing cell cycle arrest and apoptosis in endothelial cells and mononuclear cells; inhibition of wound closure was also revealed. P-cresol also stimulated ROS, PAI-1, and suPAR production (and less on PGF2α, production) in vascular cells, which may explain the presence of vascular thrombosis and hemorrhage in uremic patients. Understanding the toxicological effect of p-cresol may be helpful in identifying means to prevent and treat of patients with p-cresol intoxication and uremia.
Acknowledgments
The authors would like to thank Miss Yi-Hui Su for her technical assistance.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.
Funding Statement
This study was supported by grants from National Science Council, Taiwan, R.O.C. (NSC102-2314-B-255-003-MY2, NSC102-2628-B-255-001-MY3, NSC101-2320-B-255-002, NSC-100-2314-B-002-094, and NSC-101-2320-B-255-002) and Chang Gung Memorial Hospital (CMRPF-3B0191, CMRPG-3B0192, NMRPF3C0061, NMRPF3C0091, and NMRPF3B0071). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Buckley JR (1904) The chemistry of pulp decomposition with rational treatment of this condition and its sequelae. J Am Dent Assoc 3:764–771. [Google Scholar]
- 2. Myers DR, Kenneth H, Dirksen TR, Pashley DH, Whitford GM, et al. (1978) Distribution of 14C-formaldehyde after pulpotomy with formocresol. J Am Dent Assoc 96:805–813. [DOI] [PubMed] [Google Scholar]
- 3. Ranley DM, Garcia GF, Horn DA (1988) A comparison of the effects of cresol and eugenol on bovine pulp. Endod Dent Traumatol 4:70–75. [DOI] [PubMed] [Google Scholar]
- 4. Ohara P, Torabinejad M, Kettering JD (1993) Antibacterial effects of various endodontic medicaments on selected anaerobic bacteria. J Endod 19:498–500. [DOI] [PubMed] [Google Scholar]
- 5. Geypens B, Claus D, Evenepoel P, Hiele M, Maes B, et al. (1997) Influence of dietary protein supplements on the formation of bacterial metabolites in the colon. Gut 41:70–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Curtius HC, Mettler M, Ettlinger L (1976) Study of the intestinal tyrosine metabolism using stable isotopes and gas chromatography-mass spectrometry. J Chromatogr 126:569–580. [DOI] [PubMed] [Google Scholar]
- 7. Dietz D (1991) NTP technical report on the toxicity studies of cresols in F344/N rats and B6C3F1 mice (Feed studies). Toxic Report Service 9:1–128. [PubMed] [Google Scholar]
- 8. Sequeira DJ, Eyer CS, Cawley GF, Nick TG, Backes WL (1992) Ethylbenzene-mediated induction of cytochrome P450 isoenzymes in male and female rats. Biochem Pharmacol 44:1171–1182. [DOI] [PubMed] [Google Scholar]
- 9. Madyastha KM, Raj CP (1992) Metabolic date of methofuran in rats. Novel oxidative pathways. Drug Metab Dispos 20:295–301. [PubMed] [Google Scholar]
- 10. Kamijo Y, Soma K, Kokuto M, Ohbu M, Fuke C, et al. (2003) Hepatocellular injury with hyperaminotransferasemia after cresol ingestion. Arch Pathol Lab Med 127:364–366. [DOI] [PubMed] [Google Scholar]
- 11. Vanholder R, De Smet R, Lesaffer G (1999) p-Cresol: a toxin revealing many neglected but relevant aspects of uraemic toxicity. Nephrol Dial Transplant 14:2813–2815. [DOI] [PubMed] [Google Scholar]
- 12. Meijers BK, Bammens B, De Moor B, Verbeke K, Vanrenterqhem Y, et al. (2008) Free p-cresol is associated with cardiovascular disease in hemodialysis patients. Kidney Int 73:1174–1180. [DOI] [PubMed] [Google Scholar]
- 13. Meijers BK, Claes K, Bammens B, de Loor H, Viaene L, et al. (2010) p-Cresol and cardiovascular risk in mild-to-moderate kidney disease. Clin J Am Soc Nephrol 5:1182–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hosler GA, Cusumano AM, Hutchins GM (2003) Thrombotic thrombocytopenic purpura and hemolytic uremic syndrome are distinct pathologic entities. A review of 56 autopsy cases. Arch Pathol Lab Med 127:834–839. [DOI] [PubMed] [Google Scholar]
- 15. Chillon JM, Mozar A, Six I, Maizel J, Bugnicourt JM, et al. (2009) Pathophysiological mechanisms and consequences of cardiovascular calcifications: role of uremic toxicity. Ann Pharm Fr 67:234–240. [DOI] [PubMed] [Google Scholar]
- 16. Jono S, Shioi A, Ikari Y, Nichizawa Y (2006) Vascular calcification in chronic kidney disease. J Bone Miner Metab 24:176–181. [DOI] [PubMed] [Google Scholar]
- 17. Recht PA, Tepedino GJ, Siecke NW, Buckley MT, Mandeville JT, et al. (2004) Oxalic acid alters intracellular calcium in endothelial cells. Atherosclerosis 173:321–328. [DOI] [PubMed] [Google Scholar]
- 18. Ketteler M, Giachelli C (2006) Novel insights into vascular calcification. Kidney Int. (Suppl) 105:S5–9. [DOI] [PubMed] [Google Scholar]
- 19. Glorieux G, Cohen G, Jankowski J, Vanholder R (2009) Platelet/leukocyte activation, inflammation and uremia. Semin Dial 22:423–427. [DOI] [PubMed] [Google Scholar]
- 20.Hirsh J, Genton E (1974) Thrombogenesis. In Physiological Pharmacology, pp 99–133. New York, Academic Press.
- 21. Westrick RJ, Eitzman DT (2007) Plasminogen activator inhibitor-1 in vascular thrombosis. Curr Drug Targets 8:966–1002. [DOI] [PubMed] [Google Scholar]
- 22. Enocsson H, Wettero J, Skogh T, Sjowall C (2013) Soluble urokinase plasminogen activator receptor levels reflect organ damage in systemic lupus erythromatosus. Transl Res 162:287–296. [DOI] [PubMed] [Google Scholar]
- 23. Merino A, Nogueras S, Buenda P, Ojeda R, Carracedo J, et al. (2008) Microinflammation and endothelial damage in hemodialysis. Contrib Nephrol 161:83–88. [DOI] [PubMed] [Google Scholar]
- 24. Kofler S, Nickel T, Weis M (2005) Role of cytokines in cardiovascular diseases: a focus on endothelial responses to inflammation. Clin Sci 108:205–213. [DOI] [PubMed] [Google Scholar]
- 25. Basu S (2010) Bioactive eicosanoids: role of prostaglandin F(2α) and F2-isoprostanes in inflammation and oxidative stress related pathology. Mol Cells 30:383–391. [DOI] [PubMed] [Google Scholar]
- 26. Malyszko J (2010) Mechanism of endothelial dysfunction in chronic kidney disease. Clin Chim Acta 411:1412–1420. [DOI] [PubMed] [Google Scholar]
- 27. Ochodnicky P, Vettoretti S, Henning RH, Buikema H, Van Dokkum RP, et al. (2006) Endothelial dysfunction in chronic kidney disease: determinant of susceptibility to end-organ damage and therapeutic response. J Nephrol 19:246–258. [PubMed] [Google Scholar]
- 28. Ghiadoni L, Cupisti A, Huang Y, Mattei P, Carcinal H, et al. (2004) Endothelial dysfunction and oxidative stress in chronic renal failure. J Nephrol 17:512–519. [PubMed] [Google Scholar]
- 29. Dou L, Cernini C, Brunet P, Guilianelli C, Maol V, et al. (2002) P-cresol, a uremic toxin, decreases endothelial cell response to inflammatory cytokines. Kidney Int 62:1999–2009. [DOI] [PubMed] [Google Scholar]
- 30. Dou L, Bertrans E, Cerini C, Faure V, Sampol J, et al. (2004) The uremic solutes p-cresol and indoxyl sulfate inhibit endothelial proliferation and wound repair. Kidney Int 65:442–451. [DOI] [PubMed] [Google Scholar]
- 31. Wratten ML, Tetta C, De Smet R, Neri R, Sereni L, et al. (1999) Uremic ultrafiltrate inhibits platelet-activating factor synthesis. Blood Purif 17:134–141. [DOI] [PubMed] [Google Scholar]
- 32. Schepers E, Meert N, Glorieux G, Goeman J, Van der Eycken J, et al. (2007) P-cresylsulphate, the main in vivo metabolite of p-cresol, activates leucocyte free radical production. Nephrol Dial Transplant 22:592–596. [DOI] [PubMed] [Google Scholar]
- 33. Faure V, Cerini C, Paul P, Berland Y, Dignat-George F, et al. (2006) The uremic solute p-cresol decreases leukocyte transendothelial migration in vitro. Int Immunol 18:1453–1459. [DOI] [PubMed] [Google Scholar]
- 34. Cerini C, Dou L, Anfosso F, Sabatier F, Moal V, et al. (2004) P-cresol, a uremic retention solute, alters the endothelial barrier function in vitro. Thromb Haemost 92:140–150. [DOI] [PubMed] [Google Scholar]
- 35. Shin WS, Szuba A, Rockson SG (2002) The role of chemokines in human cardiovascular pathology: enhanced biological insights. Atherosclerosis 160:91–102. [DOI] [PubMed] [Google Scholar]
- 36. Raghunath PN, Tomaszewski JE, Brady ST, Caron RJ, Okada SS, et al. (1995) Plasminogen activator system in human coronary atherosclerosis. Arterioscler Thromb Vasc Biol 15:1432–1443. [DOI] [PubMed] [Google Scholar]
- 37. Filiopoulos V, Viassopoulos D (2009) Inflammatory syndrome in chronic kidney disease: pathogenesis and influence on outcomes. Inflamm Allergy Drug Targets 8:369–382. [DOI] [PubMed] [Google Scholar]
- 38. Himmelfarb J (2009) Uremic toxicity, oxidative stress and hemodialysis as renal replacement therapy. Semin Dial 22:636–643. [DOI] [PubMed] [Google Scholar]
- 39. Galle J, Seibold S, Wanner C (2003) Inflammation in uremic patients: what is the link? Kidney Blood Press Res 26:65–75. [DOI] [PubMed] [Google Scholar]
- 40. Tseng SK, Chang MC, Su CY, Chi LY, Hsu ML, et al. (2012) Arecoline induced cell cycle arrest, apoptosis and cell cycle arrest in human endothelial cells. Clin Oral Invest 16:1267–1273. [DOI] [PubMed] [Google Scholar]
- 41.Tseng SK, Chang MC, Hsu ML, Su CY, Chi LY, et al. (2014) Arecoline inhibits endothelial cell growth, migration and the attachment by mononuclear cells. J Dent Sci (In press).
- 42. Chang MC, Chen LI, Chan CP, Lee JJ, Wang TM, et al. (2010) The role of reactive oxygen species and hemeoxygenase-1 expression in the cytotoxicity, cell cycle alteration and apoptosis of dental pulp cells induced by BisGMA. Biomaterials 31:8164–8171. [DOI] [PubMed] [Google Scholar]
- 43. Chang MC, Uang BJ, Wu HL, Lee JJ, Hahn LJ, et al. (2002) Inducing the cell cycle arrest and apoptosis of oral KB carcinoma cells by hydroxychavicol: Roles of glutathione and reactive oxygen species. Br J Pharmacol 135:619–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chang MC, Wu HL, Lee JJ, Lee PH, Chang HH, et al. (2004) The induction of PGE2 production, IL-6 production, cell cycle arrest and apoptosis of primary oral keratinocytes and KB cancer cells by areca nut ingredients is differentially regulated by MEK/ERK. J Biol Chem 279:50676–50683. [DOI] [PubMed] [Google Scholar]
- 45. Chang HH, Guo MK, Kasten FH, Chang MC, Huang GF, et al. (2005) Stimulation of glutathione depletion, ROS production and cell cycle arrest of dental pulp cells and gingival epithelial cells by HEMA. Biomaterials 26:745–753. [DOI] [PubMed] [Google Scholar]
- 46. Ying Y, Yang K, Liu Y, Chen QJ, Shen WF, et al. (2011) A uremic solute, P-cresol, inhibits the proliferation of endothelial progenitor cells via the p38 pathway. Cir J 75:2252–2259. [DOI] [PubMed] [Google Scholar]
- 47. Gunthner T, Jankowski V, Kretschmer A, Nierhaus M, Van de Giet M, et al. (2009) Endothelium and vascular smooth muscle cells in the context of uremia. Semin Dial 22:428–432. [DOI] [PubMed] [Google Scholar]
- 48. Noh H, Yu MR, Kim HJ, Jang EJ, Hwang ES, et al. (2014) Uremic toxin p-cresol induces Akt-pathway-selective insulin resistance in bone marrow-derived mesenchymal stem cells. Stem Cells 32:2443–2453. [DOI] [PubMed] [Google Scholar]
- 49. Chang MC, Wang TM, Yeung SY, Jeng PY, Liao CH, et al. (2011) Antiplatelet effect of p-cresol, a uremic and environmental toxicant, is related to inhibition of reactive oxygen species, ERK/p38 signaling and thromboxane A2 production. Atherosclerosis 219:559–565. [DOI] [PubMed] [Google Scholar]
- 50. Zhu JZ, Zhang J, Yang K, Du R, Jing YJ, et al. (2012) P-cresol, but not p-cresylsulphate, disrupts endothelial progenitor cell function in vitro. Nephrol Dial Transplant 27:4323–4330. [DOI] [PubMed] [Google Scholar]
- 51. De Carvalho JT, Dalboni MA, Watanabe R, Peres AT, Goes MA, et al. (2011) Effect of spermidine and p-cresol on polymorphonuclear cell apoptosis and function. Artif Organs 35:E27–32. [DOI] [PubMed] [Google Scholar]
- 52. Kawakami K, Makino I, Kato I, Uchida K, Onoue M (2009) P-cresol inhibits IL-12 production by murine macrophages stimulated with bacterial immunostimulant. Immunopharmacol Immunotoxicol 31:304–309. [DOI] [PubMed] [Google Scholar]
- 53. Vanholder R, De Smet R, Waterloos MA, Van Landschoot N, Vogeleere P, et al. (1995) Mechanisms of uremic inhibition of phagocyte reactive species production: characterization of the role of p-cresol. Kidney Int 47:510–517. [DOI] [PubMed] [Google Scholar]
- 54. Brunet P, Gondouin B, Duval-Sabatier A, Dou L, Cerini C, et al. (2011) Does uremia cause vascular dysfunction? Kidney Blood Press Res 34:284–290. [DOI] [PubMed] [Google Scholar]
- 55. Sahin G, Yalcin AU, Akcar N (2007) Effect of N-acetylcysteine on endothelial dysfunction in dialysis patients. Blood Purif 25:309–315. [DOI] [PubMed] [Google Scholar]
- 56.Yuhki K, Kashiwagi H, Kojima F, Kawabe J, Ushikubi F (2010) Roles of prostanoids in the pathogenesis of cardiovascular diseases. Int Angiol 29 (2 suppl): 19–27. [PubMed]
- 57. Xu Y, Hagege J, Mougenot B, Sraer JD, Ronne E, et al. (1996) Differential expression of the plasminogen activation system in renal thrombotic microangiopathy and the normal human kidney. Kidney Int 50:2011–2019. [DOI] [PubMed] [Google Scholar]
- 58. Bifulco K, Longanesi-Cattani I, Liguori E, Arra C, Rea D, et al. (2013) A urokinase receptor-derived peptide inhibiting VEGF-dependent directional migration and vascular sprouting. Mol Cancer Ther 12:1981–1993. [DOI] [PubMed] [Google Scholar]
- 59. Sloand EM, Pfannes L, Scheinberg P, More K, Wu CO, et al. (2008) Increased soluble urokinase plasminogen activator receptor (suPAR) is associated with thrombosis and inhibition of plasmin generation in paroxysmal nocturnal hemoglobinuria (PNH) patients. Exp Hematol 36:1616–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Eddy AA (2009) Serine proteases, inhibitors and receptors in renal fibrosis. Thromb Haemost 101:656–664. [PMC free article] [PubMed] [Google Scholar]
- 61. Edsfeldt A, Nitulescu M, Gratman H, Gronberg C, Persson A, et al. (2012) Soluble urokinase plasminogen activator receptor is associated with inflammation in the vulnerable human atherosclerotic plaque. Stroke 43:3305–3312. [DOI] [PubMed] [Google Scholar]
- 62. Zhang G, Eddy AA (2008) Urokinase and its receptors in chronic kidney disease. Front Biosci 13:5462–5478. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.