Abstract
Polyploidy has been reported in several animal cells, as well as within humans; however the mechanism of developmental regulation of this process remains poorly understood. Polyploidy occurs in normal biologic processes as well as in pathologic states. Decidual polyploid cells are terminally differentiated cells with a critical role in continued uterine development during embryo implantation and growth. Here we review the mechanisms involved in polyploidy cell formation in normal developmental processes, with focus on known regulatory aspects in decidual cells.
Keywords: Embryo Implantation, Decidualization, Polyploidy, Bi-Nucleation, Mitochondria, Cell Cycle, Review
2. INTRODUCTION
Infertility affects approximately 15% of couples during the reproductive years with implantation failure contributing largely to this statistic (1). In early pregnancy, ovarian estrogen and progesterone drive uterine differentiation, which is critical for embryonic growth and successful implantation (2, 3). Uterine differentiation in the receptive uterus is characterized by transformation of uterine stromal cells into decidual cells (decidualization) Defective decidualization at the site of implantation is one mechanism responsible for implantation failure resulting in infertility (4–6). Development of functional decidua begins with widespread stromal proliferation, followed by localized differentiation into these specialized cells, and subsequent development of polyploidy cells (7, 8). Polyploidization is the hallmark of mature decidual cells characterized by large mono- or binuclear cells with multiple copies of chromosomes and has been well recognized in rodents (9–11) and recently in the humans (Hirota Y and Dey SK, unpublished observation). The physiologic significance of this process in decidual cells appears to be crucial for successful embryo implantation and the support of embryo growth during the early pregnancy (1).
The development of polyploid cells is widely reported in nature occurring in a variety of plant and animal models. In mammals, several cells including hepatocytes, cardiac myocytes, arterial smooth muscle cells, megakaryocytes, trophoblasts, and decidual cells can all acquire varying degrees of polyploidy during their cell lifespan (6, 12–14). In addition, various biologic mechanisms occur in conjunction with polyploidy development, including cellular differentiation (8, 15), tissue regeneration (16), nutritional/metabolic activity (16, 17), and embryo implantation (1). The developmental mechanisms and function of polyploidy still remain largely unknown. Loss of regulation during the cell cycle is one possible mechanism for polyploidy development, by which the cell undergoes continuous DNA synthesis in the absence of cytokinesis resulting in formation of mono- or binucleated cells, containing DNA with multiple copies of the haploid complement (6, 12–14, 17). Here we focus on the mechanisms involved in polyploid cell generation as well as the function of polyploidy in decidualization.
2.1. Characteristics of polyploid cells
Several characteristics are endemic to polyploidy cells including cell size, DNA content, and metabolic activity. Here we briefly review these characteristics. Polyploidy commonly is associated with the termination of a highly proliferative phase in many tissues both during pathologic and normal physiologic processes including the uterine decidual bed (7, 8, 12). Polyploidization may be developmentally advantageous during rapid growth, modifying the cell cycle to utilize less energy expenditure by increasing cell volume and to bypass large cell surface area needed for several generations of cell division (16). Polyploid cells have increased cell size proportional to increase in DNA content (18). The cell size appears to be a result of the rate of both cell growth and cell division. Furthermore, the cell volume is similar between binucleated 2 × 2n and mononucleated 4n or binucleated 2 × 4n or mononucleated 8n (19), consistent with evolutionary conservation in eukaryotes between DNA content and cell volume (20). Polyploid cell formation from controlled mechanisms in normal physiology has specific characteristics that differ from those processes causing polyploidization in pathologic processes. Polyploid cells resulting from endoreplication produces cells where the DNA content is in integral multiples of the normal diploid, where abortive cell cycle processes associated with tumor formation produce cells with DNA content that varies continuously from 2N to significantly greater values (21). Moreover, terminally differentiated polyploid cells from endoreplication maintain viability for long periods of time whereas polyploidy from DNA damage activates apoptotic pathways (22). These processes characteristic to polyploid cells are likely implemented in time of rapid cell growth (i.e. decidualization) or when energy availability is limited; nevertheless adaption of cell growth and cell cycle regulation appear to be well controlled for directed purpose specifically to support embryo implantation within the endometrium.
2.2. Characteristics of decidual polyploidy
At the site of the implanting blastocyst, decidualizing stromal cells undergo extensive proliferation and differentiation. In the mouse model, localized stromal cell differentiation into specialized cells (decidual cells) occurs on day 5 of pregnancy (day 1 = vaginal plug) with polyploidization occurring days 6–8 of pregnancy (7, 8). Differentiated stromal cells adjacent to the implanting blastocyst form the primary decidual zone (PDZ) at the antimesometrial pole by the afternoon on day 5. The PDZ is composed primarily of epithelioid cells and is avascular in nature (23). By day 6, the secondary decidual zone (SDZ), comprised of proliferating and differentiated polyploidy stromal cells, forms adjacent to the PDZ. By day 7 afternoon, the SDZ is fully developed and polyploidy development now gradually spreading, occurring at the antimesometrial pole and at the lateral junctional region between the mesometrial and antimesometrial poles (Figure 1) (8). By day 8, the cells comprising the PDZ have progressively degenerated by apoptosis and mostly disappeared. The mesometrial pole, between day 7 and 8, is characterized by stromal cell proliferation and differentiation, but forms a non-polyploid decidual zone prior to placentation. Cell-cycle molecules, growth-factors, signaling mediators, and homeobox transcription factors have been shown to regulate decidualization as well as decidual cell polyploid formation (4–6, 24–26). In decidual cells, polyploidy cells have DNA content >4N, while non-polyploidy cells are composed of DNA content of 2N (Figure 2). Recently, differential regulation of genes were investigated to characterize gene expression patterns in decidual polyploidy cells as compared to nonpolyploidy decidual cells by microarray studies (Figure 3) (27). The physiologic importance of decidual polyploidy remains unclear however it is speculated that polyploidy formation limits the lifespan of the decidual cells to allow for growth of the implanting embryo as well as increased quantity of protein synthesis from endoreplication that is needed for embryo growth (6).
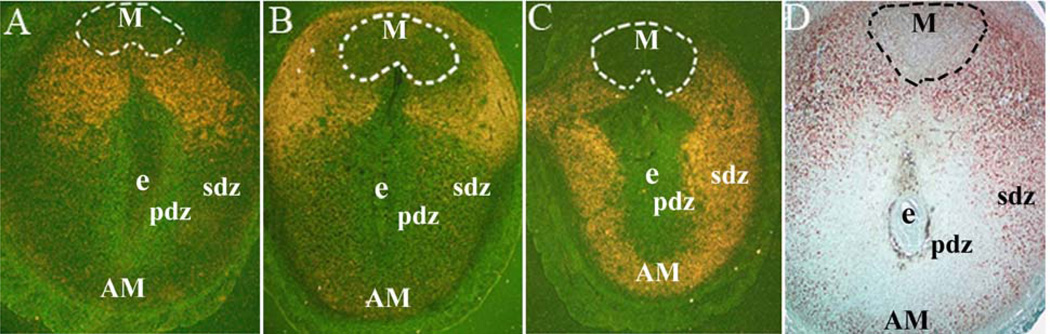
Figure 1.
Regional distribution of expression for up-regulated polyploidy genes during decidualization at the site of implantation. In situ hybridization: Expression of Nox4 (A), Nsbp1 (B), and Tdo2 (C) genes at the embryo implantation sites on day 7 of pregnancy. Dark-field photomicrographs of representative cross-sections hybridized with antisense probes are shown at 40X. Immunohistochemical analysis: Localization of CyclinD3 (D) at the embryo implantation sites on day 8. M, mesometrial pole; AM, anti-mesometrial pole; e, embryo; pdz, primary decidual zone; sdz, secondary decidual zone. (Reproduced with permission from, ref# 49).
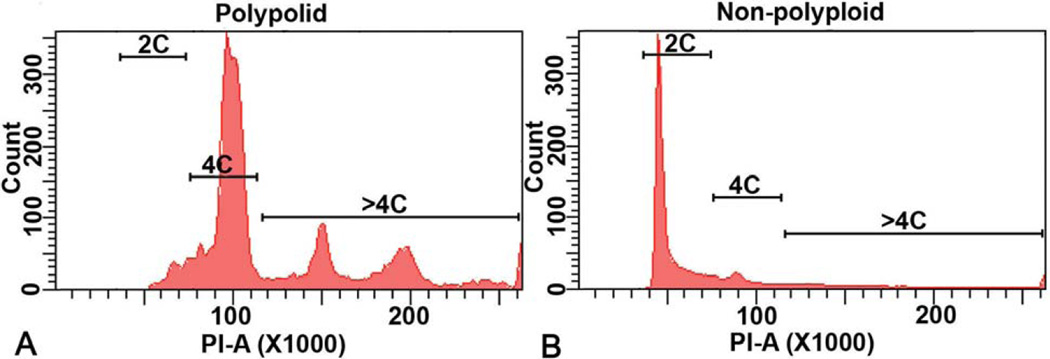
Figure 2.
Analysis of polyploid and non-polyploid cells. Deciduomal cells collected on day 7 of pseudopregnancy. Flow cytometric analyses of the DNA content for a representative polyploid (A) and non-polyploid (B) population are shown. Note: The polyploid fractions are enriched with DNA content > 4N, while the non-polyploid fractions are primarily devoid of cells with DNA content > 4N. (Reproduced with permission from, ref# 49).
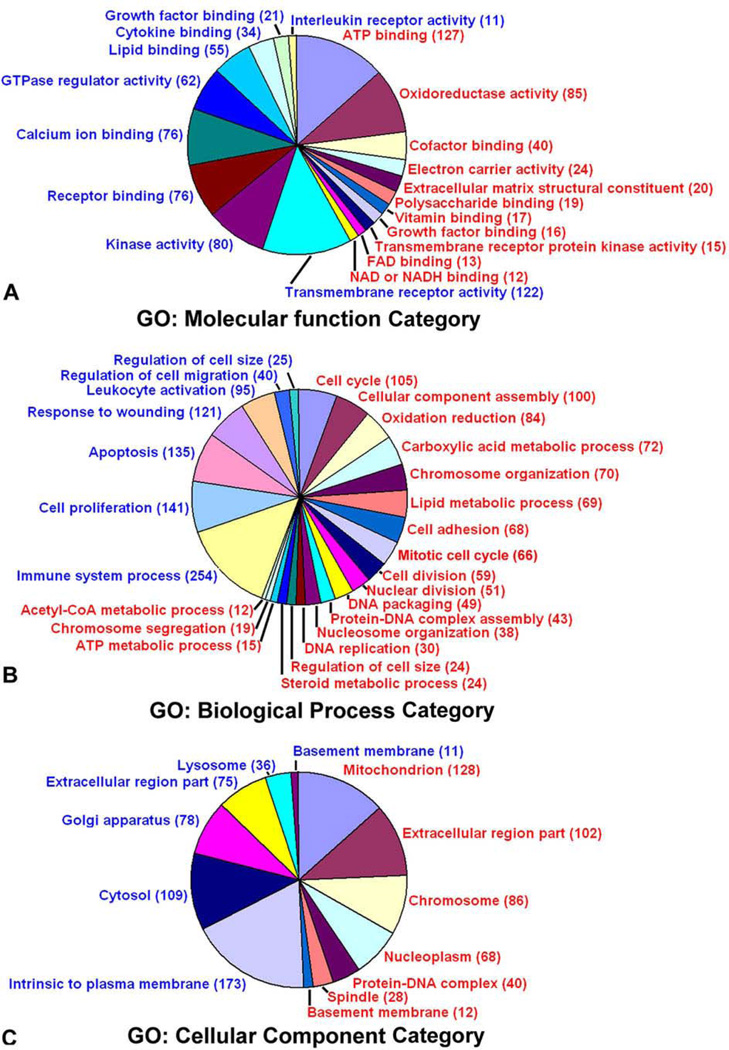
Figure 3.
Functional categorization of differentially expressed genes in the polyploid decidual cells. The up-regulated (red) and down-regulated (blue) genes are defined by the gene ontology (GO) terms: molecular function, biological processes, and cellular component categories. The number within the parenthesis represents the total genes modulated under a category. (Reproduced with permission from, ref# 49).
3. MECHANISMS OF POLYPLOIDIZATION
Within the normal cell cycle, the cell must obtain a complete copy of chromosomes with each cell division; otherwise the genomic stability within the cell can be compromised. Polyploidy occurs from deviations from the normal G1-S-G2-M cell cycle that allows genome replication without cell division (16). Several general mechanisms are considered for polyploidy cell formation occurring in diploid species, including: cell fusion, endoreplication (endomitosis), and an abortive cell cycle (20). In normal mammalian developmental processes, endoreplication appears to be the main mechanism leading to polyploidy generation (17). Abortive cell cycle involving defects in DNA replication, separation of sister chromatids, mitotic spindle checkpoints, and cytokinesis which leads to genomic instability (both polyploidy and aneuploidy) typically involved in pathologic states (20). However, polyploidy occurring in normal hepatocytes development may result from both incomplete cell division as well as endoreplication (12, 28). Here we review the proposed mechanisms involved in polyploidy cell generation most likely involved in decidual polyploidy formation by focusing on those processes involved in normal biologic processes.
3.1. Regulatory mechanisms of the endocycle
Endoreplication generates polyploid cells by a regulated cycle uncoupling DNA replication from cell division (17). In proliferating cells, the cell cycle is regulated by Cyclin-dependent kinases (Cdks), determining entry into both mitosis and S phase. Cdk1 is an important mediator of mitotic control. A and B type Cyclins and Cdc25 type phosphatases are activators of Cdk1 for mitotic phase regulation (29). Some endoreplicating cells may bypass mitosis, in these cells expression of Cdk1 and/or activators of Cdk1 may be diminished or aberrant indicating that Cdk activity may determine the degree of mitotic function within the endoreplicating cell (17). Initiation and progression of the S phase is regulated by Cdk2 by forming complexes with Cyclins A and E. The D type Cyclins with Cdk4 and Cdk6 mediate G0 to G1 transition. Fluctuations in Cyclin/Cdk levels through the normal cell cycle have been observed to maintain normal DNA replication and equal cell division (30). Low Cdk activity during G1 allows for formation of pre-replicating complexes (preRCs) necessary to signal that another round of DNA replication can be initiated. Cdks activity then increases in S and G2 phases inhibiting preRCs binding therefore blocking DNA replication during these cycles. During endoreplication, it is thought that Cdk activity oscillates between low and high levels to allow for multiple rounds of DNA replication and generation of polyploidy.
Cdk inhibitors (Ckis), which bind to and inhibit Cdk activity, are likely candidates for other regulators of endoreplication cycles by contributing to oscillations of Cdk/Cyclin levels. In addition, Ckis likely protect against inappropriate re-replication (29). Several Ckis, p57, p27, and p21, in vertebrates have been found to control cell cycles via inhibition of Cdk phosphorylation. Levels of Ckis oscillate during the cell cycle as well in order to allow for endoreplication (31). Overall, the specific interactions and expression levels of Cdks, Cyclins, and Ckis are important to the development of polyploidy in decidual cells as well as other cell types (Table 1).
Table 1.
Cell cycle regulatory proteins involved in polyploidy in normal biologic processes
| Category | Regulatory protein | Expression profile during endocycle | Stage of cell cycle | Cell types |
|---|---|---|---|---|
| Cdks | Cdk 1 | Down-regulated | M | Decidual cell, trophoblasts, megakaryocytes |
| Cdk2 | Up-regulated | S,G2 | Decidual cell, trophoblasts, megakaryocytes | |
| Cdk4 | Up-regulated | G1 | Megakaryocytes | |
| Cdk6 | Up-regulated | G1, S, G2 | Decidual cell, megakaryocytes | |
| Ckis | p21 | Up-regulated | G1, S, G2 | Decidual cell |
| p57 | Up-regulated | G1 | Trophoblast | |
| Cyclins | Cyclin A | Up-regulated | S, G2 | Decidual cell |
| Cyclin B | Down-regulated | M | Decidual cell | |
| Cyclin D3 | Up-regulated | G1, S, G2 | Decidual cell, trophoblasts, megakaryocytes | |
| Cyclin E | Up-regulated | G1/S transition | Decidual cell, trophoblasts, megakaryocytes |
3.1.1. The endocycle of decidual cells
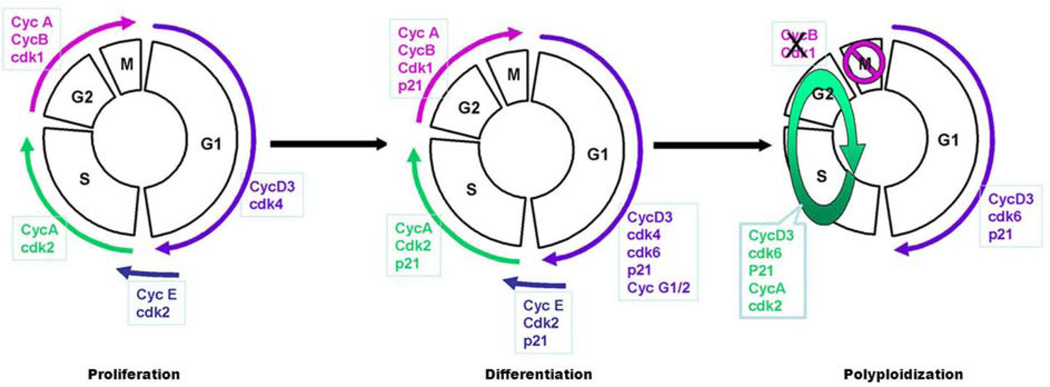
The polyploid decidual cells are thought to be terminally differentiated cells that develop via endoreplication cycle with repeated rounds of DNA replication without cytokinesis (6). The cell cycle is tightly regulated during decidualization with interplay between Cyclins, Cdks, and Ckis controlling proliferation and differentiation (Figure 4). More specifically, CyclinD3, Cdk4, Cdk6, and the Ckis p21 and p57 expression appear to play a role in directing decidualization (8). CyclinD3 has low expression within murine stromal cells on the morning of day 4, however its expression is dramatically up-regulated in decidualizing stromal cells following the initiation of implantation (32) whereas CyclinD1 and D2 have low expression prior to and during decidualization (8). CyclinD3 plays an important role in stromal proliferation, differentiation, and polyploidy formation in a stage-specific progression with temporal relationship with blastocyst implantation. CyclinD3 co-expression with Cdk4 is up-regulated in proliferating decidual cells in both the PDZ and the SDZ; however after proliferation is complete, Cdk4 down-regulation occurs in these zones (8) suggesting proliferation is controlled by CyclinD3 and Cdk4 co-activity. Furthermore, in polyploidy decidual cells we observed the overexpression of CyclinD3 with p21 and Cdk6, but not Cdk4, indicating that a complex of CyclinD3/Cdk6/p21 is implicated in polyploidy cell formation in decidual cells. Investigation of other Cyclins and Cdks during decidual polyploidy found that a large number of polyploidy cells demonstrate a heightened expression of CyclinA but not of CyclinB. Furthermore, Cdk1 and Cdk2 have similar expression patterns as CyclinB and A, respectively. Elevated CyclinE expression was also noted in polyploidy cells in the SDZ on days 6 and 7 of pregnancy. Together suggesting that CyclinB and Cdk1 down-regulation as well as up-regulation of CyclinA, CyclinE, and Cdk2 are also important in the regulation of signaling in the decidual cell endoreplication cycle (8). Although several cell cycle regulators are involved in uterine decidualization, CyclinD3 is the most investigated with studies demonstrating that other regulators of decidualization i.e. HB-EGF (heparin binding EGF-like growth factor) (33), DEDD (death-effector domain-containing protein) (1), Hoxa-10 (homeobox A10) (7, 32), Stathmin (34), IL11/IL11ra/BIRC5 (35), BTEB1 (basic transcription element-binding protein-1) (36), utilize CyclinD3 as a downstream controller of uterine decidualization and polyploidy formation.
Figure 4.
A proposed model for stromal cell proliferation, differentiation, and polyploidization. (Reproduced with permission from, ref# 6).
HB-EGF, an early marker of implantation (37), controls stromal cell polyploidy and binucleation by interacting with cell cycle regulatory molecules, specifically HB-EGF causes a marked overexpression of CyclinD3 in decidual cells (33). Polyploid decidual cells in in vitro culture demonstrated co-localization of CyclinD3/Cdk6/p21 in HB-EGF cultured cells but not in cells cultured in the absence of HB-EGF. In addition, in vivo experiments using HB-EGF-soaked beads transferred into pseudo-pregnant mouse uteri demonstrated presence of large mono- or binucleated cells in SDZ with expression of CyclinD3/Cdk6/p21 co-localized activity but this was not seen with BSA-soaked control beads. In addition, treatment of uterine stromal culture cells with antisense CyclinD3 and HB-EGF was completely inhibitory to polyploidy formation, and in vivo administration of CyclinD3 antisense adenovirus demonstrated reduced uterine weights on both days 7 and 8 of pregnancy (33). The CyclinD3 deficient mice also result in decidualization defect at the site of implantation with sub-fertility phenotype (6). Similarly, the loss of HB-EGF in mice also exhibits defects in the aspects of decidualization phenotype in early pregnancy (38). Together, these results suggest that CyclinD3 is crucial to stromal cell polyploidy formation regulated by HB-EGF both in vitro and in vivo (33).
DEDD, a member of DED-containing protein family, is found to regulate a variety of cellular signaling pathways, and has recently been implicated in uterine decidualization in the murine model (1). DEDD is associated with CyclinB1/Cdk1 activity; specially decreasing the activity of Cdk1 allowing for sufficient ribosomal RNA/protein synthesis and cell growth prior to cell division by inhibiting Cdk1 regulated mitotic progression (39, 40). DEDD also interacts with Akt and S6K1 within the PI3K signaling cascade (41, 42). Dedd null mice are completely sterile, and the infertility is likely secondary to decidualization defects. Dedd null mice exhibit normal implantation as compared with wild-type animals with similar weights on day 4.5 of pregnancy. However, the number of viable embryos rapidly declined starting on day 5.5, and by day 9.5 no living embryos were detected in Dedd−/− uteri (1). In addition, in Dedd null mice both immunostaining and mRNA expression levels for common decidualization markers were significantly reduced starting on day 5.5. Attenuation of decidual polyploidy was also noted in Dedd null mice. Expression of both Akt and CyclinD3 levels were reduced in these mice resulting in reduced polyploidy formation. The overexpression of both Akt and CyclinD3 in in vitro Dedd null stromal culture resulted in an increased proportion of polyploid cell formation. Additionally, DEDD/CyclinD3/Akt were co-localized in the decidual zones in vivo, with DEDD co-precipitated with CyclinD3 as well as CyclinD3/Cdk4 and CyclinD3/Cdk6. These finding suggest that DEDD plays an important role in uterine decidualization and polyploidization possible via Akt regulation as well as direct interaction with key cell cycle regulators (1). Mediators of cell cycle regulation play a significant role in stromal cell decidualization and polyploidy formation; however a complete understanding of all the mechanisms involved in decidual polyploidy must still be determined.
Hoxa-10, a member of the homeobox family of transcription factors (43), exhibits abundant expression in the uterine stroma during the receptive phase. The expression pattern is consistent with the status of proliferation and differentiation with the onset of decidualization (44). The loss of Hoxa-10 in mice have resulted in a complete failure of pregnancy, with a defective decidualization phenotype (45), and studies have shown that CyclinD3 expression remains downregulated in Hoxa-10−/− uteri during the progression of decidualization (32, 33). Overall, these results suggest that the loss of Hoxa-10 impairs decidualization primarily due to the deficiency of CyclinD3 at the site of implantation. Stathmin, a cytosolic phosphoprotein, has been shown to express in the antimesometrial decidual bed of the implantation site in mice and its deficiency in mice showed downregulation of CyclinD3 in the decidual bed with reduced fertility (34). BTEB1, a member of the Sp/Krüppel-like family of transcription factors, has been shown to interact with PR isoforms and express during decidualization in a PR-A-dependent fashion (36). Interestingly, Bteb−/− mice also show sub-fertility with predominant downregulation of CyclinD3 and Hoxa10 during decidualization (36). Moreover, recent studies also provided evidence that the critical decidualization signal via IL11/IL11ra (46, 47) also utilizes cyclin D3/p21 in conjunction with BIRC5 as downstream targets at the site of implantation (35). Despite the existing link of the above regulators with CyclinD3/p21, their roles in polyploidy development remain unknown and should be the subject for future study.
Additionally, uterine-specific p53 (a tumor suppressor gene) deficiency demonstrated impaired decidualization. In these mice, an elevated expression of p21 was noted with an increase in the number of decidual polyploidy cells. Typically p21 is up-regulated by p53 after DNA damage, but p21 can also be induced independent of p53. These results suggest that the loss of p53 enhances decidual cell polyploidy formation via stimulation of endocycle pushing these cells to terminal differentiation (48).
Recently, gene expression profiling comparing decidual polyploidy and non-polyploidy cells revealed a large group of genes (2222 genes total) that are differentially expressed, which suggests that a dramatic alteration of gene expression is necessary to potentiate the transition from a non-polyploidy state to a polyploidy state (49). Genetic expression analysis revealed several altered cell cycle genes, specifically 105 up-regulated and 11 down-regulated genes were found, confirming the importance cell cycle regulatory molecules in decidual cell polyploidy. A significant number of genes involved in the mitotic phase (72 total) were altered as well as p57, an inhibitor of Cdk1, which is associated with polyploidy in trophoblast giants cells (21). Several genes involved in nuclear division were also up-regulated and several genes related to bi-nucleated polyploidy were noted. Specifically, Td02 and Nsbp1 were detected in the bi-nucleated polyploidy cells within the decidual bed (49). Several minichromosome maintenance protein complexes (Mcm) that serve as eukaryotic helicase in DNA replication were up-regulated in polyploid decidual cells including Mcm2–5, and Mcm7.
Overall, endoreplication thus occurs with modification of the cell cycle to promote controlled repeat DNA replication producing polyploid cells. Multiple mechanisms in various species exist to allow for endoreplication to occur; and different cell types utilize different mechanisms. Although the specific mechanisms utilized by decidual cells remains unclear, understanding mechanisms in endocycles of other cells may help to uncover the gaps in knowledge of decidual cell polyploidy.
3.1.2. The endocycle of other cells
In Drosophila, continuous expression of CycleE/Cdk 2 or CyclinE genetic mutation results in cessations of endocycles (50–52). While triggering S phase, CyclinE/Cdk2 activity is also responsible for ensuring that only one DNA replication occurs during the S phase by directing separation of pre-RCs from origins of replication during oscillations of activity (53). Additionally, Cdk4 is the target of CyclinD3 and directs cell growth in Drosophila (54).
Several mammalian cells utilize the above mechanisms to undergo endoreplication during normal physiologic processes. CyclinE activity is required for endoreplication in rodent trophoblasts and megakaryocytes (55) with the Cyclin E/Cdk2 complex playing a major role in both mammal and insect models (16). Megakaryocytes require CyclinD3 for polyploidy formation, with CyclinD3 overexpression causing increased polyploidy in these cells (56). Reduced CyclinD3 levels show inhibition of polyploidy with thrombopoietin controlling expression of CyclinD3 in these cells (56). Cdk4 and Cdk6 are possibly the downstream target of CyclinD3 in megakaryocytes, however, this has not been extensively investigated. In megakaryocytes, cells complete anaphase A with separation of sister chromatids but will not complete anaphase B. This process therefore results in replicated chromosomal copies within the same nucleus, skipping cytokinesis completely (17). Decreased levels of CyclinB and CyclinB/Cdk1 have been found in endocycles of these cells, and excess degradation of CyclinB may drive deviation from anaphase B and cytokinesis (57).
Mammalian trophoblastic cells undergo cyclic DNA replication with certain mitotic features. In these cells, during endocycles, CyclinD1 is induced, high levels of CyclinE and A are noted, but CyclinB levels are diminished consistent with other findings in mammalian endoreplication cycles (58). Endoreplication is severely impaired in CyclinE deficient trophoblastic mouse cells by failing to incorporate MCM proteins into DNA replication origins (55). In addition, P57 levels in rodent trophoblasts are up-regulated in the G-phase and it acts via Cdk1 suppression to stimulate endoreplication (21).
3.2. Other mechanisms in polyploidization
3.2.1. Spindle checkpoints
Mitosis is the final stage of the cell cycle. During this stage, duplicated chromosomes from the S phase are separated into two equal daughter cells. For equal segregation of chromosomes, which have random distribution within the nucleus during interphase, alignment at the spindle equator in mitosis must occur prior to the initiation of sister chromatid separation. The spindle checkpoint is a regulatory mechanism that delays entry into anaphase until each chromosome is on the way to the spindle equator and all chromosomal kinetochores are attached to the spindle (59). Failure of checkpoint mechanisms results in polyploidy and aneuploidy that have been well-studied in tumorigenesis, but also occurs in some normal cell processes for endoduplication (59).
The spindle checkpoint is activated in the presence of kinetochores unattached to the spindle microtubules to prevent entry into anaphase. Mad (mitotic arrest deficient) 1, 2, 3, Bub (budding uninhibited by benzimidazole) 1, and 3 are identified genetic factors involved in spindle checkpoint. Mad2 is one of the most important components of checkpoint activation (60, 61) by forming a complex with Cdc20 (cell-cell division protein 20) preventing activation of Cdc20-APC/C (Anaphase Promoting Complex or Cyclosome) which activates Separase to cleave the two sister chromatids (62). Overexpression of Mad2 can delay anaphase, but in eukaryotes this overexpression induces tetraploidy likely because cells eventually transition from mitosis without sister chromatid separation (59). In addition, transgenic mice with overexpression Mad2 demonstrated chromosome instability with resulting tetraploidy (63). In this regard, a recent study by our group demonstrated in decidual polyploidy cells up-regulation of several genes involved in nuclear division (49). Among these were genes encoding proteins such as Mad2L1, Mad2L2, Bub1, and Bub1B which are involved in spindle assembly checkpoint and maintenance of chromosomal stability (64).
3.2.2. Cytokinesis failure
Cytokinesis occurs by sequential progression through four stages: determining the cleavage plane, ingression of the cleave furrow, formation of the midbody, and abscission (65). Improper completion of any stage can lead to failure of cytokinesis resulting in polyploidy formation, and some proteins regulate multiple steps in cytokinesis thus alterations in these proteins may make cells prone to incomplete cytokinesis. Failure of cytokinesis has historically been associated with production of tetraploid cells that give rise to tumors; however recently cytokinesis failure has been associated with normal physiologic processes (28, 66). The RhoA pathway is essential for efficient and correct furrow formation to occur in animal models (65, 67). Localized activation of RhoA is vital to microtubules in delivering signals in the furrow; aberrations of these signals by spindle disruption can lead to cytokinesis failure. Failure during the initial stage of cytokinesis by disrupted spindle elongation or positioning has recently been reported in normal physiology of hepatocytes (28, 66). In rat hepatocytes, failure of cytokinesis in diploid, mononucleated cells results in polyploidy cells secondary to failure of contractile ring formation (28) during mitosis and thus cells fail to undergo anaphase (66). Processes in cytokinesis fall under control of Cdk regulators (68). Cdk1 inactivation must occur for progression of cytokinesis, whereas Polo kinase and Aurora B kinase must be activated following Cdk1 inactivation to stimulate cytokinesis (65). Cdk1 inactivation may be the trigger for cytokinesis initiation via regulation of the RhoA pathway; Polo kinase also functions by regulating the Rho A pathway. Interestingly, some of the regulators have been found to upregulated in polyploid decidual cells (49), however, the role of these specific regulators in decidual cells is unclear and require further research to better understand the mechanisms in decidual polyploidy.
4. FURTHER CHARACTERIZATION OF DECIDUAL POLYPLOIDY
Uterine stromal cell polyploidization is crucial in directing decidualization and early implantation within the mouse model (1, 48). In our recent study, we utilize pure polyploid decidual cells as a model for further characterization of mouse decidual cell polyploidy formation by characterizing gene expression patterns within this model (49).
4.1. The mitochondrial role in decidual polyploidization
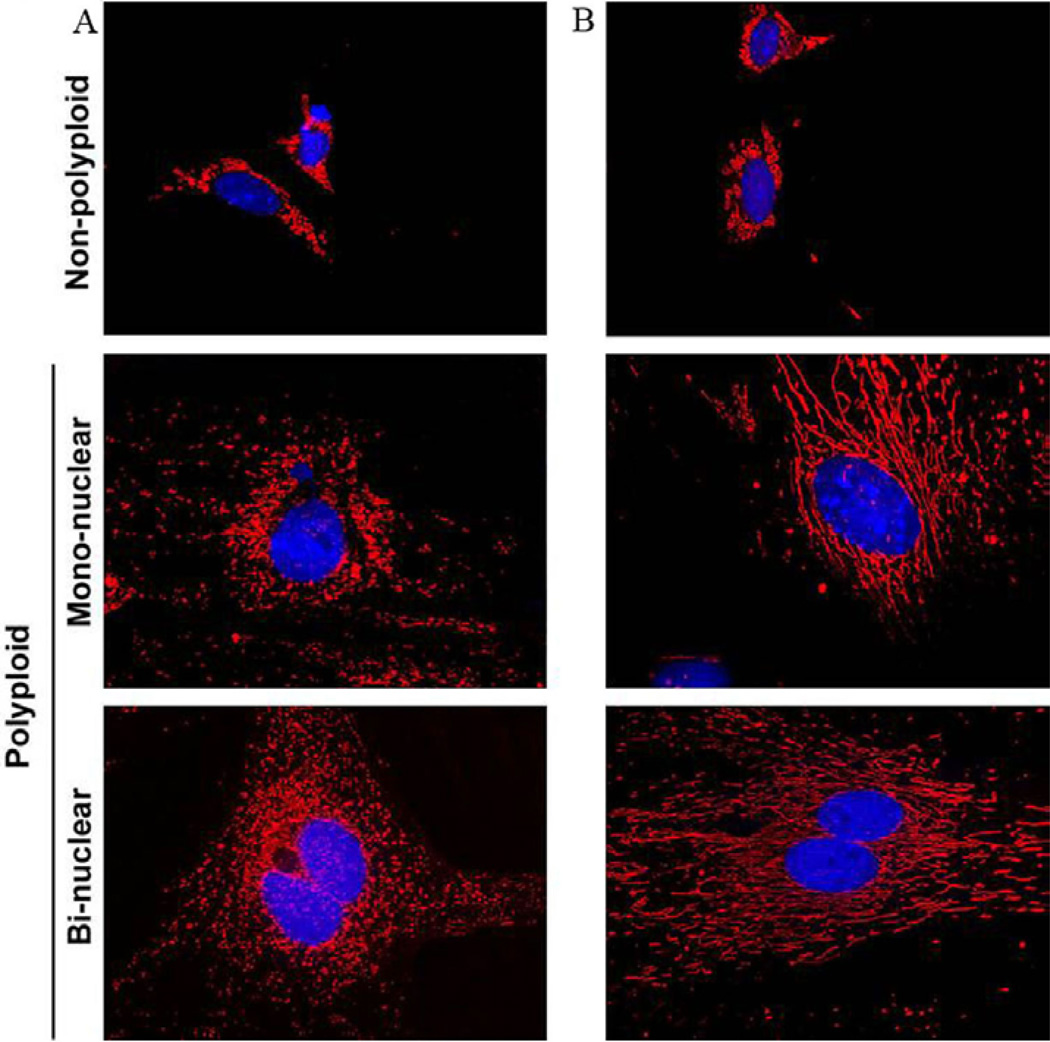
The development of polyploidy via endoreplication occurs in various cells during times of tissue regeneration and stress to promote growth and proliferation. During time of oxidative stress there is an increase in ROS production via Nox4 (NADPH oxidase 4) enzymatic activity (69–72). Hyperactivation of mitochondrial activity stimulated increased ROS production (73); and Nox4 has been shown to be associated with polyploidy development (69). High energy driven mitochondrial activity has been shown to be important in polyploid decidual cell development (49), in order to support both uterine differentiation and embryo implantation. Global gene expression profiling of polyploid decidual cells, as compared to non-polyploid decidual cells via microarray analysis revealed 7 mitochondrial gene networks including lipid metabolism, carbohydrate metabolism, cellular assembly and organization, genetic disorder, metabolic disease, small molecule biochemistry, and drug metabolism involved in polyploidy development. More specifically, Nox4 was found to be up-regulated in polyploid cells (Figure 1) and is expressed in the decidual bed consistent with polyploidy association (49). Several other identified genes with mitochondria and binuclear characteristic were up-regulated in the decidual bed in a regional pattern, consistent polyploidy expression (49). Marked increase in mitochondrial mass in both binuclear and mononuclear polyploidy cells was noted using confocal microscopy in both in vivo and in vitro models, as compared to non-polyploid cells (Figure 5). To confirm the role of mitochondria in polyploidization, we uncoupled the respiratory chain and oxidative phosphorylation in decidualizing stromal cell in vitro; and found a dramatic inhibition of binucleation and polyploidy (49). In conclusion, mitochondrial activity appears to be crucial to the development of decidual cell polyploidy, an essential process for successful pregnancy (1). Furthermore, abnormal mitochondrial function may impair decidual cell polyploidization causing infertility.
Figure 5.
Analysis of mitochondria in relation to decidual cell polyploidy. Mitochondrial mass analysis by confocal microscopy. (A) Pure polyploid and non-polyploid decidual cells isolated from day 7 deciduomal tissues. (B) Day 4 uterine stromal cells in culture were used to induce decidualization in vitro. Polyploid and non-polyploid cells were collected on day-5 following the induction of decidualization. Both cell populations derived in vivo and in vitro were cyto-spun onto slides and analyzed by staining with Mitotracker Red. (Reproduced with permission from, ref# 49).
4.2. The role of immune cells and apoptosis in decidual polyploidy
Previously, it has been reported that the implantation site demonstrates a reduction in the immune response (74, 75) suggesting that implantation is protected against maternal immunological response during pregnancy. Although maternal natural killer cells are recruited to the attachment site after embryo attachment (76), other immunological cells including leukocytes and other bone marrow-derived cells migrate from the blastocyst implantation site (75). Consistent with these finding, a large number of immune-related genes are down-regulated within decidual polyploidy cells when compared to non-polyploidy cells (49). Furthermore, it has previously been shown that apoptosis is minimally detected in uterine polyploid decidual cells (77), and gene expression analysis demonstrated that genes associated with apoptosis are consistently suppressed in decidual polyploidy cells when compared to non-polyploidy cells (49). Overall, it can be suggested that decidual polyploid cells primarily lack apoptosis and immune suppression properties.
5. PERSPECTIVES
Implantation and pregnancy rates even with assisted reproductive technologies still remain disappointingly low likely secondary to poorly understood implantation and decidualization defects. Several restraints exists limiting human uterine-embryo interactions; however animal models with similar processes (i.e. decidualization) to humans provide understanding of mechanisms involved in early embryo-uterine interactions. Continued research of these processes is vital to alleviating problems associated with infertility. Stromal polyploidy formation is a critical step in normal decidualization in both human and rodents. Understanding the physiologic functions of specific factors involved in polyploidy mechanistically via overexpression, silencing, or target gene deletions studies will provide valuable information to improved understanding human decidualization and early pregnancy.
ACKNOWLEDGEMENTS
This work was supported in parts by NIH grants: HD056044 and ES007814.
Abbreviations
- PDZ
primary decidual zone
- SDZ
secondary decidual zone
- cdks
cyclin-dependent kinases
- preRCs
pre-replicating complexes
- Ckis
cdk inhibitors
- DEDD
death-effector domain-containing protein
- Hoxa-10
homeobox A10
- BTEB1
basic transcription element-binding protein-1
- MCM
minichromosome maintenance protein complexes
- Mad
mitotic arrest deficient
- Bub
budding uninhibited by benzimidazole
- APC/C
Anaphase Promoting Complex or Cyclosome
- Nox4
NADPH oxidase 4
REFERENCES
- 1.Mori M, Kitazume M, Ose R, Kurokawa J, Koga K, Osuga Y, Arai S, Miyazaki T. Death effector domain-containing protein (DEDD) is required for uterine decidualization during early pregnancy in mice. J Clin Invest. 2011;121:318–327. doi: 10.1172/JCI44723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paria BC, Huet-Hudson YM, Dey SK. Blastocyst's state of activity determines the "window" of implantation in the receptive mouse uterus. Proc Natl Acad Sci U S A. 1993;90:10159–10162. doi: 10.1073/pnas.90.21.10159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Psychoyos A. Hormonal control of ovoimplantation. Vitam Horm. 1973;31:201–256. doi: 10.1016/s0083-6729(08)60999-1. [DOI] [PubMed] [Google Scholar]
- 4.Wang H, Dey SK. Roadmap to embryo implantation: clues from mouse models. Nat Rev Genet. 2006;7:185–199. doi: 10.1038/nrg1808. [DOI] [PubMed] [Google Scholar]
- 5.Dey SK, Lim H, Das SK, Reese J, Paria BC, Daikoku T, Wang H. Molecular cues to implantation. Endocr Rev. 2004;25:341–373. doi: 10.1210/er.2003-0020. [DOI] [PubMed] [Google Scholar]
- 6.Das SK. Cell cycle regulatory control for uterine stromal cell decidualization in implantation. Reproduction. 2009;137:889–899. doi: 10.1530/REP-08-0539. [DOI] [PubMed] [Google Scholar]
- 7.Rahman MA, Li M, Li P, Wang H, Dey SK, Das SK. Hoxa-10 deficiency alters region-specific gene expression and perturbs differentiation of natural killer cells during decidualization. Dev Biol. 2006;290:105–117. doi: 10.1016/j.ydbio.2005.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tan J, Raja S, Davis MK, Tawfik O, Dey SK, Das SK. Evidence for coordinated interaction of cyclin D3 with p21 and cdk6 in directing the development of uterine stromal cell decidualization and polyploidy during implantation. Mech Dev. 2002;111:99–113. doi: 10.1016/s0925-4773(01)00614-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sachs L, Shelesnyak MC. The development and suppression of polyploidy in the developing and suppressed deciduoma in the rat. J Endocrinol. 1955;12:146–151. doi: 10.1677/joe.0.0120146. [DOI] [PubMed] [Google Scholar]
- 10.Ansell JD, Barlow PW, McLaren A. Binucleate and polyploid cells in the decidua of the mouse. J Embryol Exp Morphol. 1974;31:223–227. [PubMed] [Google Scholar]
- 11.Moulton BC. Effect of progesterone on DNA, RNA and protein synthesis of deciduoma cell fractions separated by velocity sedimentation. Biol Reprod. 1979;21:667–672. doi: 10.1095/biolreprod21.3.667. [DOI] [PubMed] [Google Scholar]
- 12.Celton-Morizur S, Desdouets C. Polyploidization of liver cells. Adv Exp Med Biol. 2010;676:123–135. doi: 10.1007/978-1-4419-6199-0_8. [DOI] [PubMed] [Google Scholar]
- 13.Hu D, Cross JC. Development and function of trophoblast giant cells in the rodent placenta. Int J Dev Biol. 2010;54:341–354. doi: 10.1387/ijdb.082768dh. [DOI] [PubMed] [Google Scholar]
- 14.Nguyen HG, Ravid K. Polyploidy: mechanisms and cancer promotion in hematopoietic and other cells. Adv Exp Med Biol. 2010;676:105–122. doi: 10.1007/978-1-4419-6199-0_7. [DOI] [PubMed] [Google Scholar]
- 15.Cross JC. Genetic insights into trophoblast differentiation and placental morphogenesis. Semin Cell Dev Biol. 2000;11:105–113. doi: 10.1006/scdb.2000.0156. [DOI] [PubMed] [Google Scholar]
- 16.Lee HO, Davidson JM, Duronio RJ. Endoreplication: polyploidy with purpose. Genes Dev. 2009;23:2461–2477. doi: 10.1101/gad.1829209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edgar BA, Orr-Weaver TL. Endoreplication cell cycles: more for less. Cell. 2001;105:297–306. doi: 10.1016/s0092-8674(01)00334-8. [DOI] [PubMed] [Google Scholar]
- 18.Epstein CJ. Cell size, nuclear content, and the development of polyploidy in the Mammalian liver. Proc Natl Acad Sci U S A. 1967;57:327–334. doi: 10.1073/pnas.57.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin NC, McCullough CT, Bush PG, Sharp L, Hall AC, Harrison DJ. Functional analysis of mouse hepatocytes differing in DNA content: volume, receptor expression, and effect of IFNgamma. J Cell Physiol. 2002;191:138–144. doi: 10.1002/jcp.10057. [DOI] [PubMed] [Google Scholar]
- 20.Storchova Z, Pellman D. From polyploidy to aneuploidy, genome instability and cancer. Nat Rev Mol Cell Biol. 2004;5:45–54. doi: 10.1038/nrm1276. [DOI] [PubMed] [Google Scholar]
- 21.Ullah Z, Kohn MJ, Yagi R, Vassilev LT, DePamphilis ML. Differentiation of trophoblast stem cells into giant cells is triggered by p57/Kip2 inhibition of CDK1 activity. Genes Dev. 2008;22:3024–3036. doi: 10.1101/gad.1718108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu W, Dutta A. An ATR- and BRCA1-mediated Fanconi anemia pathway is required for activating the G2/M checkpoint and DNA damage repair upon rereplication. Mol Cell Biol. 2006;26:4601–4611. doi: 10.1128/MCB.02141-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paria BC, Zhao X, Das SK, Dey SK, Yoshinaga K. Zonula occludens-1 and E-cadherin are coordinately expressed in the mouse uterus with the initiation of implantation and decidualization. Dev Biol. 1999;208:488–501. doi: 10.1006/dbio.1999.9206. [DOI] [PubMed] [Google Scholar]
- 24.Kennedy TG, Gillio-Meina C, Phang SH. Prostaglandins and the initiation of blastocyst implantation and decidualization. Reproduction. 2007;134:635–643. doi: 10.1530/REP-07-0328. [DOI] [PubMed] [Google Scholar]
- 25.Lee KY, Jeong JW, Tsai SY, Lydon JP, DeMayo FJ. Mouse models of implantation. Trends Endocrinol Metab. 2007;18:234–239. doi: 10.1016/j.tem.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 26.Das SK. Regional development of uterine decidualization: molecular signaling by Hoxa-10. Mol Reprod Dev. 2010;77:387–396. doi: 10.1002/mrd.21133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cakmak H, Taylor HS. Implantation failure: molecular mechanisms and clinical treatment. Hum Reprod Update. 2011;17:242–253. doi: 10.1093/humupd/dmq037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guidotti JE, Bregerie O, Robert A, Debey P, Brechot C, Desdouets C. Liver cell polyploidization: a pivotal role for binuclear hepatocytes. J Biol Chem. 2003;278:19095–19101. doi: 10.1074/jbc.M300982200. [DOI] [PubMed] [Google Scholar]
- 29.Morgan DO. Cyclin-dependent kinases: engines, clocks, and microprocessors. Annu Rev Cell Dev Biol. 1997;13:261–291. doi: 10.1146/annurev.cellbio.13.1.261. [DOI] [PubMed] [Google Scholar]
- 30.Donaldson AD, Blow JJ. The regulation of replication origin activation. Curr Opin Genet Dev. 1999;9:62–68. doi: 10.1016/s0959-437x(99)80009-4. [DOI] [PubMed] [Google Scholar]
- 31.Hattori N, Davies TC, Anson-Cartwright L, Cross JC. Periodic expression of the cyclin-dependent kinase inhibitor p57(Kip2) in trophoblast giant cells defines a G2-like gap phase of the endocycle. Mol Biol Cell. 2000;11:1037–1045. doi: 10.1091/mbc.11.3.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Das SK, Lim H, Paria BC, Dey SK. Cyclin D3 in the mouse uterus is associated with the decidualization process during early pregnancy. J Mol Endocrinol. 1999;22:91–101. doi: 10.1677/jme.0.0220091. [DOI] [PubMed] [Google Scholar]
- 33.Tan Y, Li M, Cox S, Davis MK, Tawfik O, Paria BC, Das SK. HB-EGF directs stromal cell polyploidy and decidualization via cyclin D3 during implantation. Dev Biol. 2004;265:181–195. doi: 10.1016/j.ydbio.2003.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yoshie M, Tamura K, Hara T, Kogo H. Expression of stathmin family genes in the murine uterus during early pregnancy. Mol Reprod Dev. 2006;73:164–172. doi: 10.1002/mrd.20408. [DOI] [PubMed] [Google Scholar]
- 35.Li F, Devi YS, Bao L, Mao J, Gibori G. Involvement of cyclin D3, CDKN1A (p21), and BIRC5 (Survivin) in interleukin 11 stimulation of decidualization in mice. Biol Reprod. 2008;78:127–133. doi: 10.1095/biolreprod.107.063313. [DOI] [PubMed] [Google Scholar]
- 36.Simmen RC, Eason RR, McQuown JR, Linz AL, Kang TJ, Chatman L, Jr, Till SR, Fujii-Kuriyama Y, Simmen FA, Oh SP. Subfertility, uterine hypoplasia, and partial progesterone resistance in mice lacking the Kruppel-like factor 9/basic transcription element-binding protein-1 (Bteb1) gene. J Biol Chem. 2004;279:29286–29294. doi: 10.1074/jbc.M403139200. [DOI] [PubMed] [Google Scholar]
- 37.Das SK, Wang XN, Paria BC, Damm D, Abraham JA, Klagsbrun M, Andrews GK, Dey SK. Heparin-binding EGF-like growth factor gene is induced in the mouse uterus temporally by the blastocyst solely at the site of its apposition: a possible ligand for interaction with blastocyst EGF-receptor in implantation. Development. 1994;120:1071–1083. doi: 10.1242/dev.120.5.1071. [DOI] [PubMed] [Google Scholar]
- 38.Das SK, Dey SK. Embryo-uterine interactions during implantation: potential sites of interference by environmental toxins. Chapter 11.21. In: Richburg J, Hoyer P, editors. Comprehensive Toxicology. New York: Academic Press; 2010. [Google Scholar]
- 39.Arai S, Miyake K, Voit R, Nemoto S, Wakeland EK, Grummt I, Miyazaki T. Death-effector domain-containing protein DEDD is an inhibitor of mitotic Cdk1/cyclin B1. Proc Natl Acad Sci U S A. 2007;104:2289–2294. doi: 10.1073/pnas.0611167104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miyazaki T, Arai S. Two distinct controls of mitotic cdk1/cyclin B1 activity requisite for cell growth prior to cell division. Cell Cycle. 2007;6:1419–1425. [PubMed] [Google Scholar]
- 41.Kurabe N, Arai S, Nishijima A, Kubota N, Suizu F, Mori M, Kurokawa J, Kondo-Miyazaki M, Ide T, Murakami K, Miyake K, Ueki K, Koga H, Yatomi Y, Tashiro F, Noguchi M, Kadowaki T, Miyazaki T. The death effector domain-containing DEDD supports S6K1 activity via preventing Cdk1-dependent inhibitory phosphorylation. J Biol Chem. 2009;284:5050–5055. doi: 10.1074/jbc.M808598200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kurabe N, Mori M, Kurokawa J, Taniguchi K, Aoyama H, Atsuda K, Nishijima A, Odawara N, Harada S, Nakashima K, Arai S, Miyazaki T. The death effector domain-containing DEDD forms a complex with Akt and Hsp90, and supports their stability. Biochem Biophys Res Commun. 2010;391:1708–1713. doi: 10.1016/j.bbrc.2009.12.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Satokata I, Benson G, Maas R. Sexually dimorphic sterility phenotypes in Hoxa10-deficient mice. Nature. 1995;374:460–463. doi: 10.1038/374460a0. [DOI] [PubMed] [Google Scholar]
- 44.Daikoku T, Song H, Guo Y, Riesewijk A, Mosselman S, Das SK, Dey SK. Uterine Msx-1 and Wnt4 signaling becomes aberrant in mice with the loss of leukemia inhibitory factor or Hoxa-10: evidence for a novel cytokine-homeobox-Wnt signaling in implantation. Mol Endocrinol. 2004;18:1238–1250. doi: 10.1210/me.2003-0403. [DOI] [PubMed] [Google Scholar]
- 45.Benson GV, Lim H, Paria BC, Satokata I, Dey SK, Maas RL. Mechanisms of reduced fertility in Hoxa-10 mutant mice: uterine homeosis and loss of maternal Hoxa-10 expression. Development. 1996;122:2687–2696. doi: 10.1242/dev.122.9.2687. [DOI] [PubMed] [Google Scholar]
- 46.Bilinski P, Roopenian D, Gossler A. Maternal IL-11Ralpha function is required for normal decidua and fetoplacental development in mice. Genes Dev. 1998;12:2234–2243. doi: 10.1101/gad.12.14.2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Robb L, Li R, Hartley L, Nandurkar HH, Koentgen F, Begley CG. Infertility in female mice lacking the receptor for interleukin 11 is due to a defective uterine response to implantation. Nat Med. 1998;4:303–308. doi: 10.1038/nm0398-303. [DOI] [PubMed] [Google Scholar]
- 48.Hirota Y, Daikoku T, Tranguch S, Xie H, Bradshaw HB, Dey SK. Uterine-specific p53 deficiency confers premature uterine senescence and promotes preterm birth in mice. J Clin Invest. 2010;120:803–815. doi: 10.1172/JCI40051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ma X, Gao F, Rusie A, Hemingway J, Ostmann AB, Sroga JM, Jegga AG, Das SK. Decidual cell polyploidization necessitates mitochondrial activity. PLoS One. 2011;6:e26774. doi: 10.1371/journal.pone.0026774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Follette PJ, Duronio RJ, O'Farrell PH. Fluctuations in cyclin E levels are required for multiple rounds of endocycle S phase in Drosophila. Curr Biol. 1998;8:235–238. doi: 10.1016/s0960-9822(98)70089-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weiss A, Herzig A, Jacobs H, Lehner CF. Continuous Cyclin E expression inhibits progression through endoreduplication cycles in Drosophila. Curr Biol. 1998;8:239–242. doi: 10.1016/s0960-9822(98)70090-9. [DOI] [PubMed] [Google Scholar]
- 52.Knoblich JA, Sauer K, Jones L, Richardson H, Saint R, Lehner CF. Cyclin E controls S phase progression and its down-regulation during Drosophila embryogenesis is required for the arrest of cell proliferation. Cell. 1994;77:107–120. doi: 10.1016/0092-8674(94)90239-9. [DOI] [PubMed] [Google Scholar]
- 53.Arias EE, Walter JC. Strength in numbers: preventing rereplication via multiple mechanisms in eukaryotic cells. Genes Dev. 2007;21:497–518. doi: 10.1101/gad.1508907. [DOI] [PubMed] [Google Scholar]
- 54.Datar SA, Jacobs HW, de la Cruz AF, Lehner CF, Edgar BA. The Drosophila cyclin D-Cdk4 complex promotes cellular growth. EMBO J. 2000;19:4543–4554. doi: 10.1093/emboj/19.17.4543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Geng Y, Yu Q, Sicinska E, Das M, Schneider JE, Bhattacharya S, Rideout WM, Bronson RT, Gardner H, Sicinski P. Cyclin E ablation in the mouse. Cell. 2003;114:431–443. doi: 10.1016/s0092-8674(03)00645-7. [DOI] [PubMed] [Google Scholar]
- 56.Zimmet JM, Ladd D, Jackson CW, Stenberg PE, Ravid K. A role for cyclin D3 in the endomitotic cell cycle. Mol Cell Biol. 1997;17:7248–7259. doi: 10.1128/mcb.17.12.7248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang Y, Wang Z, Liu DX, Pagano M, Ravid K. Ubiquitin-dependent degradation of cyclin B is accelerated in polyploid megakaryocytes. J Biol Chem. 1998;273:1387–1392. doi: 10.1074/jbc.273.3.1387. [DOI] [PubMed] [Google Scholar]
- 58.MacAuley A, Cross JC, Werb Z. Reprogramming the cell cycle for endoreduplication in rodent trophoblast cells. Mol Biol Cell. 1998;9:795–807. doi: 10.1091/mbc.9.4.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ito D, Matsumoto T. Molecular mechanisms and function of the spindle checkpoint, a guardian of the chromosome stability. Adv Exp Med Biol. 2010;676:15–26. doi: 10.1007/978-1-4419-6199-0_2. [DOI] [PubMed] [Google Scholar]
- 60.Hwang LH, Lau LF, Smith DL, Mistrot CA, Hardwick KG, Hwang ES, Amon A, Murray AW. Budding yeast Cdc20: a target of the spindle checkpoint. Science. 1998;279:1041–1044. doi: 10.1126/science.279.5353.1041. [DOI] [PubMed] [Google Scholar]
- 61.Kim SH, Lin DP, Matsumoto S, Kitazono A, Matsumoto T. Fission yeast Slp1: an effector of the Mad2-dependent spindle checkpoint. Science. 1998;279:1045–1047. doi: 10.1126/science.279.5353.1045. [DOI] [PubMed] [Google Scholar]
- 62.Peters JM. The anaphase promoting complex/cyclosome: a machine designed to destroy. Nat Rev Mol Cell Biol. 2006;7:644–656. doi: 10.1038/nrm1988. [DOI] [PubMed] [Google Scholar]
- 63.Sotillo R, Hernando E, Diaz-Rodriguez E, Teruya-Feldstein J, Cordon-Cardo C, Lowe SW, Benezra R. Mad2 overexpression promotes aneuploidy and tumorigenesis in mice. Cancer Cell. 2007;11:9–23. doi: 10.1016/j.ccr.2006.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chan GK, Yen TJ. The mitotic checkpoint: a signaling pathway that allows a single unattached kinetochore to inhibit mitotic exit. Prog Cell Cycle Res. 2003;5:431–439. [PubMed] [Google Scholar]
- 65.Normand G, King RW. Understanding cytokinesis failure. Adv Exp Med Biol. 2010;676:27–55. doi: 10.1007/978-1-4419-6199-0_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Margall-Ducos G, Celton-Morizur S, Couton D, Bregerie O, Desdouets C. Liver tetraploidization is controlled by a new process of incomplete cytokinesis. J Cell Sci. 2007;120:3633–3639. doi: 10.1242/jcs.016907. [DOI] [PubMed] [Google Scholar]
- 67.Kamijo K, Ohara N, Abe M, Uchimura T, Hosoya H, Lee JS, Miki T. Dissecting the role of Rho-mediated signaling in contractile ring formation. Mol Biol Cell. 2006;17:43–55. doi: 10.1091/mbc.E05-06-0569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rieder CL, Maiato H. Stuck in division or passing through: what happens when cells cannot satisfy the spindle assembly checkpoint. Dev Cell. 2004;7:637–651. doi: 10.1016/j.devcel.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 69.McCrann DJ, Yang D, Chen H, Carroll S, Ravid K. Upregulation of Nox4 in the aging vasculature and its association with smooth muscle cell polyploidy. Cell Cycle. 2009;8:902–908. doi: 10.4161/cc.8.6.7900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McCrann DJ, Eliades A, Makitalo M, Matsuno K, Ravid K. Differential expression of NADPH oxidases in megakaryocytes and their role in polyploidy. Blood. 2009;114:1243–1249. doi: 10.1182/blood-2008-12-195883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kurata S. Selective activation of p38 MAPK cascade and mitotic arrest caused by low level oxidative stress. J Biol Chem. 2000;275:23413–23416. doi: 10.1074/jbc.C000308200. [DOI] [PubMed] [Google Scholar]
- 72.Ting CM, Lee YM, Wong CK, Wong AS, Lung HL, Lung ML, Lo KW, Wong RN, Mak NK. 2-Methoxyestradiol induces endoreduplication through the induction of mitochondrial oxidative stress and the activation of MAPK signaling pathways. Biochem Pharmacol. 2010;79:825–841. doi: 10.1016/j.bcp.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 73.Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Reese J, Das SK, Paria BC, Lim H, Song H, Matsumoto H, Knudtson KL, DuBois RN, Dey SK. Global gene expression analysis to identify molecular markers of uterine receptivity and embryo implantation. J Biol Chem. 2001;276:44137–44145. doi: 10.1074/jbc.M107563200. [DOI] [PubMed] [Google Scholar]
- 75.McMaster MT, Newton RC, Dey SK, Andrews GK. Activation and distribution of inflammatory cells in the mouse uterus during the preimplantation period. J Immunol. 1992;148:1699–1705. [PubMed] [Google Scholar]
- 76.Head JR. Uterine natural killer cells during pregnancy in rodents. Nat Immun. 1996;15:7–21. [PubMed] [Google Scholar]
- 77.Yue L, Daikoku T, Hou X, Li M, Wang H, Nojima H, Dey SK, Das SK. Cyclin G1 and cyclin G2 are expressed in the periimplantation mouse uterus in a cell-specific and progesterone-dependent manner: evidence for aberrant regulation with Hoxa-10 deficiency. Endocrinology. 2005;146:2424–2433. doi: 10.1210/en.2004-1605. [DOI] [PMC free article] [PubMed] [Google Scholar]