Abstract
Uterine estrogenic actions are biphasic, early (phase I) and late (phase II) responses. However, the molecular linkage between these phases is not known. Although certain phase I responses are considered estrogen receptor (ER)α and ERβ independent, the phase II responses are ERα dependent. We previously observed that among several genes Bip is induced by estrogen in the mouse uterus in an ER-independent manner as a phase I response. Bip is a member of the chaperone family and plays roles in protein processing and confers cellular protection. However, its role in estrogen-dependent uterine biology is unknown. We show here a new function of Bip in regulating estrogen signaling in the uterus. Bip, induced during the phase I responses, molecularly interacts with ERα required for estrogen-mediated phase II growth responses. Utilizing in vivo and in vitro model systems, we found that adenovirus-driven suppression of Bip antagonizes ERα-mediated uterine gene transcription. Importantly, down-regulation of Bip compromises estrogen-dependent phase II growth responses with sustained phase I responses. In conclusion, Bip is critical for coordinating estrogen-elicited biphasic responses and serves as a molecular link between ERα-indepen-dent and -dependent estrogenic responses in the uterus.
Estrogens regulate various functions in diverse biological processes, including reproductive and developmental aspects, cardiovascular functions, and bone metabolism (1). Estrogen signaling is considered to be primarily regulated by nuclear estrogen receptors-α and -β (ERα and ERβ), which are ligand-in-ducible transcription factors (2). In the mouse uterus, estrogenic effects are biphasic: early (phase I) and late (phase II) responses. Phase I responses that occur within 6 h are characterized by water imbibition, macromolecular uptake, and alteration in genes involved in vascular permeability, whereas the phase II responses occur between 18–30 h and are defined by increased epithelial cell proliferation (3). Female mice missing ERα show negligible classical responses to estrogen for the late growth phase (1). However, we and others have previously shown that ERα null mice or wild-type mice in which ER functions are silenced by an ER antagonist ICI 182,780 manifest early expression of certain genes in the uterus in response to estrogen, suggesting alternative estrogen-signaling pathway (4–7). In brief, accumulating evidence suggests that estrogen regulates diverse but interdependent signaling pathways in uterine biology via ER-dependent and -independent manners.
The classical mechanism of estrogen action is that estrogen first interacts with ER and then the ligand-bound ER complex homodimerizes before its binding to cis-acting estrogen response elements (EREs) in the target genes. The EREs for transcription activators are usually present in the 5’-flanking region of specific genes (8). A palindromic consensus sequence, AGGT-CA(nnn)TGACCT, fulfills the requirement of an ERE (9). However, most EREs in vivo are imperfect palindromes and provide less than maximal transcriptional regulation (8). In addition, evidence indicates that ERs can homodimerize on direct repeats of ERE half-sites and produce a transcriptional efficiency about equal to that of perfect palindromes (10). There are also estrogen-responsive genes that contain no recognizable ERE (11). Thus, the contention that estrogens function only by interacting with nuclear receptors to serve as transcription activators for specific DNA response elements is no longer tenable. Some of the complexities in regulating gene expression by estrogens, including many of the protein-protein interactions mediated via ER and coregulators, have been described previously (12). It is also known that signals generated by interactions between effectors and receptors can converge and produce effects on target genes, presumably by modulating receptor function at the chromatin level. Furthermore, emerging evidence suggests that estrogens exert nongenomic effects in target tissues. These effects involve rapid (<30min) activation of intracellular signaling pathways; a similarity of these effects to those induced by growth factors supports the contention that a putative membrane ER(s) is involved. Although controversial, evidence suggests that non-genomic signaling of estrogen is mediated by a novel form of ER after its translocation to the specialized structure in the plasma membrane or by a receptor identified as G protein-coupled receptor GPR30 (13–15). The physiological significance of these receptors in uterine biology remains to be seen.
A general consensus is that ligands elicit a conformational change leading to activation of steroid receptors. However, prior interaction of selective heat shock proteins (HSPs) to newly synthesized steroid receptors is considered a crucial step for the receptor protein processing and for directing new receptor conformation for ligand recognition functional activation (16). Previous studies have shown that expression of Bip (also known as grp78 encoded by Hspa5), a member of the heat shock protein (HSP)70 chaperone family, is induced by estrogenic compounds in a nonconventional manner in the mouse uterus (4). It is speculated that protein processing is a downstream signaling pathway regulated by estrogen in the mouse uterus. Bip is a resident protein in the endoplasmic reticulum (ER) where folding and assembly of newly synthesized peptide chains occur during the processes of secretion and translocation of proteins (17, 18). Bip is capable of binding with newly synthesized peptide, but not the native protein, with an associated ATPase activity, and plays a major role in the above processes occurring in the ER (17). Remarkably, expression of Bip occurs under variety of conditions, including oxidative stress, chemical toxicity, treatment with Ca2+ ionophores, and inhibitors of glycosylation that all modulate function of the ER (18). Studies have shown that Bip is an abundant protein under growth-regulatory conditions (19). Overexpression of Bip is associated with the protection against cell death caused by the disturbance of the ER homeostasis or cytotoxic drug-induced apoptosis (20).
We hypothesized that uterine regulation of Bip represents one of the early molecular events that is necessary to prepare the uterus for multiple physiological responses of estrogen. In this respect, one of the functions for Bip could be to process newly synthesized ERα for its competency to respond to estrogen in regulating uterine biology. Using multiple approaches, we show here that estrogen-dependent rapid regulation of uterine Bip molecularly interacts with ERα, and this interaction is critical to late uterine growth responses.
RESULTS
Differential Up-Regulation of Bip and ERα, and Their Molecular Association by Estrogen in the Mouse Uterus
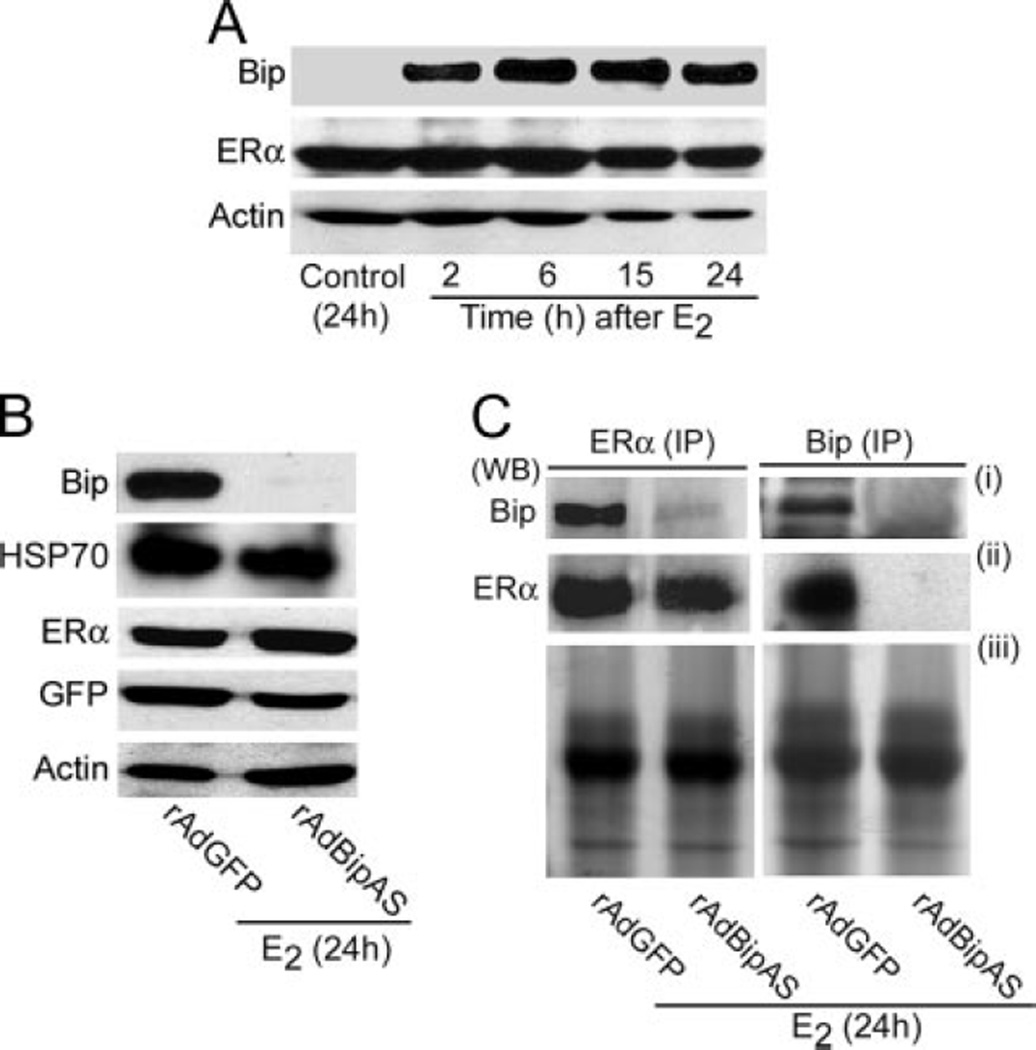
Our previous studies showed that estrogen regulates expression of several early responsive genes without involving ER in uteri of both wild-type and ERα(−/−) mice (4). One of those genes, Bip, is rapidly up-regulated after estrogen stimulation, suggesting estrogen’s influence in regulating protein processing in an early regulatory event in the uterus. This observation led us to examine first whether this gene expression is reflected at the protein level. Adult ovariectomized wild-type mice were given a single injection of 17β-estra-diol (E2), and uterine cell extracts were analyzed by Western blotting at indicated times. As shown in Fig. 1A, our results show that the levels of Bip are dramatically up-regulated by E2 at 6 and 24 h. Because Bip’s role is primarily mediated through protein-protein interaction, we initially hypothesized that estrogenic control of uterine Bip involves the regulation of ERα function through a molecular interaction. Indeed, we provide evidence that there is an interaction between Bip and ERα under the direction of E2 in the wild-type uterus. Uterine tissue extracts were subjected to coimmunoprecipitation analysis using either Bip- (Fig. 1, B and C) or ERα- (data not shown) specific antibody. Immunoprecipitates were analyzed by Western blotting to detect associated protein complex between Bip and ERα. Consistent with the above results, we further observed that E2 up-regulates immunoprecipitable Bip within 0.5 h, and the level is maintained through 24 h after an E2 injection (Fig. 1B). Furthermore, our results show that Bip is physically associated with ERα ba-sally at 0 h after an E2 injection. However, whereas this complex becomes undetectable at 0.5 and 2 h (Fig. 1C), its formation is again seen at 6, 12, and 24 h after E2 injection (Fig. 1C). Similar results were obtained when immunoprecipitation was performed with ERα antibody and analyzed by Western blotting for Bip and ERα. The interaction between these two proteins appears to be specific, because Bip-specific antibody was unable to pull down PR, an estrogen-responsive gene in the mouse uterus (21). Additionally, immunoprecipitation using preimmune serum did not detect any specific bands for Bip and ERα by Western blotting (data not shown). The absence of Bip-ERα-asso-ciated complex at early time points (0.5 and 2 h) is interesting, because levels of Bip persisted during these time periods. Moreover, our results of Western blotting of the whole uterine tissue extracts show that the presence of ERα in this tissue during these time periods is not a limiting factor for its interaction with Bip (Fig. 1D). Overall, these results suggest that rapid up-regulation of Bip forms a specific complex with ERα under the direction of estrogen in a timely fashion, in particular, at the end of the phase I response of estrogen in the uterus. To our knowledge, this is the first evidence for such an interaction between these two molecules in any biological system.
Fig. 1. Analysis of Estrogen-Dependent Regulation of Bip and ERα Proteins in Uteri of Wild-Type Mice.
Adult ovariectomized wild-type mice were given a single injection (sc) of E2 (100 ng/mouse) and killed at indicated times. A, Uterine tissue extracts were analyzed by Western blotting using the primary antibodies for Bip and actin. B and C, Uterine tissue extracts were immunoprecipitated with Bip-specific primary antibody, and then analyzed by Western blotting using Bip- (B) or ERα- (C) specific antibodies. The intense band detected at approximately 55 kDa in all lanes represents heavy chain subunit of IgG (this shown as an internal loading control). Arrow denotes the position of the detected protein band. In our control experiments, immunoprecipitation using normal goat serum did not detect any specific bands for Bip or ERα by Western blotting (data not shown). D, Uterine tissue extracts were analyzed by Western blotting using the primary antibody for ERα and actin. IP, Immunoprecipitation; WB, Western blot.
Bip Is Required for ERα-Mediated Gene Transactivation by Estrogen
We speculated that one potential mechanism for estrogen-directed molecular association between Bip and ERα in the uterus is to regulate proper conformation of ER necessary for functional activation after ligand binding. To address this possibility, we first sought to determine whether estrogen is capable of regulating expression and molecular association of Bip and ERα in a simple model system utilizing the primary culture of mouse uterine stromal cells; uterine stromal cells express BIP in response to estrogen (4). Thus, cells were examined for Bip and ERα expression at indicated times (Fig. 2A). Our results show that E2 rapidly up-regulates Bip within 2 h, and thereafter the levels remain unchanged until 24 h. In contrast, ERα levels remain elevated throughout the culture period with E2.
Fig. 2. Estrogen-Dependent Regulation of Bip and ERα in Vitro.
A, Primary culture of uterine stromal cells (at 70–80% confluence) were treated with E2 (10 nm) or ethyl alcohol (0.002% final concentration) (as vehicle control) at indicated times and then analyzed by Western blotting for expression of Bip, ERα, and actin. B, Primary cultured cells (at 70–80% confluence) were subjected to infection (~10 pfu/cell) with adenoviruses rAdBipAS or rAdGFP (empty control) for 12 h followed by initiation of E2 (10 nm) treatment for 24 h. Cellular extracts were analyzed by Western blotting for expression of Bip, HSP70, ERα, GFP, and actin. C, Experimental conditions were same as described in panel B. Cell extracts were analyzed after immunoprecipitation using primary antibodies against ERα and Bip followed by Western blotting for Bip (i) and ERα (ii). Coomassie stain gel pictures are shown as loading controls (iii). WB, Western blot; IP, immunoprecipitation.
To address the role of Bip in regulating ERα activity, we used an adenovirus-driven approach to inhibit Bip expression in vitro. The recombinant adenovirus particles carrying Bip antisense gene (rAdBipAS) and the empty vector (rAdGFP) (as control) were used. These constructs are equipped with a green fluorescent protein (GFP) expression system under a separate cytomegalovirus (CMV) promoter. Because estrogen treatment is needed to induce Bip expression in vitro, we first initiated the infection of cells with viruses at approximately 10 plaque-forming units (pfu)/cell for 12 h before examining their estrogenic effects after addition of E2 (10 nm) for 24 h. Our analysis shows that rAdBipAS suppresses Bip, whereas the control is ineffective in this response (Fig. 2B). Furthermore, suppression of Bip does not alter the levels of ERα or HSP70, suggesting a gene-specific regulation (Fig. 2B). The expression of green fluorescent protein (GFP) by either type of viruses suggests that expression of the transgene was appropriately regulated in uterine cells in vitro (Fig. 2B). We also analyzed the status of molecular association between Bip and ERα under similar conditions in vitro. Cell lysates were coimmunopre-cipitated with ERα or Bip antibodies and subsequently analyzed by Western blotting for Bip and ERα (Fig. 2C). Our results are consistent with the fact that estrogen-regulated Bip is capable of binding to ERα, whereas this is abolished after suppression of Bip expression (Fig. 2C). Furthermore, our results show that there is no detectable interaction between Bip with ERα in the absence of estrogen in vitro (data not shown). This is also consistent with our observation of lack of expression of Bip without estrogen in vitro (Fig. 2A).
We next examined whether suppression of endogenous Bip regulates estrogen-dependent endogenous ERα-mediated gene transactivation function in vitro. The results show that E2-dependent luciferase activity is induced at least 8-fold over the basal control level (Fig. 3A). However, the application of rAdBipAs virus attenuates the reporter activity in a dose-dependent manner; the control virus did not alter the basal response. Because Bip regulates ER-dependent gene transcription, we also analyzed whether suppression of Bip interferes with the cellular growth or viability. Our results show that Bip is required for estrogen-dependent growth and maintenance of cell viability in vitro (Figs. 3, B and C). Collectively, these results provide evidence that estrogen-induced Bip expression controls cell growth-regulatory functions that are dependent on functional activation of ERα.
Fig. 3. Analysis of Estrogen-Dependent Effects after Perturbation of Bip Expression in the Primary Culture of Uterine Stromal Cells.
A, Determination of endogenous ERα-mediated transactivation of luciferase activity. Cell extracts were analyzed by luciferase activity as described in Materials and Methods. Data (as percent) are represented by mean ± sem of relative luciferase activity from four independent experiments. Data were analyzed using Student’s paired t test (P < 0.01). B, Analysis of cellular growth. Primary cultured cells were subjected to infection with 1 pfu/cell of adenoviruses (rAdBipAs or rAdGFP) for 12 h, followed by the treatment of E2 (10 nm) for indicated times. Noninfected cells were also analyzed in parallel with or without addition of estrogen. Cellular proliferation was assayed as described in Materials and Methods. The asterisks (**) indicate statistically different (P < 0.01; ANOVA followed by Newman-Keul’s multiple-range test) as compared with corresponding control group on particular days. C, Analysis of cellular viability. Cells on d 3 as examined in panel B were visualized by fluorescence microscopy after staining with 4’,6-diamidino-2-phenylindole (DAPI) and propidium iodide (PI). Viable cells exclude PI, whereas dead cells stain with PI (red) and DAPI (blue).
Uterine Bip Is Necessary to Mediate Estrogen-Dependent Recruitment of ERα to Endogenous Gene Promoters
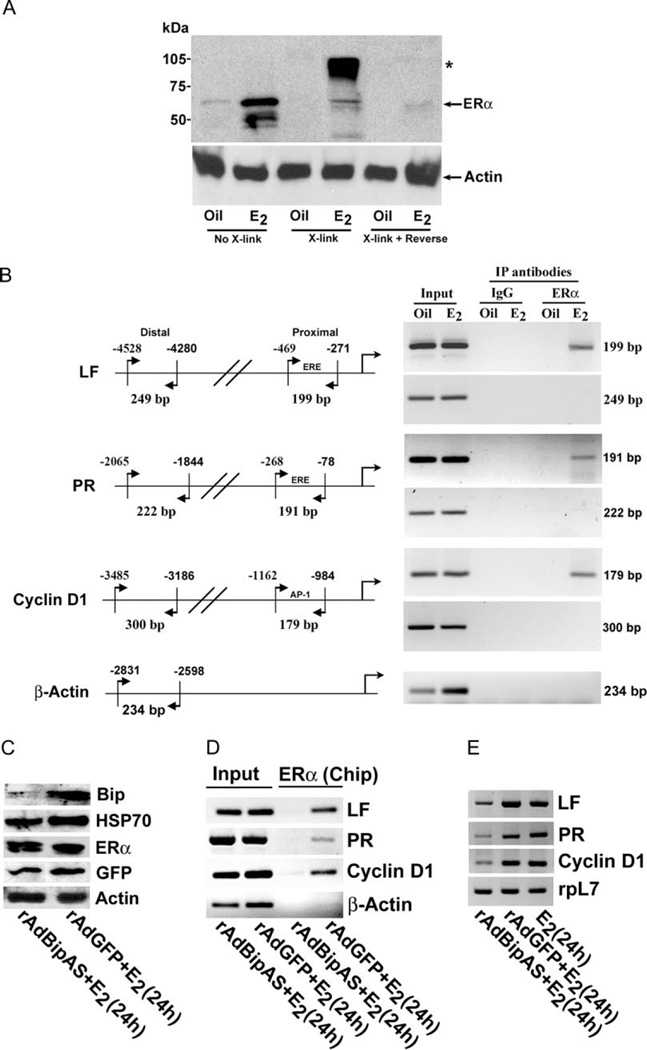
We next examined whether in vivo inhibition of estrogen-induced uterine Bip expression affects ERα-me-diated endogenous gene transcriptional regulation. To accomplish this objective, we first analyzed estrogen-dependent recruitment of endogenous ERα to the promoter of estrogen-responsive genes in the mouse uterus by chromatin immuoprecipitation (Chip). In our initial studies for the fixation of ERα in situ (Fig. 4A), we observed that treatment with 1% formaldehyde essentially cross-links E2-induced ERα to a large macromo-lecular assembly (asterisk), whereas free ERα is also detected (arrow). Analysis of oil-treated samples in parallel did not show such changes. Furthermore, estrogen-induced association is reversed by heat treatment (Fig. 4A), establishing critical requirements for Chip experiments in the uterus and allowed us to further examine whether ERα recruitment occurs to gene-specific promoters by estrogen. Estrogen-responsive gene promoters, as illustrated in Fig. 4B, helped us in designing primers around the binding of ERα (proximal), and a nonspecific region (distal) for PCR studies after the application of Chip. Using ERα antibody, we were able to amplify desired products in the proximal region, but not in the distal, for the above genes after an injection of E2, whereas the control (oil injection) did not exhibit such results. The presence of the promoter DNA before the Chip assay was confirmed by PCR (Input), and Chip assays using preimmune serum (IgG) did not detect any amplified PCR, suggesting ERα-dependent DNA recovery was promoter specific. In contrast, the application of the Bip antibody for the Chip assay was unable to show any PCR products (data not shown), suggesting Bip is not recruited to the DNA. Collectively, these results suggest that estrogen-mediated recruitment of ERα is indeed recognizable by Chip assay to gene-specific promoters in the uterus.
Fig. 4. Analysis of Estrogen-Dependent Effects after Perturbation of Bip Expression in the Uterus.
A, Analysis of estrogen-induced ERα fixation to high molecular complex by Western blotting. Uterine tissues were subjected to cross-linking using 1% formaldehyde (X-link), followed by reversal of cross-linking using heat treatment (X-link + reverse). Tissue extracts at different steps of this procedure were analyzed by Western blotting using ERα-specific antibody. Arrow indicates the position of uncomplexed ERα band, whereas the asterisk (*) denotes the position of macromolecular association of ERα. Results show that ERα-associated high molecular complex with retarded migration in SDS-PAGE gel can be reversed by prolonged heat treatment. B, Analysis of estrogen-induced recruitment of ERα to the promoter of LF, PR, and cyclin D1 genes. Chip analysis was performed using ERα antibody or normal serum IgG (as control) as described in Materials and Methods. The presence of the promoter DNA before immunoprecipitation was confirmed by PCR (Input). For PCR amplification technique, the cycle parameters were the same as described elsewhere(7). C, Analysis of adenovirus-mediated expression in the uterus. Uterine tissues were collected after administration of adenoviruses rAdBipAs or rAdGFP (control) in mice as described in Materials and Methods, followed by injections of E2 (100 ng/mouse) for 24 h. Western blot analysis for the expression of Bip, Hsp70, ERα, GFP, and actin. D, Chip analysis for estrogen-induced recruitment of ERα to LF, PR, cyclin D1, and β-actin gene promoters. E, RT-PCR analysis of expression for LF, PR, cyclin D1, and ribosomal protein-L7 (rpL7) genes. rpL7 was used as a constitutive gene. IP, Immunoprecipitation.
We next wanted to analyze whether virus-driven particles, as used above for in vitro studies, can also modulate endogenous gene transcription mediated by ERα in the uterus. We first examined the status of protein expression by Western blotting using uterine tissues collected from virus-infected mice after administration of E2 for 24 h. The results show that a strong suppression for the level of uterine Bip was achieved by rAdBipAS as compared with the control (rAdGFP) (Fig. 4C). Furthermore, the virus-mediated actions are not influenced by HSP70 or ERα levels, suggesting a gene-specific effect of Bip knockdown in the uterus (Fig. 4C). Additionally, the detected levels of GFP in both virus-treated samples clearly confirm the presence of virus-driven transgene expression in the uterus (Fig. 4C).
To determine the impact of in vivo inhibition of Bip expression on endogenous ERα activity, we examined endogenous activation of ERα for its recruitment to uterine LF, PR, and cyclin D1 gene promoters by Chip analysis. Our results show that rAdBipAS-mediated perturbation of uterine Bip expression caused dramatic elimination of ERα recruitment to proximal gene promoters, whereas the control viruses did not affect this interaction mediated by estrogen (Fig. 4D). Furthermore, DNA analysis of the input for either type of virus-treated samples did show amplification of gene-specific promoters (Fig. 4D), suggesting ERα-antibody-specific recovery of promoter regions was not a limiting factor. Under similar conditions, our results are also consistent with uterine expression of the above genes (Fig. 4E). In contrast, c-fos and c-myc, also ER-dependent early responsive genes, did not show any alteration by 2 h of estrogen treatment (data not shown), suggesting Bip mediates specific regulation of late responsive genes. Furthermore, it is to be noted that the expression of uterine progesterone-responsive genes, amphiregulin (AR) and histidine decarboxylase (HDC), were also not affected by suppression of Bip (data not shown). Overall, these results suggest that uterine expression of Bip regulates gene transcriptional activity mediated by ERα under the direction of estrogen during the late phase.
Bip Regulates Estrogen-Dependent Uterine Physiological Responses
Because adenovirus-driven direct suppression of Bip negatively impacts ERα-dependent endogenous gene regulation in the uterus in response to estrogen, we next examined whether this virus-driven inhibitory action perturbs estrogen-regulated uterine biological responses that occur during the early and late responses in mice. We observed that E2-induced uterine wet weight response occurring at 6 h in mice administered with rAdBipAs was comparable to those receiving the control viruses after E2 stimulation (Fig. 5A), suggesting that the early response is not dependent on Bip expression. However, during the late phase of E2 action at 24 h, mice injected with the rAdBipAS virus demonstrated a striking increase in uterine wet weight as compared with those with control viruses (Fig. 5A). Further analysis of the results revealed that administration of rAdBipAS virus in conjunction with E2 caused a significant increase in uterine water imbibition at 24 h, whereas the control virus did not show such a response (Fig. 5B). These results were further supported by our results of E2-induced uterine uptake of BSA (Fig. 5C) and persistent expression of the permeability genes VEGF and Flk-1 (Fig. 5D) (22) during the late phase in the endometrial stoma by rAdBipAS; the control viruses were not effective in these responses. These results suggest that E2-induced early responses, at least with respect to water accumulation and vascular permeability regulators, were aberrantly prolonged by suppression of Bip expression in the mouse uterus.
Fig. 5. Analysis of Estrogen-Dependent Water Permeability Responses in Ovariectomized Mice after Administration of Adenoviruses.
Experimental conditions were same as described in Fig. 4C. A, Analysis of uterine wet weights. Uterine weights were examined at 6 and 24 h after E2 injection. Values with asterisks are statistically different (P< 0.01; ANOVA followed by Newman-Keul’s multiple-range test) as compared with their corresponding control groups. B, Analysis of uterine water uptake. The asterisk indicates statistically different (P < 0.01; ANOVA followed by Newman-Keul’s multiple-range test) as compared with control group. C, Analysis of uterine macromolecular uptake. The fluorescence for the uptake of rhodamine-BSA in uterine tissue sections was visualized by direct fluorescence (panels b and d) and phase-contrast (panels a and c) microscope. le, Luminal epithelium; s, stroma. Bars, 100 µm. D, Analysis of uterine permeability gene expression. Frozen uterine sections were analyzed by in situ hybridization of VEGF and Flk-1. le, Luminal epithelium; myo, myometrium; s, stroma. Bars, 100 µm. VEGF, Vascular endothelial growth factor; Flk-1, VEGF receptor 2.
We next examined the effectiveness of these viruses in the regulation of uterine growth (a late effect) induced by E2 at 24 h. This was performed by analyzing uterine cell proliferation and apoptosis, because these processes are involved in growth regulation. Our results show that the presence of rAdBipAs virus is antagonistic to E2-dependent uterine epithelial cell proliferation (Fig. 6, A and B) and inhibition of apoptosis in epithelial and stromal cells (Fig. 6, C and D). These findings provide evidence that Bip is critical to appropriate regulation of both early and late responses in the uterus mediated by estrogen.
Fig. 6. Analysis of Estrogen-Dependent Regulation of Uterine Cell Growth in Ovariectomized Mice after Administration of Adenoviruses.
Uterine tissues were collected from mice after administration of adenoviruses and E2 as described in Fig. 5. A, BrdU immunostaining. Reddish-brown nuclear deposits indicate the sites of positive immunostaining. le, Luminal epithelium; ge, glandular epithelium; s, stroma. Bars, 50 µm. Dramatic induction of uterine edema within stromal compartment (shown by arrow) is clearly visible in rAdBipAS+E2 group. B, Quantitation of BrdU-positive cells in separate uterine compartments as shown in panel A. Approximately 500 cells were counted in each uterine compartment. The data presented here after the analysis of at least 10 different mice from each group. Values with asterisks are statistically different (P < 0.01; ANOVA followed by Newman-Keul’s multiple-range test) as compared with their corresponding control groups. C, TUNEL staining. Uterine cross-sections were incubated with TUNEL reaction mixture as described by Yue et al. (45). After a brief rinse in PBS, sections were incubated in streptavidin-HRP solution, followed by incubation in diaminobenzidine solution for color development. Sections were viewed after light hematoxylin counterstaining. Apoptotic cells are detected by dark brown staining (arrows). A representative oil-treated section is provided as control. le, Luminal epithelium; ge, glandular epithelium; s, stroma. Bars, 50 µm. D, Quantitation of TUNEL-positive cells in different uterine compartments as shown in panel C. Approximately 500 cells were counted in each uterine compartment. The data are presented here after the analysis of at least 10 different mice from each group. Values with asterisks are statistically different (P< 0.01; ANOVA followed by Newman-Keul’s multiple-range test) as compared with their corresponding control groups.
DISCUSSION
The uterus is a major target of estrogen action during the estrous cycle and pregnancy. Previous studies from our laboratory have shown that the expression of Bip is tightly regulated at the site of embryo implantation, an event that is regulated by estrogen in mice (23). Furthermore, Bip is associated with uterine stromal cell decidualization in rats (19). Although these studies provide circumstantial evidence for a functional role of Bip in regulating uterine cell growth and differentiation, the definitive role of Bip in uterine functions and the molecular mechanism by which it influences estrogen-regulated uterine biology remain unknown. Here, we provide evidence for the first time that Bip plays a critical role in mediating estrogen-dependent uterine physiological responses in mice, primarily through its molecular association with ERα. Using both in vitro and in vivo model systems, we specifically show that Bip is required for the maintenance of the functional activity of ERα in regulating gene transcription. Further studies revealed that down-regulation of uterine Bip is strongly correlated with compromised estrogen-dependent late growth responses, whereas the onset of early estrogenic responses remains unaffected but abnormally prolonged, suggesting Bip is necessary to coordinate the phase I and phase II responses.
Molecular chaperones primarily function as a part of the multiprotein heterocomplex to direct appropriate protein folding and assembly, intracellular trafficking, and targeting of proteins for proteosomal degradation (16). There is also evidence that these proteins are involved in signal transduction pathways (24). For steroid hormone receptors, the chaperone protein plays a major role in ligand-dependent modulation of the receptor for gene transcription (16). Thus, molecular chaperones are considered as potential targets not only for regulating steroid hormone receptor actions, but are also targets for therapeutic interventions in pathological situations (25). In addition, overexpression of specific chaperones is shown to be beneficial in certain disorders resulting from protein misfolding (26). Thus the underpinning theme is that chaperone proteins are important for functional maturation of steroid hormone receptors.
Our findings of molecular association of Bip and ERα in response to estrogen provide evidence that these genes are differentially but mutually regulated by E2 in the uterus. The elevated levels of uterine Bip during the phase I and phase II responses, and its interaction with ERα at the end of phase I and continuing through phase II are consistent with the suggestion that Bip-mediated chaperone activity partici-pates in ERα processing required for uterine growth responses under the direction of estrogen. In addition, our observation of estrogen-dependent rapid and sustained up-regulation of Bip, as opposed to constitutive levels of ERα in primary cultures of uterine stromal cells with or without estrogen (Fig. 2A), strongly suggests that cellular stability of ERα is not particularly regulated by the presence of Bip. This speculation is further supported by the fact that adenovirus-driven suppression of Bip expression in vitro does not attenuate the level of ERα (Fig. 2, B and C). This observation further suggests that Bip-mediated regulation of ERα exists at the level of functional activity of ERα. Indeed, our in vitro transactivation studies show that estrogen-dependent regulation of Bip is involved in regulating ERα-mediated luciferase-reporter gene transcription under the direction of estrogen (Fig. 3A), suggesting that functional activity of ERα is regulated by Bip. The concentration of E2 (10 nm) we selected for our in vitro studies approximates 2.7 ng/ml and was based on published studies by other investigators to observe in vitro effects of estrogen. This concentration is apparently higher than the circulating levels of E2 detected in mice at the proestrous stage (27). However, it is not known how circulating levels of E2 are translated locally at the tissue levels and whether E2 responses in vivo and in vitro are comparable, because pharmacokinetics are likely to be different under these conditions. Whereas it is known that Bip acts as a chaper-one for many interacting cellular proteins during their processing, the lack of interaction between cellular Bip and luciferase reporter proteins has been reported previously (28). Our previous studies have shown that estrogen induces Bip and ERα expression in uterine epithelial and subluminal stromal cells in mice (4, 29), suggesting Bip influences ERα activity in both cell types. Bip is known to be exclusively localized to the ER, where it binds transiently to proteins as they are processed (18). We therefore speculate that estrogen-dependent regulation of Bip and ERα also occurs in the ER of uterine cells. However, the mechanisms of Bip-mediated regulation of ERα in the ER and subsequent maintenance of nuclear activity of ERα for gene transcription are not clearly understood. In this regard, recent studies show that ATF6, a transcription factor, exists in the ER as a membrane anchored protein, but can be activated by ER-stress response. It is interesting to note that ATF6 normally binds to Bip, but dissociates after the onset of stress response to further undergo processing and trafficking in the Golgi before its translocation to the nucleus (30). A similar scenario could be envisioned for ERα translocation from ER to the nucleus. Our future goal is to explore this possibility.
It has been shown that LF, PR, and cyclin D1 are regulated by ER via interaction with defined consensus sites in the promoter (31–33). Estrogen-regulated expression of LF and cyclin D1 genes in the mouse uterus primarily occurs in the epithelium, whereas that of progesterone receptor (PR) is operative in the epithelium and subluminal stroma (21, 34), suggesting these genes could be considered as potential transcriptional targets acting downstream of Bip and ERα interaction regulated by estrogen in the uterus. Indeed, our in vivo Bip suppression studies demonstrating elimination of either gene-specific interaction of ERα and gene-specific expression (Fig. 4, D and E), support a notion that Bip plays a critical role for uterine gene transcription via functional activation of endogenous ERα in the mouse uterus.
Although estrogen-regulated biphasic uterine responses are well recognized, a long sought question as to how the early and late responses are molecularly linked remains unanswered. It has previously been shown that estrogenic compounds elicit early biological responses in the mouse uterus with or without involving ERα or ERβ, whereas the late growth responses are dependent on the participation of ERα (4–7). Because the levels of ERβ in the uterus are extremely low and because this receptor isoform is not involved in influencing uterine biology (35, 36), our present study focused on ERα function in the uterus. Our in vivo observation of prolonged expression of two vascular permeability-related genes, seemingly leading to exaggerated water uptake in the absence of true uterine growth after adenovirus-driven down-regulation of Bip, suggests that Bip-mediated ERα activity is critical to coordinating the phase I responses with those of phase II for regulated growth response by estrogen in the uterus. Estrogen is known to stimulate uterine epithelial cell proliferation (3). Our present findings provide strong evidence that Bip is a major player in this estrogen-mediated response (Fig. 6, A and B). In this regard, it is to be noted that our analyses show that Bip is not regulatory to estrogen in other estrogen-responsive mouse tissues, i.e. liver, heart, and a breast cancer cell line (MCF-7) (data not shown), suggesting uterine actions appear to be specific. It is known that estrogen prevents uterine cell apoptosis in ovariecto-mized mice (37). In this respect, overexpression of Bip also provides protection against cellular death (20). We also observed that estrogen-dependent uterine cell protection against apoptosis utilizes Bip in cooperation with ERα (Fig. 6, C and D). In conclusion, our present study presents Bip-ERα signaling axis as a molecular link that coordinates biphasic estrogenic responses in the uterus for its appropriate growth. This remarkable finding of ERα-independent early Bip expression contributing to ERα-dependent events in the mouse uterus adds new insights to our understanding of uterine biology.
MATERIALS AND METHODS
Animals and Injections
Adult CD-1 (Charles River Laboratories, Wilmington, MA) mice were housed in our institutional animal care facility according to NIH and institutional guidelines for laboratory animals. In general, 8- to 10-wk-old adult mice were ovari-ectomized and rested for 7 d before they received any injections. Mice were injected (0.1 ml/mouse) with sesame seed oil (control) or E2 (100 ng/mouse) dissolved in oil sc and killed at indicated times. In some studies, wild-type littermates were analyzed in parallel with ERα(−/−) mice (38). Both mice (C57BL/6J/129/J) were produced by crossing of heterozygous females and males in our animal facility.
Antibodies and Other Reagents
The affinity-purified polyclonal antibodies for Bip (catalog no. sc-1050), ERα (catalog no. sc-542), actin (catalog no. sc-1615), PR (catalog no. sc-539) and GFP (catalog no. sc-1050) were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Anti-Hsp70 polyclonal antibody was purchased from Stressgen (Victoria, British Columbia, Canada).
Immunohistochemistry, Immunoprecipitation, and Western Blotting
These procedures were the same as previously described (39). Proteins were extracted in RIPA lysis buffer (Santa Cruz).
Primary Culture and Cell Proliferation Assay
The primary culture of uterine stromal cells was followed as described elsewhere (40). Cells (~5 × 104) were seeded in 96-well plates, and the growth was analyzed by CellTiter 96R aqueous one solution cell proliferation assay kit (Promega Corp., Madison, WI) according to manufacturer’s instruction.
Luciferase Gene Transactivation
ERα-dependent luciferase activity in total cellular extracts was measured using a Luciferase kit according to the manufacturer’s instruction (Promega). Primary cultured cells (in triplicate set for each experiment) were subjected to coinfection with ERE-luciferase adenovirus (rAdERE-luc, 1 pfu/cell) and increasing amounts (1, 5, 10, and 20 pfu/cell) of adenoviruses rAdBipAs or rAdGFP (empty control) for 12 h, followed by treatment with E2 (10 nm) or ethyl alcohol (0.002% final concentration) for an additional 24 h. Cells (in triplicate) infected with similar concentration of rAdERE-luc alone were used as a control. The luciferase activity, as obtained in the absence of estrogen, in the control was considered basal at 100%. The activity (as percent) in relation to other treatments, with or without addition of estrogen, was calculated based on the basal level.
Recombinant Adenoviral Plasmids and Generation of Viral Particles
The full-length coding region of mouse Bip cDNA was generated by RT-PCR using mouse d 5 pregnant uterine total RNA. Primers carrying the linkers for EcoRV at 5’-ends were used for RT-PCR as follows: 5’-GCCCCGGGATGATGAAGT-TCACTGTG-3’ (sense) and 5’-GCCCCGGGCTACAACT-CATCTTTTTC-3’ (antisense). The amplified DNA fragment was inserted into a shuttle vector pAd-track CMV at EcoRV restriction site, in a direction of the antisense orientation with respect to CMV promoter. The selected clone was sequenced to confirm its identity. Both the resultant clone and the empty shuttle vector possess an additional CMV promoter, which drives green fluorescence protein (GFP) independently. Plasmid DNAs were linearized with PmeI and subsequently cotransfected with pAdEasy-1 for recombination into Escherichia coli BJ5183. The recombinant plasmid clones, harboring either BipAs DNA or none (empty control), were confirmed by restriction cutting using Pacl and by sequencing. Recombinant adenoviral ERE-luciferase construct (rAdERE-Luc) was obtained from Dr. Larry Jameson (Northwestern University Medical School, Chicago, IL) (41). Viral packaging of these plasmids was carried out in HEK293 cells as described elsewhere (42). Virus particles were purified through CsCl density gradient centrifugation and stored at −70 C.
Adenoviral Infection in Vitro
This procedure was followed essentially as described previously (7).
In Vivo Delivery of Adenoviruses
In our initial studies, we have seen that intraluminal virus delivery in addition to injection through tail vein, as reported previously (7), enhances infection efficiency to luminal and subluminal stromal cells in the uterus. Thus, in the present study, virus particles were first inoculated directly into uterine lumen of both horns (~20 µl solution in saline containing 1 × 1011 virus particles per horn) from the oviductal-end just before ovariectomy. They were given rest for 7 d before they received the second inoculum (~100 µl solution in saline containing 1 × 1011 virus particles) through tail vein. They were again rested for 2 more days before receiving injections of estrogen or oil for indicated times. Uterine tissues were appropriately collected for subsequent analysis.
RT-PCR
RT-PCRs have previously been described (4). Mouse-specific primers were designed for AR, c-fos, c-myc, cyclin D1, HDC, PR, LF, and rpL7 cDNAs. Amplified fragments were separated by electrophoresis on 2% agarose gels and visualized by ethidium bromide staining.
Probes and in Situ Hybridization Technique
cRNA probes were generated from mouse-specific cDNA clones. In situ hybridization technique was performed as previously described (43).
Chip and PCR Analysis
Uterine tissues were collected in small pieces (~0.3 cm) and cross-linked with 1% formaldehyde (catalog no: FX0418–1; EMD Chemicals, Inc., Gibbstown, NJ) at room temperature for 15 min. The reaction was terminated by addition of 0.1% glycine. Fixed tissue pieces were then pelleted, resuspended in lysis buffer (Protein Master, Geno-Tech, St. Louis, MO) containing 1 × protease inhibitor (protease arrest; catalog no. 786–108, Geno-Tech) and subjected to cell lysis by continuous vortex at high speed for 40 min in the presence of acid-washed glass beads (G-1277; Sigma Chemical Co., St. Louis, MO) on ice. The broken cell suspension was then collected into a new tube by passing through a hole at the bottom and subjected to sonication to derive smaller chromatin fragments of approximately 500 bp, using MicroUltra-sonic cell disrupture (Kontes, Vineland, NJ), at a maximal input for each 15-sec burst (five times). The final lysate was used for immunoprecipitation using either ERα antibody or normal serum IgG (as control). The bound protein was eluted with immunoprecipitation elution buffer (1% sodium dodecyl sulfate, 0.1m NaHCO3) at least three times, pooled, and heated at 50 C for 5 h to reverse the cross-linking between DNA and protein. DNA and protein was precipitated with addition of 100% ethanol for overnight at −20 C. The pellet was then dissolved in Tris-EDTA buffer in the presence of proteinase K by incubation at 50 C for 30 min. DNA was then extracted once with phenol-chloroform-isoamyl alcohol (24: 24:1 vol/vol), once with chloroform and precipitated with alcohol. Finally, the DNA pellet was dissolved in Tris-EDTA buffer and analyzed by PCR. The primers named as “proximal” were designed around this specific binding region of these genes, whereas arbitrary regions located further upstream of gene promoters with no recognizable ERα binding sites were chosen for designing of “distal” primers. The primers used for PCR were as follows: LF (proximal), 5’-tctaggct-gactccgctctc-3’ (sense) and 5’-tagaggtgggacatggggta-3’ (antisense); LF (distal), 5’-catgtgcatgtatgtgagatgaa-3’ (sense) and 5’-atcccctgtcagtcagtgccttc-3’ (antisense); PR (proximal), 5’-ccagcttgctccagctactt-3’ (sense) and 5’-atat-aggggcagagggagga-3’ (antisense); PR (distal), 5’-actgtcca-gaatgcctccac-3’ (sense) and 5’-atcaccagggaggtgctaca-3’ (antisense); cyclin D1 (proximal), 5’-aggtggagaaacaccac-cac-3’ (sense) and 5’-cggtttgcccaagaaaaata-3’ (antisense); cyclin D1 (distal), 5’-aaatctccgctctttgga-3’ (sense) and 5’-aaatctcgtggcaggaactg-3’ (antisense). The anticipated product sizes for LF, PR, and cyclin D1 genes were 199,191, and 179 bp for the proximal, and 249, 222, and 300 bp for the distal regions, respectively. β-Actin primers (sense: 5’-ttgaat-gtccccaggagaag-3’ and antisense: 5’-ccagagaactttgccct-cac-3’) designed from the promoter region were used as a control, and the anticipated product size was 234 bp. PCR products were resolved in 2% agarose gels containing ethidium bromide.
Analysis of Phase I and Phase II Responses in the Uterus
To examine the effects of E2 on uterine biphasic responses in mice, uterine wet weights were initially recorded. The early uterine effects were determined by examination of water accumulation, macromolecules uptake [viz. after injection (iv) of rhodamine-tagged BSA 15 min before killing (44)] and expression for permeability genes VEGF and Flk-1 (22), whereas the late uterine effects were analyzed by bromode-oxyuridine (BrdU) incorporation into DNA. For studies with BrdU incorporation, mice were injected (sc) with BrdU (50 mg/kg bd wt) 2 h before killing. Frozen tissue sections fixed in 10% formaldehyde for BrdU staining as described (7). The biotinylated BrdU antibody (catalog no. 93–3943) was purchased from Zymed Laboratories, Inc. (South San Francisco, CA).
TUNEL (Terminal Deoxynucleotide Transferase-Mediated Deoxyuridine Triphosphate-Biotin Nick End Labeling) Assay
The TUNEL kit was purchase from Promega (DeadEnd Col-orimetric TUNEL System; catalog no. G7130). The experimental protocol was same as described elsewhere (45). For positive controls, the sections were treated with deoxyribo-nuclease I (5–10 U/ml) for 10 min at room temperature before incubation with the TUNEL reaction mixture.
Acknowledgments
We thank Dr. Sudhansu K. Dey for his continuous encouragement during the course of this study and for his critical reading of this manuscript. We thank Dr. Bert Vogelstein for providing reagents to generate recombinant adenoviral clones, and Dr. Larry Jameson for providing recombinant adenoviral ERE-luciferase construct (rAdERE-Luc).
This work was supported by National Institutes of Health Grants (ES07814, HD37830, HD12304, and HD33994).
Abbreviations
- BrdU
Bromodeoxyuridine
- Chip
chromatin immunoprecipitation
- CMV
cytomegalovirus
- E2
17β-estra-diol
- ER
estrogen receptor
- ER
endoplasmic reticulum
- ERE
estrogen response element
- GFP
green fluorescent protein
- HSP
heat shock protein
- PR
progesterone receptor
- rAdBi-pAs
recombinant adenovirus particles carrying Bip antisense gene
- TUNEL
terminal deoxynucleotide transferase-medi-ated deoxyuridine triphosphate-biotin nick end labeling
Footnotes
The authors listed in the manuscript (S.R., X.H., H.-E.Z., H.W., S.K.D.) have nothing to declare.
REFERENCES
- 1.Couse JF, Korach KS. Estrogen receptor null mice: what have we learned and where will they lead us? Endocr Rev. 1999;20:358–417. doi: 10.1210/edrv.20.3.0370. [DOI] [PubMed] [Google Scholar]
- 2.Tsai MJ, O’Malley BW. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu Rev Biochem. 1994;63:451–486. doi: 10.1146/annurev.bi.63.070194.002315. [DOI] [PubMed] [Google Scholar]
- 3.Dey SK, Lim H, Das SK, Reese J, Paria BC, Daikoku T, Wang H. Molecular cues to implantation. Endocr Rev. 2004;25:341–373. doi: 10.1210/er.2003-0020. [DOI] [PubMed] [Google Scholar]
- 4.Das SK, Tan J, Raja S, Halder J, Paria BC, Dey SK. Estrogen targets genes involved in protein processing, calcium homeostasis and Wnt signaling in the mouse uterus independent of estrogen receptor-α and -β. J Biol Chem. 2000;275:28834–28842. doi: 10.1074/jbc.M003827200. [DOI] [PubMed] [Google Scholar]
- 5.Hewitt SC, Deroo BJ, Hansen K, Collins J, Grissom S, Afshari CA, Korach KS. Estrogen receptor-dependent genomic responses in the uterus mirror the biphasic physiological response to estrogen. Mol Endocrinol. 2003;17:2070–2083. doi: 10.1210/me.2003-0146. [DOI] [PubMed] [Google Scholar]
- 6.Watanabe H, Suzuki A, Kobayashi M, Takahashi E, Ita-moto M, Lubahn DB, Handa H, Iguchi T. Analysis of temporal changes in the expression of estrogen-regulated genes in the uterus. J Mol Endocrinol. 2003;30:347–358. doi: 10.1677/jme.0.0300347. [DOI] [PubMed] [Google Scholar]
- 7.Hou X, Tan Y, Li M, Dey SK, Das SK. Canonical Wnt signaling is critical to estrogen mediated uterine growth. Mol Endocrinol. 2004;18:3035–3049. doi: 10.1210/me.2004-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stancel G, Boettger-Tong HL, Chiappetta C, Hyder SM, Kirkland JL, Murthy L, Loose-Mitchell DS. Toxicity of endogenous and environmental estrogens: what is the role of elemental interactions? Environ Health Perspect. 1995;103(Suppl 7):29–33. doi: 10.1289/ehp.95103s729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klein-Hitpass L, Ryfel GU, Heitlinger E, Cato ACB. A 13 bp palindrome is a functional estrogen response element and interacts specifically with estrogen receptor. Nucleic Acids Res. 1988;16:647–663. doi: 10.1093/nar/16.2.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aumais JP, Lee HS, DeGannes C, Horsford J, White JH. Function of directly repeated half-sites as response elements for steroid hormone receptors. J Biol Chem. 1996;271:12568–12577. doi: 10.1074/jbc.271.21.12568. [DOI] [PubMed] [Google Scholar]
- 11.Sukovich DA, Mukherjee R, Benfield PA. A novel, cell-type-specific mechanism for estrogen receptor-mediated gene activation in the absence of an estrogen-responsive element. Mol Cell Biol. 1994;14:7134–7143. doi: 10.1128/mcb.14.11.7134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith CL, O’Malley BW. Coregulator function: a key to understanding tissue specificity of selective receptor modulators. Endocr Rev. 2004;25:45–71. doi: 10.1210/er.2003-0023. [DOI] [PubMed] [Google Scholar]
- 13.Levin ER. Cell localization, physiology, and non-genomic actions of estrogen receptors. J Appl Physiol. 2001;91:1860–1867. doi: 10.1152/jappl.2001.91.4.1860. [DOI] [PubMed] [Google Scholar]
- 14.Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science. 2005;307:1625–1630. doi: 10.1126/science.1106943. [DOI] [PubMed] [Google Scholar]
- 15.Thomas P, Pang Y, Filardo EJ, Dong J. Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells. Endocrinology. 2005;146:624–632. doi: 10.1210/en.2004-1064. [DOI] [PubMed] [Google Scholar]
- 16.Pratt WB, Toft DO. Steroid receptor interactions with heat shock protein and immunophilin chaperones. Endocr Rev. 1997;18:306–360. doi: 10.1210/edrv.18.3.0303. [DOI] [PubMed] [Google Scholar]
- 17.Hartl FU. Molecular chaperones in cellular protein folding. Nature. 1996;381:571–579. doi: 10.1038/381571a0. [DOI] [PubMed] [Google Scholar]
- 18.Kaufman RJ. Stress signaling from the lumen of the endoplasmic reticulum: coordination of gene transcriptional and translational controls. Genes Dev. 1999;13:1211–1233. doi: 10.1101/gad.13.10.1211. [DOI] [PubMed] [Google Scholar]
- 19.Simmons DG, Kennedy TG. Induction of glucose-regulated protein 78 in rat uterine glandular epithelium during uterine sensitization for the decidual cell reaction. Biol Reprod. 2000;62:1168–1176. doi: 10.1095/biolreprod62.5.1168. [DOI] [PubMed] [Google Scholar]
- 20.Reddy RK, Mao C, Baumeister P, Austin RC, Kaufman RJ, Lee AS. Endoplasmic reticulum chaperone protein GRP78 protects cells from apoptosis induced by topoisomerase inhibitors. J Biol Chem. 2003;278:20915–20924. doi: 10.1074/jbc.M212328200. [DOI] [PubMed] [Google Scholar]
- 21.Das SK, Tan J, Johnson DC, Dey SK. Differential spatiotemporal regulation of lactoferrin and progesterone receptor genes in the mouse uterus by primary estrogen, catechol estrogen, and xenoestrogen. Endocrinology. 1998;139:2905–2915. doi: 10.1210/endo.139.6.6051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma W-G, Tan J, Matsumoto H, Robert B, Abrahamson DR, Das SK, Dey SK. Adult tissue angiogenesis: evidence for negative regulation by estrogen in the uterus. Mol Endocrinol. 2001;15:1983–1992. doi: 10.1210/mend.15.11.0734. [DOI] [PubMed] [Google Scholar]
- 23.Reese J, Das SK, Paria BC, Lim H, Song H, Matsumoto HM, Knudtson KL, DuBois RN, Dey SK. Global gene expression analysis to identify molecular markers of uterine receptivity and embryo implantation. J Biol Chem. 2001;276:44137–44145. doi: 10.1074/jbc.M107563200. [DOI] [PubMed] [Google Scholar]
- 24.Aligue R, Akhavan-Niak H, Russell P. A role for Hsp90 in cell cycle control: Wee1 tyrosine kinase activity requires interaction with Hsp90. EMBO J. 1994;13:6099–6106. doi: 10.1002/j.1460-2075.1994.tb06956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bagatell R, Khan O, Paine-Murrieta G, Taylor CW, Aki-naga S, Whitesell L. Destabilization of steroid receptors by heat shock protein 90-binding drugs: a li-gand-independent approach to hormonal therapy of breast cancer. Clin Cancer Res. 2001;7:2076–2084. [PubMed] [Google Scholar]
- 26.Heinlein CA, Chang C. Role of chaperones in nuclear translocation and transactivation of steroid receptors. Endocrine. 2001;14:143–149. doi: 10.1385/ENDO:14:2:143. [DOI] [PubMed] [Google Scholar]
- 27.Jablonka-Shariff A, Ravi S, Beltsos AN, Murphy LL, Olson LM. Abnormal estrous cyclicity after disruption of endothelial and inducible nitric oxide synthase in mice. Biol Reprod. 1999;61:171–177. doi: 10.1095/biolreprod61.1.171. [DOI] [PubMed] [Google Scholar]
- 28.Winge I, Pryme IF. Sodium butyrate stimulates the synthesis of firefly luciferase in transfected CHO cells but levels of BiP chaperone are unaffected. Cell Biol Int. 2002;26:489–494. doi: 10.1006/cbir.2002.0881. [DOI] [PubMed] [Google Scholar]
- 29.Wang H, Tranguch S, Xie H, Hanley G, Das SK, Dey SK. Variation in commercial rodent diets induces disparate molecular and physiological changes in the mouse uterus. Proc Natl Acad Sci USA. 2005;102:9960–9965. doi: 10.1073/pnas.0501632102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shen J, Chen X, Hendershot L, Prywes R. ER stress regulation of ATF6 localization by dissociation of BiP/ GRP78 binding and unmasking of Golgi localization signals. Dev Cell. 2002;3:99–111. doi: 10.1016/s1534-5807(02)00203-4. [DOI] [PubMed] [Google Scholar]
- 31.Liu YH, Teng CT. Estrogen response module of the mouse lactoferrin gene contains overlapping chicken ovalbumin upstream promoter transcription factor and estrogen receptor-binding elements. Mol Endocrinol. 1992;6:355–364. doi: 10.1210/mend.6.3.1584212. [DOI] [PubMed] [Google Scholar]
- 32.Planas-Silva MD, Shang Y, Donaher JL, Brown M, Wein-berg RA. AIB1 enhances estrogen-dependent induction of cyclin D1 expression. Cancer Res. 2001;61:3858–3862. [PubMed] [Google Scholar]
- 33.Petz LN, Ziegler YS, Schultz JR, Kim H, Kemper JK, Nardulli A, Petz LN, Ziegler YS, Schultz JR, Kim H, Kem-per JK, Nardulli AM. Differential regulation of the human progesterone receptor gene through an estrogen response element half site and Sp1 sites. J Steroid Bio-chem Mol Biol. 2004;88:113–122. doi: 10.1016/j.jsbmb.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 34.Tong W, Pollard JW. Progesterone inhibits estrogen-induced cyclin D1 and cdk4 nuclear translocation, cyclin E- and cyclin A-cdk2 kinase activation, and cell proliferation in uterine epithelial cells in mice. Mol Cell Biol. 1999;19:2251–2264. doi: 10.1128/mcb.19.3.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tan J, Paria BC, Dey SK, Das SK. Differential uterine expression of estrogen and progesterone receptors correlates with uterine preparation for implantation and de-cidualization in the mouse. Endocrinology. 1999;140:5310–5321. doi: 10.1210/endo.140.11.7148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krege JH, Hodgin JB, Couse JF, Enmark E, Warner M, Mahler JF, Sar M, Korach KS, Gustafsson JA, Smithies O. Generation and reproductive phenotypes of mice lacking estrogen receptor β. Proc Natl Acad Sci USA. 1998;95:15677–15682. doi: 10.1073/pnas.95.26.15677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sato T, Fukazawa Y, Kojima H, Ohta Y, Iguchi T. Multiple mechanisms are involved in apoptotic cell death in the mouse uterus and vagina after ovariectomy. Re-prod Toxicol. 2003;17:289–297. doi: 10.1016/s0890-6238(03)00011-x. [DOI] [PubMed] [Google Scholar]
- 38.Lubahn DB, Moyer JS, Golding TS, Couse JF, Korach KS, Smithies O. Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proc Natl Acad Sci USA. 1993;90:11162–11166. doi: 10.1073/pnas.90.23.11162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tan J, Raja S, Davis MK, Tawfik O, Dey SK, Das SK. Evidence for coordinated interaction of cyclin D3 with p21 and cdk6 in directing the development of uterine stromal cell decidualization and polyploidy during implantation. Mech Dev. 2002;111:99–113. doi: 10.1016/s0925-4773(01)00614-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tan Y, Li M, Cox S, Davis MK, Tawfik O, Paria BC, Das SK. HB-EGF directs stromal cell polyploidy and decidualization via Cyclin D3 during implantation. Dev Biol. 2004;265:181–195. doi: 10.1016/j.ydbio.2003.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee EJ, Jakacka M, Duan WR, Chien PY, Martinson F, Gehm BD, Jameson JL. Adenovirus-directed expression of dominant negative estrogen receptor induces apoptosis in breast cancer cells and regression of tumors in nude mice. Mol Med. 2001;7:773–782. [PMC free article] [PubMed] [Google Scholar]
- 42.He TC, Zhou S, da Costa LT, Yu J, Kinzler KW, Vogelstein B. A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci USA. 1998;95:2509–2514. doi: 10.1073/pnas.95.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Das SK, Wang XN, Paria BC, Damm D, Abraham JA, Klagsbrun M, Andrews GK, Dey SK. Heparin-bind-ing EGF-like growth factor gene is induced in the mouse uterus temporally by the blastocyst solely at the site of its apposition: a possible ligand for interaction with blastocyst EGF-receptor in implantation. Development. 1994;120:1071–1083. doi: 10.1242/dev.120.5.1071. [DOI] [PubMed] [Google Scholar]
- 44.Das SK, Taylor JA, Korach KS, Paria BC, Dey SK, Lubahn DB. Estrogenic responses in estrogen receptor-α deficient mice reveal a novel estrogen signaling pathway. Proc Natl Acad Sci USA. 1997;94:12786–12791. doi: 10.1073/pnas.94.24.12786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yue L, Daikoku T, Hou X, Li M, Wang H, Noji H, Dey SK, Das SK. Cyclin G1 and cyclin G2 are expressed in the periimplantation mouse uterus in a cell-specific and progesterone-dependent manner: evidence for aberrant regulation with Hoxa-10 deficiency. Endocrinology. 2005;146:2424–2433. doi: 10.1210/en.2004-1605. [DOI] [PMC free article] [PubMed] [Google Scholar]