Abstract
Objective
Despite numerous studies reporting increased cardiovascular disease (CVD) in patients with rheumatoid arthritis (RA), the impact of RA on managing modifiable CVD risk factors remains understudied. We tested the hypothesis that RA is a risk factor for not receiving a hypertension diagnosis.
Methods
Using a cohort design, we studied adult patients with and without RA/inflammatory arthritis from a large academic multispecialty practice. All were seen regularly in primary care and met clinical guideline hypertension criteria but lacked prior hypertension diagnosis/treatment. The primary outcome was time to ICD-9 code for hypertension or elevated blood pressure, or antihypertensive medication prescription. Kaplan Meier (KM) Survival and Cox proportional hazard modeling were used to examine the impact of RA on diagnosis of hypertension.
Results
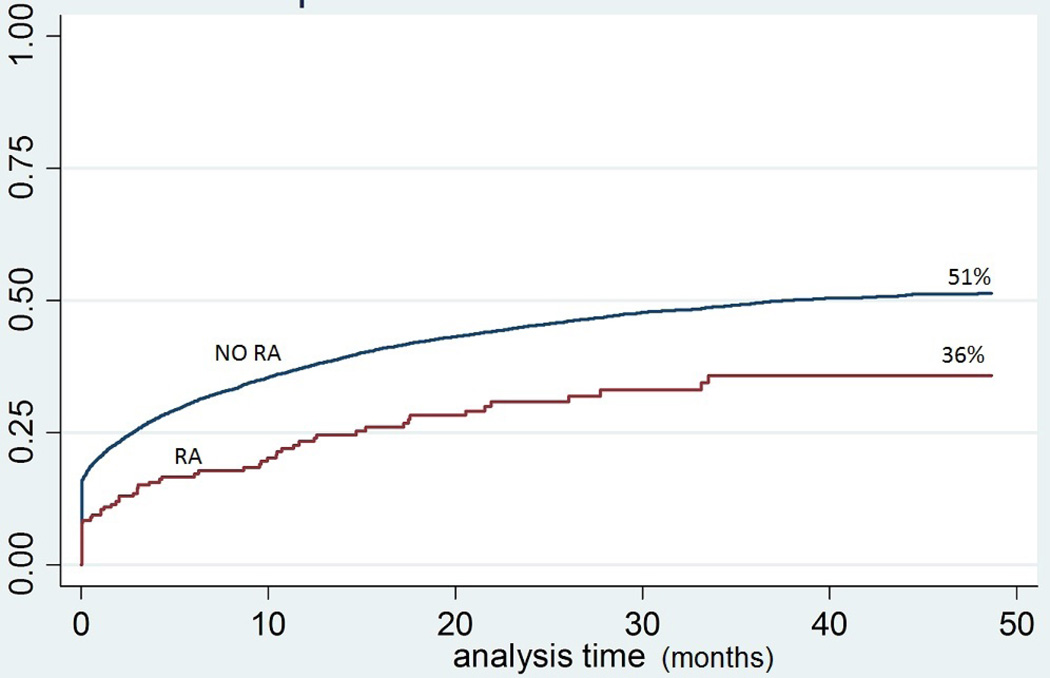
Among 14,974 patients with undiagnosed hypertension, 201 patients had RA codes. RA patients had equivalent primary care visits and more total visits compared to patients without RA. At study end the likelihood of hypertension diagnosis was 36% in RA patients compared to 51% without RA. In adjusted Cox models, RA patients had 29% lower hypertension diagnosis hazard [Hazard Ratio 0.71, 0.55–0.93], reflecting more undiagnosed hypertension than with other comorbidities.
Conclusion
Among patients meeting guideline-based hypertension criteria, RA patients were less likely to be diagnosed despite more visits than those without RA. Given heightened CVD risks in RA, and the importance of hypertension diagnosis as a first step toward controlling risk, rheumatologists should collaborate to improve rates of diagnosis for this modifiable CVD risk factor.
Introduction
Patients with rheumatoid arthritis (RA) have 50–60% increased incidence of cardiovascular disease (CVD) events and premature death as compared to those without this disease due to both RA itself and traditional CVD risk factors (1, 2). Mortality has declined in the general population in recent decades, with much of that reduction attributed to improved CVD preventative care (3). Nearly half of the survival gains in the US general population in the last 20 years have been credited to lowering systolic blood pressures and cholesterol levels. However, the mortality gap between RA patients and the general population has widened over the last decades (4) for unclear reasons.
Based upon our prior work demonstrating low lipid testing in Medicare RA patients, (5, 6) we broadly hypothesized that RA patients, despite increased CVD risk, have not received as much CVD preventive care as non-RA peers. Specifically, in the present work we focus on hypertension, a prevalent and modifiable risk factor for increased CVD in both RA patients and the general population. Several studies cite hazards for the impact of hypertension on CVD events in RA patients ranging from 2.8 to 3.8 (7, 8). Prior studies have reported wide variations in prevalence of hypertension among RA patients ranging from 3.8% to 73% (9). Such studies have rarely applied standard clinical guidelines like the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure (JNC-7) criteria and have rarely simultaneously examined non-RA comparisons (10–17). Given that the clinical standard for a hypertension diagnosis in the US involves serial measurements and interpretation per JNC-7 guidelines, an integrated primary care and multispecialty health system is an optimal setting to examine the impact of RA upon hypertension diagnosis during routine clinical care. Therefore, in a population of patients with and without RA who received regular primary care and met definitions for incident JNC-7 hypertension, we tested the hypothesis that RA is a risk factor for missed hypertension diagnosis, and examined the predictors of an initial diagnosis of hypertension.
Patients and Methods
Sample Definition
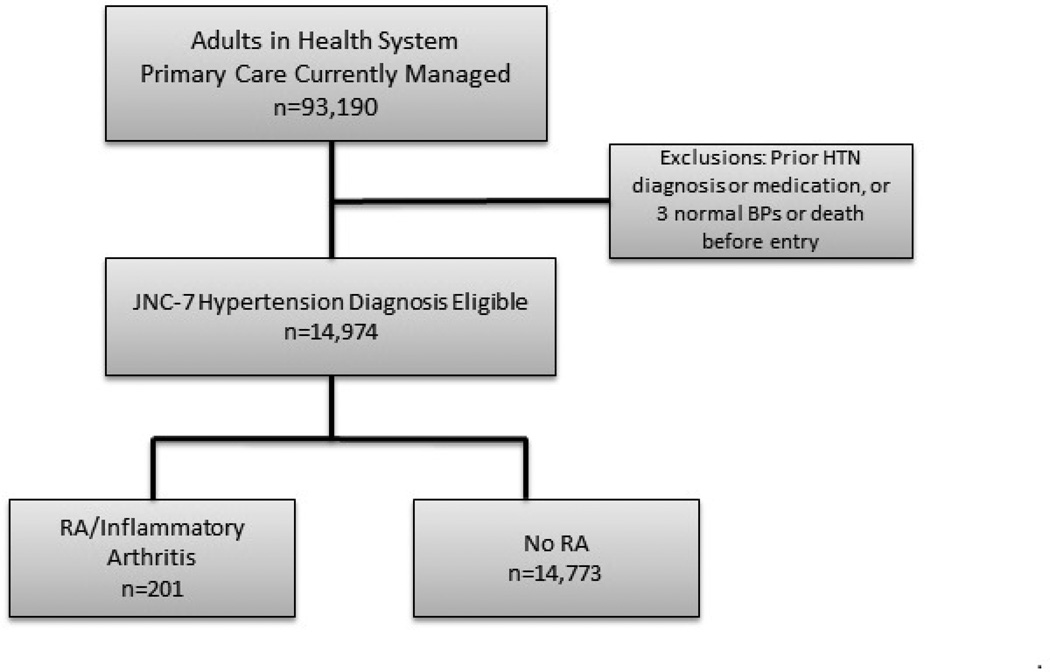
In a large academic multispecialty practice with a shared electronic health record (EHR), we defined a cohort of all adults ≥18 years old who were regularly seen in primary care and met the definition of guideline-based hypertension using an EHR algorithm, but lacked prior hypertension diagnosis or treatment. Using this EHR search-defined cohort we compared the likelihood of receiving a new diagnosis in patients with and without RA (Figure 1); similarly we used automated EHR searches for RA status and diagnosis to minimize potential for ascertainment bias. Regular primary care was defined as having 2 or more network primary care ambulatory visits in 36 months with at least 1 of those visits in the most recent 24 months per a published algorithm from our group used for statewide reporting (18). This definition performs well against other definitions of “regular primary care.” This definition guarantees that an individual included in the study was receiving both specialist and generalist care, facilitating continuous blood pressure trend review over time. Using JNC-7 criteria, the first date of hypertension diagnosis eligibility was defined as the second date of ambulatory blood pressures (BP) greater than 160/100 mmHg, or third greater than 140/90 mmHg. Elevated readings had to be at least 30 days apart and within 2 years (19). The study entry date reflected the first date patients met both the hypertension diagnosis eligibility and regular primary care criteria during our observation period (2008–2011). Patients were excluded if they had previously diagnosed hypertension (20), or were previously prescribed antihypertensive medications. Patients were also excluded if their blood pressure normalized before cohort entry (defined as 3 consecutive normal BP’s <130/80 mmHg with diabetes or kidney disease, otherwise <140/90 mmHg), (19) or if they died prior to entry. We received Institutional Review Board expedited approval with Health Insurance Portability and Accountability Act (HIPPA) waiver of consent for this medical records study.
Figure 1.
Flow diagram demonstrating selection of patients with regular primary care who were eligible for diagnosis of hypertension with and without RA.
Outcome Definitions
From study entry, patients were followed for up to four years. Our primary outcome was the date of first hypertension diagnosis indicated by: (a) the first ICD-9 diagnosis code for hypertension (401.1, 401.9, 402.10, 402.90, 404.10, 404.90, 405.11, 405.19, 405.91, 405.99) (20) OR “elevated blood pressure without hypertension” (796.2) (21) OR (b) prescription of any antihypertensive medication if it preceded the first hypertension code. Per the conventions of survival analysis, censoring to account for potentially incomplete data after a certain time point occurred for the following: end of the last calendar year if 24 months had passed without primary care contact (i.e. patient was no longer current with primary care), blood pressure normalization after cohort entry, end of study, or death.
Explanatory Variables
Our primary independent variable was RA/inflammatory arthritis status, assessed using algorithms requiring two ICD-9 claims of 714 in 24 months at any inpatient or outpatient visit (22). Control variables included socio-demographics, comorbidities, and healthcare visit utilization assessed over a 12 month baseline period. Sociodemographic factors included age, race, marriage status, tobacco history, language, and ever being enrolled in Medicaid. Comorbidities examined included diabetes (23), chronic kidney disease/end stage renal failure (24), TIA/Stroke (25), ischemic heart disease (25), congestive heart failure (26), peripheral vascular disease (27), hyperlipidemia (26), hypothyroidism, anxiety/psychosis (28), and depression (29), all defined using established algorithms. Body Mass Index (BMI) was also included. To control for composite disease burden and health needs for individual patients with and without RA, we used a validated single score calculated via The Johns Hopkins ACG (Adjusted Clinical Group) Case-Mix System (Version 10)(30). ACG calculates a score based on common morbidity patterns determined by 224 different clinical groupings. In this system an average individual scores 1, and an individual scoring >1 would be predicted to have greater than average composite health needs or illness burden. Visit utilization was also assessed by including the number of primary care MD/DO or NP/PA visits to Internal Medicine, Family Medicine, OB-Gynecology, Pediatrics or Geriatrics clinics and the number of non-primary care provider visits with BP assessments from the baseline year (31). Primary care provider (PCP) specialty was also included in the model. Correlation matrices showed no evidence of collinearity for covariates in the full model.
Analysis
To calculate descriptive statistics we used chi square testing for categorical variables and ANOVA for continuous variables. We considered a p value of <0.05 significant. Controlled Kaplan Meier survival analysis was performed to assess time to hypertension diagnosis. For our final multivariate adjusted regression analysis, we used Cox proportional hazards modeling including age, sex, marital status, race/ethnicity, language, Medicaid status, tobacco use, BMI, comorbidities, healthcare utilization, and primary care provider type to examine predictors of hypertension diagnosis. Hazard ratios (HR) and 95% confidence intervals (CI) were calculated using robust estimates of variance. Patient sample selection and data variable creation was conducted using SAS version 9.1.3 (SAS Institute, Inc., Cary, NC); statistical analysis was conducted using Stata version 12.0 (StataCorp, College Station, TX) (32).
Results
Descriptive Characteristics of Cohort
A total of 14,974 patients with incident hypertension seen regularly in primary care were identified including 201 patients with RA (1.3%). At baseline, RA patients with hypertension were older (mean 56.3 vs. 49.5 years, p≤0.0001), more often female (71% vs. 49%, p ≤0.0001), more often married (64% vs. 59%, p 0.006) and English speaking (97% vs. 92%, p 0.021) compared to hypertensive non-RA patients (Table 1). There was no significant difference between the RA and non-RA groups in rates of individual comorbidities (p >0.01 for all). However, composite complexity was higher in the RA group as indicated by higher ACG mean in RA patients with hypertension versus lower than average in non-RA patients with hypertension (ACG mean 1.1 ± 0.5 vs. 0.7 ± 0.5). RA patients had essentially an equivalent number of primary care visits when compared to non-RA patients (2.5 vs. 2.6). However, the RA patients had more non-primary care visits, (5.4 compared to 2.3), including on average 2.6 rheumatology visits annually. RA patients were more likely to see an internist than other specialties for primary care.
Table 1.
Baseline characteristics of hypertension diagnosis eligible, with and without RA
| CHARACTERISTIC | ALL N=14,974 |
RA N=201 |
Non RA N=14,773 |
P-value | |
|---|---|---|---|---|---|
| Age (Mean, SD) | 50 +/− 15 | 56 +/− 13 | 50 +/− 15 | ||
| 20–39 | 26% | 10% | 26% | <0.0001 | |
| 40–59 | 49% | 49% | 49% | ||
| 60–79 | 22% | 35% | 21% | ||
| >80 | 3% | 5% | 3% | ||
| Female | 50% | 71% | 49% | <0.0001 | |
| Married/partnered | 60% | 64% | 59% | 0.01 | |
| Race | White | 88% | 93% | 88% | 0.12 |
| Black | 5% | 2% | 5% | ||
| Other/Unknown | 7% | 5% | 7% | ||
| Language | English | 92% | 97% | 92% | 0.02 |
| Non-English | 8% | 3% | 8% | ||
| Medicaid (ever) | 10% | 9% | 10% | 0.73 | |
| BMI (mean) | 31 +/− 8 | 30 +/− 7 | 31 +/− 8 | 0.1 | |
| Tobacco | Never | 43% | 43% | 43% | 0.44 |
| Current | 17% | 14% | 17% | ||
| Former | 25% | 29% | 25% | ||
| Missing/Passive | 16% | 14% | 16% | ||
| Condition Depression | 8% | 10% | 8% | 0.28 | |
| Anxiety/Psychosis | 15% | 13% | 15% | 0.59 | |
| Hypothyroidism | 4% | 5% | 3% | 0.25 | |
| Inflammatory Bowel Disease | 1% | 1% | 1% | 0.56 | |
| Hyperlipidemia | 20% | 18% | 20% | 0.51 | |
| Diabetes | 5% | 4% | 5% | 0.45 | |
| Chronic Kidney Disease | 1% | 2% | 1% | 0.23 | |
| Atrial Fibrillation | 0.4% | 0% | 0.4% | 0.36 | |
| Ischemic Heart Disease | 2% | 2% | 2% | 0.47 | |
| Peripheral Vascular Disease | 2% | 3% | 1% | 0.08 | |
| CHF | 0.4% | 1% | 0.4% | 0.17 | |
| TIA/Stroke | 3% | 2% | 3% | 0.82 | |
| ACG Score (Mean, SD) | 0.7 +/− 0.5 | 1.1 +/− 0.5 | 0.7 +/− 0.5 | <0.0001 | |
| PCP | Internal Medicine | 37% | 46% | 37% | <0.0001 |
| Family Medicine | 50% | 39% | 50% | ||
| OB/Gynecology | 1% | 4% | 1% | ||
| Other (Geriatrics, Peds) | 12% | 11% | 12% | ||
| PC Visit Count (Mean, SD) | 2.6 +/− 2.5 | 2.5 +/− 2.1 | 2.6 +/− 2.5 | 0.84 | |
| Non-PC Visit (Mean, SD) | 2.3 +/− 3.1 | 5.4 +/− 4.3 | 2.3 +/− 3.0 | <0.0001 | |
| Rheum Visit (Mean, SD) | 0.1 +/− 0.6 | 2.6 +/− 2.3 | 0.1 +/− 0.4 | <0.0001 | |
Abbreviation Legend: ACG=Adjusted Clinical Groups, BMI=body mass index, CHF=congestive heart failure, PCP=primary care provider, SD=standard deviation, TIA=transient ischemic attack
Hypertension diagnosis rates and predictors
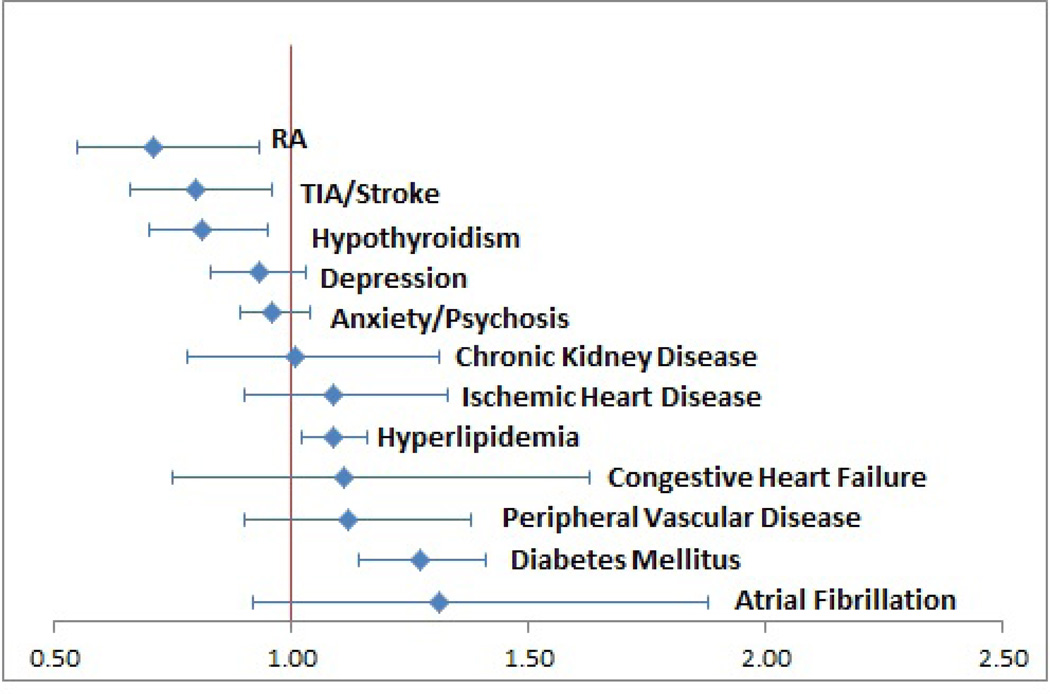
Adjusted Kaplan Meier models showed that only 36% of RA patients compared to 51% of controls were eventually diagnosed with hypertension at the end of follow-up (mean follow-up 14 mos., maximum 4 years) (See Figure 2). For those eventually diagnosed, time to diagnosis was not different for patients with or without RA (3 vs 1.4 months, p=0.1 as shown in Table 2). However again, in the final adjusted Cox proportional hazards model, RA predicted nearly 30% lower hazard of hypertension diagnosis (HR 0.71, CI 0.55–0.93, where a lower hazard of diagnosis reflects a lower likelihood of appropriate diagnosis and more undiagnosed cases). As shown in Figure 3, the RA hazard was worse than other specific comorbidities. In contrast, patients with comorbid diabetes or hyperlipidemia were more likely to be rapidly diagnosed with hypertension (HR 1.27 and 1.09 respectively). When comparing the impact of various comorbidities on hypertension diagnosis, patients with RA were least likely to receive a diagnosis as indicated by the lowest hazards of diagnosis.
Figure 2.
Kaplan Meier survival curve for hypertension diagnosis with and without RA.
Table 2.
Impact of RA on observed hypertension diagnosis and Kaplan Meier diagnosis 2008–11
| Unadjusted | Time to diagnosis, Among diagnosed patients (months) |
||||
|---|---|---|---|---|---|
| Sample N | % Diagnosed with Hypertension |
Median | Mean, SD | p-value | |
| All Patients | 14,974 | 44% | 1.5 | 5.6 ± 8.4 | |
| RA | 201 | 29% | 3 | 7.5 ± 9.0 | 0.1 |
| Non-RA | 14,773 | 44% | 1.4 | 5.6 ± 8.4 | |
Figure 3.
Tornado plot of impact of comorbidities on hypertension diagnosis hazards. Recall, HR >1 is interpreted as greater hypertension diagnosis hazard (fewer remaining undiagnosed).
In the final adjusted Cox model, younger age, white race, non-English primary language, higher Adjusted Clinical Group quartiles, and more total visits predicted lower hazard of hypertension recognition (See Table 3). Hypertension diagnosis was higher for those who were older, non-white, married, and upper BMI quartiles. Age was the greatest predictor of higher diagnosis hazard. Patients between the ages of 60–79 years had the highest hazard of diagnosis (HR 1.42, 1.31–1.54). Black race had the next highest boost in hypertension diagnosis hazard (HR 1.28, 1.14–1.44). Those with increasing ACG health needs had lower hazard of diagnosis. Patients in the highest ACG quartiles had a significant decrease in hypertension diagnosis hazards (HR 0.87 and 0.80). Additionally, current smokers had lower hazard of hypertension diagnosis (HR 0.88, 0.82–0.96).
Table 3.
Observed and Cox proportional hazards for impact of sociodemographic factors and utilization on hypertension diagnosis
| CHARACTERISTIC | Unadjusted % Diagnosed |
Adjusted | ||
|---|---|---|---|---|
| HR* | 95% CI | |||
| Age | 20–39 | 36.7 | 1.00 | - |
| 40–59 | 46.0 | 1.34 | 1.26–1.43 | |
| 60–79 | 46.5 | 1.42 | 1.31–1.54 | |
| >80 | 40.5 | 1.26 | 1.07–1.49 | |
| Sex | Male | 44.7 | 1.00 | - |
| Female | 42.3 | 0.96 | 0.91–1.02 | |
| Married/partnered | 44.9 | 1.00 | - | |
| Single | 39.6 | 0.95 | 0.90–1.02 | |
| Divorced/widowed | 45.3 | 1.08 | 1.001–1.17 | |
| Race | White | 43.1 | 1.00 | - |
| Black | 47.8 | 1.28 | 1.14–1.44 | |
| Other/unknown | 45.3 | 1.14 | 1.04–1.26 | |
| Language | English | 44.1 | 1.00 | - |
| Non-English | 36.2 | 0.79 | 0.71–0.87 | |
| Medicaid (ever) | 40.3 | 1.09 | 0.99–1.19 | |
| BMI | Lowest Quartile | 38.9 | 1.00 | - |
| Second Quartile | 42.8 | 1.07 | 0.99–1.15 | |
| Third Quartile | 45.2 | 1.13 | 1.05–1.22 | |
| Highest Quartile | 46.2 | 1.18 | 1.10–1.26 | |
| Tobacco | Never | 43.7 | 1.00 | - |
| Current | 39.2 | 0.88 | 0.82–0.96 | |
| Former | 43.3 | 1.01 | 0.95–1.08 | |
| Not asked | 48.1 | 0.96 | 0.90–1.03 | |
| ACG | First Quartile | 51.6 | 1.00 | - |
| Second Quartile | 46.4 | 0.94 | 0.88–1.01 | |
| Third Quartile | 42.7 | 0.87 | 0.81–0.94 | |
| Highest Quartile | 34.7 | 0.80 | 0.73–0.88 | |
| PCP | Internal Medicine | 44.1 | 1.00 | - |
| Family Medicine | 43.8 | 1.04 | 0.99–1.10 | |
| OB/Gyn | 37.2 | 0.81 | 0.65–1.02 | |
| Other (Geriatrics, Peds) | 41.3 | 0.96 | 0.88–1.04 | |
Note HR >1 is interpreted as greater hypertension diagnosis (few undiagnosed).
Model also includes utilization and comordibities demonstrated in Fig 3.
Abbreviation Legend: ACG=Adjusted Clinical Groups, BMI=body mass index, CHF=congestive heart failure, PCP=primary care provider
Discussion
In this population of patients with incident hypertension seen regularly in primary care, we observed that patients with RA had 29% lower hazard of receiving a hypertension diagnosis compared to those without RA. This finding is concerning given that receiving a diagnosis is the first step toward monitoring and controlling blood pressure for subsequent prevention of CVD. Older patients, those of black race, and those with highest BMI had higher diagnosis rates likely reflecting provider knowledge that these groups have higher hypertension prevalence. Patients with diabetes and hyperlipidemia also experienced higher diagnosis, likely reflecting concordant CVD risk perceptions (33), and well-known, guideline-driven practices for CVD risk management. RA patients, in contrast, may be suffering from the lack of provider awareness regarding RA as a concordant CVD risk (34). Lower hypertension diagnosis in active smokers was particularly concerning given that health care providers should be more vigilant to traditional CVD risk factors like hypertension in patients who smoke.
Our direct comparison of diagnosis of hypertension in those with and without RA using standard clinical guideline criteria offers solid support for under-diagnosis of this CVD risk factor in RA. In their UK cross-sectional RA study, Panoulas et al reported that approximately 40% of RA patients with hypertension were undiagnosed and untreated (15), although they lacked a non-RA comparison. In our longitudinal study, examining only incident hypertension cases, 64% of RA patients (compared to 49% without RA) with incident hypertension remained undiagnosed/untreated. Our results highlight that undiagnosed hypertension is a pervasive problem that is compounded for RA patients--suggesting gaps in current clinical care much like our previous work reporting low lipid testing in Medicare patients with RA (5, 6).
Prior studies and expert recommendations have suggested that strategies to reduce CVD risk in RA patients should include both a focus on controlling RA disease and traditional CVD risk factors, including hypertension (16, 35). Recommendations from the European League Against Rheumatism call for annual cardiovascular risk assessments in RA patients including blood pressure review (36). While RA quality guidelines in the US have not as aggressively advocated comprehensive traditional CVD risk assessment, efforts in defining and measuring CVD risk management as a component of US lupus care quality show similar gaps (37, 38). More universally, the Centers for Disease Control has recently named hypertension as “public enemy #2” (behind only smoking) as a modifiable risk factor for CVD that should be addressed in all patients. We would argue that heightened attention to hypertension diagnosis and treatment is needed particularly in the RA population.
Currently, primary care provider and rheumatologists’ practices for managing RA patients’ CVD risk vary widely. A 2007 survey found that 92.8% of rheumatologists reported that they routinely screen their patients for hypertension; however, only 31% were willing to initiate antihypertensive treatment (34). Rheumatologists who believed that their patient had inadequate primary care were more likely to initiate treatment. In the present work, we note poor diagnosis even among RA patients with regular primary care. Another survey reported that as few as 32% of UK PCP’s recognized that RA is an independent CVD risk factor (39). In the US, a recent report showed that rheumatologists managed CVD risks less often than PCP’s, but that PCP’s delivered less care to RA patients than general patients (40). These collective findings suggest that there needs to be better collaboration between rheumatologists and primary care for management of hypertension and other CVD risk factors (34).
Further work to examine causes for gaps in diagnosis of hypertension among RA patients may illustrate rational solutions. If, for instance, PCP’s are less familiar with RA-specific CVD risk, or have concerns regarding medication interactions with disease modifying drugs, then educational interventions could help. If diagnostic overshadowing by RA (41), or dispersion of care across specialty clinics results in less trend monitoring, then electronic health record alerts or other system supports might help. Alternatively, if rheumatologists are most frequently encountering RA patients but believe CVD prevention falls outside their scope of care, CVD prevention roles may need to be re-examined. Particularly in the era of Accountable Care (42) when healthcare systems are responsible for BP management quality not only in primary care clinics, but at each contact, systems will be incentivized to implement supports like care coordination agreements or algorithm-driven hypertension management across clinics (43, 44). In accordance with EULAR recommendations for comprehensive annual CVD risk assessment in RA patients, some groups have reported rheumatology clinic CVD risk screening results (45) and one recent international study piloted nurse outreach for CVD risk screening in RA (46). That study noted that 74% of diabetic RA patients and 45% of other RA patients with known hypertension had uncontrolled blood pressures. They then referred back the patients at highest CVD risk, including those with risk scores above 5%, those with blood pressure above the recommended level, total cholesterol above 8 mmol/L or fasting glucose of ≥ 6 mmol/L for general practitioner follow-up. Standard cardiovascular guidelines state that hypertension should be actively managed in virtually all patients, including timely follow up, therapy adjustments and titration but systematically coordinating this across specialty and primary care is challenging. Locally, our rheumatology division is piloting an electronic health record tool to help facilitate CVD risk management for RA patients that includes longitudinal system-wide blood pressure trend monitoring along with RA disease activity measures (Materials available through the University of Wisconsin Health Innovation Program at www.HIPxChange.org). The impact of these and other intervention strategies merits further investigation.
Despite strengths of this study including the natural experiment of a multispecialty practice with longitudinal blood pressures to compare the diagnosis of hypertension in RA patients with non-RA controls using standard clinical criteria, one must also consider limitations. First, questions regarding generalizability of findings to other health systems or freestanding clinics emerge, yet the benefits of this cohort were that it encompassed patients receiving both primary and rheumatologic care in a single system. Second, our sample represented a relatively small number of RA/inflammatory arthritis patients, and individual cases were not validated by American College of Rheumatology RA criteria. Sensitivity analyses using stricter RA definitions requiring ≥2 encounters with ICD-9 714.0–714.33, 714.4, 714.80, 714.81, 714.89 yielded similar estimates (47). Other comorbidities were also assessed using established algorithms to improve code based diagnostic accuracy, although we acknowledge the limits of coded data. Moreover recognizing that hypertension is often under-coded, we included antihypertensive medications and codes for elevated blood pressure without hypertension that are known to improve sensitivity for the diagnosis of hypertension (21). Lastly, ambulatory blood pressure measurement was not standardized, however this again reflects usual clinical practice.
Future work should examine the causes of lower clinical hypertension diagnosis in RA, and strategies to improve recognition and control. Studies also should aim to examine and optimize the communication and roles of rheumatologists, primary care providers, and health systems to develop evidence-based interventions for actively managing and controlling modifiable CVD risks in RA.
Conclusion
Among currently primary care managed patients meeting criteria for hypertension, those with RA had 29% lower hypertension diagnosis hazard despite more clinic visits than those without RA. Given that both hypertension and RA increase CVD event risk, rheumatologists should collaborate to improve hypertension diagnosis as a key first step to modify CVD risk in RA patients.
Significance and Innovation.
RA patients had 29% lower hypertension diagnosis hazard despite more total clinic visits than those without RA.
Patients with and without RA had equivalent numbers of primary care encounters, and RA patients had rheumatology visits in equal proportion to primary care visits.
Future work should investigate how rheumatologists can collaborate with primary care to diagnose and manage hypertension in RA patients to reduce modifiable CVD risk.
Acknowledgements
Katie Ronk PhD and Patrick Ferguson MS for meticulous data preparation and Courtney Maxcy for manuscript preparation.
Sources of support: Research reported in this publication was primarily supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, part of the National Institutes of Health (NIH), under Award Number K23AR062381 (Bartels). Additional support from NIH-NCATS 9U54TR000021 the Health Innovation Program/Community-Academic Partnerships core of UW ICTR-CTSA, NIH-NHLBI 1 K23 HL112907 (Johnson), and Shapiro Research Program. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Department of Veterans Affairs.
References
- 1.Avina-Zubieta JA, Choi HK, Sadatsafavi M, Etminan M, Esdaile JM, Lacaille D. Risk of cardiovascular mortality in patients with rheumatoid arthritis: a meta-analysis of observational studies. Arthritis Rheum. 2008;59:1690–1697. doi: 10.1002/art.24092. [DOI] [PubMed] [Google Scholar]
- 2.Meune C, Touze E, Trinquart L, Allanore Y. Trends in cardiovascular mortality in patients with rheumatoid arthritis over 50 years: a systematic review and meta-analysis of cohort studies. Rheumatology (Oxford) 2009;48:1309–1313. doi: 10.1093/rheumatology/kep252. [DOI] [PubMed] [Google Scholar]
- 3.Ford ES, Ajani UA, Croft JB, Critchley JA, Labarthe DR, Kottke TE, et al. Explaining the decrease in U.S. deaths from coronary disease, 1980–2000. N Engl J Med. 2007;356:2388–2398. doi: 10.1056/NEJMsa053935. [DOI] [PubMed] [Google Scholar]
- 4.Gonzalez A, Maradit Kremers H, Crowson CS, Nicola PJ, Davis JM, 3rd, Therneau TM, et al. The widening mortality gap between rheumatoid arthritis patients and the general population. Arthritis Rheum. 2007;56:3583–3587. doi: 10.1002/art.22979. [DOI] [PubMed] [Google Scholar]
- 5.Bartels CM, Kind AJ, Everett C, Mell M, McBride P, Smith M. Low frequency of primary lipid screening among medicare patients with rheumatoid arthritis. Arthritis Rheum. 2011;63:1221–1230. doi: 10.1002/art.30239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bartels CM, Kind AJ, Thorpe CT, Everett CM, Cook RJ, McBride PE, et al. Lipid testing in patients with rheumatoid arthritis and key cardiovascular-related comorbidities: a medicare analysis. Semin Arthritis Rheum. 2012;42:9–16. doi: 10.1016/j.semarthrit.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Innala L, Moller B, Ljung L, Magnusson S, Smedby T, Sodergren A, et al. Cardiovascular events in early RA are a result of inflammatory burden and traditional risk factors: a five year prospective study. Arthritis Res Ther. 2011;13:R131. doi: 10.1186/ar3442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Serelis J, Panagiotakos DB, Mavrommati M, Skopouli FN. Cardiovascular disease is related to hypertension in patients with rheumatoid arthritis: a greek cohort study. J Rheumatol. 2011;38:236–241. doi: 10.3899/jrheum.100564. [DOI] [PubMed] [Google Scholar]
- 9.Panoulas VF, Metsios GS, Pace AV, John H, Treharne GJ, Banks MJ, et al. Hypertension in rheumatoid arthritis. Rheumatology (Oxford) 2008;47:1286–1298. doi: 10.1093/rheumatology/ken159. [DOI] [PubMed] [Google Scholar]
- 10.Bild DE, Detrano R, Peterson D, Guerci A, Liu K, Shahar E, et al. Ethnic differences in coronary calcification: the Multi-Ethnic Study of Atherosclerosis (MESA) Circulation. 2005;111:1313–1320. doi: 10.1161/01.CIR.0000157730.94423.4B. [DOI] [PubMed] [Google Scholar]
- 11.Chung CP, Giles JT, Petri M, Szklo M, Post W, Blumenthal RS, et al. Prevalence of traditional modifiable cardiovascular risk factors in patients with rheumatoid arthritis: comparison with control subjects from the multi-ethnic study of atherosclerosis. Semin Arthritis Rheum. 2012;41:535–544. doi: 10.1016/j.semarthrit.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crowson CS, Matteson EL, Roger VL, Therneau TM, Gabriel SE. Usefulness of risk scores to estimate the risk of cardiovascular disease in patients with rheumatoid arthritis. The American journal of cardiology. 2012;110:420–424. doi: 10.1016/j.amjcard.2012.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crowson CS, Nicola PJ, Kremers HM, O'Fallon WM, Therneau TM, Jacobsen SJ, et al. How much of the increased incidence of heart failure in rheumatoid arthritis is attributable to traditional cardiovascular risk factors and ischemic heart disease? Arthritis Rheum. 2005;52:3039–3044. doi: 10.1002/art.21349. [DOI] [PubMed] [Google Scholar]
- 14.Dessein PH, Joffe BI, Veller MG, Stevens BA, Tobias M, Reddi K, et al. Traditional and nontraditional cardiovascular risk factors are associated with atherosclerosis in rheumatoid arthritis. J Rheumatol. 2005;32:435–442. [PubMed] [Google Scholar]
- 15.Panoulas VF, Douglas KM, Milionis HJ, Stavropoulos-Kalinglou A, Nightingale P, Kita MD, et al. Prevalence and associations of hypertension and its control in patients with rheumatoid arthritis. Rheumatology (Oxford) 2007;46:1477–1482. doi: 10.1093/rheumatology/kem169. [DOI] [PubMed] [Google Scholar]
- 16.Solomon DH, Kremer J, Curtis JR, Hochberg MC, Reed G, Tsao P, et al. Explaining the cardiovascular risk associated with rheumatoid arthritis: traditional risk factors versus markers of rheumatoid arthritis severity. Ann Rheum Dis. 2010;69:1920–1925. doi: 10.1136/ard.2009.122226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soubrier M, Zerkak D, Dougados M. Indications for lowering LDL cholesterol in rheumatoid arthritis: an unrecognized problem. J Rheumatol. 2006;33:1766–1769. [PubMed] [Google Scholar]
- 18.Thorpe CT, Flood GE, Kraft SA, Everett CM, Smith MA. Effect of patient selection method on provider group performance estimates. Med Care. 2011;49:780–785. doi: 10.1097/MLR.0b013e31821b3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 20.Tu K, Campbell NR, Chen ZL, Cauch-Dudek KJ, McAlister FA. Accuracy of administrative databases in identifying patients with hypertension. Open Med. 2007;1:e18–e26. [PMC free article] [PubMed] [Google Scholar]
- 21.Hansen ML, Gunn PW, Kaelber DC. Underdiagnosis of hypertension in children and adolescents. JAMA : the journal of the American Medical Association. 2007;298:874–879. doi: 10.1001/jama.298.8.874. [DOI] [PubMed] [Google Scholar]
- 22.MacLean CH, Louie R, Leake B, McCaffrey DF, Paulus HE, Brook RH, et al. Quality of care for patients with rheumatoid arthritis. JAMA. 2000;284:984–992. doi: 10.1001/jama.284.8.984. [DOI] [PubMed] [Google Scholar]
- 23.Hebert PL, Geiss LS, Tierney EF, Engelgau MM, Yawn BP, McBean AM. Identifying persons with diabetes using Medicare claims data. Am J Med Qual. 1999;14:270–277. doi: 10.1177/106286069901400607. [DOI] [PubMed] [Google Scholar]
- 24.Foley RN, Murray AM, Li S, Herzog CA, McBean AM, Eggers PW, et al. Chronic kidney disease and the risk for cardiovascular disease, renal replacement, and death in the United States Medicare population, 1998 to 1999. J Am Soc Nephrol. 2005;16:489–495. doi: 10.1681/ASN.2004030203. [DOI] [PubMed] [Google Scholar]
- 25.2011 Chronic Condition Reference List. Warrenton, VA: Buccaneer Computer Systems and Services, Inc; 2011. URL: http://wwwccwdataorg/cs/groups/public/documents/document/ccw_conditionreferencelist2011pdf. [Google Scholar]
- 26.Borzecki AM, Wong AT, Hickey EC, Ash AS, Berlowitz DR. Identifying hypertension-related comorbidities from administrative data: what's the optimal approach? Am J Med Qual. 2004;19:201–206. doi: 10.1177/106286060401900504. [DOI] [PubMed] [Google Scholar]
- 27.Newton KM, Wagner EH, Ramsey SD, McCulloch D, Evans R, Sandhu N, et al. The use of automated data to identify complications and comorbidities of diabetes: a validation study. J Clin Epidemiol. 1999;52:199–207. doi: 10.1016/s0895-4356(98)00161-9. [DOI] [PubMed] [Google Scholar]
- 28.Marciniak MD, Lage MJ, Dunayevich E, Russell JM, Bowman L, Landbloom RP, et al. The cost of treating anxiety: the medical and demographic correlates that impact total medical costs. Depress Anxiety. 2005;21:178–184. doi: 10.1002/da.20074. [DOI] [PubMed] [Google Scholar]
- 29.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 30.Reid RJ, Roos NP, MacWilliam L, Frohlich N, Black C. Assessing population health care need using a claims-based ACG morbidity measure: a validation analysis in the Province of Manitoba. Health Serv Res. 2002;37:1345–1364. doi: 10.1111/1475-6773.01029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pham HH, Schrag D, Hargraves JL, Bach PB. Delivery of preventive services to older adults by primary care physicians. JAMA. 2005;294:473–481. doi: 10.1001/jama.294.4.473. [DOI] [PubMed] [Google Scholar]
- 32.StataCorp. Stata Statistical Software: Release 12. College Station, TX: StataCorp; 2011. [Google Scholar]
- 33.Piette JD, Kerr EA. The impact of comorbid chronic conditions on diabetes care. Diabetes Care. 2006;29:725–731. doi: 10.2337/diacare.29.03.06.dc05-2078. [DOI] [PubMed] [Google Scholar]
- 34.Nguyen-Oghalai TU, Hunnicutt SE, Smith ST, Maganti R, McNearney TA. Factors that impact decision making among rheumatologists in the initiation of treatment for hypertension in rheumatoid arthritis. J Clin Rheumatol. 2007;13:307–312. doi: 10.1097/RHU.0b013e318156bcc2. [DOI] [PubMed] [Google Scholar]
- 35.Symmons DP, Gabriel SE. Epidemiology of CVD in rheumatic disease, with a focus on RA and SLE. Nat Rev Rheumatol. 2011;7:399–408. doi: 10.1038/nrrheum.2011.75. [DOI] [PubMed] [Google Scholar]
- 36.Peters MJ, Symmons DP, McCarey D, Dijkmans BA, Nicola P, Kvien TK, et al. EULAR evidence-based recommendations for cardiovascular risk management in patients with rheumatoid arthritis and other forms of inflammatory arthritis. Ann Rheum Dis. 2010;69:325–331. doi: 10.1136/ard.2009.113696. [DOI] [PubMed] [Google Scholar]
- 37.Yazdany J, Panopalis P, Gillis JZ, Schmajuk G, MacLean CH, Wofsy D, et al. A quality indicator set for systemic lupus erythematosus. Arthritis Rheum. 2009;61:370–377. doi: 10.1002/art.24356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yazdany J, Trupin L, Tonner C, Dudley RA, Zell J, Panopalis P, et al. Quality of care in systemic lupus erythematosus: application of quality measures to understand gaps in care. J Gen Intern Med. 2012;27:1326–1333. doi: 10.1007/s11606-012-2071-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bell C, Rowe IF. The recognition and assessment of cardiovascular risk in people with rheumatoid arthritis in primary care: a questionnaire-based study of general practitioners. Musculoskeletal Care. 2011;9:69–74. doi: 10.1002/msc.196. [DOI] [PubMed] [Google Scholar]
- 40.Desai SS, Myles JD, Kaplan MJ. Suboptimal cardiovascular risk factor identification and management in patients with rheumatoid arthritis: a cohort analysis. Arthritis Res Ther. 2012;14:R270. doi: 10.1186/ar4118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jopp DA, Keys CB. Diagnostic overshadowing reviewed and reconsidered. Am J Ment Retard. 2001;106:416–433. doi: 10.1352/0895-8017(2001)106<0416:DORAR>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 42.Fisher ES, McClellan MB, Bertko J, Lieberman SM, Lee JJ, Lewis JL, et al. Fostering accountable health care: moving forward in medicare. Health affairs. 2009;28:w219–w231. doi: 10.1377/hlthaff.28.2.w219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carrier E, Dowling MK, Pham HH. Care coordination agreements: barriers, facilitators, and lessons learned. The American journal of managed care. 2012;18:e398–e404. [PubMed] [Google Scholar]
- 44.Feldman RD, Zou GY, Vandervoort MK, Wong CJ, Nelson SA, Feagan BG. A simplified approach to the treatment of uncomplicated hypertension: a cluster randomized, controlled trial. Hypertension. 2009;53:646–653. doi: 10.1161/HYPERTENSIONAHA.108.123455. [DOI] [PubMed] [Google Scholar]
- 45.Gomez-Vaquero C, Robustillo M, Narvaez J, Rodriguez-Moreno J, Gonzalez-Juanatey C, Llorca J, et al. Assessment of cardiovascular risk in rheumatoid arthritis: impact of the new EULAR recommendations on the score cardiovascular risk index. Clin Rheumatol. 2012;31:35–39. doi: 10.1007/s10067-011-1774-6. [DOI] [PubMed] [Google Scholar]
- 46.Primdahl J, Clausen J, Horslev-Petersen K. Results from systematic screening for cardiovascular risk in outpatients with rheumatoid arthritis in accordance with the EULAR recommendations. Ann Rheum Dis. 2013 doi: 10.1136/annrheumdis-2013-203682. [DOI] [PubMed] [Google Scholar]
- 47.Katz JN, Barrett J, Liang MH, Bacon AM, Kaplan H, Kieval RI, et al. Sensitivity and positive predictive value of Medicare Part B physician claims for rheumatologic diagnoses and procedures. Arthritis Rheum. 1997;40:1594–1600. doi: 10.1002/art.1780400908. [DOI] [PubMed] [Google Scholar]