SUMMARY
T cell-mediated hypersensitivity to metal cations is common in humans. How the T cell antigen receptor (TCR) recognizes these cations bound to a major histocompatibility complex (MHC) protein and self-peptide is unknown. Individuals carrying the MHCII allele, HLA-DP2, are at risk for chronic beryllium disease (CBD), a debilitating inflammatory lung condition caused by the reaction of CD4 T cells to inhaled beryllium. We show here that the T cell ligand is created when a Be2+ cation becomes buried in an HLA-DP2/peptide complex, where it is coordinated by both MHC and peptide acidic amino acids. Surprisingly, the TCR does not interact with the Be2+ itself, but rather with surface changes induced by the firmly bound Be2+ and an accompanying Na+ cation. Thus, CBD, by creating a new antigen by indirectly modifying the structure of pre-existing self MHC-peptide complex, lies on the border between allergic hypersensitivity and autoimmunity.
INTRODUCTION
The majority of αβ T cell antigen receptors (TCRs) recognize ligands in which a classical major histocompatibility complex (MHC) protein presents a peptide secured in its peptide binding groove. To prevent responses to self-peptides presented by self-MHC alleles, an elaborate system of checkpoints has evolved to control self-reactive T cells, either by deleting them while they develop in the thymus (Kappler et al., 1987; Kisielow et al., 1988) or by suppressing their response in peripheral organs (Fontenot and Rudensky, 2004). These mechanisms steer T cell responses either toward foreign peptides, e.g. those derived from foreign pathogens, bound to self-MHC, or toward peptides bound to foreign MHC alleles, e.g. those expressed on grafted foreign tissues. Experimental work over several decades has created a deep understanding of the nature of these types of MHC-peptide antigens and their recognition by TCRs (Gras et al., 2008; Marrack et al., 2008; Wilson and Garcia, 1997).
However, there are other ligands for T cells that also involve the classical self-MHC molecules, but remain poorly understood. These are often involved in inflammatory immunological diseases. For example, despite the numerous checkpoints, T cells can become activated in some individuals by MHC bound to self-peptides, causing serious tissue-specific autoimmune diseases such as type-1 diabetes, rheumatoid arthritis and multiple sclerosis. Furthermore, T cell mediated allergic hypersensitivities involve a wide variety of non-peptide environmental chemicals that include metal cations, as well as plant, industrial and pharmaceutical organic compounds (Fontenot and Amicosante, 2008; Louis-Dit-Sully and Schamel, 2014; Pichler et al., 2006; Thierse et al., 2005). Most evidence indicates that these agents must become associated with a self MHC-peptide complex in order to elicit a T cell response, but how they bind to the complex and how the TCR recognizes their presence is largely unknown. A common hypothesis is that they occupy a position on the surface of the MHCII-peptide complex and are recognized directly by the TCR in a hapten-like manner, but other mechanisms have been proposed (Illing et al., 2013; Pichler et al., 2006).
Beryllium hypersensitivity is an example of this latter type of T cell recognition. Because of its unique chemical properties, industrial importance and high toxicity, beryllium has been extensively studied by clinicians, biologists, chemists and physicists for many years. Chronic beryllium disease (CBD) is a lifelong CD4 T cell mediated lung inflammatory illness, common among certain industrial workers who handle the beryllium metal (Dai et al., 2013). It is highly associated with certain human class II MHC (MHCII) alleles, especially HLA-DP2 (DP2), that have a glutamic acid at position 69 of the β chain (β69E). The active agent in the disease is the Be2+ cation. Since the Be2+ cation has such a high charge density, it immediately forms higher order complexes in biological solutions involving electron donors such as water, hydroxyl ions, carbonate, phosphate, etc (Everest, 1964; Sutton and Burastero, 2003). It has been suggested that β69E captures such a Be2+ containing complex which is then recognized on the DP2 surface by CD4 T cells, but the structural details of this complex or its recognition are unknown.
Numerous Be2+-specific CD4 T cell lines and clones have been isolated from the lungs of CBD patients that express DP2 (Bowerman et al., 2011; Falta et al., 2013; Fontenot et al., 1999; Saltini et al., 1989). In vitro these T cells respond to Be2+, but not other metal cations, presented by autologous or other DP2-expressing antigen presenting cells (APCs). This response is dependent not only on Be2+ and DP2, but also on particular DP2 bound self-peptides (Falta et al., 2013). Using positional scanning peptide libraries, we recently identified a set of peptide mimotopes, and subsequently a set of related natural self-peptides, that can complete the DP2/peptide/Be2+ ligand for one of these T cells, AV22, as well as other T cells from the same patient or from non-related DP2+ patients (Falta et al., 2013). Here we report the functional, biophysical and structural properties of the AV22 TCR/DP2/peptide/Be2+ quaternary complex, and define the changes that occur in the DP2-peptide complex upon Be2+ binding that enable TCR recognition. Remarkably, the structures show that the immunogenic form of beryllium is not a beryllium containing compound, but rather the Be2+ cation itself, which is buried within the DP2-peptide structure. The TCR does not interact directly with the Be2+ ion, but instead recognizes changes in the surface of DP2/peptide complex surface induced by the internally bound Be2+.
RESULTS
Common features of DP2-peptide complexes
Listed at the top of Fig. 1A are three peptides that are well-represented among those naturally bound to DP2 (Diaz et al., 2005), yet do not present Be2+ to the Be2+ specific TCR, AV22, or any other Be2+ reactive T cell tested so far (Dai et al., 2010; Falta et al., 2013). We previously solved the structure of one of these non-presenting peptides, DRa, bound to DP2 (Dai et al., 2010). We have now solved the structures of DP2 bound to the other two non-presenting peptides, A28 and RAS. Although they do not present Be2+, the structures of these complexes reveal two common features of DP2 that are relevant to Be2+ presentation. First, in all three structures the important β69E lies in an acidic pocket that includes two other DP2 acidic amino acids, β26E and β68E (Fig. 1B). We have shown previously that these three amino acids are important in Be2+ presentation (Dai et al., 2010). Secondly, the wide gap (11.0Å) between the peptide backbone and the DP2 beta chain alpha helix that we reported previously for the DP2-DRa structure is also present in the DP2-A28 (10.3Å) and DP2-RAS (10.5Å) structures (Fig. S1). This gap is several angstroms wider than generally seen in the structures of other MHCII alleles (Dai et al., 2010). Consequently, this extra space frees the side chains of the p4 and p7 amino acids that flank the acidic pocket to assume rotamers that can enter the pocket. For example, in the A28 structure the p4K interacts with DP2 β26E and β69E. Similarly, in the RAS structure, p4Q and p7H interact with DP2 β26E and β69E, respectively. These observations suggested to us that, depending on the peptide sequence, the particular amino acids at p4 and p7 of the peptide might influence the ability of the pocket to capture Be2+ from the solvent. We therefore set out to test this idea.
Figure 1. Unique properties of the DP2 acidic pocket.
(A) Three groups of DP2-binding peptides are listed. The top group are self-peptides that are commonly found bound to DP2 (Díaz et al., 2005), but cannot present Be2+ to AV22. The middle group contains examples from a series of mimotope peptides found in a positional scanning library (Falta et al., 2013) that can present Be2+ to AV22. The bottom group contains examples of self-peptides that can present Be2+ to AV22, identified by their similarity to the mimotope peptides (Falta et al., 2013).
(B) Details of the acidic pocket of DP2 occupied by each of three peptides at the top of (A), pDRa, pA28, pRAS. Ribbons: DP2α cyan; DP2β magenta. Wireframe with CPK coloring: Peptide and side chains of DP2 β26E, β68E, and β69E. Potential H-bond/salt bridges, green lines.
See also Figure S1 and Table S1.
Unique properties of the peptides that allow Be2+ presentation by DP2
Some of the previously identified mimotopes and self-peptides that, when bound to DP2, allow presentation of Be2+ to AV22 and related T cells are also listed in Fig. 1A in the middle and bottom sections, respectively (Falta et al., 2013). These Be2+ presenting peptides all share a p4D and p7E. Given the results described above, we hypothesized that these two acidic amino acids interact with DP2 to capture and coordinate Be2+ such that the combination is recognized by Be2+ specific TCRs. Therefore, we investigated their role in the properties of mimotope-2 (M2) bound to DP2 in the presence and absence of Be2+.
We prepared two mutated forms of the DP2-M2 complex: DP2-M2(NQ), in which the p4D and p7E of M2 were changed to the nearly isomorphous amides, N and Q, and DP2(β69K)-M2, in which the DP2 high-risk amino acid for CBD, β69E, was changed to lysine, the amino acid found at position β69 in HLA-DP4, a DP allele negatively associated with CBD (Maier et al., 2003). We assessed the ability of the wild type and mutated complexes to stimulate the AV22 T cell transfectoma with or without Be2+ (Fig. 2A). As a negative control, we used an irrelevant HLA-DR52c-peptide complex (Yin et al., 2012). None of the complexes stimulated AV22 in the absence of Be2+. In the presence of Be2+, AV22 responded strongly to the DP2-M2 complex, but there was no response to either of the mutant DP2-M2 complexes or the control DR52c complex, indicating the importance of β69E and the requirement for the carboxylates of the p4D and p7E side chains for Be2+ presentation.
Figure 2. The acidic pocket is required for the Be2+-dependent functional and biophysical changes in DP2-peptide complexes.
(A) Equivalent amounts of biotinylated MHCII-peptide complexes (DP2-M2, DP2-M2(NQ), DP2(β69K)-M2, and HLA-DR52c-pHIR) were immobilized in separate culture wells and used to stimulate the AV22 transfectoma T cell with or without various concentrations of BeSO4. IL-2 secretion was assessed at 24 hr. The experiment was repeated with equivalent results.
(B) Biotinylated DP2-M2 (Approximately 2,000 RU) was captured in a flowcell of an SA-biosensor chip in a BIAcore 2000 instrument. Various concentrations of soluble AV22 TCR were injected for 140s through the flowcell, before and after loading of the immobilized DP2-M2 with Be2+ . The surface plasmon resonance signal (RU) was followed both during and after the injection and the binding kinetics analyzed with BIAcore BIAevaluation 4.1 software. The experiment was repeated four times with similar results.
(C) The plateau equilibrium RU data in (B) were used to create a Scatchard plot. The dissociation constant (KD) and regression coefficient (R) were calculated from the linear least-squares fit to the data.
(D) Same as (B) except that biotinylated DP2-M2(NQ) and DP2(β69K)-M2 were immobilized in separate flowcells. Only data for the injection with 5 µM AV22 TCR is shown. The experiment was repeated with similar results.
(E) The thermal stability of the series of soluble MHCII-peptide complexes shown was assessed in the presence or absence of Be2+ using differential scanning fluorimetry with the fluorescent dye SYPRO orange. The melting temperature (Tm) was defined as the temperature needed to achieve the half maximal increase in fluorescence. Results are pooled and averaged from six experiments. Each melting curve was performed at least three times among the experiments.
We confirmed these results with surface plasmon resonance TCR binding experiments. Equal amounts of the soluble, biotinylated DP2-M2 or the mutant complexes were captured in the flow cells of a BIAcore streptavidin BIAsensor chip and tested for binding of soluble AV22 TCR. In the absence of Be2+ the unmutated DP2-M2 complex failed to bind the TCR (Fig. 2B, left panel). As we previously have demonstrated (Falta et al., 2013), in the presence of Be2+ the same complex bound AV22 TCR with high affinity (3–6µM) and first order kinetics (Figs. 2B and C). In contrast, neither the DP2-M2(NQ) nor the DP2(β69K)-M2 mutated versions of the complex, with or without Be2+, bound the AV22 TCR (Fig. 2D). Taken together these results confirm our previous conclusion that the acidic properties of p4D, p7E and β69E are essential for Be2+ presentation.
Finally, using differential scanning fluorimetry (Niesen et al., 2007), we determined the effect of Be2+ on the thermal stability of various MHCII-peptide complexes, using the binding of the fluorescent dye, SYPRO Orange, to follow protein denaturation (Fig. 2E). We examined four DP2-peptide complexes, two with peptides, M2 and Plx4, that could present Be2+ to AV22 (Fig. 1A), one with the self-peptide, A28, that could not present Be2+ and finally the DP2(β69K)-M2 mutant described above. As a negative control, we used the well-studied mouse MHCII-peptide complex, IAb-3K (Liu et al., 2002). The stability of each complex was defined by the melting temperature (Tm), i.e. the temperature causing the half maximal increase in SYPRO Orange fluorescence. In the absence of Be2+, the Tm of the control IAb-3K complex, as well as those of the DP2-A28, DP2-M2 and DP2-Plx4 complexes ranged from 54.4–62.5°C. As expected, the addition of Be2+ had little effect on the Tm of IAb-3K or the DP2-A28 complex. However, addition of Be2+ raised the Tm of DP2-M2 and DP2-Plx4 complex to more than 70°C, a greater than 10°C increase. Unexpectedly, in the DP2(β69K)-M2 complex, substitution of lysine for the critical DP2 β69E led to a similar increase in stability, whether or not Be2+ was added. This result suggests that the positively charged ε-amino group of β69K might substitute for Be2+ in the stabilization of the complex, but could not create the ligand recognized by AV22 (Fig 2A).
These findings strongly implicated the cluster of acidic amino acids from both DP2 and the presenting peptides as the site of Be2+ binding, since both were involved in stabilizing the complex in the presence of Be2+ and both were needed to create a Be2+ dependent ligand that could be recognized by the AV22 T cell.
Be2+ and an accompanying Na+ cation bind internally in the acidic pocket of DP2-M2
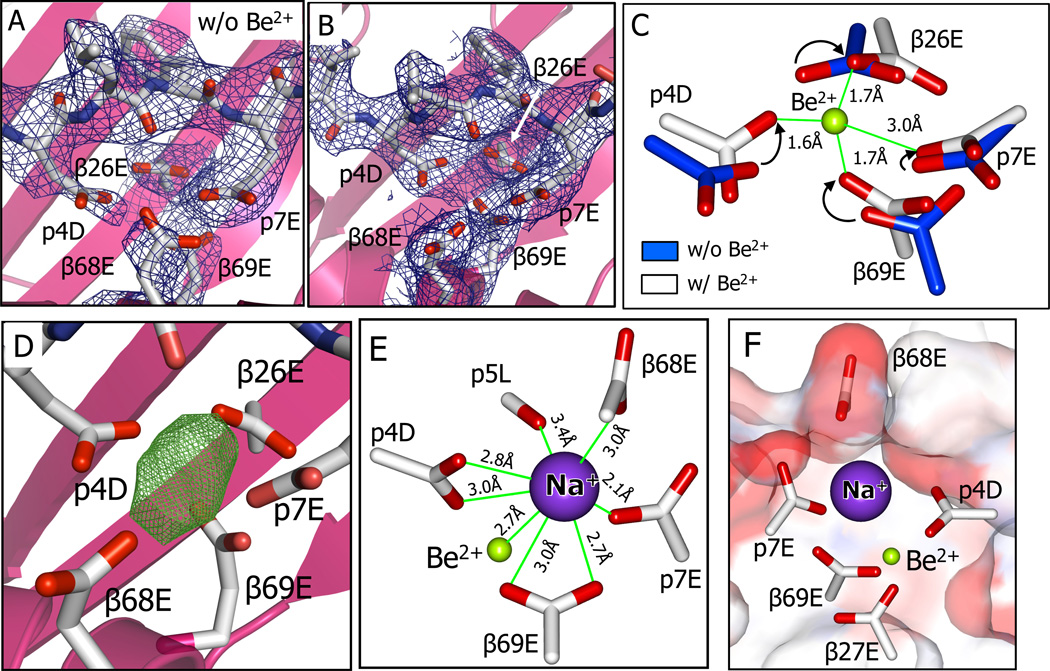
To understand the nature of the Be2+ moiety that binds to DP2-M2, how the DP-M2 structure changes after binding Be2+, and how the AV22 TCR recognizes these changes, we solved the structures of DP2-M2 in the absence of Be2+ and of the DP2-M2-Be2+ complex bound by the AV22 TCR (relevant methods and statistics for data collection and refinement for all structures reported in the paper are given in Supplemental Experimental Procedures and Table S1). We first compared the structure of the acidic pocket in the free DP2-M2 complex to that in the Be2+ loaded DP2-M2 bound by the AV22 TCR.
In the DP2-M2 complex, electron density in the acidic pocket clearly shows that the side chains of p4D and p7E have entered the acidic pocket, both prior to (Fig. 3A) and after (Fig. 3B) Be2+ addition. However, their side chain configurations and those of the other acidic amino acids in the pocket have changed in the presence of Be2+, indicating that Be2+ has been captured within the pocket. To deduce the position of the Be2+, we relied on previous analyses of numerous small molecule structures containing Be2+ coordinated by oxygens, as well as larger protein structures in which an aspartic acid carboxylate has captured BeF3. The coordination chemistry of Be2+ is well documented in these studies (Everest, 1964; Keizer et al., 2005). The high charge density if Be2+ creates four short, semi-covalent bonds (1.5 to 1.7 Å) to its ligands distributed in a tetrahedral arrangement with bond angles of about 110°. Because there are but two electrons in Be2+, in most x-ray structures the position of the Be2+ in the complex cannot be deduced from the Be2+ electron density alone, but rather from the position where the electron densities of its ligands converge.
Figure 3. Be2+ and an accompanying Na+ cation captured in the acidic pocket are shielded from the solvent.
(A and B) View from above looking into the acidic pocket of DP2-M2-Be2+ with the side chains of β26E, β68E, β69E, p4D, and p7E shown with 2Fo-Fc (1σ) electron density before (A) and after (B) capture of Be2.
(C) Overlay of the carboxylates of β26E, β69E, p4D and p7E before (carbon, blue) and after (carbon, white) binding of Be2+. Arrows show the Be2+ induced movement of the relevant carboxylate oxygens. Be2+ (green) is modeled within the cluster of oxygens based on small molecule structures of Be2+ coordinated by carboxylates (Alerghi et al., 1999; Schmidbaur et al., 1991; Schmidt et al., 1997) and the Be-O bond lengths are shown.
(D) Unaccounted for electron density in the Fo-Fc (6σ) map in the acidic pocket of DP2-M2-Be2+.
(E) A Na+ cation is shown modeled into Fo-Fc density of (D). Distances of the Na+ from Be2+ and from oxygens of the carboxylates of 68E, β69E, p4D, and p7E and the backbone carbonyl oxygen of p5L are shown.
(F) A view looking through the M2 peptide toward the DP2 β1 alpha helix showing the water accessible surface of the DP2-M2-Be2+ colored by the contributing atoms (oxygen-red, carbon-white). The surface contributed by the DP2 α1 alpha helix has been clipped to reveal the carboxylates of the five acidic amino acids in the pocket along with Be2+ and Na+ among which only β68E, p4D, and p7E are surface accessible.
Based on the genetic, mutational, functional and biophysical data, we clearly predicted that a carboxylate oxygen from β69E would be one of these ligands. In the absence of Be2+, separate densities were seen for each of the five carboxylates in the acidic pocket (Fig. 3A), but their oxygens were not in the correct positions to join with β69E in tetrahedral Be2+ ligation. However, in the presence of Be2+, the density for the carboxylates converged (Fig. 3B) and carboxylate oxygens from p4D and β26E were brought into position to ligate an β69E captured Be2+ with the expected Be-O bond lengths of 1.6–1.7Å and O-Be-O bond angles of 110–115° (Fig. 3C). Also movement of the p7E carboxylate placed a fourth oxygen at an angle such that it could be the fourth Be2+ ligand. However, the 3Å distance from this oxygen to the Be2+ is too far to allow a conventional Be-O bond (Fig. 3C). Since there are no published structures of Be2+ coordinated solely by protein amino acid carboxylates, we cannot say if this type of Be2+ ligation in a protein is unusual, although small molecules containing a Be2+ with only three ligands do exist (Diyabalanage et al., 2008) and deviations from ideal ligation have been seen for other cations incorporated into protein structures (Evans, 2007; Harding, 2002). Therefore, this positioning of Be2+ among these four oxygens was consistent both with its known coordination chemistry and with our data with mutations of β26E β69E, p4D and p7E presented here and previously (Dai et al., 2010; Falta et al., 2013).
The Fo-Fc electron density map of the complex suggested the presence of another larger atom(s) in the acidic pocket in addition to Be2+ (Fig. 3D). We considered that this density might be due to an hydroxyl ion acting as a fourth Be2+ ligand, but the position and distance of this oxygen in relation to Be2+ would be inconsistent with Be2+ coordination geometry. It is more likely that this density was due to a second cation, often seen attracted to Be2+ complexes because of the net negative charge of the ligated Be2+. We could not determine the nature of this cation from the density alone. However, no ordered cation was seen in the structure of the free DP2-M2 complex in the absence of Be2+ (Fig 3A) and the mother liquor used in crystallizing the AV22-DP2-M2-Be2+ complex contained 300mM Na+ as the only cation. Therefore, by default, we modeled Na+ into this density (Fig. 3E). Placed in this position the Na+ is surrounded by oxygens from the carboxylates of p4D, p7E, β68E and β69E and from the carbonyl of the p5L backbone, consistent with the analyses of M. Harding (Harding, 2002) documenting a predilection for Na+ to interact with up to 8 oxygens in proteins. In order to accommodate this Na+ the carboxylate of β68E has been pushed up on the surface of the DP2-M2 complex and undergone a 90° rotation, bringing its negatively charged oxygen into alignment with the Na+ cation.
While our data support the conclusion that the cation captured in this crystal is Na+, our structure here does not rule out the possibility that, in vivo or tissue culture, cations other than Na+ might occupy this position and may even complete the TCR ligand. Mg2+ would be a possibility, but so would Ca2+ and K+, also abundant in biological fluids and tissue culture media.
In summary, upon Be2+ binding, the DP2-M2 acidic pocket undergoes a conformational rearrangement in order to capture the Be2+ and an accompanying Na+ cation through a network of interactions via the β69E carboxylates and other DP2 and M2 oxygens, thus dramatically increasing the thermal stability of the complex. There are two unexpected features of this complex. First, the Be2+ moiety captured is the Be2+ cation itself, not a larger complex of Be2+ already partially ligated by solvent derived electron donors (water, hydroxyl, carbonate, etc.). This answers a long-standing question in this field regarding the chemical form of Be2+ that is captured by DP2. Secondly, both the Be2+ and Na+ are not accessible on the surface of the complex, so that the AV22 TCR must recognize some change in the complex surface induced indirectly by the Be2+ and Na+. (Fig. 3F). Therefore, we analyzed the interface between the AV22 TCR and the DP2-M2-Be2+ complex, especially in the region over the inaccessible Be2+ and Na+. A summary of the atom-to-atom contacts and the complete details of the contacts are listed in Table 1 and Table S2, respectively.
Table 1.
Contacts between AV22 TCR and DP2-M2-Be2+
| AV22 TCR Contacts to DP2-M2-Be2+ |
DP2-M2-Be2+ Contacts to AV22 TCR |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| V Domain |
CDR Loop |
Amino Acid |
# of Atom to Atom Contacts to |
Ligand | Amino Acid |
# of Atom to Atom Contacts to |
|||||
| DP2α | M2 | DP2β | Vα | Vβ | |||||||
| 30Y | – | 6 | 45 | 55E | 7 | – | |||||
| 1 | 32S | – | – | 3 | 57Q | 4 | – | ||||
| DP2α | |||||||||||
| 49K | – | – | 1 | 58G | 4 | – | |||||
| 2 | 51T | – | – | 3 | 65I | – | 4 | ||||
| 52K | – | – | 2 | 1F | 1 | – | |||||
| Vα | |||||||||||
| 95Y | – | 59 | – | 2W | 54 | – | |||||
| 97G | 3 | – | – | 3I | 24 | – | |||||
| 3 | 98A | 12 | 11 | – | M2 | 5L | 11 | 10 | |||
| 99G | – | 14 | – | 6F | – | 4 | |||||
| 101Y | – | – | 19 | 7E | – | 6 | |||||
| 1 | 30R | – | 10 | – | 8T | 17 | |||||
| 50F | – | 1 | – | 58Y | – | 3 | |||||
| 2 | 55R | – | 3 | – | 62Q | – | 4 | ||||
| 95L | – | – | 12 | 64D | – | 22 | |||||
| 96A | – | – | 10 | 65I | – | 4 | |||||
| Vβ | 97Q | 4 | 23 | 5 | 67E | 2 | – | ||||
| 3 | 98G | – | – | 6 | 68E | – | 20 | ||||
| 99G | 1 | DP2β | 71A | 2 | – | ||||||
| 100E | – | – | 10 | 74D | 4 | – | |||||
| 101T | – | – | 9 | 75R | 24 | 8 | |||||
| 103Y | – | – | 8 | 78R | 15 | – | |||||
| 79H | 20 | – | |||||||||
| 82E | 6 | – | |||||||||
| Total | 19 | 127 | 134 | Total | 178 | 102 | |||||
General features of the AV22 TCR binding to the DP2-M2-Be2+ ligand
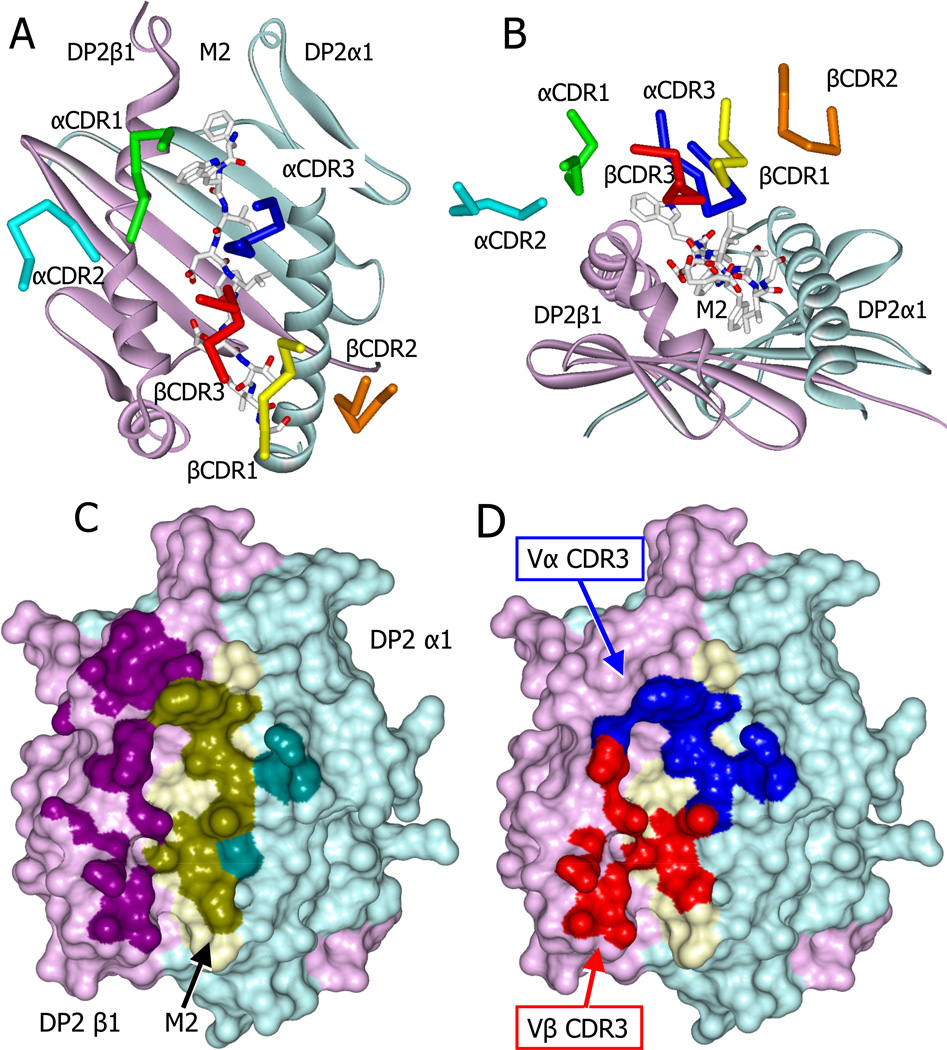
Typically, TCRs bind to MHCII ligands in a diagonal mode that places the CDR3 loops of the V domains over the peptide, while CDR1 and CDR2 loops make multiple interactions with the MHCII helices [e.g. see (Marrack et al., 2008; Wilson and Garcia, 1997)]. The AV22 TCR also takes this diagonal orientation on the DP2 complex (Fig. 4A), but there are some unusual features of the interface. The TCR is tilted toward the DP2 beta chain helix (Fig. 4B) resulting in extensive contact with the entire length of the M2 peptide and DP2 beta chain helix, at the expense of the DP2 alpha chain helix (Fig. 4C, Table 1). Furthermore, atypically, the hypervariable CDR3 loops of the TCR dominate the binding (Fig. 4D, Table 1), providing almost three quarters of the contacts with the ligand. The CDR1 and CDR2 loops of the TCR contribute little to the interface with the exception of a single TCR Vα amino acid, CDR1 α30Y, which binds extensively to the C-terminal end of DP2 β chain helix (Table 1, Fig. 4C). The focus of the AV22 TCR on the bound peptide and DP2 beta chain helix is consistent with our previous extensive mutational analysis of DP2 and the AV22 TCR (Bill et al., 2005; Bowerman et al., 2011). Despite this tilted conformation, the footprint of the AV22 TCR on DP2-M2 involves 280 atom-to-atom contacts (Table 1) and covers 1288 Å2, well within the range typically seen for CD4 T cell TCR contact with MHC-peptide ligands. Moreover, the TCR has a KD of ~5µM when binding to the complex (Falta et al., 2013) and Fig. 2B,C, among the highest affinities seen for a TCR from a CD4 T cell.
Figure 4. Skewed docking of the AV22 TCR on the DP2-M2-Be2+ complex.
(A) View from above showing the diagonal docking of the six AV22 TCR CDR loops over the DP2-M2-Be2+ complex.
(B) View looking down the peptide binding groove from the peptide C terminus showing the tilt and shift of the AV22 TCR CDR loops toward the DP2 beta chain helix.
(C) Water accessible surface of the DP2-M2-Be2+ complex, DP2α (cyan), DP2β (magenta), and M2 (yellow). The footprint of the AV22 TCR on the complex is shown with darker versions of the same colors. Complex atoms were defined to be part of the footprint if they were within 4.5 Å of a TCR atom.
(D) The parts of the footprint created by the AV22 TCR CDR3α (blue) and CDR3β (red) are shown.
See also Table S1 and Table S2.
TheAV22 TCR interacts with changes in DP2-M2 induced by Be2+
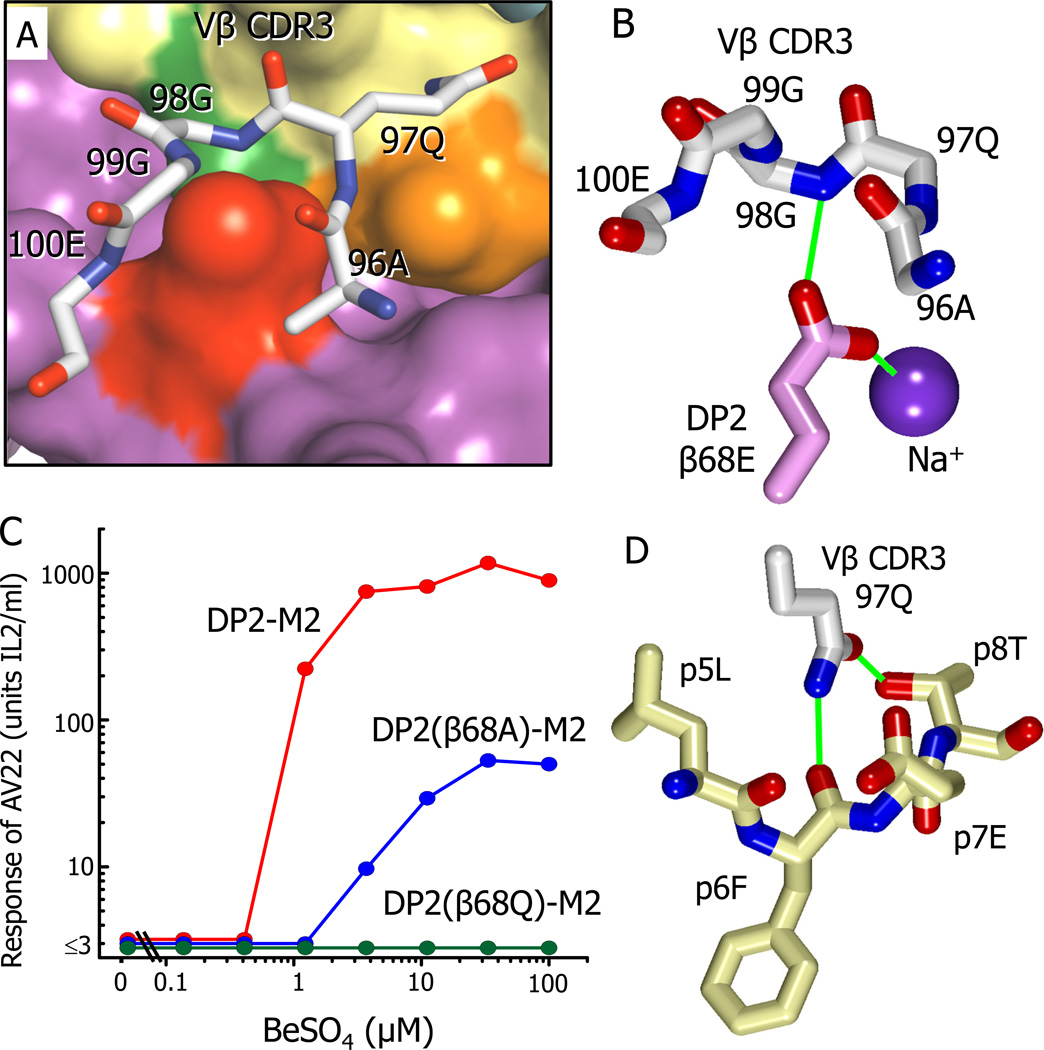
As predicted by their internal positions in the ligand structure, the sheltered Be2+, Na+, β26E and β69E have no direct TCR contact (Table S2), but there is a large area of TCR contact, especially via Vβ CDR3, with the surface of DP2-M2 that includes the re-positioned β68E carboxylate, as well as surrounding DP2 and M2 amino acids (Fig. 5A, Fig.4D, Table 1, Table S2). The re-positioned β68E carboxylate bridges the hidden Na+ and Vβ CDR3 via an H-bond to the backbone N of Vβ 98G (Fig. 5B, Table S2).
Figure 5. Interaction of AV22 VβCDR3 with the surface of DP2-M2 above the Be2+ binding site.
(A) A wireframe representation is shown of the backbone of the AV22 TCR VβCDR3 loop and the side chain of Vβ97Q interacting with the surface of DP2-M2.
(B) The orientation of the side chain carboxylate of DP2 β68E between the VβCDR3 loop of the AV22 TCR and the internal Na cation is shown. Potential H-bond/salt bridges are shown as green lines.
(C) The response of AV22 without or with various concentrations of BeSO4 presented by the wild-type DP2-M2 complex or the complex mutated at β68E to glutamine or alanine. The experiment was repeated with similar results.
(D) Interactions of the carboxamide of Vβ CDR3 97Q with the M2 peptide are shown. Potential H-bonds are shown as green lines.
See also Figure S2 and Table S2.
In a previous study we showed that mutation of β68E to A virtually eliminated the Be2+ response of a line of lung T cells (containing T cells bearing various TCRs) from the patient that provided the AV22 T cell. In these experiments the presenting DP2 was on the surface of an APC line and contained the usual array of naturally processed self-peptides (Dai et al., 2010). Therefore, we tested the effect of mutating β68E on the recognition by the AV22 of our soluble DP2, in this case uniformly occupied by M2 and Be2+. We tested the mutation of β68E to A (eliminating its side chain) but also to Q (eliminating only the negative charge of its carboxylate). The β68E to Q change completely eliminated the ability of DP2-M2 to present Be2+ to AV22 (Fig. 5C) or to be bound by the soluble AV22 TCR in the presence of Be2+ (Fig. S2). However, in this case, mutation of β68E to A only partially reduced the response (Fig. 5C). It also decreased by twofold the half-life of the AV22 TCR complex with DP2-M2-Be2+, but not the overall affinity of the TCR for the ligand (Fig. S2). These results suggest that the direct interactions of the Vβ CDR3 with the side chain of β68E contribute to, but are not required for, a response, depending on the specific TCR and peptide presenting the Be2+. However, more importantly, when this side chain is present, the Be2+, the accompanying Na+ and the negative charge of β68E carboxylate may all be required for the proper re-alignment of the carboxylate. Removing the β68E charge by its mutation to Q, must somehow untether the side chain to interfere with TCR binding.
Another area of extensive interaction of the Vβ CDR3 loop is via the carboxamide of the Vβ 97Q side chain, which lies across the peptide making substantial van der Waals’ and H-bond contacts with the M2 peptide (p5L, p6F p7E and p8T) (Fig. 5D and Table 1 and Table S2). These aspects of the interaction agree with our previous mutation analysis of the AV22 TCR, where an alanine scan of the Vβ CDR3 loop showed that the side chain of β97Q was required for Be2+ recognition (Bowerman et al., 2011).
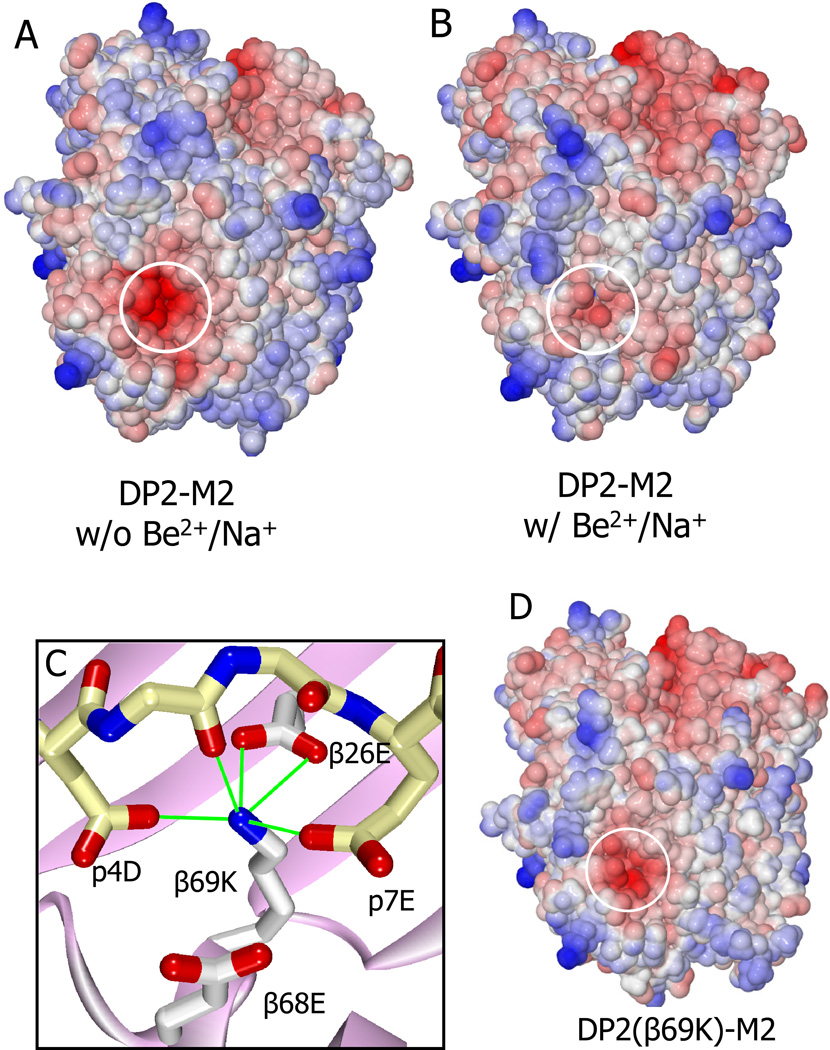
In addition to the conformational changes, the most dramatic difference between the DP2-M2 complex before and after Be2+ and Na+ binding is the change in the predicted electrostatic surface potential (Fig. 6A,B). In the absence of Be2+ and Na+ the surface potential in the entire region around the acidic pocket is very negative, but it becomes substantially neutralized by the addition of the positively charged Be2+ and Na+ to the acidic pocket. This electrostatic difference would be expected to influence the ability of the Vβ CDR3 loop to approach and interact with this surface. As mentioned above, we can’t rule out that, in vivo or tissue culture, the cation accompanying Be2+ might be an abundant divalent cation, such as Mg2+ or Ca2+, rather than Na+. In this case we would predict that the negative electrostatic surface potential would be reduced even further.
Figure 6. Specific changes in the electrostatic surface potential of the DP2-M2 complex caused by binding Be2+/Na+.
(A and B) Water accessible surface of the DP-M2 complex in the absence (A) or presence (B) of Be2+ and Na+, colored by the electrostatic surface potential (red, negative; blue, positive). Area overlaying the acidic pocket is circled.
(C) The interaction of the β69K lysine ε-amino group in the DP2(β69K)-M2 mutant structure with amino acids in the acidic pocket is shown. Potential H-bond/salt bridges are shown as green lines.
(D) The electrostatic surface potential of the DP2(β69K)-M2 mutant complex is shown as in (A).
See also Table S1 and Table S2.
The structure of the DP2(β69K) complex supports the importance of these surface changes induced by Be2+ and Na+ binding. This mutated complex cannot present Be2+ to AV22 (Fig. 2A), but its thermal stability is equal to that of the wild type complex in the presence of Be2+ (Fig. 2E). In the structure, the β69K positively charged ε-amino group has been drawn into the acidic pocket in a position similar to the Be2+ in the wild type structure and is involved in a network of H-bonds and salt bridges to p4D, p7E, DP2 β26E and the peptide backbone (Fig. 6C), accounting for the thermal stability of the complex and predicting that this complex could not bind Be2+. However, there is no evidence of a captured cation and the β68E side chain is aligned similarly to that of the DP2-M2 complex without Be2+. Furthermore the predicted electrostatic surface potential of the mutant complex is only slightly reduced by the lysine single positive charge (Fig. 6D). These differences from the DP-M2-Be2+ complex most likely explain the lack of recognition by AV22.
In summary, after binding to DP2-M2, Be2+ and Na+ are not exposed for TCR interaction, but rather induce both a reduction in the electrostatic surface potential and a subtle change in surface topology of the DP2-M2 over the cation binding site where the AV22 TCR Vβ CDR3 interacts. Our data indicate that both of these changes probably contribute to creation of the AV22 ligand, but we cannot at present estimate the relative contributions of the two alterations.
DISCUSSION
Dozens of structures of TCRs interacting with MHC-peptide ligands have been published and there are a number of structures detailing how TCRs bind to non-classical MHC molecules specialized to present particular non-peptide or peptide ligands (http://www.imgt.org/3Dstructure-DB/). However, T cell mediated hypersensitivity to non-peptide environmental agents such as drugs, plant products and metal cations are widespread in the human population. There is very little known structurally about how these agents become associated with MHC molecules and how T cells recognize their presence. The work presented here answers a long-standing question about how a metal cation, in this case Be2+, becomes associated with a self MHC-peptide complex to create the ligand for a pathogenic T cell.
The most common hypothesis for how non-peptide moieties are recognized by T cells has been that they act as haptens, i.e. they become associated with or covalently bound to the surface of an MHC-peptide complex and offer a new site for TCR interaction, in the same way that a mutation in one of the upwardly pointing amino acids of the MHC or bound peptide would create a new antigen. Earlier studies with T cell reactivity to MHC-peptide complexes modified with chemically reactive forms of trinitrophenol and fluorescein or the urushiols produced by plants such as poison ivy were interpreted this way (Kalish, 1990; Martin and Weltzien, 1994).
Our X-ray diffraction data as well as mutational and biophysical results, show that Be2+ is clearly not working in this way, but, rather, becomes part of the internal structure of the MHC-peptide complex, indirectly causing structural and biophysical changes on the surface of the complex that are now recognized by the T cell. The Pichler group has argued for some years for a concept they term “pharmacological interaction with immune receptors” or the “p-i” concept (Pichler et al., 2006), in which drugs can interact with TCRs or MHC-peptide complexes to promote an interaction without necessarily placing the drug at the interface between the two. Perhaps the most dramatic evidence for this phenomenon is seen in the severe allergic response to the drugs, abacavir and carbamazepine. These reactions are strongly associated with particular MHCI HLA-B alleles (Illing et al., 2013). Recent structural and biochemical data (Illing et al., 2012) indicate that, unlike Be2+, these drugs work by binding inside the MHC peptide binding groove to drastically change the repertoire of self-peptides bound, generating a robust CD8 T cell response similar to an allo-MHC response.
In our studies here Be2+ also binds internally within the peptide binding groove of DP2 without becoming part of the TCR interface, yet it creates the TCR ligand without changing the DP2 bound peptide. Also supporting this conclusion is our previous study using DP2+ antigen presenting cells to present Be2+ to CBD T cells. To show that Be2+ dependent loading of new peptides into DP2 was not required, we fixed the antigen presenting cells prior to Be2+ exposure without compromising the T cell response to Be2+ (Fontenot et al., 2006). Nevertheless, the DP2 complex becomes much more stable in the presence of Be2+ and the appropriate mimotope or self-peptide (Fig. 2E). Therefore, it is possible that the continuous presence of Be2+ in vivo in CBD, may promote the loading or stability of peptides that present Be2+ in the MHCII endosomal peptide loading compartment, eventually skewing the lung DP2 bound peptide repertoire toward Be2+ presenting peptides.
The nature of the Be2+ containing compounds recognized by the immune system has been discussed for many years. Because of its ability to form higher ordered complexes in aqueous solutions (Everest, 1964; Sutton and Burastero, 2003), it was natural to consider that the Be compound captured by DP2 β69E might already contain multiple Be ligands, thus creating a surface exposed hapten-like moiety for T cell recognition. Our data presented here clearly shows that this is not the case. Rather, it is the Be2+ cation itself that is captured by the DP2-M2 complex. This explains why individual T cell clones, such as AV22, can be activated by a wide variety of soluble Be containing compounds (Dai et al., 2010). The Be2+ binding acidic pocket in the DP2-peptide complex appears to compete extremely well with other potential ligands for Be2+.
AV22 and related T cells found in the lungs of multiple CBD patients respond to Be2+ presented by DP2 containing a variety of related self or library generated peptides (Falta et al., 2013) and Fig. 1A. The common elements in all of the peptides, besides a DP2 binding motif, are p4D and p7E. However, these peptides do not satisfy all Be2+ reactive T cells (Falta et al., 2013). Given the extensive interaction of the AV22 TCR with areas of the DP2-M2 surface away from the DP2 acidic pocket (Table I), it is likely that these other T cells will require TCR contact with different peptide amino acids in these other areas. However, we predict that they will have to retain this p4D/p7E or a similar acidic motif in order to effectively capture Be2+.
CBD is a particularly dramatic example of blurring the line between T cell allergic hypersensitivity and autoimmunity. Similar to many of the well-studied autoimmune diseases, CBD risk is tightly associated with certain MHCII alleles, in this case with an existing DP2-self-peptide complex that has been subtly changed by the internally bound Be2+. Also, as structural information begins to accumulate for the actual targets of autoimmune diseases, enzymatic post-translational modifications (Cordova et al., 2013; Scally et al., 2013; Sollid and Jabri, 2011) and organ-specific peptide processing have been implicated in generating the relevant ligands (Crawford et al., 2011; Mohan and Unanue, 2013; Stadinski et al., 2010). These are often not recognized by the autoimmune T cells directly, but instead affect the way the peptide binds to MHCII. In the same way, Be2+ binding could be thought of as a special form of “post translational modification” that converts a self-MHCII-peptide complex to which the immune system is tolerant into an immunogenic one. In this light, it is perhaps more useful to think of T cell allergic hypersensitivities vs. autoimmunity as involving a continuum of peptides from truly foreign ones through various levels of modifications of self-peptides mediated by both foreign and endogenous mechanisms.
EXPERIMENTAL PROCEDURES
More experimental details are in the Supplemental Material
Protein expression and purification
All MHC proteins were expressed in baculovirus infected insect cells as soluble proteins as previously described (Crawford et al., 1998; Dai et al., 2010). All contained an antigenic peptide covalently attached via a C-terminal flexible linker to the N-terminus of the MHCII beta chain (Kozono et al., 1994). All had a stabilizing C terminal acid-base leucine zipper (O'Shea et al., 1993) and a peptide tag for enzymatic biotinylation (Crawford et al., 1998), which were removed proteolytically with either papain or thrombin for crystallography and differential scanning fluorimetry.
The V domains of the AV22 TCR were cloned as previously described into a bacterial expression vector in frame with sequence encoding the extracellular portions of human Cα or Cβ (Dai et al., 2008; Kjer-Nielsen et al., 2003). Inclusion bodies containing the separate chains were prepared in E. coli BL21 Rosetta cells (EMD Biosciences). The solubilized proteins were mixed and refolded as previously described (Dai et al., 2008; Kjer-Nielsen et al., 2003).
T cell stimulation
A T cell transfectoma bearing the AV22 TCR (Bowerman et al., 2011) was stimulated in culture alone or with various concentrations of BeSO4. The surface of the culture wells had various biotinylated MHCII-peptide combinations captured via Extravidin (Sigma). The extent of AV22 activation was assessed by IL-2 in the culture supernatants after 24 hrs (White et al., 2000).
Surface plasmon resonance
The ability of the soluble AV22 TCR to bind to various combinations of MHCII-peptide with or without Be2+ was assessed with surface plasmon resonance using a BIAcore 2000 instrument. The biotinylated MHCII-peptide (~2000RU) was immobilized in flowcells of a streptavidin BIAsensor chip and various concentrations of the soluble AV22 TCR injected before and after exposure of the MHCII to 50µM Be2+. Binding kinetics were calculated using instrument software (BIAevaluation 4.1).
Differential scanning fluorimetry
The thermal stabilities of various MHCII-peptide complexes were determined by differential scanning fluorimetry using either a Strategene MX3005p (Agilent) or CFX96 RCT-PCR (BIORAD) instrument. Proteins were mixed with the dye SYPRO orange and the fluorescence of the dye bound to the protein was followed as the temperature was increased from 20°C to 100°C. The melting temperature (Tm) was defined as the temperature at which the fluorescence increase was 50% of the maximal increase.
Crystallography
Crystals grown in hanging or sitting drop chambers were cryopreserved in liquid nitrogen. Diffraction data were collected at Brookhaven x25, APS 24 ID-C and Berkley 822. Details of crystallization, cryo-preservation, data collection, data processing and model building and refinement are in the Supplemental Information and Table S1.
Structure analysis and representations
Atom-to-atom interactions between the AV22 TCR and DP2-M2-Be2+ were calculated with the ncont program in the CCP4 program suite. Electrostatic surface potential was calculated using PDB2PQR and APBS (Baker et al., 2001; Dolinsky et al., 2007; Dolinsky et al., 2004). Graphical representations of structures were constructed with PyMol, (PyMol), WebLab Viewer Pro 4.1 (Accelrys) and JMol (JMol).
Supplementary Material
ACKNOWLEDGEMENTS
We thank Igor Kurinov for data collection assistance at APS Argonne National Laboratory, Beamline 24 ID-C and Corie Ralston at ALS Lawrence Berkley National Laboratory, Beamline 822. Oliver Smart (Global Phasing) assisted us with beryllium definitions in AUTOBuster, and Mario Santiago made the BIORAD CFX96 RCT-PCR instrument available to us. We also thank Randy Anselment at the Molecular Resource Center, National Jewish Health, for oligonucleotide synthesis and DNA sequencing. This work was supported in part by NIH grant AI17785 (PCM), by NIH grants HL62410, HL92997, and ES011810 (to A.P.F.), by CCTSI KL2TR000156 and KL1TR000155 and the Boettcher Foundation (to S.D.) and NIH Grant AI17134 (to J.W.K)..
Footnotes
ACCESSION NUMBERS
The coordinate and structure factor files for the five new structures reported here have been deposited in the RCSB Protein Database with accession nos. 4P4K, 4P4R, 4P57, 4P5M and 4P5K.
SUPPLEMENTAL INFORMATION
Supplemental Information includes Extended Experimental Procedures, two figures and 2 tables.
AUTHOR CONTROBUTIONS
G.M.C., Y.W., S.D. and J.W.K. designed the studies. G.M.C., F.C. and J.W.K. prepared the expression constructs. G.M.C., F.C. Y.W., S.D. and A.N. produced and purified the proteins. G.M.C., Y.W., and S.D. performed the crystallography and solved the structures. G.M.C., Y.W., S.D., B.T.W. and J.S.K. collected the diffraction data. G.M.C., F.C. and J.W.K. performed the biophysical studies. M.T.F., N.A.B. and A.P.F. provided peptides and mutational data. G.M.C., Y.W., P.C.M., S.D., and J.W.K. wrote the paper with help from the other authors.
References
- Baker NA, Sept D, Joseph S, Holst MJ, McCammon JA. Electrostatics of nanosystems: application to microtubules and the ribosome. Proc Natl Acad Sci U S A. 2001;98:10037–10041. doi: 10.1073/pnas.181342398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bill JR, Mack DG, Falta MT, Maier LA, Sullivan AK, Joslin FG, Martin AK, Freed BM, Kotzin BL, Fontenot AP. Beryllium presentation to CD4+ T cells is dependent on a single amino acid residue of the MHC class II beta-chain. J Immunol. 2005;175:7029–7037. doi: 10.4049/jimmunol.175.10.7029. [DOI] [PubMed] [Google Scholar]
- Bowerman NA, Falta MT, Mack DG, Kappler JW, Fontenot AP. Mutagenesis of beryllium-specific TCRs suggests an unusual binding topology for antigen recognition. J Immunol. 2011;187:3694–3703. doi: 10.4049/jimmunol.1101872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordova KN, Willis VC, Haskins K, Holers VM. A citrullinated fibrinogen-specific T cell line enhances autoimmune arthritis in a mouse model of rheumatoid arthritis. J Immunol. 2013;190:1457–1465. doi: 10.4049/jimmunol.1201517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford F, Kozono H, White J, Marrack P, Kappler J. Detection of antigen-specific T cells with multivalent soluble class II MHC covalent peptide complexes. Immunity. 1998;8:675–682. doi: 10.1016/s1074-7613(00)80572-5. [DOI] [PubMed] [Google Scholar]
- Crawford F, Stadinski B, Jin N, Michels A, Nakayama M, Pratt P, Marrack P, Eisenbarth G, Kappler JW. Specificity and detection of insulin-reactive CD4+ T cells in type 1 diabetes in the nonobese diabetic (NOD) mouse. Proc Natl Acad Sci U S A. 2011;108:16729–16734. doi: 10.1073/pnas.1113954108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai S, Falta MT, Bowerman NA, McKee AS, Fontenot AP. T cell recognition of beryllium. Curr Opin Immunol. 2013;25:775–780. doi: 10.1016/j.coi.2013.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai S, Huseby ES, Rubtsova K, Scott-Browne J, Crawford F, Macdonald WA, Marrack P, Kappler JW. Crossreactive T Cells spotlight the germline rules for alphabeta T cell-receptor interactions with MHC molecules. Immunity. 2008;28:324–334. doi: 10.1016/j.immuni.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai S, Murphy GA, Crawford F, Mack DG, Falta MT, Marrack P, Kappler JW, Fontenot AP. Crystal structure of HLA-DP2 and implications for chronic beryllium disease. Proc Natl Acad Sci U S A. 2010;107:7425–7430. doi: 10.1073/pnas.1001772107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz G, Canas B, Vazquez J, Nombela C, Arroyo J. Characterization of natural peptide ligands from HLA-DP2: new insights into HLA-DP peptide-binding motifs. Immunogenetics. 2005;56:754–759. doi: 10.1007/s00251-004-0735-5. [DOI] [PubMed] [Google Scholar]
- Diyabalanage HV, Ganguly K, Ehler DS, Collis GE, Scott BL, Chaudhary A, Burrell AK, McCleskey TM. Three-coordinate ligand for physiological beryllium imaging by fluorescence. Angew Chem Int Ed Engl. 2008;47:7332–7334. doi: 10.1002/anie.200801965. [DOI] [PubMed] [Google Scholar]
- Dolinsky TJ, Czodrowski P, Li H, Nielsen JE, Jensen JH, Klebe G, Baker NA. PDB2PQR: expanding and upgrading automated preparation of biomolecular structures for molecular simulations. Nucleic Acids Res. 2007;35:W522–W525. doi: 10.1093/nar/gkm276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolinsky TJ, Nielsen JE, McCammon JA, Baker NA. PDB2PQR: an automated pipeline for the setup of Poisson-Boltzmann electrostatics calculations. Nucleic Acids Res. 2004;32:W665–W667. doi: 10.1093/nar/gkh381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans PR. An introduction to stereochemical restraints. Acta Crystallogr D Biol Crystallogr. 2007;63:58–61. doi: 10.1107/S090744490604604X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everest DA. The Chemistry of Beryllium. In: Robinson PL, editor. In Topics in Inorganic and General Chemistry. Vol. 1. 52 Vanderbilt Avenue, New York, N.Y.: American Elsevier Publishing Company, Inc.; 1964. [Google Scholar]
- Falta MT, Pinilla C, Mack DG, Tinega AN, Crawford F, Giulianotti M, Santos R, Clayton GM, Wang Y, Zhang X, et al. Identification of berylliumdependent peptides recognized by CD4+ T cells in chronic beryllium disease. The Journal of experimental medicine. 2013;210:1403–1418. doi: 10.1084/jem.20122426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontenot AP, Amicosante M. Metal-induced diffuse lung disease. Semin Respir Crit Care Med. 2008;29:662–669. doi: 10.1055/s-0028-1101276. [DOI] [PubMed] [Google Scholar]
- Fontenot AP, Falta MT, Freed BM, Newman LS, Kotzin BL. Identification of pathogenic T cells in patients with beryllium-induced lung disease. J Immunol. 1999;163:1019–1026. [PubMed] [Google Scholar]
- Fontenot AP, Keizer TS, McCleskey M, Mack DG, Meza-Romero R, Huan J, Edwards DM, Chou YK, Vandenbark AA, Scott B, et al. Recombinant HLA-DP2 Binds Beryllium and Tolerizes Beryllium-Specific Pathogenic CD4+ T Cells. J Immunol. 2006;177:3874–3883. doi: 10.4049/jimmunol.177.6.3874. [DOI] [PubMed] [Google Scholar]
- Fontenot JD, Rudensky AY. Molecular aspects of regulatory T cell development. Seminars in immunology. 2004;16:73–80. doi: 10.1016/j.smim.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Gras S, Kjer-Nielsen L, Burrows SR, McCluskey J, Rossjohn J. T-cell receptor bias and immunity. Curr Opin Immunol. 2008;20:119–125. doi: 10.1016/j.coi.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Harding MM. Metal-ligand geometry relevant to proteins and in proteins: sodium and potassium. Acta Crystallogr D Biol Crystallogr. 2002;58:872–874. doi: 10.1107/s0907444902003712. [DOI] [PubMed] [Google Scholar]
- Illing PT, Vivian JP, Dudek NL, Kostenko L, Chen Z, Bharadwaj M, Miles JJ, Kjer-Nielsen L, Gras S, Williamson NA, et al. Immune self-reactivity triggered by drug-modified HLA-peptide repertoire. Nature. 2012;486:554–558. doi: 10.1038/nature11147. [DOI] [PubMed] [Google Scholar]
- Illing PT, Vivian JP, Purcell AW, Rossjohn J, McCluskey J. Human leukocyte antigen-associated drug hypersensitivity. Curr Opin Immunol. 2013;25:81–89. doi: 10.1016/j.coi.2012.10.002. [DOI] [PubMed] [Google Scholar]
- Kalish RS. The use of human T-lymphocyte clones to study T-cell function in allergic contact dermatitis to urushiol. J Invest Dermatol. 1990;94:108S–111S. doi: 10.1111/1523-1747.ep12876061. [DOI] [PubMed] [Google Scholar]
- Kappler JW, Roehm N, Marrack P. T cell tolerance by clonal elimination in the thymus. Cell. 1987;49:273–280. doi: 10.1016/0092-8674(87)90568-x. [DOI] [PubMed] [Google Scholar]
- Keizer TS, Sauer NN, McCleskey TM. Beryllium binding at neutral pH: the importance of the Be-O-Be motif. J Inorg Biochem. 2005;99:1174–1181. doi: 10.1016/j.jinorgbio.2005.02.017. [DOI] [PubMed] [Google Scholar]
- Kisielow P, Bluthmann H, Staerz UD, Steinmetz M, von Boehmer H. Tolerance in T-cell-receptor transgenic mice involves deletion of nonmature CD4+8+ thymocytes. Nature. 1988;333:742–746. doi: 10.1038/333742a0. [DOI] [PubMed] [Google Scholar]
- Kjer-Nielsen L, Clements CS, Purcell AW, Brooks AG, Whisstock JC, Burrows SR, McCluskey J, Rossjohn J. A structural basis for the selection of dominant alphabeta T cell receptors in antiviral immunity. Immunity. 2003;18:53–64. doi: 10.1016/s1074-7613(02)00513-7. [DOI] [PubMed] [Google Scholar]
- Kozono H, White J, Clements J, Marrack P, Kappler J. Production of soluble MHC class II proteins with covalently bound single peptides. Nature. 1994;369:151–154. doi: 10.1038/369151a0. [DOI] [PubMed] [Google Scholar]
- Liu X, Dai S, Crawford F, Fruge R, Marrack P, Kappler J. Alternate interactions define the binding of peptides to the MHC molecule IA(b) Proc Natl Acad Sci U S A. 2002;99:8820–8825. doi: 10.1073/pnas.132272099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis-Dit-Sully C, Schamel WW. Activation of the TCR complex by small chemical compounds. EXS. 2014;104:25–39. doi: 10.1007/978-3-0348-0726-5_3. [DOI] [PubMed] [Google Scholar]
- Maier LA, McGrath DS, Sato H, Lympany P, Welsh K, Du Bois R, Silveira L, Fontenot AP, Sawyer RT, Wilcox E, et al. Influence of MHC class II in susceptibility to beryllium sensitization and chronic beryllium disease. J Immunol. 2003;171:6910–6918. doi: 10.4049/jimmunol.171.12.6910. [DOI] [PubMed] [Google Scholar]
- Marrack P, Rubtsova K, Scott-Browne J, Kappler JW. T cell receptor specificity for major histocompatibility complex proteins. Curr Opin Immunol. 2008;20:203–207. doi: 10.1016/j.coi.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin S, Weltzien HU. T cell recognition of haptens, a molecular view. Int Arch Allergy Immunol. 1994;104:10–16. doi: 10.1159/000236703. [DOI] [PubMed] [Google Scholar]
- Mohan JF, Unanue ER. A novel pathway of presentation by class II-MHC molecules involving peptides or denatured proteins important in autoimmunity. Mol Immunol. 2013;55:166–168. doi: 10.1016/j.molimm.2012.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niesen FH, Berglund H, Vedadi M. The use of differential scanning fluorimetry to detect ligand interactions that promote protein stability. Nat Protoc. 2007;2:2212–2221. doi: 10.1038/nprot.2007.321. [DOI] [PubMed] [Google Scholar]
- O'Shea EK, Lumb KJ, Kim PS. Peptide 'Velcro": design of a heterodimeric coiled coil. Current Biology. 1993;3:658–667. doi: 10.1016/0960-9822(93)90063-t. [DOI] [PubMed] [Google Scholar]
- Pichler WJ, Beeler A, Keller M, Lerch M, Posadas S, Schmid D, Spanou Z, Zawodniak A, Gerber B. Pharmacological interaction of drugs with immune receptors: the p-i concept. Allergol Int. 2006;55:17–25. doi: 10.2332/allergolint.55.17. [DOI] [PubMed] [Google Scholar]
- Saltini C, Winestock K, Kirby M, Pinkston P, Crystal RG. Maintenance of alveolitis in patients with chronic beryllium disease by beryllium-specific helper T cells. The New England journal of medicine. 1989;320:1103–1109. doi: 10.1056/NEJM198904273201702. [DOI] [PubMed] [Google Scholar]
- Scally SW, Petersen J, Law SC, Dudek NL, Nel HJ, Loh KL, Wijeyewickrema LC, Eckle SB, van Heemst J, Pike RN, et al. A molecular basis for the association of the HLA-DRB1 locus, citrullination, and rheumatoid arthritis. The Journal of experimental medicine. 2013;210:2569–2582. doi: 10.1084/jem.20131241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sollid LM, Jabri B. Celiac disease and transglutaminase 2: a model for posttranslational modification of antigens and HLA association in the pathogenesis of autoimmune disorders. Curr Opin Immunol. 2011;23:732–738. doi: 10.1016/j.coi.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadinski BD, Delong T, Reisdorph N, Reisdorph R, Powell RL, Armstrong M, Piganelli JD, Barbour G, Bradley B, Crawford F, et al. Chromogranin A is an autoantigen in type 1 diabetes. Nat Immunol. 2010;11:225–231. doi: 10.1038/ni.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton M, Burastero SR. Beryllium chemical speciation in elemental human biological fluids. Chem Res Toxicol. 2003;16:1145–1154. doi: 10.1021/tx0256477. [DOI] [PubMed] [Google Scholar]
- Thierse HJ, Gamerdinger K, Junkes C, Guerreiro N, Weltzien HU. T cell receptor (TCR) interaction with haptens: metal ions as non-classical haptens. Toxicology. 2005;209:101–107. doi: 10.1016/j.tox.2004.12.015. [DOI] [PubMed] [Google Scholar]
- White J, Kappler J, Marrack P. Production and characterization of T cell hybridomas. Methods Mol Biol. 2000;134:185–193. doi: 10.1385/1-59259-682-7:185. [DOI] [PubMed] [Google Scholar]
- Wilson IA, Garcia KC. T-cell receptor structure and TCR complexes. Curr Opin Struct Biol. 1997;7:839–848. doi: 10.1016/s0959-440x(97)80156-x. [DOI] [PubMed] [Google Scholar]
- Yin L, Crawford F, Marrack P, Kappler JW, Dai S. T-cell receptor (TCR) interaction with peptides that mimic nickel offers insight into nickel contact allergy. Proc Natl Acad Sci U S A. 2012;109:18517–18522. doi: 10.1073/pnas.1215928109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.