Abstract
Progressive retinal degenerations are among the most common causes of blindness both in human and in dogs. Canine progressive retinal atrophy (PRA) resembles human retinitis pigmentosa (RP) and is typically characterized by a progressive loss of rod photoreceptors followed by a loss of cone function. The disease gradually progress from the loss of night and day vision to a complete blindness. We have recently described a unique form of retinopathy characterized by the multifocal gray/brown discoloration and thinning of the retina in the Swedish Vallhund (SV) breed. We aimed to identify the genetic cause by performing a genome wide association analysis in a cohort of 18 affected and 10 healthy control dogs using Illumina's canine 22k SNP array. We mapped the disease to canine chromosome 17 (p = 7.7×10−5) and found a 6.1 Mb shared homozygous region in the affected dogs. A combined analysis of the GWAS and replication data with additional 60 dogs confirmed the association (p = 4.3×10−8, OR = 11.2 for homozygosity). A targeted resequencing of the entire associated region in four cases and four controls with opposite risk haplotypes identified several variants in the coding region of functional candidate genes, such as a known retinopathy gene, MERTK. However, none of the identified coding variants followed a compelling case- or breed-specific segregation pattern. The expression analyses of four candidate genes in the region, MERTK, NPHP1, ANAPC1 and KRCC1, revealed specific upregulation of MERTK in the retina of the affected dogs. Collectively, these results indicate that the retinopathy is associated with overexpression of MERTK, however further investigation is needed to discover the regulatory mutation for the better understanding of the disease pathogenesis. Our study establishes a novel gain-of-function model for the MERTK biology and provides a therapy model for retinopathy MERTK inhibitors. Meanwhile, a marker-based genetic counseling can be developed to revise breeding programs.
Introduction
Dogs suffer from hundreds of hereditary disorders according to the Online Mendelian Inheritance in Animal database (OMIA, http://omia.angis.org.au/home/) and many of them represent clinically and physiologically relevant models for human conditions. Examples include several retinal conditions, such as canine multifocal retinopathies (cmr) [1]–[2] and Leber congenital amaurosis (canine LCA) [3]. Progressive retinal degenerations form a heterogeneous group of disorders that affect different retinal cells such as photoreceptors or retinal pigment epithelium (RPE), resulting in the impairment or complete loss of vision (RetNet; http://www.sph.uth.tmc.edu/Retnet/). Retinitis pigmentosa (RP) is one of the most common incurable blindness worldwide [4]. In RP, the degenerative process typically starts from rod photoreceptors and expands to cone cells leading to a progressive loss of both night- and day light vision before complete blindness [5].
Canine progressive retinal degenerations resemble human RP and are commonly referred as progressive retinal atrophies (PRA). PRA affects many breeds with remarkable variation in the etiology, progression and onset. Careful characterization of these conditions across breeds is not only important for the health of the dogs but could also provide valuable information about the genetics, retinal biology, molecular pathogenesis of RPs and possible environmental factors complementing existing human studies. Furthermore, gene discoveries would establish large animal models for retinal gene therapies [6]–[7]. Today, over dozen PRA genes have been described in dogs [1], [3], [8]–[23], and many remain still to be found.
We have recently characterized a unique type of retinal degeneration in the Swedish Vallhund (SV) breed [24]. (S1 Figure). The phenotype of this disease differs from most known forms of PRA with a multifocal rather than diffuse degeneration of the retina. Furthermore, age of onset and rate of progression vary considerably even in the littermates. Clinical signs progress in three stages ranging from diffuse multifocal red/brown discoloration of the tapetal fundus without associated visual deficits (Stage 1), to geographic retinal thinning/degeneration with mild to moderate signs of night-blindness (Stage 2), to more diffuse retinal thinning/degeneration affecting most of the tapetal fundus and associated with night-vision loss and severely impaired day-vision (Stage 3) [24]. This disease affects both the RPE and rod and cone photoreceptors with an excessive accumulation of autofluorescent material within the RPE [24]. Since the known canine PRA genes did not associate with the disease [24], we embarked a study here to identify the genetic cause.
Materials and Methods
Study cohort
Blood samples from SVs across various countries were collected to the canine DNA bank at the University of Helsinki, Finland with owner's consent and under the permission of animal ethical committee of County Administrative Board of Southern Finland (ESAVI/6054/04.10.03/2012). Altogether 436 samples were collected, including 93 cases and 76 controls. All affected dogs were examined by certified veterinary ophthalmologists at least once in Finland, Sweden or USA and diagnosed with SV retinopathy. All the control dogs used in the genome-wide association analysis were over 7 years of age at the time of eye examination by veterinary ophthalmologists and none of them were diagnosed with any retinal abnormalities. Genomic DNA was extracted from EDTA blood samples using Chemagic Magnetic Separation Module I (MSM I) (Chemagen Biopolymer-Technologie AG, Baeswieler, Germany) according to the manufacturer's instructions.
Retinal samples from four affected SVs and a PRA-free Australian Cattle Dog and a Belgian Shepherd became available due to euthaniziation for unrelated causes, and were collected post mortem with owners' consents. RNA was extracted using RNeasy Mini Kit (Qiagen) according to manufacturer's instructions.
A pedigree (S2 Figure) modified from related manuscript, Cooper et al. [24] (S1 Figure) was constructed around the affected dogs using Genopro software and the genealogical data available in public canine registries such as the Finnish Kennel Club's Koiranet, the Swedish Kennel Club's Hunddata databases or as informed by the owners.
Genome wide association study
To map the retinopathy locus in SVs a genome-wide association mapping was performed with 18 cases and 10 controls using Illumina Canine SNP20 BeadChip array (San Diego, CA, USA). Genotyping was performed at the FIMM Technology Center. The genotyping data was analyzed using PLINK 1.07 analysis software [25]. A total of 22,362 markers were initially included for the analysis. No individual were removed for low genotyping success of 95%. Missingness test of 95% removed 87 SNPs and the average genotyping rate per individual remained at 99.9%. A total of 8,078 SNPs had minor allele frequency of less than 5% and were removed. None of the SNPs deviated from Hardy-Weinberg equilibrium based of HWE test of P< = 0.0001. After frequency and genotyping pruning, 13,699 SNPs remained in the analysis.
To compare the affected dogs and healthy control dogs an allelic case-control association test was performed and significance values from this analysis were used to generate a whole-genome association plot using R-program [26]. Identity-by-state (IBS) clustering and CMH meta-analysis (PLINK) were used to adjust for population stratification. Genome-wide corrected empirical p-values were determined applying 50,000 permutations to the data. Besided PLINK the data was analyzed using R-implemented GenABEL software [27] (data not shown).
Replication study
A replication study for the best associated SNPs at CFA17 (BICF2G630207991) was performed in additional 34 cases and 26 controls. We performed a standard PCR, including 1.2 U Biotools DNA Polymerase (Biotools, Madrid, Spain), 1.5 mM MgCl2 (Biotools, Madrid, Spain), 200 µM dNTPs (Finnzymes, Espoo, Finland), 1× Biotools PCR buffer (Biotools, Madrid, Spain), 0.83 µM forward GCTGCTTCCTTTTTGCTCAT and reverse GGTGCTACGTTTGACAGCAA primers (Sigma Aldrich, St. Louis, USA) and 10 ng template genomic DNA. Reaction mixtures were subjected to a thermal cycling program of 95°C for 10 min, 35 cycles of 95°C for 30 s, 30 s 58°C, 72°C for 60 s and a final elongation stage of 72°C for 10 min. ExoSap (USB Corporation, Ohio, USA) purified fragments were sequenced at the FIMM Technology Center using ABI 3730xl DNA analyzer (Applied Biosystems, Foster City, California, USA). Sequence analysis was performed using Variant Reporter software (Applied Biosystems, Foster City, California, USA).
Targeted capture and re-sequencing
The associated region of 6.1 Mb on CFA17 (37.53–43.64 Mb, CanFam2.0) was resequenced in four affected SVs, four healthy SVs and 16 dogs from two other breeds, Dandie Dinmont Terrier and Staffordshire Bull Terrier. The SV samples were selected based on the opposite haplotypes in cases and controls ( Fig. 1B ). The region was captured using custom designed probes (according to the build 2.1 of the canine genome reference sequence) and Roche Nimblegen solution based capture method followed by paired-end sequencing using the Illumina HiSeq2000.
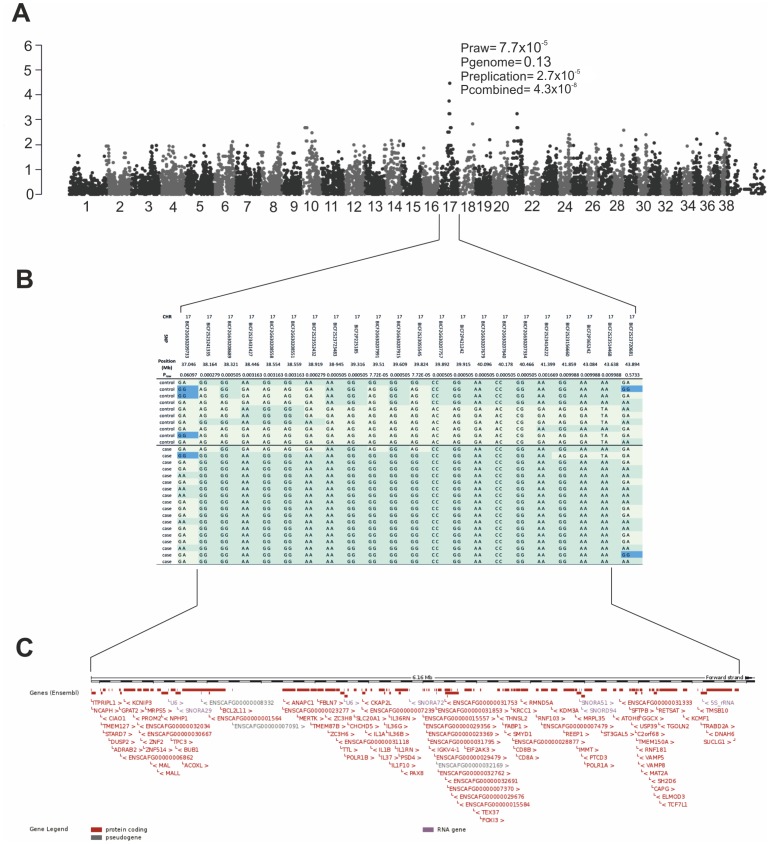
Figure 1. Results of the genome-wide association study.
Genome-wide association analysis identifies the retinopathy locus in the SV breed. A) Manhattan plot with tentative association on CFA17 (praw = 7.7×10−5), replication analysis supported the association (prepl = 2.7×10−5) and was confirmed by combined analysis (p = 4.3×10−8). CFA39 represent the X chromosome. B) A close-up of the associated region on CFA17, which spans from 38.16 Mb to 43.64 Mb. C) The associated region harbors over hundred genes, including a known PRA gene, MERTK.
The data analysis included quality control, alignment, variant calling and annotation of the variants. Quality control was performed using FASTX toolkit (http://hannonlab.cshl.edu/fastx_toolkit/index.html) to remove the low quality bases called by the sequencing machine. Base call accuracy of 99% i.e. bases with Phred scores <Q20, were trimmed to reduce false positives during variant calling. The quality passed paired-end reads were aligned to the build 3.1 of the canine genome reference sequence with Burrows-Wheeler (BWA) aligner tool with default parameters [28]. After mapping, the reads that mapped to the targeted region were extracted followed by the removal of potential PCR duplicate reads using Samtools 0.1.18 [29]. Local realignment around potential indel sites and base quality scores recalibration were implemented using GATK [30] and fix mate-pair information using Samtools [29] to improve the quality of the sequence data before variant calling. The sequence alignments were then directed to the variant calling programs GATK [30] and Samtools [29] to identify the SNPs and short indels present within the samples. Pindel program was further used to identify indels and structural variants [31]. Finally, the identified variants were annotated against NCBI and UCSC databases to find out the variants present in the coding and non-coding regions. The annotation was performed using SnpEff [32] and in-house custom R-scripts. The dbSNP131 database was utilized to identify known polymorphic variants. To identify the case-specific variant, the data was filtered against the controls and 16 other dogs from two breeds under a recessive model using our in-house R-scripts. To study the CNVs and repeat elements (SINE, LINE), a heatmap analysis was performed by comparing normalized read depths in each position between cases and controls.
Validation of candidate causative variants
The tentative disease causing variants identified by resequencing, were genotyped in a large sample cohort of SVs ( Table 1 ) and from eight unaffected dogs from two other breeds for the breed specificity. The identified variants were categorized based on type and position: non-synonymous first, followed by variants in the non-coding RNAs, UTR regions, and conserved intergenic and intronic regions, respectively ( Table 1 ). The primers for the validated markers are available upon request. The association of each variant with the disease was calculated by PLINK [25].
Table 1. Markers selected for further studies based on the targeted resequencing data.
| CODING | ||||||||||||||||
| Gene | Type | Position † | Ref | Alt | Protein | Polyphen2/SIFT prediction | No. Case | No. Control | P | OR | CHISQ | HMM based conservation score | A1 | F_A | F_U | A2 |
| NPHP1 | SNV | 35128247 | A | G | p.H86R | probably damaging | 51 | 33 | 5.2×10−6 | 5.7 | 20.8 | 0.4 | G | 0.9 | 0.6 | A |
| ANAPC1 | SNV | 36279583 | G | A | p.V734I | benign | 51 | 33 | 0.09 | 1.7 | 2.8 | 0.3 | A | 0.7 | 0.6 | G |
| MERTK | SNV | 36405419 | G | C | p.A369P | benign | 51 | 33 | 2.8×10−6 | 6.2 | 21.9 | 1.0 | C | 0.9 | 0.6 | G |
| KRCC1 | SNV | 38321727 | T | G | p.L97R | probably damaging | 51 | 33 | 6.0×10−4 | 4.8 | 11.9 | 0.5 | G | 0.9 | 0.7 | T |
| NON-CODING | ||||||||||||||||
| BCL2L11 | INDEL | 35627062–35627089 | ACACTTTCAGTTCTTTTGGATATCTAT | - | 23 | 22 | 0.01 | 3.2 | 6.7 | 0.01 | del/del | 0.8 | 0.5 | ref | ||
| MERTK | SNV | 36348610 | A | G | 30 | 33 | 0.04 | 2.5 | 4.1 | - | G | 0.9 | 0.7 | A | ||
| MERTK | INDEL | 36348691 | - | TCTG | 23 | 23 | 0.001 | 4.5 | 10.6 | - | ins/ins | 0.8 | 0.5 | ref | ||
| MERTK | SNV | 36410244 | C | A | 23 | 23 | 0.002 | 3.9 | 9.1 | 0.02 | A | 0.8 | 0.5 | C | ||
| MERTK | SNV | 36410251 | T | C | 23 | 23 | 0.004 | 3.8 | 8.2 | 0.02 | C | 0.8 | 0.5 | T | ||
| SLC20A1 | SNV | 36872452 | C | T | 22 | 20 | 0.003 | 3.8 | 8.9 | 0.007 | T | 0.8 | 0.5 | C | ||
| PSD4 | SNV | 37267152 | A | T | 21 | 18 | 0.05 | 2.8 | 3.8 | 0.2 | T | 0.8 | 0.6 | A | ||
| ncRNA | SNV | 37464374 | A | G | 10 | 6 | 0.0003 | 5.8 | 13.3 | 0.007 | A | 0.7 | 0.3 | G | ||
| ENSCAFG00000028877 | SNV | 38310169 | A | G | 19 | 19 | 0.009 | 5.4 | 6.7 | 0.05 | A | 0.9 | 0.7 | G | ||
| ENSCAFG00000028877 | SNV | 38310174 | G | A | 36 | 31 | 0.002 | 6.1 | 9.4 | 0.06 | G | 1.0 | 0.8 | A | ||
| ENSCAFG00000028877 | SNV | 38310216 | G | A | 20 | 18 | 0.02 | 4.0 | 5.8 | 0.005 | G | 0.9 | 0.6 | A | ||
| ENSCAFG00000028877 | SNV | 38310235 | A | T | 39 | 27 | 0.006 | 3.7 | 7.5 | 0.2 | A | 0.9 | 0.7 | T | ||
| ENSCAFG00000028877 | SNV | 38310249 | T | C | 39 | 32 | NA | NA | NA | 0.2 | C | 1 | 1 | T | ||
| RNF103 | SNV | 38568101 | A | G | 20 | 20 | NA | NA | NA | 0.09 | A | 1 | 1 | G | ||
| RNF103 | SNV | 38569756 | A | C | 21 | 21 | 0.1 | 2.1 | 2.6 | 0.007 | C | 0.7 | 0.6 | A | ||
| RNF103 | SNV | 38570219 | G | A | 20 | 20 | 0.1 | 2.2 | 2.7 | 0.3 | A | 0.8 | 0.6 | G | ||
| KDM3A | INDEL | 38601421 | - | GTGGA | 23 | 22 | 0.2 | 1.7 | 1.9 | 0.04 | ins/ins | 0.7 | 0.5 | ref | ||
| KDM3A | SNV | 38753172 | G | A | 28 | 24 | 0.2 | 1.7 | 1.6 | 0.05 | A | 0.7 | 0.5 | G | ||
| KDM3A | SNV | 38753853 | G | A | 23 | 22 | 0.2 | 1.7 | 1.6 | 0.02 | A | 0.7 | 0.5 | G | ||
| KDM3A | SNV | 38753998 | A | G | 23 | 22 | 0.2 | 1.7 | 1.6 | 0.1 | G | 0.7 | 0.5 | A | ||
| Gene | Type | Position † | Ref | Alt | Protein | Polyphen2/SIFT prediction | No. Case | No. Control | P | OR | CHISQ | HMM based conservation score | A1 | F_A | F_U | A2 |
| - | INDEL | 38785175 | AGGT | - | 15 | 16 | 0.6 | 0.8 | 0.2 | 0.01 | ref | 0.6 | 0.6 | del/del | ||
| REEP1 | INDEL | 38906987–38906990 | TACT | - | 23 | 23 | 0.3 | 1.6 | 1.2 | 0.9 | del/del | 0.7 | 0.6 | ref | ||
| POLR1A | SNV | 39044115 | C | T | 26 | 24 | 0.4 | 1.4 | 0.8 | 0.1 | T | 0.6 | 0.6 | C | ||
| POLR1A | SNV | 39044233 | G | A | 26 | 22 | 0.3 | 1.6 | 1.1 | 0.2 | A | 0.7 | 0.6 | G | ||
| POLR1A | INDEL | 39048757 | - | A | 23 | 23 | 0.3 | 1.6 | 1.2 | 0 | ins/ins | 0.7 | 0.6 | ref | ||
| SH2D6 | SNV | 39603031 | A | T | 23 | 21 | 0.6 | 1.7 | 0.3 | 0.2 | T | 1 | 0.9 | A | ||
| SH2D6 | SNV | 39603158 | C | A | 31 | 23 | 0.2 | 4.3 | 2.8 | 0 | A | 1 | 0.9 | C | ||
| SH2D6 | SNV | 39613315 | G | A | 22 | 21 | 0.6 | 1.4 | 0.2 | 0.009 | A | 0.9 | 0.9 | G | ||
| SH2D6 | SNV | 39613514 | T | C | 20 | 19 | 0.1 | 4.6 | 2.1 | 0.007 | C | 1 | 0.9 | T | ||
| TGOLN2 | SNV | 39685675 | C | T | 23 | 22 | 0.3 | 2.3 | 1.3 | 0.03 | T | 0.9 | 0.8 | C |
The position is based on canine reference sequence CanFam3.1.
The best association was found in the MERTK gene.
Expression analyses
Retinal RNA samples were studied to screen MERTK transcript for mutations and to quantitate transcript levels of four retinal candidate genes, MERTK, NPHP1, ANAPC1 and KRCC1 between the affected (n = 4) and unaffected (n = 2) dogs. The MERTK mRNA sequence (XM_005630437.1) was amplified using Biotools (Biotools, Madrid, Spain)) polymerase and PCR protocol described in the replication section with annealing temperature of 60°C. Amplicons were Sanger sequenced for mutations.
To quantitate transcript levels, a real time PCR was performed using Applied Biosystems'7500 Fast Real-Time PCR machine and Universal SYBR Green Master (Roche). RT-PCR was carried out on equal amounts of retinal RNA in each sample by using the High Capasity RNA-to-cDNA kit (Applied Biosystems). RT-PCR was carried out in 0.25 µM of forward and reverse primes in a total reaction volume of 20 µl. The primers used for mRNA amplification and real time PCR are available upon request. A housekeeping gene, GAPDH was used as a normalization control, and triplicate samples were used for all reactions. The efficiency of the reaction was calculated from a seven-point dilution series. No significant differences were detected in the efficiencies between the housekeeping and target reactions, and the comparative ΔΔCt-method could be used to determine relative expression differences. Statistical significance of the expression differences was calculated by using the Student's t-test on normalized mean cycle threshold (Ct) -values. PASW Statistics 18 software SPSS (IBM) was used to perform the statistical tests.
Results
We have recently identified a novel type of retinal degeneration in SVs with a likely recessive mode of inheritance [24] and aimed to map the disease locus here by expanding the SV study cohort across different countries in Europe and the US. We performed a GWAS in a cohort of 18 cases and 10 controls using Illumina's canine 22k SNP array. Genotyping data was analyzed by a linear regression (PLINK) and mixed model approaches (GenAbel, data not shown). No significant population structure was identified by genome wide IBS clustering (λ = 1.1). We identified a tentative locus on CFA17 (praw = 7.7×10−5, pgenome = 0.13) with the most highly associated SNP, BICF2G630207991 ( Fig. 1A ).
To confirm the association, we genotyped the best associated SNP on CFA17 in additional 34 cases and 26 controls (prepl = 2.7×10−5). The combined analysis (GWAS and replication) supported the association (pcom = 4.3×10−8) ( Fig. 1A ).
According to the combined analysis, 82% (41/50) of the cases and 30.5% (11/36) of controls were homozygous for the risk allele at CFA17 given the (OR = 11.2). Eleven unaffected dogs that were homozygous for the risk allele were all eye examined healthy after 6 years of age, however, it is possible that the dogs become affected later as the age of onset and disease progression varies even in the littermates [24]. Reduced penetrance of the disease may be linked to the large phenotypic variation or possible genetic or environmental modifiers [24].
Candidate gene analysis
The associated region has a homozygous risk haplotype in the cases (17/18), spanning a 6.1 Mb region from 37.5 Mb to 43.60 Mb ( Fig. 1B ). One of the cases clearly carries a different haplotype from the rest of the cases and could be a phenocopies due to other reasons ( Fig. 1B ). The region contains 102 genes of which anaphase promoting complex subunit 1 (ANAPC1) [33], c-mer proto-oncogene tyrosine kinase (MERTK) [34]–[41] and nephronophthisis 1 (NPHP1) [42]–[43] have been previously associated with retinal disease or retinal function ( Fig. 1C ). These genes were selected for exonic mutation screening, revealing three non-synonymous coding variants p.A369P (g.36405419) in MERTK, p.H86R (g.3512824) in NPHP1 and p.V734I (g.36279583) in the ANAPC1 ( Table 1 ).
The pathogenicity of the coding variants was predicted based on the bioinformatics prediction softwares Polyphen2.0 [44] and SIFT [45], which evaluate the evolutionary conservation of the residues across species. The MERTK p.A369P and the ANAPC1 p.V734I variants were predicted to be benign while NPHP1 variant as likely pathogenic. However, when we genotyped all three coding variants in 33 eye examined unaffected SVs ( Table 1 ) and eight dogs from two other unaffected breeds, Whippet and Finnish Lapphund, we found all of them in unaffected SVs and in the two other breed with a moderate frequency, suggesting them as polymorphisms rather than causative.
Targeted resequencing
To identify the causative mutation, we performed a targeted resequencing of the entire 6.1 Mb associated locus in eight SVs and 16 other dogs from Dandie Dinmont Terrier and Staffordshire Bull Terrier breeds. We reached an average 98.8% sequence coverage across the locus in each dog and found altogether 86,160 single nucleotide variants (SNVs) and 2,294 indels (S1 Table). After filtering the variants under the recessive model altogether 408 SNVs and 70 indels were shared between the SV cases (S1 Table). The canine reference sequence (CanFam3.1) was used to annotate the identified variants. Although 102 genes have been annotated in the capture region, targeted resequencing revealed only one additional new case specific coding variant, p.L97R, in the lysine-rich coiled-coil 1 (KRCC1) gene (XP_005630523.1). Further analysis of this variant in 51 additional affected and 33 unaffected SVs and in two other unaffected breeds, Whippet and Finnish Lapphund, did not support segregation and indicated that is found in other non-affected breeds ( Table 1 ).
Since the identified coding variants did not segregate with the disease, we next selected 30 non-coding variants across conserved (UCSC conservation scores) [46] regions of the top candidate genes for further analyses in additional samples ( Table 1 ). However, none of these variants segregated either with the disease.
Possible presence of case-specific CNVs and repeats such as, SINEs and LINEs were studied by a read depth-based heatmap analysis between the cases and controls. No case-specific differences were found in the read depths across the associated region (data not shown).
Collectively, these genomic analyses did not reveal a causative mutation but identify the strongest association with the SNP (BICF2G630207991) in the intron of the MERTK gene. This marker was therefore further tested in 400 SVs from our DNA bank revealing a very high carrier frequency (59.9%) and a significant risk for homozygosity (p = 6.3×10−27, OR = 18.5 with 95%CI = 10.3–33.2).
MERTK transcript analysis
Because our genomic analyses did not reveal the causative mutation, we decided to sequence the MERTK mRNA for possible abnormal splicing or other events from the retinal samples, including the neuro-retina and RPE of two affected SVs and an unaffected dog available. Sequencing revealed the p.A369P variant found in the genomic analyses in the affected dogs but did not reveal any additional variants or abnormal splicing events.
Expression analysis of the retinal candidate genes
We obtained retinal tissue samples from four retinopathy affected SVs and two control dogs and used them to quantitate transcript levels of the four retinal candidate genes, MERTK, NPHP1, ANAPC1 and KRCC1 in the critical region. Real-time PCR revealed a 6.5-fold upregulation of the MERTK gene in the affected dogs while no difference was found in the NPHP1, ANAPC1 or KRCC1 genes ( Fig. 2 ). This result suggests that a regulatory mutation outside the coding region results in the overexpression of MERTK, which then leads to retinopathy in the affected dogs.
Figure 2. Retinal upregulation of MERTK in the affected SVs.
The retinal mRNA levels of MERTK, NPHP1, ANAPC1 and KRCC1 genes were compared between affected (n = 4) and unaffected dogs (n = 2). A specific overexpression of MERTK was found in the affected SVs. The relative mRNA expression levels are represented as a fold change. Error bars denote the standard error of normalized Ct-values. *p≤0.0001 (two-tailed t-test p-value).
Discussion
We have previously identified a unique form of PRA in the SV breed and show here genetic and functional evidence that the disease is associated with a known human RP gene, MERTK. The SV breed is small worldwide, which is reflected with extensive linkage disequilibrium of the associated region. The region harbors over 100 genes, including some that have been associated with retinal function or retinal degeneration in human and rodent models, such as ANAPC1, MERTK and NPHP1. Although we found several coding and regulatory variants in these retinal candidate genes, none of them followed appropriate segregation pattern and were present in other unaffected breeds, excluding them as candidates for the disease. However, while the causative variant remains to be found, positional and functional evidence support the role of MERTK in the SV retinopathy.
First, our genetic data identified the best association within the MERTK gene, and this was 100 times stronger than in any other regions of the gene-rich locus. This result strongly suggests that the cause of the disease lies in the identified region within or nearby MERTK. Second, from the three retinal genes in the critical region, MERTK appears as the most likely candidate based on its function and clinical significance in the retinal degeneration in human and rat [35], [39], [41], [47]. The MERTK protein is a member of the TAM receptor tyrosine kinase and is expressed in the retinal pigment epithelium (RPE) plasma membrane [48]. It participates in apoptotic cell clearance and cytoskeleton regulation with anti-inflammatory properties [49]. Human recessive MERTK mutations have been associated with several phenotypes including RP, rod cone dystrophy and early onset, childhood blindness [34], [36]–[38], [47], [50]–[57]. Progressive loss of photoreceptor cells in MERTK-deficient Royal College of Surgeon (RCS) rats was suggested to result from the lack of the cooperation between the photoreceptor and the RPE cells [35].
The analysis of the MERTK transcript isolated from the affected retinas indicated an intact transcript sequence without additional coding sequence or splicing abnormalities. However, an unexpected 6.5-fold up-regulation of the MERTK transcript was found in the affected retina. This was specific to MERTK, since no changes were found in the transcript levels of the other three candidate genes, NPHP1, ANAPC1 and KRCC1 in the same retinal tissues ( Fig. 2 ).
Potential explanations of the increased transcription of MERTK might include i) the presence of a regulatory mutation in the MERTK gene, ii) a massive infiltration of MERTK positive inflammatory cells into the RPE [49], iii) normal variation in the MERTK expression due to circadian rhythm of the RPE phagocytosis peaking at the daily onset of light [58] or iv) the presence of a regulatory variation outside MERTK affecting its ligands or MERTK-induced cellular physiology [59]. The hypotheses ii and iii appear unlikely, since our routine histopathology was not indicative of invasion of inflammatory cells in the affected retina [24], and the retinal samples for the transcript quantification were harvested several hours after the phagocytosis peak when MERTK should not be active and elevated. Our genetic data points to a cause within MERTK not outside. The presence of a MERTK-specific regulatory mutation is more likely and our future efforts will focus on the MERTK gene and surrounding regions, including the promoter region, upstream enhancers and possible UTR miRNA binding sites. Overexpression of MERTK has been associated with the variants in the 3UTR miRNA binding sites [60].
Previous MERTK-related retinal degenerations have been associated with recessive loss-of-function mutations [34], [36]–[38], [47], [50]–[57]. The question how overexpression of MERTK may lead to the multifocal retinopathy in the affected SVs remains unknown, although several possible hypotheses can be speculated based on the diversity of the MERTK functions in various models.
First, MERTK can activate several intracellular canonical signaling pathways, including phosphoinositide 3 kinase, PI3K/AKT, pathway including phospholipase C, ERK1/2, Ras, and MAP kinase and JAK/STAT pathway [61], which could lead to increased or abnormal apoptosis in the local regions of the RPE.
Second, overexpression of MERTK has been shown to induce efferocytosis in cancer cell lines [59]. Similarly, overactive MERTK in the RPE could increase the efferocytosis of the POS, lead to an accumulation of photoreceptor debris in the RPE and subsequent degeneration of photoreceptors in the affected dogs. Human MERTK patients show deposit of autofluorescence consisting of photoreceptor outer segment (POS) membranes and their by-products, including accumulating lipofuscin in the outer retina [41], [51]. Similarly in RCS rats, an abnormal accumulation of outer segment debris between photoreceptor outer segment layer and the RPE occurs, prior to photoreceptor cell death [62]–[64]. In the RCS rats, RPE cells fail to engulf POS, which causes the accumulation of POS debris in the subretinal space [65]. The SV retinopathy affects both the RPE and rod and cone photoreceptors with an excessive accumulation of autofluorescent lipofuscin-like material within the RPE [24].
Third, the MERTK receptor has a soluble form, sMer [66] with an antagonistic role to full-length MERTK. sMer can bind to Gas6 and inhibit Gas6-mediated MERTK activation [66], which in turn could result in the impaired phagocytosis and retinopathy. sMer is posttranslationally generated from the MERTK receptor by the ADAM17 cleavage [66] and its expression patterns in the affected retina and RPE should be studied at the protein level.
Fourth, MERTK overexpression has been shown to result in an altered localization of the MERTK protein in the nuclear compartment [67]. In the RPE, this model could lead to a net loss-of-function effect of the MERTK activity as seen in the recessive RP cases.
Finally, MERTK is expressed in macrophages that participate in the phagocytosis [68]. Constitutive overexpression of MERTK in the macrophages could result in the enhanced local efferocytosis in the RPE, which in turn, would further promote MERTK activity and undesired apoptosis and loss of photoreceptors.
Upon confirmation of the MERTK overexpression with subsequent downstream mechanisms, existing MERTK inhibitors [69] may provide a therapeutic option for the retinopathy dogs. MERTK overexpression is characteristic to several cancers and inhibition in those models has been found efficient [69]–[70]. A clinical trial with MERTK inhibitors remains as an exciting possibility not only to treat canine patients but also to better understand the related disease mechanisms and MERTK biology in the eye.
The uniqueness of the SV retinopathy is based on the retinal disease phenotype, the variation in age of onset and in the rate of disease progression. This suggests that genetic and/or environmental disease modifiers likely contribute to the disease phenotype. Our GWAS data with a modest samples size revealed a single locus with a possible reduced penetrance. The vast majority of the affected dogs are homozygous for the risk haplotype, however, the phenotypic variability in SVs may be related to the particular type of regulatory mutation causing the upregulation of MERTK in combination with possible environmental factors.
Three additional coding variants were found in other genes. A predicted pathological variant, p.H86R, was identified in the NPHP1 gene, which encodes for nephronophthisis 1. NPHP1 is widely expressed in many tissues and localized to the photoreceptor-connecting cilia at the junction of the inner segment and outer segment [71]. The gene has been implicated in an autosomal recessive, juvenile nephronophthisis 1 [72] with infrequent retinal degeneration [73]. Nphp1-deficient mice present an early-onset rapidly progressing degeneration of the outer and inner segments and nuclei, losing the photoreceptors within the first 8 months of life [74]. Although the p.H86R variant was predicted to be pathological, its homozygous presence in other unaffected SVs and breeds exclude its causative role.
The third coding variant (p.V734I) was identified in the ANAPC1 gene. This gene has been implicated in the normal eye development in Drosophila. The shattered (shtd) mutation in the fly leads to a failure in G1 arrest during the mitosis, causing a defective arrangement of photoreceptor cells and other developmental problems in the eye [75]. Again the identified variant in our SVs was not case- or breed-specific ruling it out as the cause of the disease.
The fourth non-synonymous coding variant in the associated region, p.L97R, was found in the KRCC1 gene, which encodes a lysine-rich coiled-coin protein 1, with an unknown function. However, the KRCC1 variant was also present in other unaffected dogs and did not segregate with the disease.
Similarly, a large number of potential regulatory variants were found in the conserved regions across the associated region but validation experiments for 30 of them did not support an appropriate segregation pattern. We may have missed the causative mutation in MERTK due to technical reasons despite high quality resequencing data. Targeted capture is not efficient in repetitive regions, which are often lost already at the target design. Another challenge relates to possible larger structural variants such as CNVs, which are not easy to pinpoint in the resequencing data. It is possible that some coding variants have been missed in our analysis, however, the annotations around MERTK locus were identical in both species and it is unlikely that coding variants were missed in the critical region. We will consider resequencing of the whole genomes of SVs to avoid capture-related obstacles.
In conclusion, we have mapped the cause of retinopathy in SVs and show the involvement of the up-regulated MERTK gene in the affected dogs. Our study establishes a novel gain-of-function model for the MERTK physiology in the retina. Future studies will include the search for the regulatory mutation and study of overexpression-related disease mechanisms with a possibility for a therapeutic option with MERTK inhibitors. Meanwhile, a genetic marker test can be developed for breeding purposes to help the future SV population to reduce the high-risk allele frequency in the breed.
Supporting Information
A pedigree from a related manuscript, Cooper et al., indicates clinically studied dogs [24] .
(TIF)
Pedigree indicates the dogs that were used in the GWAS study (marked yellow). Disease segregation suggests an autosomal recessive mode of inheritance.
(TIF)
Summary of the targeted resequencing data. The 6.1 Mb associated region was captured and resequenced in four cases and four control SVs with opposite risk haplotypes to identify the causative mutation.
(XLSX)
Acknowledgments
We would like to thank all veterinarians and owners that donated samples to our DNA-bank from their dogs. The Swedish Vallhund Clubs in Finland, USA and Sweden are thanked for their co-operation. Ann E. Cooper and Jessica S. Rowlan (University of Pennsylvania), Christine D. Harman (Michigan State University) and Minna Virta, Ranja Eklund, Reetta Hänninen and Sini Karjalainen (University of Helsinki) are thanked for their technical support and sample processing. We thank devoted SV breeders Inga Kahlman and Marja-Liisa Lainepää for encouraging SV owners to participate. Special thanks to Uma Aaltonen, who was starting the project but sadly passed away during this study.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.
Funding Statement
The study has been supported partly by the Sigrid Juselius Foundation, the Jane and Aatos Erkko Foundation, ERCStG-260997, the Academy of Finland, Biocentrum Helsinki, University of Helsinki Research Funds, private donations, and NIH grants K12-EY15398 and R01-EY019304. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Guziewicz KE, Zangerl B, Lindauer SJ, Mullins RF, Sandmeyer LS, et al. (2007) Bestrophin gene mutations cause canine multifocal retinopathy: A novel animal model for best disease. Invest Ophthalmol Vis Sci 48:1959–1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zangerl B, Wickstrom K, Slavik J, Lindauer SJ, Ahonen S, et al. (2010) Assessment of canine BEST1 variations identifies new mutations and establishes an independent bestrophinopathy model (cmr3). Mol Vis 16:2791–2804. [PMC free article] [PubMed] [Google Scholar]
- 3. Aguirre GD, Baldwin V, Pearce-Kelling S, Ray K, Acland GM (1998) Congenital stationary night blindness in the dog: Common mutation in the RPE65 gene indicates founder effect. Mol Vis 4:23. [PubMed] [Google Scholar]
- 4. Hartong DT, Berson EL, Dryja TP (2006) Retinitis pigmentosa. Lancet 368:1795–1809. [DOI] [PubMed] [Google Scholar]
- 5. Pagon RA (1988) Retinitis pigmentosa. Surv Ophthalmol 33:137–177. [DOI] [PubMed] [Google Scholar]
- 6. Acland GM, Aguirre GD, Ray J, Zhang Q, Aleman TS, et al. (2001) Gene therapy restores vision in a canine model of childhood blindness. Nat Genet 28:92–95. [DOI] [PubMed] [Google Scholar]
- 7. Komaromy AM, Alexander JJ, Rowlan JS, Garcia MM, Chiodo VA, et al. (2010) Gene therapy rescues cone function in congenital achromatopsia. Hum Mol Genet 19:2581–2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ahonen SJ, Arumilli M, Lohi H (2013) A CNGB1 frameshift mutation in Papillon and Phaléne dogs with progressive retinal atrophy. PLOS One 8:e72122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Downs L, Bell J, Freeman J, Hartley C, Hayward L, et al. (2012) Late-onset progressive retinal atrophy in the Gordon and Irish Setter breeds is associated with a frameshift mutation in C2orf71. Anim Genet 44:169–77. [DOI] [PubMed] [Google Scholar]
- 10. Downs LM, Wallin-Håkansson B, Boursnell M, Marklund S, Hedhammar Å, et al. (2011) A frameshift mutation in Golden Retriever dogs with progressive retinal atrophy endorses SLC4A3 as a candidate gene for human retinal degenerations. PLOS One 6:e21452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Goldstein O, Kukekova AV, Aguirre GD, Acland GM (2010) Exonic SINE insertion in STK38L causes canine early retinal degeneration (erd). Genomics 96:362–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Goldstein O, Mezey JG, Boyko AR, Gao C, Wang W, et al. (2010) An ADAM9 mutation in canine cone-rod dystrophy 3 establishes homology with human cone-rod dystrophy 9. Molecular vision 16:1549. [PMC free article] [PubMed] [Google Scholar]
- 13. Kijas JW, Cideciyan AV, Aleman TS, Pianta MJ, Pearce-Kelling SE, et al. (2002) Naturally occurring rhodopsin mutation in the dog causes retinal dysfunction and degeneration mimicking human dominant retinitis pigmentosa. Proceedings of the National Academy of Sciences 99:6328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kukekova AV, Goldstein O, Johnson JL, Richardson MA, Pearce-Kelling SE, et al. (2009) Canine RD3 mutation establishes rod-cone dysplasia type 2 (rcd2) as ortholog of human and murine rd3. Mammalian Genome 20:109–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Petersen–Jones SM, Entz DD, Sargan DR (1999) cGMP phosphodiesterase-α mutation causes progressive retinal atrophy in the Cardigan Welsh Corgi dog. Invest Ophthalmol Vis Sci 40:1637. [PubMed] [Google Scholar]
- 16. Sidjanin DJ, Lowe JK, McElwee JL, Milne BS, Phippen TM, et al. (2002) Canine CNGB3 mutations establish cone degeneration as orthologous to the human achromatopsia locus ACHM3. Hum Mol Genet 11:1823. [DOI] [PubMed] [Google Scholar]
- 17. Suber ML, Pittler SJ, Qin N, Wright GC, Holcombe V, et al. (1993) Irish Setter dogs affected with rod/cone dysplasia contain a nonsense mutation in the rod cGMP phosphodiesterase beta-subunit gene. Proceedings of the National Academy of Sciences 90:3968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wiik AC, Wade C, Biagi T, Ropstad EO, Bjerkås E, et al. (2008) A deletion in nephronophthisis 4 (NPHP4) is associated with recessive cone-rod dystrophy in Standard Wire-Haired Dachshund. Genome Res 18:1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zangerl B, Goldstein O, Philp AR, Lindauer SJ, Pearce-Kelling SE, et al. (2006) Identical mutation in a novel retinal gene causes progressive rod-cone degeneration in dogs and retinitis pigmentosa in humans. Genomics 88:551–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang Q, Acland GM, Wu WX, Johnson JL, Pearce-Kelling S, et al. (2002) Different RPGR exon ORF15 mutations in canids provide insights into photoreceptor cell degeneration. Hum Mol Genet 11:993–1003. [DOI] [PubMed] [Google Scholar]
- 21. Dekomien G, Runte M, Gödde R, Epplen J (2000) Generalized progressive retinal atrophy of Sloughi dogs is due to an 8-bp insertion in exon 21 of the PDE6B gene. Cytogenetic and Genome Research 90:261–267. [DOI] [PubMed] [Google Scholar]
- 22. Mellersh C, Boursnell M, Pettitt L, Ryder E, Holmes N, et al. (2006) Canine RPGRIP1 mutation establishes cone–rod dystrophy in Miniature Longhaired Dachshunds as a homologue of human Leber Congenital Amaurosis. Genomics 88:293–301. [DOI] [PubMed] [Google Scholar]
- 23. Vilboux T, Chaudieu G, Jeannin P, Delattre D, Hedan B, et al. (2008) Progressive retinal atrophy in the Border Collie: A new XLPRA. BMC veterinary research 4:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cooper AE, Ahonen S, Rowlan JS, Duncan A, Seppälä EH, et al. (2014) A novel form of progressive retinal atrophy in Swedish Vallhund dogs. PLOS One 8 9:e106610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, et al. (2007) PLINK: A tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 81:559–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.R Development Core Team, R foundation for Statistical Computing (2009) R: A language and enviroment for statistical computing.
- 27. Aulchenko YS, Ripke S, Isaacs A, van Duijn CM (2007) GenABEL: An R library for genome-wide association analysis. Bioinformatics 23:1294–1296. [DOI] [PubMed] [Google Scholar]
- 28. Li H, Durbin R (2010) Fast and accurate long-read alignment with Burrows–Wheeler transform. Bioinformatics 26:589–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, et al. (2009) The sequence alignment/map format and SAMtools. Bioinformatics 25:2078–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, et al. (2010) The genome analysis toolkit: A MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res 20:1297–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ye K, Schulz MH, Long Q, Apweiler R, Ning Z (2009) Pindel: A pattern growth approach to detect break points of large deletions and medium sized insertions from paired-end short reads. Bioinformatics 25:2865–2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cingolani P, Platts A, Wang LL, Coon M, Nguyen T, et al. (2012) A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of drosophila melanogaster strain w1118; iso-2; iso-3. Fly 6:80–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jörgensen PM, Gräslund S, Betz R, Ståhl S, Larsson C, et al. (2001) Characterisation of the human APC1, the largest subunit of the anaphase-promoting complex. Gene 262:51–59. [DOI] [PubMed] [Google Scholar]
- 34. Gal A, Li Y, Thompson DA, Weir J, Orth U, et al. (2000) Mutations in MERTK, the human orthologue of the RCS rat retinal dystrophy gene, cause retinitis pigmentosa. Nat Genet 26:270–271. [DOI] [PubMed] [Google Scholar]
- 35. D'Cruz PM, Yasumura D, Weir J, Matthes MT, Abderrahim H, et al. (2000) Mutation of the receptor tyrosine kinase gene mertk in the retinal dystrophic RCS rat. Hum Mol Genet 9:645–651. [DOI] [PubMed] [Google Scholar]
- 36. McHenry CL, Liu Y, Feng W, Nair AR, Feathers KL, et al. (2004) MERTK arginine-844-cysteine in a patient with severe rod–cone dystrophy: Loss of mutant protein function in transfected cells. Invest Ophthalmol Vis Sci 45:1456–1463. [DOI] [PubMed] [Google Scholar]
- 37. Brea-Fernandez A, Pomares E, Brion M, Marfany G, Blanco M, et al. (2008) Novel splice donor site mutation in MERTK gene associated with retinitis pigmentosa. Br J Ophthalmol 92:1419–1423. [DOI] [PubMed] [Google Scholar]
- 38. Mackay DS, Henderson RH, Sergouniotis PI, Li Z, Moradi P, et al. (2010) Novel mutations in MERTK associated with childhood onset rod-cone dystrophy. Molecular vision 16:369. [PMC free article] [PubMed] [Google Scholar]
- 39. Ostergaard E, Duno M, Batbayli M, Vilhelmsen K, Rosenberg T (2011) A novel MERTK deletion is a common founder mutation in the Faroe islands and is responsible for a high proportion of retinitis pigmentosa cases. Molecular vision 17:1485. [PMC free article] [PubMed] [Google Scholar]
- 40. Siemiatkowska AM, Arimadyo K, Moruz LM, Astuti GD, de Castro-Miro M, et al. (2011) Molecular genetic analysis of retinitis pigmentosa in Indonesia using genome-wide homozygosity mapping. Molecular vision 17:3013. [PMC free article] [PubMed] [Google Scholar]
- 41. Ksantini M, Lafont E, Bocquet B, Meunier I, Hamel CP (2012) Homozygous mutation in MERTK causes severe autosomal recessive retinitis pigmentosa. Eur J Ophthalmol 22:647–653. [DOI] [PubMed] [Google Scholar]
- 42. Parisi MA, Bennett CL, Eckert ML, Dobyns WB, Gleeson JG, et al. (2004) The NPHP1 gene deletion associated with juvenile nephronophthisis is present in a subset of individuals with Joubert syndrome. The American Journal of Human Genetics 75:82–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Otto EA, Helou J, Allen SJ, O'Toole JF, Wise EL, et al. (2008) Mutation analysis in nephronophthisis using a combined approach of homozygosity mapping, CEL I endonuclease cleavage, and direct sequencing. Hum Mutat 29:418–426. [DOI] [PubMed] [Google Scholar]
- 44. Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, et al. (2010) A method and server for predicting damaging missense mutations. Nature methods 7:248–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ng PC, Henikoff S (2001) Predicting deleterious amino acid substitutions. Genome Res 11:863–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Siepel A, Bejerano G, Pedersen JS, Hinrichs AS, Hou M, et al. (2005) Evolutionarily conserved elements in vertebrate, insect, worm, and yeast genomes. Genome Res 15:1034–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Shahzadi A, Riazuddin SA, Ali S, Li D, Khan SN, et al. (2010) Nonsense mutation in MERTK causes autosomal recessive retinitis pigmentosa in a consanguineous Pakistani family. Br J Ophthalmol 94:1094–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Prasad D, Rothlin CV, Burrola P, Burstyn-Cohen T, Lu Q, et al. (2006) TAM receptor function in the retinal pigment epithelium. Molecular and Cellular Neuroscience 33:96–108. [DOI] [PubMed] [Google Scholar]
- 49. Tibrewal N, Wu Y, D'mello V, Akakura R, George TC, et al. (2008) Autophosphorylation docking site tyr-867 in mer receptor tyrosine kinase allows for dissociation of multiple signaling pathways for phagocytosis of apoptotic cells and down-modulation of lipopolysaccharide-inducible NF-κB transcriptional activation. J Biol Chem 283:3618–3627. [DOI] [PubMed] [Google Scholar]
- 50. Thompson DA, McHenry CL, Li Y, Richards JE, Othman MI, et al. (2002) Retinal dystrophy due to paternal isodisomy for chromosome 1 or chromosome 2, with homoallelism for mutations in RPE65 or MERTK, respectively. The American Journal of Human Genetics 70:224–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tschernutter M, Jenkins S, Waseem N, Saihan Z, Holder G, et al. (2006) Clinical characterisation of a family with retinal dystrophy caused by mutation in the mertk gene. Br J Ophthalmol 90:718–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ebermann I, Walger M, Scholl HP, Issa PC, Lüke C, et al. (2007) Truncating mutation of the DFNB59 gene causes cochlear hearing impairment and central vestibular dysfunction. Hum Mutat 28:571–577. [DOI] [PubMed] [Google Scholar]
- 53. Issa PC, Bolz HJ, Ebermann I, Domeier E, Holz FG, et al. (2009) Characterisation of severe rod–cone dystrophy in a consanguineous family with a splice site mutation in the MERTK gene. Br J Ophthalmol 93:920–925. [DOI] [PubMed] [Google Scholar]
- 54. Yzer S, Leroy BP, De Baere E, de Ravel TJ, Zonneveld MN, et al. (2006) Microarray-based mutation detection and phenotypic characterization of patients with Leber Congenital Amaurosis. Invest Ophthalmol Vis Sci 47:1167–1176. [DOI] [PubMed] [Google Scholar]
- 55. Simonelli F, Ziviello C, Testa F, Rossi S, Fazzi E, et al. (2007) Clinical and molecular genetics of Leber's Congenital Amaurosis: A multicenter study of Italian patients. Invest Ophthalmol Vis Sci 48:4284–4290. [DOI] [PubMed] [Google Scholar]
- 56. Vallespin E, Cantalapiedra D, Riveiro-Alvarez R, Wilke R, Aguirre-Lamban J, et al. (2007) Mutation screening of 299 Spanish families with retinal dystrophies by Leber Congenital Amaurosis genotyping microarray. Invest Ophthalmol Vis Sci 48:5653–5661. [DOI] [PubMed] [Google Scholar]
- 57. Henderson RH, Waseem N, Searle R, van der Spuy J, Russell-Eggitt I, et al. (2007) An assessment of the apex microarray technology in genotyping patients with Leber Congenital Amaurosis and early-onset severe retinal dystrophy. Invest Ophthalmol Vis Sci 48:5684–5689. [DOI] [PubMed] [Google Scholar]
- 58. LaVail MM (1976) Rod outer segment disk shedding in rat retina: Relationship to cyclic lighting. Science 194:1071–1074. [DOI] [PubMed] [Google Scholar]
- 59. Nguyen KQ, Tsou WI, Calarese DA, Kimani SG, Singh S, et al. (2014) Overexpression of MERTK receptor tyrosine kinase in epithelial cancer cells drives efferocytosis in a gain-of-function capacity. J Biol Chem 289:25737–25749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Png KJ, Halberg N, Yoshida M, Tavazoie SF (2012) A microRNA regulon that mediates endothelial recruitment and metastasis by cancer cells. Nature 481:190–194. [DOI] [PubMed] [Google Scholar]
- 61. Lemke G (2013) Biology of the TAM receptors. Cold Spring Harb Perspect Biol 5:a009076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Dowling JE, Sidman RL (1962) Inherited retinal dystrophy in the rat. J Cell Biol 14:73–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Herron WL, Riegel BW, Myers OE, Rubin ML (1969) Retinal dystrophy in the rat—a pigment epithelial disease. Invest Ophthalmol 8:595–604. [PubMed] [Google Scholar]
- 64. Bok D, Hall MO (1971) The role of the pigment epithelium in the etiology of inherited retinal dystrophy in the rat. J Cell Biol 49:664–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Mullen RJ, LaVail MM (1976) Inherited retinal dystrophy: Primary defect in pigment epithelium determined with experimental rat chimeras. Science 192:799–801. [DOI] [PubMed] [Google Scholar]
- 66. Sather S, Kenyon KD, Lefkowitz JB, Liang X, Varnum BC, et al. (2007) A soluble form of the mer receptor tyrosine kinase inhibits macrophage clearance of apoptotic cells and platelet aggregation. Blood 109:1026–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Migdall-Wilson J, Bates C, Schlegel J, Brandão L, Linger RM, et al. (2012) Prolonged exposure to a mer ligand in leukemia: Gas6 favors expression of a partial mer glycoform and reveals a novel role for mer in the nucleus. PLOS one 7:e31635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Scott RS, McMahon EJ, Pop SM, Reap EA, Caricchio R, et al. (2001) Phagocytosis and clearance of apoptotic cells is mediated by MER. Nature 411:207–211. [DOI] [PubMed] [Google Scholar]
- 69. Knubel KH, Pernu BM, Sufit A, Nelson S, Pierce AM, et al. (2014) MerTK inhibition is a novel therapeutic approach for glioblastoma multiforme. Oncotarget 5:1338–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Rogers A, Le J, Sather S, Pernu B, Graham D, et al. (2011) Mer receptor tyrosine kinase inhibition impedes glioblastoma multiforme migration and alters cellular morphology. Oncogene 31:4171–4181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Fliegauf M, Horvath J, von Schnakenburg C, Olbrich H, Müller D, et al. (2006) Nephrocystin specifically localizes to the transition zone of renal and respiratory cilia and photoreceptor connecting cilia. Journal of the American Society of Nephrology 17:2424–2433. [DOI] [PubMed] [Google Scholar]
- 72. Hildebrandt F, Otto E, Rensing C, Nothwang HG, Vollmer M, et al. (1997) A novel gene encoding an SH3 domain protein is mutated in nephronophthisis type 1. Nat Genet 17:149–153. [DOI] [PubMed] [Google Scholar]
- 73. Hildebrandt F, Zhou W (2007) Nephronophthisis-associated ciliopathies. Journal of the American Society of Nephrology 18:1855–1871. [DOI] [PubMed] [Google Scholar]
- 74. Jiang S, Chiou Y, Wang E, Chien Y, Ho H, et al. (2009) Essential role of nephrocystin in photoreceptor intraflagellar transport in mouse. Hum Mol Genet 18:1566–1577. [DOI] [PubMed] [Google Scholar]
- 75. Tanaka-Matakatsu M, Thomas BJ, Du W (2007) Mutation of the Apc1 homologue shattered disrupts normal eye development by disrupting G1 cell cycle arrest and progression through mitosis. Dev Biol 309:222–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A pedigree from a related manuscript, Cooper et al., indicates clinically studied dogs [24] .
(TIF)
Pedigree indicates the dogs that were used in the GWAS study (marked yellow). Disease segregation suggests an autosomal recessive mode of inheritance.
(TIF)
Summary of the targeted resequencing data. The 6.1 Mb associated region was captured and resequenced in four cases and four control SVs with opposite risk haplotypes to identify the causative mutation.
(XLSX)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.