Abstract
Background
Although prevention of complications in diabetes requires careful control over many years, little is known about which patients persistently fail to get recommended care.
Objective
To determine the frequency and correlates of persistent long-term gaps in diabetes care.
Methods
Patient surveys and reviews of medical records were used to assess preventive care services for diabetes among 8392 patients who were continuously enrolled in 10 US managed care plans from 1999 to 2002. Demographic and socioeconomic characteristics, access to care, social support, and mental and physical health were determined by interview. Five preventive care services of diabetes care (testing of hemoglobin A1c, cholesterol, and albuminuria, dilated eye exams, and foot exams) were assessed by survey and chart abstraction for a 3-year period (1999–2002). We defined a “persistent lapse” as a participant’s missing a preventive care service for the entire 3 years.
Results
In all, 70% of patients had no persistent lapses, 22% had 1, 6% had 2, and 2% had ≥ 3. Persistent lapses occurred most often for lipid testing (11.6%), microalbuminuria testing (9.7%), and eye exams (9.0%), but less frequently for foot exams (6.9%) and A1c tests (4.2%). In multivariate analyses, the odds of a persistent lapse in care was 42% higher for young (age 18–44) than middle aged persons and 26% higher among lean than very obese persons. In addition, the odds of a persistent lapse was 26% higher for those of low income, 29% higher among employed persons, 18% higher for smokers, 27% higher in those with fewer than 5 years of diabetes than those with > 15 years, and 42% higher for persons with zero or 1 comorbid conditions (compared to ≥ 3). In addition, non-Hispanic blacks were particularly likely to miss lipid tests (15.3%) and those not taking medications were especially likely to miss foot exams (7.1%), A1c tests (10.6%), and proteinuria tests (10.8%). Sex, education, marital status, family demands, transportation, trust in physicians, and mental health were not associated with lapses in care.
Conclusions
Even in an insured cohort, 3 in 10 participants had 1 or more persistent lapses in diabetes care. Patients with lower income, younger age, having fewer co-morbidities, taking fewer medications and poor health behaviors are particularly vulnerable to persistent lapses in care and a group who warrant targeted interventions to improve preventive diabetes care.
Introduction
The growing burden of diabetes and its complications, combined with the availability of several promising interventions, has made its control a priority for health systems (1). Accordingly, various initiatives have been undertaken to improve the care of patients with diabetes, including close management of glycemic control and regular screening for complications (e.g., microalbuminuria) and cardiovascular disease risk factors, and use of disease management programs(1–4). Many earlier studies have documented inadequate diabetes care (5–7), but virtually all have been cross-sectional in design, representing at most a one-year period. As a result, little is known about whether patients who do not receive recommended services at one point in time are likely to receive them later on or to continue to miss these services over a more extended period. We refer to an extended period of not receiving services as a ‘persistent lapse’ in care. These persistent lapses represent missed opportunities to maintain good glycemic control and to improve profiles for cardiovascular risk, and they may put patients at particular risk for developing cardiovascular disease and other complications (8–10).
Suboptimal diabetes care has been attributed to barriers in health access, health systems, and variation in individual provider practices (11). However, considerable variation in measured receipt of preventive care services can stem from patient-level factors (5, 12, 13). Lower socioeconomic status may be associated with persistent lapses in care due to cost barriers or competing demands, or lack of education about preventive care. Psychological and mental status variables may indicate isolation and withdrawal from activities and could increase risk for lapses in care, or alternatively, could reduce lapses in care due to increased contact with the health system. Self-care behaviors may be markers of poor self-efficacy or inattention to preventive health. Finally, demographic factors such as age and sex may be markers of other unmeasured social factors that serve as practical ways of identifying people at risk. Identifying the key patient-level predictors of persistent lapses in receipt of preventive care services can help health care organizations aim interventions at persons who are most at risk. We report here an analysis of the patient demographic, socioeconomic, and health factors and other non-financial barriers to access to care associated with having persistent lapses in receipt of preventive services over a 3-year period among persons with diabetes enrolled in ten U.S. health plans.
Methods
Study Design and Sample
Translating Research Into Action for Diabetes (TRIAD) is a multicenter longitudinal study designed to systematically examine the factors that influence the processes and outcomes of diabetes care in managed care settings (14). The details of the study sample, design, and recruitment have been described previously. Briefly, 6 research centers around the United States (US) used administrative data to recruit community-dwelling persons aged ≥ 18 years with previously diagnosed diabetes from 10 health plans. Participants were considered eligible for these analyses if they had been continuously enrolled in the health plan for at least 3 years and submitted at least 1 claim in the first 18 months, and were not pregnant. Ninety percent of the people who were contacted and found eligible responded to the survey. A total of 11,928 persons participated in the baseline surveys (from July 2000 to October 2001), of whom 8,790 (73%) participated in follow-up surveys over a second consecutive 18 months later (between April 2002 and March 2003). Three hundred ninety-eight persons with probable type 1 diabetes were excluded, leaving an analytic sample of 8392. Of the 8392 in the analytic sample, 1838 (16%) were missing chart review data at wave 1, 2600 (31%) were missing chart review data at wave 2 (31%) and 1275 were missing chart review at both times (15.2%). To reduce bias associated with missing data, we conducted multiple imputation (methods described below), resulting in a complete analytic sample of 8,392 patients.
Independent Variables
The categories of independent variables included demographic and socioeconomic factors, non-financial barriers to access, physical and mental health variables, and health behaviors. Demographic and socioeconomic factors included age, sex, race/ethnicity, marital status, income, and education. Non-financial barriers included competing demands for time, as assessed by self-reported employment status and presence of small children or older adults requiring special care living in the same household. These variables, along with transportation barriers and trust in health care providers, were assessed using a standard questionnaire (15). Physical and mental health status variables included body mass index (BMI, kg/m2), duration of diabetes, diabetes medications, and presence of coronary heart disease (CHD). Duration was based on both self-report and chart abstraction. Medications, presence of CHD, and frequency of visits were abstracted from the medical chart. The Short Form-12 (SF12) was used to assess mental (MCS-12) and physical health (PCS-12) (16), and the 9-item Patient Health Questionnaire (PHQ) was employed to assess symptoms of depression (17). The Charlson comorbidity score was calculated using data abstracted from the medical record (18). To assess health behaviors, participants were asked about current smoking (defined as smoking “some days” or “every day” during the past year) and how many times per day per week and how many times per day they usually check their own blood glucose.
Outcome Variables
We used survey and medical record data to determine receipt of 5 preventive services over the 3-year analytic period: hemoglobin A1c (A1C) testing, lipid profile, urinary albumin, dilated eye exam, and foot exam. The determination of whether A1C, lipid tests, and testing for urinary albumin tests were received was based solely on abstraction of the chart, while the dilated eye exam and foot exam were considered to have been received if they were either self-reported or recorded in the medical record. The primary study outcome, defined more specifically below, was the probability of missing preventive services over the entire 3 years of the study.
Statistical Methods
To compare the prevalence of persistent lapses between different exposure groups, we used a GEE marginal logistic regression to model the probability of a lapse (19). Each patient provided 5 observations, one for each service. We examined both independent and exchangeable working correlation structures and found little difference between these two approaches. Results reported here are from models using an exchangeable structure. Within health plan structure was modeled using fixed effects. Service was included as a main effect and as part of an interaction with each of the independent variables. All independent variables were evaluated simultaneously in a single reduced multivariate model along with all possible interactions with type of preventive service. Significant (p < .05) interactions were retained for a final multivariate model that was used for hypothesis testing.
We used predicted margins from the model with all interactions to report an estimate of effect size and to examine the collective likelihood of persistently missing services. Predictive margins are a type of direct standardization in which predicted values from logistic regression models are averaged over the covariate distribution in the population (20). This statistic has the advantage of providing a measure of absolute difference rather than relative difference. We also computed an overall predicted margin, which refers to the adjusted probability (or predicted probability) of any single lapse in care, averaged over the 5 preventive care services.
Analyses were performed using the RLOGIST module in SAS callable SUDAAN (release 9.0.1, 2005, Research Triangle Institute, Research Triangle Park, NC). Multiple imputation (MI) was used to generate missing outcomes and demographic and socioeconomic covariates at baseline and follow-up visits (21). Imputed values were generated using IVEWARE (version 2.0) and the results here summarize the imputations (22). IVEware uses a sequence of multiple regressions to impute missing values using all of the observed values. The types of regressions depend upon the distribution of the imputed variable, with logistic regressions used for binary variables and generalized logit regressions used for categorical variables. Imputed missing values are used cyclically to update previously imputed values until stable predictions are achieved. We used 20 iterations, and appropriate restrictions and bounds on imputed values are incorporated. Estimates were pooled according to the formulas of Rubin and Schenker (23). The TRIAD study was reviewed and approved by Human Subjects Oversight Committees at each of the collaborating institutions and all patients gave informed consent for participation in surveys and for the access to their medical and administrative health information.
Results
The characteristics of the 8,392 TRIAD participants are shown in Table 1 The analytic sample was very similar to the TRIAD full sample with regard to demographic and health status characteristics, with the only notable difference that the analytic sample had a slightly lower proportion of persons with income < $20,000 (28% vs 35%) and less likely to be using insulin by itself. Participants missing chart review data at both times tended to be less likely to have more than a high school education among those with chart review data at both times), and more likely to be non-Hispanic black and Asian than those with complete chart review data.
TABLE 1.
Sociodemographic and Clinical Characteristics of TRIAD Analytic Sample and the Full TRIAD Sample
| TRIAD Analytic Sample (n=8392) | TRIAD Full Sample (n=11927) | TRIAD sample with medical record review at both time periods | Participants with no chart review data | |
|---|---|---|---|---|
|
| ||||
| Demographic characteristics | Mean or % | Mean or % | Mean or % | Mean or % |
| Age (mean) | 61.0 | 60.4 | 60.0 | 58.8 |
| Age group (years): | ||||
| 18–44 (%) | 9.9 | 12.4 | 12.1 | 15.1 |
| 45–54 (%) | 22.9 | 22.2 | 23.0 | 24.0 |
| 55–64 (%) | 28.0 | 26.5 | 28.0 | 27.6 |
| 65–74 (%) | 26.7 | 25.7 | 25.3 | 21.6 |
| ≥75 (%) | 12.6 | 13.1 | 11.6 | 11.6 |
| Female (%) | 53.1 | 53.3 | 52.5 | 53.1 |
| Education: | ||||
| < high school | 22.0 | 24.3 | 18.8 | 23.2 |
| High school graduate | 30.0 | 29.7 | 29.2 | 32.2 |
| Some college | 48.4 | 46.0 | 52.0 | 44.6 |
| Income < $20,000 (%) | 27.8 | 34.7 | ||
| Race/ethnicity: | ||||
| Hispanic | 15.9 | 16.0 | 13.6 | |
| Black non-Hispanic | 15.7 | 17.0 | 14.5 | |
| White non-Hispanic | 41.2 | 39.9 | 40.4 | |
| Asian/Pacific Islander | 18.2 | 18.1 | 11.2 | |
| Other | 9.0 | 9.1 | 8.3 | |
| Smoker (%) | 16.8 | 16.8 | 17.3 | |
| Duration of diabetes(years) | ||||
| <5 | 29.8 | 27.8 | 29.8 | |
| 5 – 14 | 45.5 | 44.1 | 43.1 | |
| ≥ 15 | 24.7 | 28.1 | 27.1 | |
| Diabetes medication: insulin only (%) | 14.7 | 18.1 | 8.5 | |
| Insulin + oral meds (%) | 12.1 | 11.8 | 11.9 | |
| Oral meds only (%) | 65.1 | 62.5 | 45.2 | |
| No medications (%) | 8.1 | 7.6 | 8.5 | |
| Coronary heart disease (%) | 17.0 | 17.8 | ||
| Fair or poor self-rated health (%) | 36.1 | 38.0 | 36.3 | |
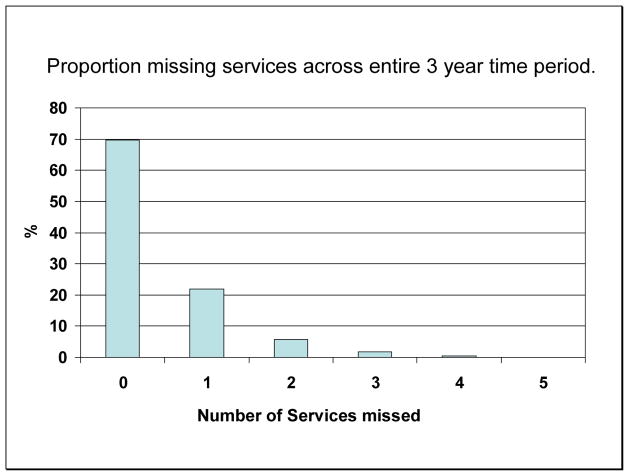
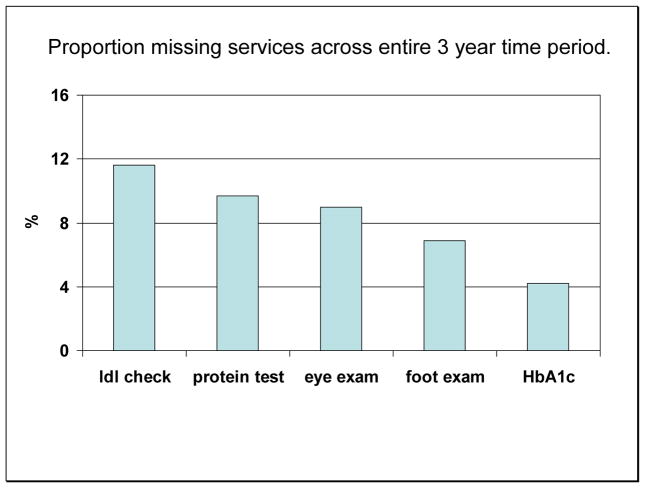
Seventy percent of patients had no persistent lapses over the 3 year period. However, 22% had a persistent lapse of 1 of the five services; 6% had 2; and the remaining 2% had 3 or more (Figure 1). The services most likely to be persistently missed were lipid panel (11.6%), urinary albumin tests (9.7%), and eye exams (9.0%), while the 2 services least likely to be missed persistently were foot exams (6.9%) and A1C tests (4.2%) (Figure 2).
Figure 1.
Proportion of participants missing preventive care services* over a 3 year period, according to number of services missed.
*Preventive care services evaluated include Hemoglobin A1c (A1C) testing, lipid profile, urinary albumin, dilated eye exam, and foot exam.
Figure 2.
Proportion of participants missing each preventive care services over a 3 year period.
The odds of an overall persistent lapses in care was 42% higher for young (age 18–44) than middle aged persons (age 55 to 64) and 26% higher for lean than very obese persons, 26% higher for those of low income. In addition persistent lapses in care were 29% more likely among employed persons than retired or unemployed persons, 18% higher for smokers, 27% higher in those with fewer than 5 years of diabetes than those with > 15 years, and 42% higher for persons with zero or 1 comorbid conditions (9.5%) (compared to ≥ 3, 7.2%). Lapses were also 14% higher for those who report less self blood glucose monitoring (Table 2).
Table 2.
Multivariate associations between demographic, socioeconomic, psychological/mental health status, social support, and health-related characteristics and persistent lapses in care.
| Odds Ratio | P value | |
|---|---|---|
| Demographic characteristics | ||
| Age group young (18–44) vs middle (55–64) | 1.42 (1.21 – 1.68) | 0.0001 |
| BMI group (kg/m2) <25 vs very obese | 1.26 (1.08 – 1.47) | 0.003 |
| Female sex | 0.98 (0.88 – 1.09) | 0.72 |
| Race/ethnicity: Hispanic | varied according to service | |
| Socioeconomic Factors | ||
| Competing family demands | 1.09 (0–.87 – 1.38) | 0.44 |
| Marital status (no vs yes) | 1.06 (0.93 – 1.91) | 0.38 |
| No Transportation (vs yes) | 1.04 (0.87 – 1.24) | 0.64 |
| Employed (vs retired) | 1.29 (1.13 – 1.46) | 0.007 |
| Trust Doctor (much vs little) | 0.93 (0.79 – 1.10) | 0.33 |
| Education Level (Low vs high) | 1.01 (0.83 – 1.23) | 0.92 |
| Annual income (low vs high) | 1.26 (1.00 – 1.56) | 0.02 |
| Health-related characteristics | ||
| Depression (vs no) | 1.12 (0.79 – 1.33) | 0.27 |
| Smoker (vs no) | 1.18 (1.04 – 0.96) | 0.03 |
| Duration of diabetes (yrs):< 5 vs > 15 | 1.27 (1.10 – 1.46) | 0.03 |
| Medications | varied according to service | |
| Daily SBGM No | 1.14 (1.02 – 1.27) | 0.03 |
| Coronary heart disease: No | 1.11 (0.908 – 1.27) | 0.14 |
| PCS-12 by quartile: (low vs high) | 1.03 (0.87 – 1.23) | 0.72 |
| MCS-12 by quartile: (low vs high) | 1.12 (0.97 – 1.29) | 0.11 |
| Charlson index: 0–1 vs > 3 | 1.42 (1.25 – 1.60) | 0.0001 |
significant (p<0.05) variables listed in bold. Note: we noted significant interactions for medications and race/ethnicity according to type of service; thus we present data for those analyses stratified according to service (Table 3).
Lapses in care were also significantly associated with race/ethnicity and type of treatment, but the nature of these associations varied by specific service as indicated by statistically significant interactions with service. For example, non-Hispanic blacks were more likely to miss lipid tests (15%), non-Hispanic blacks and Asian-Pacific Islanders were more likely to miss tests of proteinuria (10.2% and 10.8%, respectively), and non-Hispanic whites were more likely to miss eye exams (11.1%) (Table 3). Associations with overall lapses in care (i.e., overall predicted margins) tended to be driven by variation in eye exams, lipid tests, and proteinuria tests. Lapses in eye exams were most notable among young adults (13%), persons of low income (11%), recently diagnosed persons (10.3%), and those with few comorbid conditions (9.5%) (Table 3). In addition to being missed frequently among non-Hispanic blacks (15.3%), lipid tests were more frequently missed by patients who use only insulin (14.3%), and by the youngest and oldest adults (13%), and persons of low income (13%). Proteinuria tests were missed most frequently by the young (12.4%), lean patients (12.7%), and those with few comorbid conditions (12.6%). Foot exams and A1c tests were most frequently missed by those on no diabetes medication.
Table 3.
Predicted probability of persistent lapses in care for significant correlates, according to service.
| Proportion Missing Each Service | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Overall Predicted Margin* | Eye exams | Foot Exams | A1c Tests | Lipid tests | Proteinuria tests | |
| Demographic characteristics | ||||||
|
| ||||||
| Age group (years): | ||||||
| 18–44 | 10.1 | 13.4 | 7.3 | 4.5 | 13.3 | 12.4 |
| 45 – 54 | 8.7 | 10.7 | 7.3 | 4.8 | 11.3 | 9.5 |
| 55 – 64 | 7.5 | 9.0 | 6.0 | 4.0 | 10.3 | 8.5 |
| 65 – 74 | 7.7 | 6.5 | 6.3 | 3.9 | 11.7 | 9.7 |
| ≥ 75 | 8.7 | 7.2 | 8.8 | 4.0 | 13.1 | 10.5 |
|
| ||||||
| BMI (kg/m2): | ||||||
| < 25 | 9.0 | 8.7 | 8.1 | 4.3 | 11.3 | 12.7 |
| 25 to 29.9 | 8.9 | 9.8 | 7.0 | 4.6 | 12.6 | 10.9 |
| 30 to 34.9 | 8.0 | 8.6 | 7.2 | 4.0 | 11.1 | 9.2 |
| ≥ 35 | 7.5 | 8.8 | 5.6 | 4.1 | 11.0 | 7.4 |
|
| ||||||
| Smoker: | ||||||
| No | 8.1 | 8.7 | 6.6 | 4.0 | 11.5 | 9.3 |
| Yes | 9.2 | 10.0 | 8.0 | 5.3 | 11.8 | 11.5 |
|
| ||||||
| Income | ||||||
| < 15k/year | 9.1 | 11.0 | 7.0 | 5.5 | 12.5 | 9.9 |
| 15 to 44k/year | 8.3 | 9.4 | 7.0 | 4.2 | 11.0 | 9.6 |
| 45 to 74k/year | 7.8 | 7.7 | 6.8 | 3.8 | 10.9 | 9.8 |
| > 75k/year | 7.5 | 6.7 | 6.5 | 3.4 | 11.6 | 9.5 |
|
| ||||||
| Race/ethnicity | ||||||
| Hispanic | ------- | 7.2 | 6.0 | 3.5 | 10.7 | 9.0 |
| Black non-Hispanic | ------- | 7.7 | 5.0 | 5.9 | 15.3 | 10.2 |
| White non-Hispanic | ------- | 11.1 | 7.8 | 4.4 | 10.6 | 8.3 |
| Asian/Pacific Islander | ------- | 7.5 | 6.4 | 2.9 | 8.6 | 10.8 |
| Other | ------- | 10.0 | 8.7 | 3.9 | 12.6 | 9.9 |
|
| ||||||
| Employment status: | ||||||
| Employed | 9.1 | 9.9 | 7.8 | 5.1 | 12.5 | 10.3 |
| Not employed | 8.3 | 8.3 | 5.4 | 4.5 | 12.3 | 9.7 |
| Retired | 7.3 | 8.1 | 6.0 | 3.1 | 10.2 | 8.8 |
|
| ||||||
| Duration of diabetes (yrs): | ||||||
| < 5 | 9.0 | 10.3 | 7.8 | 3.9 | 11.9 | 10.7 |
| 5 – 9 | 8.2 | 9.3 | 6.5 | 4.3 | 11.6 | 9.4 |
| 10–14 | 8.4 | 9.2 | 7.5 | 4.0 | 11.8 | 9.7 |
| ≥ 15 | 7.3 | 6.6 | 5.2 | 5.0 | 10.9 | 8.6 |
|
| ||||||
| Medications: | ||||||
| None (%) | ------- | 9.8 | 10.1 | 7.1 | 10.6 | 10.8 |
| Oral meds only (%) | ------- | 9.3 | 6.8 | 4.3 | 11.1 | 10.0 |
| Insulin only (%) | ------- | 8.4 | 5.2 | 4.4 | 14.3 | 8.7 |
| Insulin + oral meds (%) | ------- | 7.5 | 6.0 | 2.1 | 10.8 | 8.0 |
|
| ||||||
| Daily SBGM | ||||||
| No | 8.6 | 9.7 | 7.5 | 4.2 | 12.1 | 9.8 |
| Yes | 7.8 | 8.1 | 5.9 | 4.3 | 8.7 | 9.0 |
|
| ||||||
| Charlson index: | ||||||
| 0–1 | 9.5 | 10.4 | 8.0 | 4.5 | 12.2 | 12.6 |
| 2 | 7.8 | 8.5 | 6.7 | 4.2 | 11.0 | 8.5 |
| ≥ 3 | 7.2 | 7.8 | 5.4 | 3.9 | 11.3 | 7.1 |
Overall predicted margin refers to the adjusted probability (or predicted probability) of a any single lapse in care, averaged over the 5 preventive care services.
Number of visits per year was a significant predictor of lapses, as those with more fewer than 8 visits over the 3 years had a 63% greater odds of a persistent lapse (9%) than those with many (> 18 visits over 3 years). However, inclusion of number of visits in the model had essentially no impact on the correlates of lapses or the magnitude of their association. Sex, competing family demands, marital status, transportation limitations, education, trust in the physician, depression, history of CHD, and physical and mental health scores were not associated with lapses in care after adjusting for all other factors.
Discussion
In this first study of persistent lapses in care, we found that, among a large diabetic cohort from ten health plans, nearly one-third of patients had at least 1 persistent lapse. Lapses were more common for lipid panel testing, urinary albumin tests, and eye exams than for HbA1c tests and foot exams. Equally important, persistent lapses were more frequent among patients who were younger, healthier, and relied on diet only to control their diabetes. They were also more frequent among patients who were lower income and had engaged in fewer preventive health behaviors.
Two design features of this study make it particularly novel. To our knowledge, it is the first study to examine extended lapses in receipt of preventive care services over a multi-year time frame. In addition, the availability of both patient surveys and chart abstractions for patients from multiple health plans permitted the evaluation of the impact of a more comprehensive set of patient factors—including issues such as availability of transportation and trust in the physician—on receipt of preventive care services than has been performed previously.
Our findings extend prior literature by showing that some of the patient-level characteristics that predict those who miss services at a single time point—being younger, healthier, having lower income, having less favorable health behaviors, or not taking insulin (5, 12)—also predict persistent lapses. Similarly, these findings also extend prior findings from TRIAD showing that younger age is associated with worse control of cardiovascular disease risk factors and that, with the exception of African Americans being less likely to have lipid levels tested and well managed, race/ethnicity is only modestly associated with lapses in care in managed care settings (23–25). Healthier patients may receive fewer preventive services because there is less need for regular changes in medication and medication refills. Persons taking no medications or insulin alone may be perceived as having milder disease and thus given less diligent care. One of the most consistent correlates of persistent lapses, however, was younger age. This may be due to a perception or judgment on the part of both clinicians and patients that these services are not needed.
Some of our hypothesized psychosocial factors, including competing demands for time, mental health, and economic barriers, did not emerge as important predictors of persistent gaps in care. This may be because we examined an insured population, as some previous analyses in broader populations has suggested [xxxxxxx]. Alternatively, this may be because we used a composite outcome (i.e, overall gaps in care), over an extended period of time. Prior TRIAD analyses found female sex to be associated with inferior CVD related processes of care and depression associated with frequency of recommended care processes, but each analysis focused on care at one point in time. Thus, our analyses raise the possibility that the patterns of correlated of long-term gaps in care differ somewhat from the patterns of traditionally assessed gaps.
On the surface, less-frequent delivery of services to younger, healthier persons may seem adaptive and better than the converse situation (i.e., sicker people receiving less care). However, the services we studied are universally accepted and disseminated standards of care that prevent long-term complications. Where debate exists about the value of the processes of care, that debate exists for older adults rather than younger adults. The greatest relative increases in diabetes prevalence are occurring among young adults and many of the most powerful long-term benefits of early initiation of care and diligent diabetes management have been observed in younger, healthier populations (26). Good glycemic control, maintenance of appropriate blood pressure, lipid management, eye care, and screening and treatment for micoalbuminuria have all been shown to be particularly cost-effective among younger, healthier persons (27, 27–30). Similarly, the observation that lean people are more likely to miss tests of proteinuria and foot exams is also concerning give that risk of amputations and renal disease are as great for lean diabetic persons as their obese counterparts. Thus, although it is perhaps understandable why these patients had persistent lapses, it is a particularly unfortunate example of clinicians or patients making short-term decisions that have big long-term implications. Patients with lower income may be more likely to miss services because they wish to avoid the costs associated with care (e.g., office co-pays, transportation costs). This pattern—lower adherence to treatment plans among lower income groups—has been documented with medication adherence in diabetes (31).
There were some limitations to our study. Nineteen percent were the medical record review data at both time points, mainly due to participant refusal at the time of survey. This presents some potential bias because several of the predictors, preventive care services, and measurement of frequency of doctor visits depended on the medical record review. Although there were not major differences according to presence of medical record data, there was a tendency for persons missing data to be Asian/Pacific Islander of non-hispanic Black and less likely to have advanced education. We attempted to reduce bias associated with these factors by using multiple imputation. In sensitivity analyses we found no substantial differences in study conclusions between the imputed analysis and the complete case analysis.
Our imputed analyses were unable to assess the potential role of visit frequency as an explanation for missing services. Our complete case analysis indicated that inclusion of visit frequency in the models did not substantively change the magnitude or nature of associations. However, since visit frequency depended on the medical record review, we were unable to assess the degree to which visit frequency was correlated with missing data. Thus, we were not able to fully assess the potential role of visit frequency as an explanation for missing service and an important remaining question is whether persistent gaps in care is more due to reduced access to doctor visits as opposed to a different pattern of services given to the patient after they are in the office.
Our analyses were restricted to insured populations with at least minimal contact with the health care system, as all patients in our analyses were continuously enrolled in the same plan for 3 consecutive years and by inclusion criteria had at least one visit. Thus, the prevalence and correlates of persistent lapses in care may be different in samples that include uninsured patients. Measurements of processes of care are subject to reporting error, whether collected by self-report or medical record review. Our analyses also did not examine system-level factors, disease management approaches or the relative impact of provider practices and perceptions on persistent lapses. Further research should examine how variation in system and physician factors interact with patient factors to lead to persistent lapses in care. Ultimately, these analyses should stimulate more comprehensive examination of the implications of variation in care. In addition, there may have been changes in the prevalence of persistent lapses during the 5 years since these surveys were conducted. National survey data suggests that quality of care has continued to improve at a modest pace; however, these analyses highlight the importance of also evaluating trends in the proportion who persistently fail to get services. Finally, despite the obvious importance of the preventive care services studied, the implications of long-term lapses in care on health outcomes have not been specifically quantified. The recent increases in diabetes prevalence among young adults raises new questions about the impact of long-term patterns of care. Thus, these analyses should stimulate new studies into the implications of persistent lapses in care as well as the impact of interventions targeting susceptible patients.
Conclusions
In our study group, we found that persistent lapses in the receipt of diabetes preventive care services happen to almost one-third of patients with diabetes. Persistent lapses were especially common among younger, healthier patients and those with limited income. Preventing a considerable portion of the risk of complications in these patients is possible, yields particularly large clinical benefits, and should be a focus of clinicians and health plans. Further research into the extent of persistent lapses in other populations and into the implications of persistent lapses for long-term outcomes is needed.
Reference List
- 1.Engelgau MM, Geiss LS, Saaddine JB, et al. The evolving diabetes burden in the United States. Ann Intern Med. 2004;140(11):945–950. doi: 10.7326/0003-4819-140-11-200406010-00035. [DOI] [PubMed] [Google Scholar]
- 2.Norris SL, Nichols PJ, Caspersen CJ, et al. The effectiveness of disease and case management for people with diabetes. A systematic review. Am J Prev Med. 2002;22(4 Suppl):15–38. doi: 10.1016/s0749-3797(02)00423-3. [DOI] [PubMed] [Google Scholar]
- 3.Fleming BB, Greenfield S, Engelgau MM, et al. The Diabetes Quality Improvement Project: moving science into health policy to gain an edge on the diabetes epidemic. Diabetes Care. 2001;24(10):1815–1820. doi: 10.2337/diacare.24.10.1815. [DOI] [PubMed] [Google Scholar]
- 4.Acton KJ, Shields R, Rith-Najarian S, et al. Applying the diabetes quality improvement project indicators in the Indian Health Service primary care setting. Diabetes Care. 2001;24(1):22–26. doi: 10.2337/diacare.24.1.22. [DOI] [PubMed] [Google Scholar]
- 5.Saaddine JB, Engelgau MM, Beckles GL, et al. A diabetes report card for the United States: quality of care in the 1990s. Ann Intern Med. 2002;136(8):565–574. doi: 10.7326/0003-4819-136-8-200204160-00005. [DOI] [PubMed] [Google Scholar]
- 6.Selby JV, Ray GT, Zhang D, et al. Excess costs of medical care for patients with diabetes in a managed care population. Diabetes Care. 1997;20(9):1396–1402. doi: 10.2337/diacare.20.9.1396. [DOI] [PubMed] [Google Scholar]
- 7.Preventive-care practices among persons with diabetes--United States, 1995 and 2001. MMWR Morb Mortal Wkly Rep. 2002;51(43):965–969. [PubMed] [Google Scholar]
- 8.Effect of intensive diabetes management on macrovascular events and risk factors in the Diabetes Control and Complications Trial. Am J Cardiol. 1995;75(14):894–903. doi: 10.1016/s0002-9149(99)80683-3. [DOI] [PubMed] [Google Scholar]
- 9.Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. BMJ. 1998;317(7160):703–713. [PMC free article] [PubMed] [Google Scholar]
- 10.Sustained effect of intensive treatment of type 1 diabetes mellitus on development and progression of diabetic nephropathy: the Epidemiology of Diabetes Interventions and Complications (EDIC) study. JAMA. 2003;290(16):2159–2167. doi: 10.1001/jama.290.16.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krein SL, Hofer TP, Kerr EA, et al. Whom should we profile? Examining diabetes care practice variation among primary care providers, provider groups, and health care facilities. Health Serv Res. 2002;37(5):1159–1180. doi: 10.1111/1475-6773.01102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gregg EW, Geiss LS, Saaddine J, et al. Use of diabetes preventive care and complications risk in two African-American communities. Am J Prev Med. 2001;21(3):197–202. doi: 10.1016/s0749-3797(01)00351-8. [DOI] [PubMed] [Google Scholar]
- 13.Saaddine JB, Cadwell B, Gregg EW, et al. Improvements in diabetes processes of care and intermediate outcomes: United States, 1988–2002. Ann Intern Med. 2006;144(7):465–474. doi: 10.7326/0003-4819-144-7-200604040-00005. [DOI] [PubMed] [Google Scholar]
- 14.The Translating Research Into Action for Diabetes (TRIAD) study: a multicenter study of diabetes in managed care. Diabetes Care. 2002;25(2):386–389. doi: 10.2337/diacare.25.2.386. [DOI] [PubMed] [Google Scholar]
- 15.Safran DG, Kosinski M, Tarlov AR, et al. The Primary Care Assessment Survey: tests of data quality and measurement performance. Med Care. 1998;36(5):728–739. doi: 10.1097/00005650-199805000-00012. [DOI] [PubMed] [Google Scholar]
- 16.Ware J, Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 17.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 19.Zeger SL, LKAPS . Models for Longitudinal Data: A Gernerlized Estimating Equation Approach. International Biometric Society; 1988. [PubMed] [Google Scholar]
- 20.Graubard BI, Korn EL. Predictive margins with survey data. Biometrics. 1999;55(2):652–659. doi: 10.1111/j.0006-341x.1999.00652.x. [DOI] [PubMed] [Google Scholar]
- 21.Schafer JL. Multiple imputation: a primer. Stat Methods Med Res. 1999;8(1):3–15. doi: 10.1177/096228029900800102. [DOI] [PubMed] [Google Scholar]
- 22.Imputation and variance estimation software. [computer program] Ann Arbor, MI: Institute for Social Research, University of Michigan; 1998. [Google Scholar]
- 23.Rubin DB, Schenker N. Multiple imputation in health-care databases: an overview and some applications. Stat Med. 1991;10(4):585–598. doi: 10.1002/sim.4780100410. [DOI] [PubMed] [Google Scholar]
- 24.Brown AF, Ettner SL, Piette J, et al. Socioeconomic Position and Health among Persons with Diabetes Mellitus: A Conceptual Framework and Review of the Literature. Epidemiol Rev. 2004;26(1):63–77. doi: 10.1093/epirev/mxh002. [DOI] [PubMed] [Google Scholar]
- 25.Selby JV, Swain BE, Gerzoff RB, et al. Understanding the gap between good processes of diabetes care and poor intermediate outcomes: Translating Research into Action for Diabetes (TRIAD) Med Care. 2007;45(12):1144–1153. doi: 10.1097/MLR.0b013e3181468e79. [DOI] [PubMed] [Google Scholar]
- 26.Chaturvedi N, Stephenson JM, Fuller JH. The relationship between smoking and microvascular complications in the EURODIAB IDDM Complications Study. Diabetes Care. 1995;18(6):785–792. doi: 10.2337/diacare.18.6.785. [DOI] [PubMed] [Google Scholar]
- 27.Cost-effectiveness of intensive glycemic control, intensified hypertension control, and serum cholesterol level reduction for type 2 diabetes. JAMA. 2002;287(19):2542–2551. doi: 10.1001/jama.287.19.2542. [DOI] [PubMed] [Google Scholar]
- 28.Zhang P, Engelgau MM, Norris SL, et al. Application of economic analysis to diabetes and diabetes care. Ann Intern Med. 2004;140(11):972–977. doi: 10.7326/0003-4819-140-11-200406010-00039. [DOI] [PubMed] [Google Scholar]
- 29.Polak BC, Crijns H, Casparie AF, et al. Cost-effectiveness of glycemic control and ophthalmological care in diabetic retinopathy. Health Policy. 2003;64(1):89–97. doi: 10.1016/s0168-8510(02)00143-4. [DOI] [PubMed] [Google Scholar]
- 30.Vijan S, Hofer TP, Hayward RA. Cost-utility analysis of screening intervals for diabetic retinopathy in patients with type 2 diabetes mellitus. JAMA. 2000;283(7):889–896. doi: 10.1001/jama.283.7.889. [DOI] [PubMed] [Google Scholar]
- 31.Tseng CW, Tierney EF, Gerzoff RB, et al. Race/ethnicity and economic differences in cost-related medication underuse among insured adults with diabetes: the Translating Research Into Action for Diabetes Study. Diabetes Care. 2008;31(2):261–266. doi: 10.2337/dc07-1341. [DOI] [PubMed] [Google Scholar]