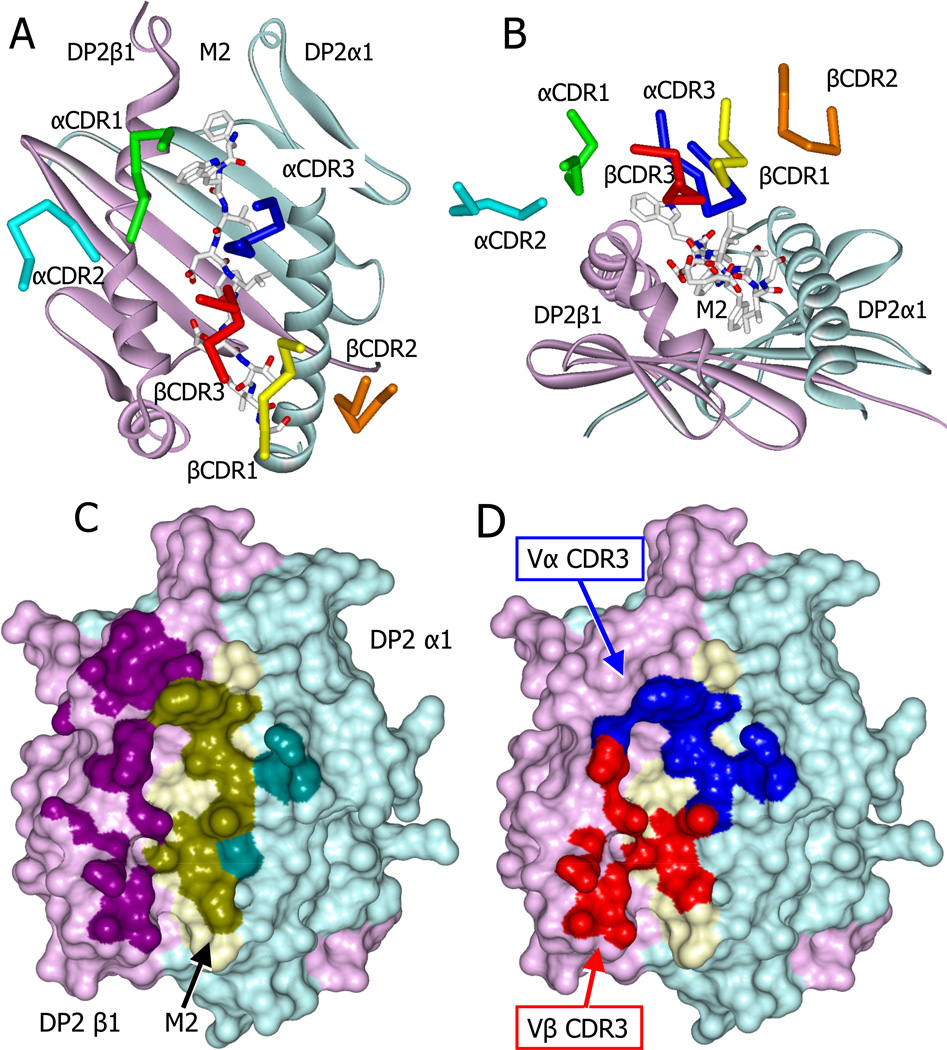
Figure 4. Skewed docking of the AV22 TCR on the DP2-M2-Be2+ complex.
(A) View from above showing the diagonal docking of the six AV22 TCR CDR loops over the DP2-M2-Be2+ complex.
(B) View looking down the peptide binding groove from the peptide C terminus showing the tilt and shift of the AV22 TCR CDR loops toward the DP2 beta chain helix.
(C) Water accessible surface of the DP2-M2-Be2+ complex, DP2α (cyan), DP2β (magenta), and M2 (yellow). The footprint of the AV22 TCR on the complex is shown with darker versions of the same colors. Complex atoms were defined to be part of the footprint if they were within 4.5 Å of a TCR atom.
(D) The parts of the footprint created by the AV22 TCR CDR3α (blue) and CDR3β (red) are shown.
See also Table S1 and Table S2.