Abstract
Prostate cancer is the most frequently diagnosed cancer and the second leading cause of death in males in the United States. Using human prostate cancer specimens, the authors have previously shown that elevated expression levels of 12-lipoxygenase (12-LOX) occurred more frequently in advanced stage, high-grade prostate cancer, suggesting that 12-LOX expression is associated with carcinoma progression and invasion. Previous reports from their group and others have shown that 12-LOX is a positive modulator of invasion and metastasis; however, the mechanism remains unclear. In this work, a new link between 12-LOX and the matrix metalloproteinase 9 (MMP9) in prostate cancer angiogenesis is reported. This study demonstrated that overexpression of 12-LOX in prostate cancer PC-3 cells resulted in elevated expression of MMP9 mRNA, protein and secretion. Exogenous addition of 12(S)-hydroxy eicosatetraenoic acid, the sole and stable end product of arachidonic acid metabolism by 12-LOX, is able to increase MMP9 expression in wild-type PC-3 cells. Furthermore, using pharmacological and genetic inhibition approaches, it was found that 12-LOX activates phosphoinositol 3 kinase (PI3K)/Akt, which results in nuclear factor-kappa B (NF-κB)-driven MMP9 expression, ensuing in enhanced chemoattraction of endothelial cells. Specific inhibitors of 12-LOX, PI3K or NF-κB inhibited MMP9 expression in 12-LOX-expressing PC-3 cells and resulted in the blockade of the migratory ability of endothelial cells. In summary, the authors have identified a new pathway by which overexpression of 12-LOX in prostate cancer cells leads to augmented production of MMP9 via activation of PI3K/Akt/NF-κB signaling. The role of 12-LOX-mediated MMP9 secretion in endothelial cell migration may account for the proangiogenic function of 12-LOX in prostate cancer.
Keywords: 12-lipoxygenase, matrix metalloproteinase, MMP9, NF-κB, prostate cancer, angiogenesis
Prostate cancer is the most frequently diagnosed cancer and the second leading cause of death in males in the United States. Eicosanoids, the metabolites of arachidonic acid (AA), are potent bioactive lipid mediators,1 whose role in cancer progression is increasingly apparent. However, the exact mechanism mediating the effects of AA and its metabolites on cancer cells has been largely unclear. The metabolism of AA is catalyzed by cyclooxygenases, cytochrome p450 or lipoxygenases. Specifically, 12-lipoxygenase (12-LOX) converts AA to hydroxy eicosatetraenoic acids [12(S)-HETE], which plays an important and well-established role in cancer.2–4 12-LOX is expressed in a variety of tumor cells as its mRNA has been detected in erythroleukemia, colon carcinoma, epidermoid carcinoma, human glioma, prostate and breast cancer cells.2 There is compelling evidence for 12-LOX as a key regulator of human cancer development. 12-LOX is overexpressed more frequently in advanced stage, high-grade prostate cancer, suggesting that 12-LOX expression is associated with carcinoma progression and invasion in vivo.3,5 Moreover, 12(S)-HETE facilitates the invasion and metastasis of tumors in a multifaceted way, such as enhancing tumor cell motility, proteinase secretion and angiogenesis.6–9 We have previously demonstrated that 12(S)-HETE is involved in the proliferation of prostate carcinoma cells10 and that inhibition of 12-LOX induces apoptosis of these cells.5,11 Furthermore, ectopic overexpression of 12-LOX in the human prostate carcinoma cell line PC-3 led to enhanced tumor growth.5,12
Our previous studies indicate that 12(S)-HETE has direct stimulatory effects on multiple processes associated with angiogenesis. For example, 12(S)-HETE has been shown to be a mitogenic factor for microvascular endothelial cells13 and to stimulate endothelial cell migration.6 Exogenous addition of 12(S)-HETE induced a reversible, nondestructive and time- and dose-dependent retraction of endothelial cells by stimulating cytoskeletal rearrangement,14 and tumor cells can synthesize endogenous 12(S)-HETE in sufficient amounts to induce microvascular endothelial cell retraction (16). In addition to our own study, an independent group also found that some of the potential of 12(S)-HETE as a significant stimulator of pathological angiogenesis may lie in its ability to induce transcription and production of vascular endothelial growth factor (VEGF).15,16 Baicalein and Nordihydroguaiaretic Acid (NDGA), the known 12-LOX inhibitors, reduced VEGF expression, confirming that the enzymatic activity of 12-LOX plays a significant role in the regulation of VEGF gene expression. Nevertheless, our studies show that 12-LOX and 12(S)-HETE regulate other important elements involved in angiogenesis.
We have previously shown that overexpression of 12-LOX and treatment with 12(S)-HETE in prostate cancer cell lines activate the nuclear factor-kappa B (NF-κB) transcription factor.17 NF-κB, a dimer of p50 and p65 subunits of Rel proteins, is held in the cytoplasm in an inactive form, bound to inhibitors such as IκBα. Phosphorylation of IκB by protein kinases, such as protein kinase C, targets IκB for ubiquitination and degradation, thus releasing NF-κB, which is then translocated to the nucleus where it regulates transcription by binding to NF-κB response elements of target genes. NF-κB plays a pivotal role in many aspects of cancer, such as survival, proliferation, cell cycle control, angiogenesis and invasiveness. 12 In fact, NF-κB has been reported to be constitutively upregulated in prostate cancer.18 The phosphoinositol 3 kinase (PI3K)/Akt and NF-κB pathways control both proliferation and resistance to apoptosis of many cancer cells. Therefore, signaling through these pathways critically regulates proliferation and survival mechanisms.
Matrix metalloproteinases (MMPs) promote tumor progression, metastasis and invasion by degradation of the extracellular matrix (ECM). In addition to ECM assisting migration of metastatic cancerous cells, ECM degradation also allows enhanced tumor growth by providing necessary expansion space. MMP9 is overexpressed in advanced stage prostate cancer cells.19 In our study, we identified that enhanced invasive ability results from an increase in MMP9 expression and secretion by 12-LOX-overexpressing PC-3 cells (nL8). Therefore, our study was undertaken to delineate the pathway by which overexpression of platelet-type 12-LOX in PC-3 cells leads to augmented production of MMP9. In addition, the finding that 12-LOX mediates the MMP9 secretion via activation of PI3K/Akt/NF-κB is unique and is the first ever report of a lipoxygenase enzyme regulating MMP9 through its arachidonate metabolite.
Material and Methods
Materials
PC-3 and LnCaP cell lines were obtained from ATCC (Manassas, VA), and human umbilical vein endothelial cells (HUVECs) were obtained from Lonza (Walkersville, MD). All other reagents were of analytical grade and were obtained from Sigma (St. Louis, MO).
Antibodies
The antibodies for Western blotting, MMP9, p-Akt and Akt, were purchased from Cell Signaling (Beverly, MA). 12-LOX antibody was obtained from Oxford Biomedical Research (Oxford, MI). NF-κB p65 and NF-κB p50 antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA), and actin antibody was obtained from Chemicon (Billerica, MA).
RNA quantification
Total RNA was extracted with Ambion reagent (Applied Biosystems, Grand Island, NY) according to the manufacturer’s instructions. The ABI Prism 7000 light cycles and a SYBR RT-PCR kit (Applied Biosystems, Grand Island, NY) were used for quantitative real-time PCR analysis. Specific primers used for RT-PCR assays were 5′-GACCTCAAGTGGCACCACCA-3′ (sense) and 5′-GTGGTACTGCACCAGGGCAA-3′ (antisense) for MMP9 and 5′-TGTTACCAACTGGGACGACA-3′ and 5′- CTGGGTCATCTTTTCACGGT-3′ for β-actin. Data are normalized to β-actin expression in each sample.
siRNA knockdown of NF-κB and measurement of MMP9
siRNA for NF-κB (p50 and p65) and scrambled siRNA sequence were purchased from (Qiagen, Germantown, MD), and the siRNA transfection reagent Dharmafect 2 was obtained from (Dharmacon, Pittsburgh, PA). For transfection, nL8 cells and vector control cells (neo-α) were plated at 1×105 cells per well of a six-well plate. After 48 hr, cells were transfected either with the NF-κB (p50 and p65)-specific siRNA or the control siRNA according to the manufacturer’s protocol. Post-transfection, cells were grown for 48 hr with complete medium followed by serum-free medium for 24 hr. Cells were lysed, and NF-κB (p50 and p65) expression level was measured by immunoblotting. The impact of siRNA knockdown on MMP9 secretion was studied by enzyme-linked immunosorbent assay (ELISA; R&D Systems, Minneapolis, MN) on conditioned medium (CM) collected at the end of 72 hr.
Total RNA extraction and RT-PCR analysis
Total RNA was isolated from neo-α cells, nL8 cells and PC-3 cells treated with 12(S)-HETE for the indicated time in 10-cm culture dishes with Ambion RNA isolation kit according to the protocol of the manufacturer. First-strand cDNA synthesis was performed with 2 µg of total RNA using random hexamers as primers in a final volume of 20 µl. The reaction was carried out at 37°C for 60 min. cDNAs encoding GAPDH and MMP9 were amplified from 3 to 5 µl of the cDNA reaction mixture using specific gene primers. Oligonucleotide primers for GAPDH and MMP9 were as follows: GAPDH (636 bp): 5′- TGACGGGGTCACCCACACTGTGCCCATCTA-3′ (sense) and 5′-CTAGAAGCATTTGCGGTGGACGATG-3′ (antisense); MMP9 (483 bp): 5′-GACCTCAAGTGGCACCACCA-3′ (sense) and 5′-GTGGTACTGCACCAGGGCAA-3′ (antisense).
SDS-PAGE and Western blotting
Cells were extracted in solubilization buffer,20 and proteins were loaded on 10% SDS-PAGE gels and transferred to nitrocellulose membranes. Blots were then probed with indicated antibodies and developed by enhanced chemiluminescence (Pierce, Rockford, IL) according to the manufacturer’s instructions.
Gelatin zymography
Gelatin zymography was performed as described previously.21 Briefly, 50 µl of serum-free cell culture supernatant was resuspended in nonreducing Laemmli sample buffer and resolved by 8% SDS-PAGE containing 1 mg/ml gelatin (Fisher, Columbia, MD). Following electrophoresis, gels were washed with 2.5% Triton X-100 to remove SDS and incubated in substrate buffer (50 mM, pH 8 Tris buffer containing 5 mM CaCl2) for 18 hr at 37°C. Gelatinase activity was visualized by staining the gels with 0.5% Coomassie blue for 30 min followed by incubation in the destaining solution (30% methanol and 7% acetic acid).
ELISA
The amount of secreted MMP9 was determined in the culture medium of neo-α cells, nL8 cells and PC-3 cells, and then the cells were treated with indicated inhibitors or siRNA of p50 and p65 domain of NF-κB. Transfected nL8 cells and PC-3 cells were treated with 12(S)-HETE for the indicated time and analyzed using a commercial ELISA kit (R&D Systems), following the instructions of the manufacturer. CM (100 µl) was applied to each well for the ELISA. Each sample was run in duplicate, and the final measurement was read using a plate reader at 450 nm. The concentration of MMP9 protein in each sample was determined according to the standards (recombinant proteins) provided with the kits. MMP9 levels in these samples were located within the linear range of the standard curves.
Endothelial cell migration assay
Cell migration and invasion assays were performed in a modified Boyden chamber using 24-well cell culture plates fitted with 8-µm transwell filters as described.22 HUVECs were harvested by trypsinization in the presence of EDTA and suspended in EBM-2 medium (Lonza, Allendale, NJ) with 2% FBS at a density of 4×105 cells per 0.5 ml. For detection of secreted chemotactic factors, the CM from 12-LOX-transfected PC-3 cells and their vector control cells were concentrated (6×) using Centricon-10 (Amicon Millipore, Billerica, MA). The concentrated culture supernatants were then diluted (10×) with EBM-2% FBS and added in the bottom chamber of the well in the presence of neutralizing antibodies against MMP9. Migration was initiated by adding cells (20 µl) to the upper chamber. After 12–18 hr, the cells on the upper side of the membrane were removed using Kimwipes, and the plate was fixed and stained. Usually five fields (100×) representing two perpendicular cross-lines of each membrane were counted. For each treatment, at least six chambers were used.
Results
MMP9 expression is upregulated by 12-LOX
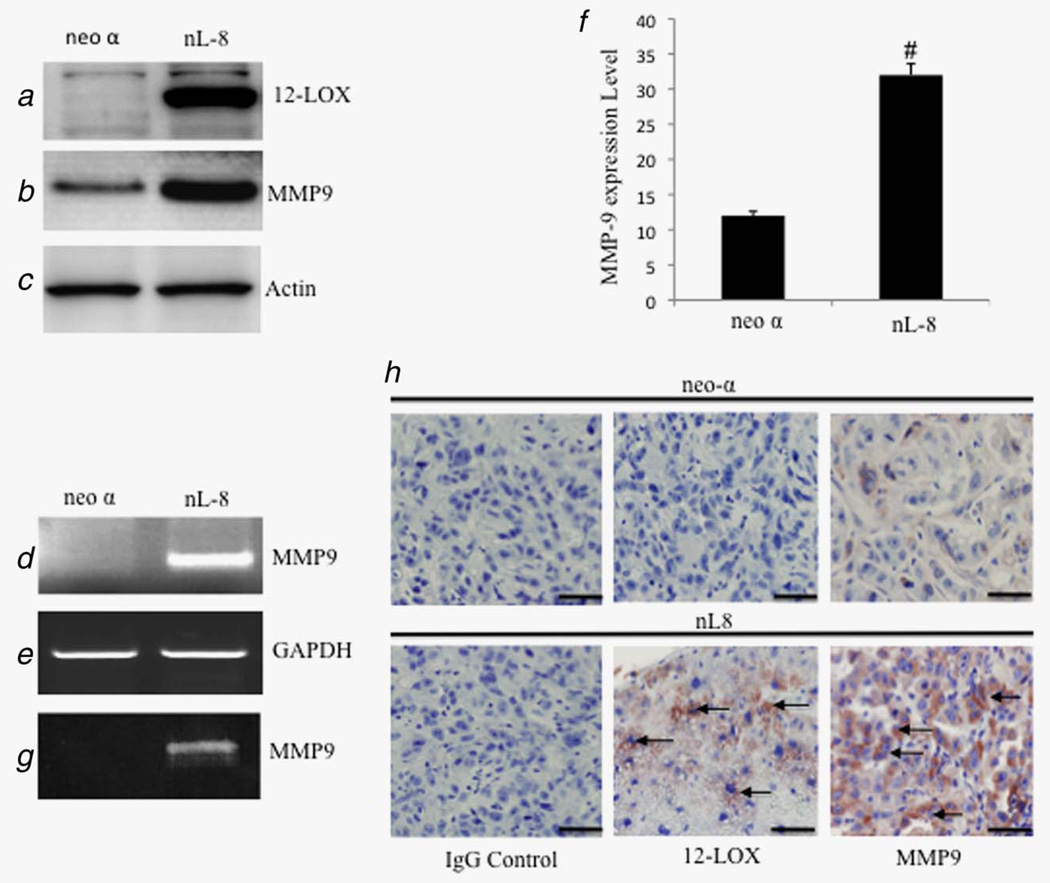
Previous studies have demonstrated clearly that 12-LOX is expressed in prostate carcinoma cells5 and that the level of 12-LOX mRNA is positively correlated with tumor grade and stage.23 Additionally, PC-3 cells overexpressing 12-LOX generate highly angiogenic tumors in mice.5 In light of these observations, we examined whether 12-LOX regulates the production of MMP9, a putative angiogenic factor in prostate carcinoma, by using stable PC3 12-LOX transfectants (nL8; Fig. 1a). In this background, MMP9 expression is increased at the level of protein production (Fig. 1b) compared to neo-α cells, mRNA expression (Fig. 1e) as assessed by real-time PCR (Fig. 1f) and an increased enzymatic activity as measured by zymography (Fig. 1g). These results demonstrate a significant elevation of MMP9 expression in nL8 cells compared to the neo-α cells. Furthermore, tumor sections from xenografts of nL8 cells or neo-α cells injected in mice5 presented brown for 12-LOX and MMP9 in nL8 cells compared to neo-α cells (Fig. 1h).
Figure 1.
Western blot analysis of nL8 cells shows higher 12-LOX (a) and MMP9 (b) protein levels compared to neo-α control cells. nL8 cells show an elevated mRNA level of MMP9 by using real-time PCR (d and f). Gelatin zymogram analysis of culture supernatant of nL8 cells demonstrated an elevated gelatinolytic activity compared to neo-α control cells (g). Tumor sections from nL8 cells or neo-α cells subcutaneously injected into mice were immunostained using antibodies specific for 12-LOX and MMP9, which show brown, positive staining compared to neo-α cell-injected tumor (h). Arrows (→) indicate positive staining of 12-LOX and MMP-9. Slides stained with secondary antibodies (IgG control) are shown as an indication of the staining specificity (scale bar: 50 µm). #p < 0.05 according to Student’s t-test. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Effect of 12(S)-HETE on MMP9 production
12-LOX metabolizes AA to 12(S)-HETE, which is converted to 12(S)-HETE. To study whether exogenous addition of 12(S)-HETE can regulate MMP9 expression, PC-3 cells were treated with graded levels of 12(S)-HETE, and the protein levels of MMP9 in culture medium were assayed by ELISA, normalized to the cell number (Fig. 2a). The AA metabolite 12(S)-HETE increased MMP9 expression in parental PC-3 cells (Fig. 2b) and stimulated MMP9 expression (Fig. 2d) from PC-3 cells within 18 hr of treatment with optimal doses of 0.5–1.0 µM. A similar result was observed in another prostate cancer cell line, LnCaP, treated with 12(S)-HETE (Supporting Information Fig. S1). The results demonstrate that a 12-LOX product can regulate the production of MMP9 in prostate cancer cells.
Figure 2.
Exogenous 12(S)-HETE induces the MMP-9 expression in PC3 cells. PC-3 wild-type cells were exposed to the indicated concentrations of 12(S)-HETE for 18 hr, after which conditioned medium was collected and measured by ELISA using MMP9 R&D assay kit (a). MMP9 protein levels increase in 12(S)-HETE-treated wild-type PC-3 cells in a dose-dependent manner (b). Gelatin zymo-gram analysis of conditioned medium of 12-HETE-treated wild-type PC-3 cells shows elevated gelatinase activity in a dose-dependent manner (d). The results are representative of at least three independent experiments. *p <0.01 according to Student’s t-test.
12-LOX regulates MMP9 through PI3K-Akt-NF-κB pathway
Previously, it has been shown that 12-LOX activates PI3K/Akt and NF-κB in prostate cancer cells,15,17 which leads to nuclear translocation of NF-κB and transcriptional activation of a large number of target genes. Inhibition of 12-LOX enzymatic activity using baicalein inhibited MMP9 expression in nL8 cells (Fig. 3a), further confirming that the effect is due to 12-LOX enzymatic activity through the synthesis of 12(S)-HETE. Furthermore, compared to the control DMSO-treated cells, MMP9 induction and gelatinolytic activity were suppressed in nL8 cells when PI3K/Akt and NF-κB were inhibited, using the LY294002 and MG-132 inhibitors, respectively (Figs. 3a and 3c). Secreted levels of MMP9 in CM were also reduced in nL8 CM after PI3K/Akt and NF-κB inhibition compared to nontreated conditions (Fig. 3d). In conclusion, these results confirmed that LnCaP cells transfected with 12-LOX (Supporting Information Fig. S2). These data show that the enhanced MMP9 expression by 12-LOX may be mediated by the activation of the NF-κB and PI3K/Akt pathways.
Figure 3.
12-LOX-induced MMP9 expression is mediated through the PI3K/Akt/NF-κB pathway. nL8 cells were exposed to PI3K inhibitor (20 µM), NF-κB inhibitor (20 µM), 12-LOX inhibitor (20 µM) and solvent control (S.C.) for 18 hr. After treatment, cells were lysed and subjected to Western blot analysis with an anti-MMP9 antibody (a). The blot was reprobed with actin as a protein loading control (b). MMP9 was detected in cell-conditioned medium by zymography (c). nL8 cells were exposed to 20 µM LY294002 or MG-132, and Baicalein shows a decrease in MMP9 levels (d) compared to vehicle control. #p < 0.05 according to Student’s t-test.
12-LOX signals MMP9 production through NF-κB
Our experimental results showed that MMP9 expression levels were inhibited by the NF-κB inhibitor, MG-132. We wanted to confirm the result using siRNA of p50 and p65 subunits of NF-κB in nL8 cells. Successful silencing of either p50 or p65 using specific siRNAs (Figs. 4a–4d) significantly blocked the 12-LOX-mediated increase in MMP9 mRNA expression and its gelatinolytic activity in nL8 cells. In addition, the siRNA silencing of p50 and p65 subunits of NF-κB in nL8 cells also prevented 12-LOX-dependent MMP9 expression compared to scrambled siRNA-transfected nL8 cells (Figs. 4e–4i). This result has been correlated with levels of secreted MMP9 in CM of nL8 cells transfected with siRNA of p50 and p65 domain of NF-κB (Fig. 4j). Taken together, these results clearly show that 12-LOX mediates the expression and secretion of MMP9 via activation of NF-κB.
Figure 4.
12-LOX induces MMP9 expression in NF-κB p50- and p65-dependent manner. nL8 cells were transfected with NF-κB p65 or p50 siRNAs or scrambled siRNA. After 72 hr, nuclear protein extracts were subject to Western blot analysis with anti-p65 and anti-p50 antibodies (a and c). Actin was used as an internal control (b and d). The effect of NF-κB p65 or p50 siRNAs on mRNA levels of MMP9 in nL8 cells was determined by RT-PCR (e). GAPDH was used as an internal control (f). Protein expression levels of MMP9 in conditioned media were determined by immunoblot analysis (g). Gelatin zymogram assay was performed on the cells transfected with siRNAs for p65 and p50 to analyze gelatinolytic activities of secreted MMP9 (i). The effect of NF-κB p65 or p50 siRNA on MMP9 levels in nL8 cells was determined by ELISA. nL8 cells were transfected with NF-κB p65 or p50 siRNAs or scrambled siRNA. After 72 hr, MMP9 levels were determined by ELISA (j). #p <0.05 according to Student’s t-test. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
12-LOX-mediated production of MMP-9 in PC3 cells impacts HUVEC migration
Overexpression of 12-LOX in PC-3 cells results in highly angiogenic tumors.5 In our study, we observed that 12-LOX overexpression in PC-3 cells enhances secretion of MMP9 in culture medium. Our observation showed that specific inhibitors of 12-LOX or PI3K and/or NF-κB inhibited MMP9 expression in nL8 cells. Therefore, these cells were treated with 12-LOX or PI3K and/or NF-κB inhibitors for 12 hr followed by collection of the CM for the stimulation of HUVEC cell migration. We found that CM from nL8 cells showed significant increase in HUVEC cell migration compared to neo-α cells. However, this increased ability was significantly reduced (p < 0.05) by pretreating the tumor cell CM with inhibitors of 12-LOX (12-LOX+Bai) or PI3K (12-LOX+LY) and/or NFκB (12-LOX+MG), as indicated in Figures 5a and 5b. These results suggest that the activation of a 12-LOX/PI3K/NF-κB signaling axis in tumor cells can enhance migration. To study whether MMP9 mediates the angiogenic activity of 12-LOX in prostate cancer cells, we evaluated the effect of neutralizing antibodies against MMP9 on endothelial cell migration, as stimulated by the CM from 12-LOX-transfected PC-3 cells (nL8). As shown in Figures 6a and 6b, in the presence of control mouse IgG, the concentrated CM from nL8 cells stimulated endothelial cell migration (p < 0.01, Student’s t-test) compared to those from neo-α cells. However, this increased ability was significantly reduced (p < 0.05) by pretreating the tumor cells CM with a MMP9-neutralizing antibody (Figs. 6a and 6b). The data suggest that enhanced MMP9 secretion from 12-LOX-overexpressing PC-3 cells can lead to an enhanced angiogenic response by stimulating endothelial cell migration.
Figure 5.
Effect of PI3K/Akt/NF-κB/12-LOX inhibitors on nL8 cells and HUVEC migration. nL8 cells were exposed to PI3K/Akt/NF-κB and 12-LOX inhibitors. After 24 hr, conditioned medium (CM) from 12-LOX-transfected PC-3 cells and vector controls were harvested and concentrated (6×) using Centricon-10. HUVEC migration assay was carried out through exposure to CM from neo-α and nL8 cells in the presence or absence of PI3K/Akt/NF-κB and 12-LOX inhibitors. CM from nL8 cells demonstrated significant increase in HUVEC migration compared to empty vector (ev). CM from nL8 cells after exposure to PI3K/Akt/NF-κB and 12-LOX inhibitors demonstrates significant decrease in HUVEC migration compared to nontreated nL8 cell CM (a). Representative phase contrast microscopy images are shown in correlation with the invasion assay (b). Scale bar: 50 µm.
Figure 6.
Stimulation of endothelial cell migration by nL8 is blocked by an MMP9-neutralizing antibody. Conditioned media (CM) from nL8 and neo-α cells were harvested and concentrated. The migrated endothelial cells in response to the CM are shown. This increased stimulation of endothelial cell migration was blocked by the pretreatment with a MMP9-neutralizing antibody. The migration of endothelial cells was expressed as the percentage of the control in which no CM was added (a). Column, the average percentage of migration compared to the medium control; bar, SD from six membranes. *p < 0.01 and #p < 0.05 according to Student’s t-test. Representative phase contrast microscopy images are shown in correlation with the invasion assay (b). Scale bar: 50 µm.
Discussion
We report for the first time that a lipoxygenase enzyme and its arachidonic metabolite positively influenced the expression levels of MMP9 in prostate PC-3 cancer cells. Similar results were also obtained from the incubation of wild-type PC-3 cells with exogenously added 12(S)-HETE, the product of AA metabolism by 12-LOX. Inhibition of 12-LOX activity using baicalein decreased the level of MMP9 protein, emphasizing the necessity for 12-LOX enzymatic activity for the MMP9 response. The signaling mechanism involved in the expression of MMP9 by 12-LOX was found to be mediated by the PI3K-Akt-NF-κB pathway, inhibition of which diminishes the ability of 12-LOX to upregulate MMP9. Finally, inhibition of NF-κB by siRNA in 12-LOX-overexpressing cells affects the secretion of MMP9 in these cells, clearly demonstrating the influence of 12-LOX in eliciting MMP9 expression via activation of the NF-κB pathway. We have previously shown that 12-LOX and 12(S)-HETE activate NF-κB.17 We also showed that 12(S)-HETE activates the PI3K pathway.24 Recently, we have identified GPR31, renamed as 12HETER, as a high-affinity receptor for 12(S)-HETE and showed that 12(S)-HETE’s binding to this receptor activates NF-κB.25 Therefore, based on our data, the sequence of events appears to be the binding of 12(S)-HETE to 12HETER which, through PI3K/Akt, activates NF-κB and then MMP9 synthesis and secretion. Regulation of MMP9 by eicosanoids is not known. Earlier studies by our group showed that the PI3K-Akt pathway is pivotal in mediating 12-LOX-induced VEGF expression and secretion.15 Our work has revealed that 12-LOX uses the same pathway in upregulating MMP9 levels.
Two independent studies show that the 12-LOX regulates tumor angiogenesis. The first study,5 from our laboratory, overexpressed 12-LOX in human prostate cancer PC-3 cells by transfection with a platelet-type 12-LOX cDNA construct. Stable transfectants, which express constitutively high levels of 12-LOX in both mRNA and protein levels, were generated and confirmed to produce more 12(S)-HETE than did the mock-transfected PC-3 cells. In vitro, the growth rates of several 12-LOX-transfectant clones were similar to those of neo-controls and PC-3 wild type in the presence of serum. However, following subcutaneous injection into nude mice, 12-LOX-transfected PC-3 cells grew faster and formed larger tumors than neo-α control cells, and the increased tumor volume was positively correlated with enhanced tumor angiogenesis.5 Another study involved breast cancer. Using a similar approach, Connolly and Rose26 overexpressed 12-LOX in breast cancer cells and found that 12-LOX enhanced tumor angiogenesis and growth in an orthotopic xenograft animal model. Taken together, these studies suggest that 12-LOX, when expressed in cancer cells, can enhance their angiogenic potential. As inhibition of angiogenesis has proven to be an effective approach to block tumor growth, inhibition of 12-LOX activity may be a novel approach to develop anticancer, antiangiogenic therapy.
Metastatic cancer cells secrete large amounts of MMPs. MMPs have been identified as agents that degrade the ECM and basement membrane, which allows their spread to distant organs. Increased expression levels of MMPs are associated with a number of cancers. Recent study demonstrates that arachidonate 5 lipoxygenase expression in papillary thyroid carcinoma promotes invasion via MMP-9 induction.27 There is compelling evidence to suggest that MMP9 plays important roles in tumor invasion and metastasis. The design of new drugs to inhibit MMP expression levels is, therefore, a priority. It was noted that the anti-MMP9-neutralizing antibody demonstrated significant anti-invasion effect.
In conclusion, we show that 12-LOX and 12(S)-HETE elevate the expression levels of MMP9 that plays an important role in prostate cancer invasion and metastasis. We also conclude that the angiogenic effects of 12-LOX and its product 12(S)-HETE may be mediated through MMP9 gene expression by the activation of PI3K/Akt/NF-κB. Blockade of 12-LOX-mediated invasive and angiogenic potential of prostate cancer cells can be achieved through the suppression of MMP9 gene transcription by inhibiting the PI3K-Akt-NF-κB pathway in 12-LOX-overexpressing cells.
What’s new?
The worst human prostate cancers frequently have elevated levels of an enzyme, 12-lipoxygenase (12-LOX). Earlier work has shown that 12-LOX assists with invasion and metastasis, but it’s not clear how it does so. In this study, the authors demonstrate that extra 12-LOX in prostate cancer cells boosts matrix metalloproteinase 9 (MMP9) by activating the PI3K/Akt/NFkB pathway. Even without overexpression of 12-LOX, simply adding its product, 12(S)-HETE, to the cells produced the same increase in MMP9. Thus it seems likely that suppressing MMP9 by blocking the PI3K/Akt/NFkB pathway could effectively halt the tumor angiogenesis driven by 12-LOX.
Acknowledgement
This work was supported by NIH/NCI CA# 29997 (K.V.H.).
Grant sponsor: NIH/NCI; Grant number: CA# 29997
Footnotes
Additional Supporting Information may be found in the online version of this article.
References
- 1.Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 2001;294:1871–1875. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- 2.Honn KV, Tang DG, Gao X, et al. 12-Lipoxygenases and 12(S)-HETE: role in cancer metastasis. Cancer Metastasis Rev. 1994;13:365–396. doi: 10.1007/BF00666105. [DOI] [PubMed] [Google Scholar]
- 3.Nie D. Cyclooxygenases and lipoxygenases in prostate and breast cancers. Front Biosci. 2007;12:1574–1585. doi: 10.2741/2170. [DOI] [PubMed] [Google Scholar]
- 4.Gao X, Honn KV. Biological properties of 12(S)-HETE in cancer metastasis. Adv Prostaglandin Thromboxane Leukot Res. 1995;23:439–444. [PubMed] [Google Scholar]
- 5.Nie D, Hillman GG, Geddes T, et al. Platelet-type 12-lipoxygenase in a human prostate carcinoma stimulates angiogenesis and tumor growth. Cancer Res. 1998;58:4047–4051. [PubMed] [Google Scholar]
- 6.Nie D, Tang K, Diglio C, et al. Eicosanoid regulation of angiogenesis: role of endothelial arachidonate 12-lipoxygenase. Blood. 2000;95:2304–2311. [PubMed] [Google Scholar]
- 7.Honn KV, Timar J, Rozhin J, et al. A lipoxygenase metabolite, 12-(S)-HETE, stimulates protein kinase C-mediated release of cathepsin B from malignant cells. Exp Cell Res. 1994;214:120–130. doi: 10.1006/excr.1994.1240. [DOI] [PubMed] [Google Scholar]
- 8.Timar J, Silletti S, Bazaz R, et al. Regulation of melanoma-cell motility by the lipoxygenase metabolite 12-(S)-HETE. Int J Cancer. 1993;55:1003–1010. doi: 10.1002/ijc.2910550621. [DOI] [PubMed] [Google Scholar]
- 9.Nappez C, Liagre B, Beneytout JL. Changes in lipoxygenase activities in human erythroleukemia (HEL) cells during diosgen-ininduced differentiation. Cancer Lett. 1995;96:133–140. doi: 10.1016/0304-3835(95)03923-k. [DOI] [PubMed] [Google Scholar]
- 10.Liu B, Maher RJ, Hannun YA, et al. 12(S)-HETE enhancement of prostate tumor cell invasion: selective role of PKCα. J Natl Cancer Inst. 1994;86:1145–1151. doi: 10.1093/jnci/86.15.1145. [DOI] [PubMed] [Google Scholar]
- 11.Wong BC, Wang WP, Cho CH, et al. 12-Lipoxygenase inhibition induced apoptosis in human gastric cancer cells. Carcinogenesis. 2001;22:1349–1354. doi: 10.1093/carcin/22.9.1349. [DOI] [PubMed] [Google Scholar]
- 12.Karin M. Nuclear factor-κB in cancer development and progression. Nature. 2006;441:431–436. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- 13.Tang DG, Renaud C, Stojakovic S, et al. 12(S)-HETE is a mitogenic factor for microvascular endothelial cells: its potential role in angiogenesis. Biochem Biophys Res Commun. 1995;211:462–468. doi: 10.1006/bbrc.1995.1836. [DOI] [PubMed] [Google Scholar]
- 14.Tang DG, Diglio CA, Honn KV. 12(S)-HETE-induced microvascular endothelial cell retraction results from PKC-dependent rearrangement of cytoskeletal elements and αVβ 3 integrins. Prostaglandins. 1993;45:249–267. doi: 10.1016/0090-6980(93)90051-8. [DOI] [PubMed] [Google Scholar]
- 15.Nie D, Krishnamoorthy S, Jin R, et al. Mechanisms regulating tumor angiogenesis by 12-lipoxygenase in prostate cancer cells. J Biol Chem. 2006;281:18601–18609. doi: 10.1074/jbc.M601887200. [DOI] [PubMed] [Google Scholar]
- 16.McCabe NP, Selman SH, Jankun J. Vascular endothelial growth factor production in human prostate cancer cells is stimulated by overexpression of platelet 12-lipoxygenase. Prostate. 2006;66:779–787. doi: 10.1002/pros.20360. [DOI] [PubMed] [Google Scholar]
- 17.Kandouz M, Nie D, Pidgeon GP, et al. Platelet-type 12-lipoxygenase activates NF--κB in prostate cancer cells. Prostaglandins Other Lipid Mediat. 2003;71:189–204. doi: 10.1016/s1098-8823(03)00042-x. [DOI] [PubMed] [Google Scholar]
- 18.Shukla S, MacLennan GT, Fu P, et al. Nuclear factor--κB/p65 (Rel A) is constitutively activated in human prostate adenocarcinoma and correlates with disease progression. Neoplasia. 2004;6:390–400. doi: 10.1593/neo.04112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nalla AK, Gorantla B, Gondi CS, et al. Targeting MMP-9, uPAR, and cathepsin B inhibits invasion, migration and activates apoptosis in prostate cancer cells. Cancer Gene Ther. 2010;17:599–613. doi: 10.1038/cgt.2010.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dilly AK, Rajala RV. Insulin growth factor 1 receptor/PI3K/Akt survival pathway in outer segment membranes of rod photoreceptors. Invest Ophthalmol Vis Sci. 2008;49:4765–4773. doi: 10.1167/iovs.08-2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herron GS, Banda MJ, Clark EJ, et al. Secretion of metalloproteinases by stimulated capillary endothelial cells. II. Expression of collagenase and stromelysin activities is regulated by endogenous inhibitors. J Biol Chem. 1986;261:2814–2818. [PubMed] [Google Scholar]
- 22.Ustach CV, Taube ME, Hurst NJ, Jr, et al. A potential oncogenic activity of platelet-derived growth factor d in prostate cancer progression. Cancer Res. 2004;64:1722–1729. doi: 10.1158/0008-5472.can-03-3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao X, Grignon DJ, Chbihi T, et al. Elevated 12-lipoxygenase mRNA expression correlates with advanced stage and poor differentiation of human prostate cancer. Urology. 1995;46:227–237. doi: 10.1016/s0090-4295(99)80198-8. [DOI] [PubMed] [Google Scholar]
- 24.Szekeres CK, Trikha M, Nie D, et al. Eicosanoid 12(S)-HETE activates phosphatidylinositol 3-kinase. Biochem Biophys Res Commun. 2000;275:690–695. doi: 10.1006/bbrc.2000.3348. [DOI] [PubMed] [Google Scholar]
- 25.Guo Y, Zhang W, Giroux C, et al. Identification of the orphan G protein-coupled receptor GPR31 as a receptor for 12-(S)-hydroxyeicosatetraenoic acid. J Biol Chem. 2011;286:33832–33840. doi: 10.1074/jbc.M110.216564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Connolly JM, Rose DP. Enhanced angiogenesis and growth of 12-lipoxygenase gene-transfected MCF-7 human breast cancer cells in athymic nude mice. Cancer Lett. 1998;132:107–112. doi: 10.1016/s0304-3835(98)00171-2. [DOI] [PubMed] [Google Scholar]
- 27.Kummer NT, Nowicki TS, Azzi JP, et al. Arachidonate 5 lipoxygenase expression in papillary thyroid carcinoma promotes invasion via MMP-9 induction. J Cell Biochem. 2012;113:1998–1908. doi: 10.1002/jcb.24069. [DOI] [PMC free article] [PubMed] [Google Scholar]