Abstract
Observational studies from different regions of the world provide valuable information in patient selection, clinical practice, and their relationship to patient and technique outcome. The present study is the first large cohort providing patient characteristics, clinical practice, patterns and their relationship to outcomes in Latin America. The objective of the present study was to characterize the cohort and to describe the main determinants of patient and technique survival, including trends over time of peritoneal dialysis (PD) initiation and treatment.
This was a nationwide cohort study in which all incident adult patients on PD from 122 centers were studied. Patient demographics, socioeconomic and laboratory values were followed from December 2004 to January 2011 and, for comparison purposes, divided into 3 groups according to the year of starting PD: 2005/06, 2007/08 and 2009/10. Patient survival and technique failure (TF) were analyzed using the competing risk model of Fine and Gray. All patients active at the end of follow-up were treated as censored. In contrast, all patients who dropped the study for any reason different from the primary event of interest were treated as competing risk. Significance was set to a p level of 0.05.
A total of 9,905 patients comprised the adult database, 7,007 were incident and 5,707 remained at least 90 days in PD. The main cause of dropout was death (54%) and of TF was peritonitis (63%). Technique survival at 1, 2, 3, 4, and 5 years was 91%, 84%, 77%, 68%, and 58%, respectively. There was no change in TF during the study period but 3 independent risk factors were identified: lower center experience, lower age, and automated PD (APD) as initial therapy. Cardiovascular disease (36%) was the main cause of death and the overall patient survival was 85%, 74%, 64%, 54%, and 48% at 1, 2, 3, 4, and 5 years, respectively. Patient survival improved along all study periods: compared to 2005/2006, patients starting at 2007/2008 had a relative risk reduction (SHR) of 0.83 (95% confidence interval [CI] 0.72 - 0.95); and starting in 2009/2010 of 0.69 (95% CI 0.57 - 0.83). The independent risk factors for mortality were diabetes, age > 65 years, previous hemodialysis, starting PD modality, white race, low body mass index (BMI), low educational level, center experience, length of pre-dialysis care, and the year of starting PD.
We observed an improvement in patient survival along the years. This finding was sustained even after correction for several confounders and using a competing risk approach. On the other hand, no changes in technique survival were found.
Keywords: Peritoneal dialysis, trends, complications, outcome, mortality
Large and representative cohorts provide the opportunity to assess a wide variety of both exposures and outcomes. The information acquired from these studies is particularly important in fields where randomized clinical trials are difficult to perform. Several cohorts from different continents, namely CANUSA (1) and USRDS (2) in North America, NECOSAD (3) and EAPOS (4) in Europe, ASPD (5) in Asia, and the ANZDATA (6) in Oceania have reported information related to patient characteristics, clinical practice patterns and their relationship to clinical outcomes. The present study was the first large and representative cohort study providing this information in Latin America, a region contributing to 25% of the global peritoneal dialysis (PD) population (7)
Another potential advantage of the analysis of large longitudinal cohort studies is related to the possibility of providing an overview of trends in patient characteristics, clinical practice and outcomes for specific populations across the globe and different time periods, defining the clinical practice patterns associated with best outcomes (2). Recent reports describe a trend towards outcome improvement in PD patients in the developed world (2,8,9). However, most of the recent growth in PD has occurred in the developing world, where studies looking at patient characteristics, clinical practice patterns, and trends in outcomes over time and in particular settings are lacking.
Therefore, the objective of the present study was to describe the characteristics of the population, clinical practice patterns, and their relationship to clinical outcome in a large nationwide prospective cohort. In addition, we aimed to analyze temporal trends in patient and technique survival in BRAZPD II.
Materials and Methods
This was a nationwide prospective cohort study launched in December 2004, enrolling all patients from dialysis centers using supplies manufactured by Baxter Healthcare and with at least 10 patients in PD. Although it was not possible to estimate the average percentage of patients per center included in the study, the level of center participation during the study was constant, and by extrapolation and comparison to the Brazilian dialysis census, the cohort represented approximately 65 - 70% of all prevalent PD patients in the country throughout the study period. Once selected for the study, each clinic submitted the research project to the local ethics committee and upon approval, at least 1 physician and 1 nurse from each center were trained by study monitors to use the specific software (PDNet, Baxter Healthcare, São Paulo, Brazil). All patients signed an informed consent agreeing to participate in the study and were followed until dropout or to the end of the study in January 2011. A software application was especially developed for data collection and was previously described (7).
Patient Population
This study included all adult patients from PD centers nationwide, reported monthly by nephrologists and nurses at the clinic using PDNet. Center participation over the study period was constant and all patients using supplies manufactured by Baxter were included in the database. The number of prevalent patients in each year corresponded to approximately 65 to 70% of all PD patients in the country. Data collection included demographic data including age (years), gender, race, cause of end-stage renal disease, history and time of pre-dialysis care, family income (minimum wages [MW] per month: 0 - 2; 3 - 5; 6 - 10; 11- 20; > 20 MW), education level (illiteracy, elementary, secondary, and higher), distance from dialysis center (< 25; 25 - 50; > 50 km), region where patients live and its Human Development Index (HDI) and center experience in patient-years. Clinical data included PD modality - continuous ambulatory PD (CAPD) or automated PD (APD), body mass index (BMI; kg/m2), blood pressure (mmHg), presence of edema, and exit-site conditions. The presence of comorbid conditions (lupus, malignancy, coronary artery disease, known left ventricular hypertrophy, stroke, peripheral artery disease, and diabetes) was registered and the Davies score calculated accordingly. Peritoneal dialysis-specific data, such as dialysis prescription, residual renal volume, and ultrafiltration volume were also collected in a subgroup of patients.
For the analysis of technique survival, the primary event was defined as definitive transfer to hemodialysis (HD) for any reason, which means the patient did not return to PD until the end of the follow-up. Dropout data were stratified as death, recovery of renal function, renal transplantation, definitive transfer to HD, and lost to follow-up. Center experience was measured by patient-years, i.e., the follow-up time of all patients from a certain center was summed and the result divided by the number of years that center participated in the study. The tertiles of center experience measured in patient-years were T1: ≤ 11; T2: 11.1 - 25; T3 > 25. For the description of trends in population characteristics, patient and technique survival, the population was divided into 3 groups according to the year of starting PD: 2005/2006, 2007/2008, and 2009/2010.
Statistical Analysis
Continuous variables were expressed as mean ± standard deviation (SD) or median and range, while categorical variables (e.g., gender, race, primary renal disease, presence of comorbid conditions, initial therapy, current PD modality) were expressed as frequencies or percentages. Comparison between continuous variables at baseline was performed using ANOVA test while categorical variables were compared by chi-square test. For adjusted multivariate patient and technique survival, we considered the influence of competing risks and, considering that any patient can experience only 1 type of event, either death for patient survival or transfer to HD for technique survival, we used a competing risk model based on Fine and Gray. All patients active at the end of follow-up were treated as censored. In contrast, all patients who dropped the study for any reason different from the primary event of interest were treated as competing risk. Transplantation was not considered as technique failure but as an event that competed with the primary event. We modeled competing risk survival using a cumulative incidence function rather than survival. Differences between cumulative incidence curves were compared using Fine and Gray’s method. All analysis was adjusted for covariates. Finally, collinearity among variables was tested and if statistically significant interactions were present, 1 of them was excluded. Covariates were included in the model when a p value lower than 0.20 in the univariate analysis was found. Statistical significance was set at p < 0.05. All statistical descriptive analyses were performed with SPSS 20.0 (SPSS, Chicago, USA). The competing risk analysis was performed using STATA 12 (StataCorp LP, College Station, TX, USA) and the package cmprsk: Subdistribution Analysis for Competing Risks, R version 3.0.2 (R Foundation for Statistical Computing).
Results
From December 2004 to January 2011 a total of 9,905 adult patients with valid data from 122 national centers were recruited in the study and included in the database. The center participation over the study period was constant and all patients using supplies manufactured by Baxter were recruited. Although it was not possible to capture the percentage of patients for each center, by extrapolation and comparing the number of prevalent patients in each year to the Brazilian Census of dialysis corresponded to approximately 65 - 70% of all PD patients in the country during the study. In this cohort, 7,007 were incident patients on PD, of which 5,707 remained at least 90 days on PD and in 3,311, PD was the first ever renal replacement therapy (Figure 1).
Figure 1 —
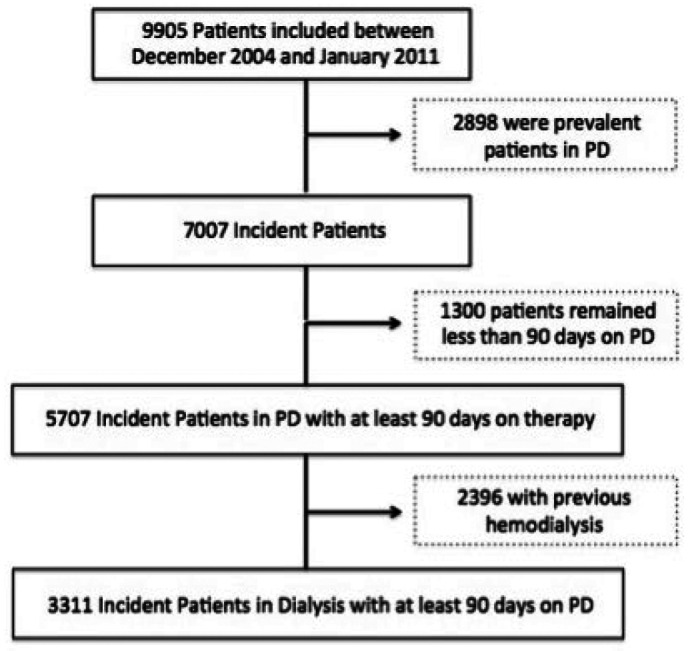
Characterization of BRAZPD II cohort. PD = peritoneal dialysis.
Baseline Characteristics
In Table 1, we present the main characteristics of the study population, divided into a description of the overall population (including prevalent and incident patients), and the incident population. The mean age of patients was 58.9 ± 16 years, 48% were males, 64% were white, and 57% lived 25 km from the PD center or closer. Reflecting the characteristics of the Brazilian population, 10% were illiterate, 33% had a family income less than 2 Brazilian minimum wage (MW), only 50% received predialysis care and 9% of patients lived more than 25 km away from the dialysis center. Regarding comorbidities, diabetes was present in 43%, and hypertension in 72%; Davies score was 0 (no comorbidities) in 37%, 1 - 2 in 57% and > 2 in 6%. Information regarding laboratory parameters and its behavior after commencing PD can be found in a supplementary file.
TABLE 1.
Clinical and Demographic Characteristics of the BRAZPD II Cohort
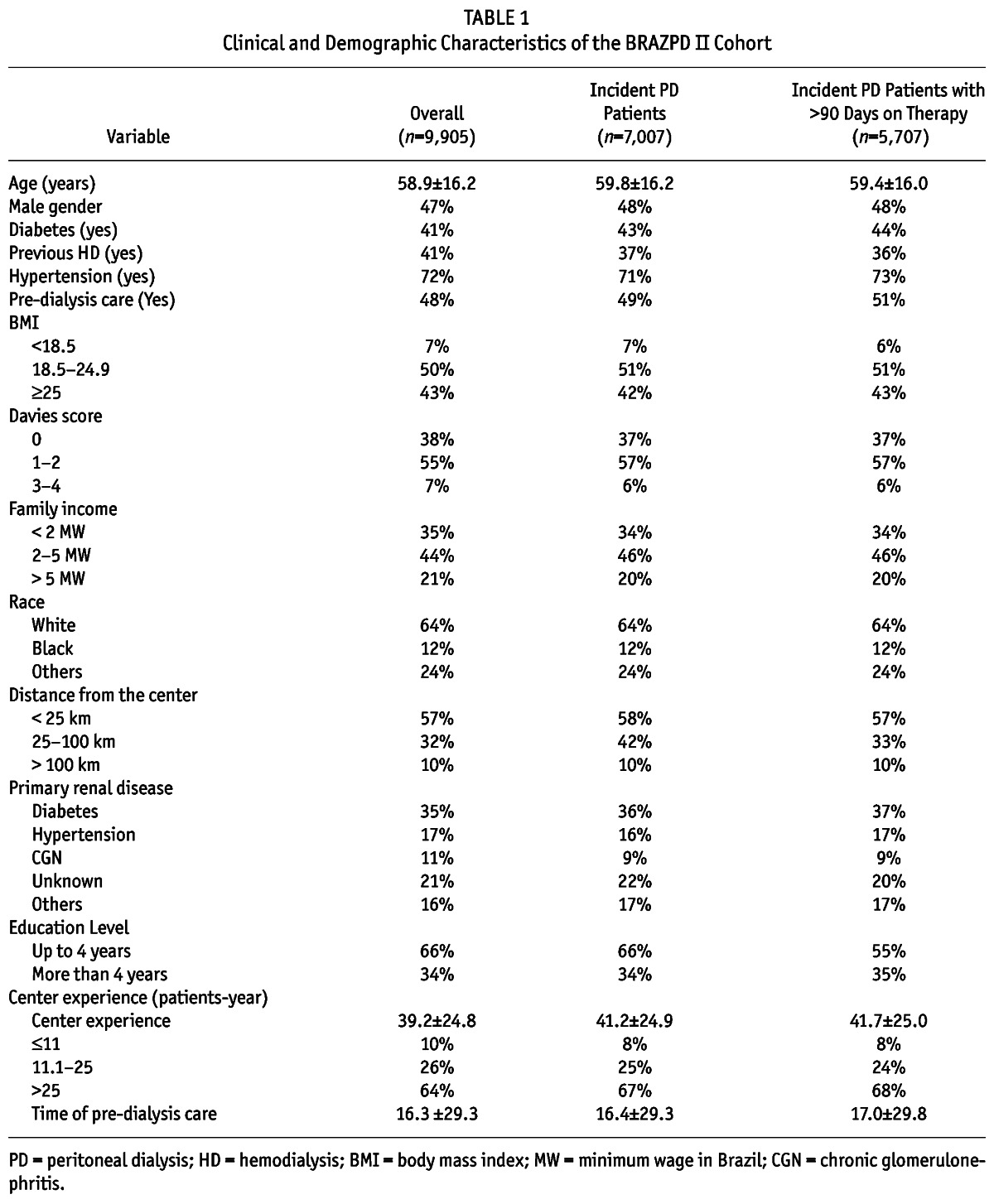
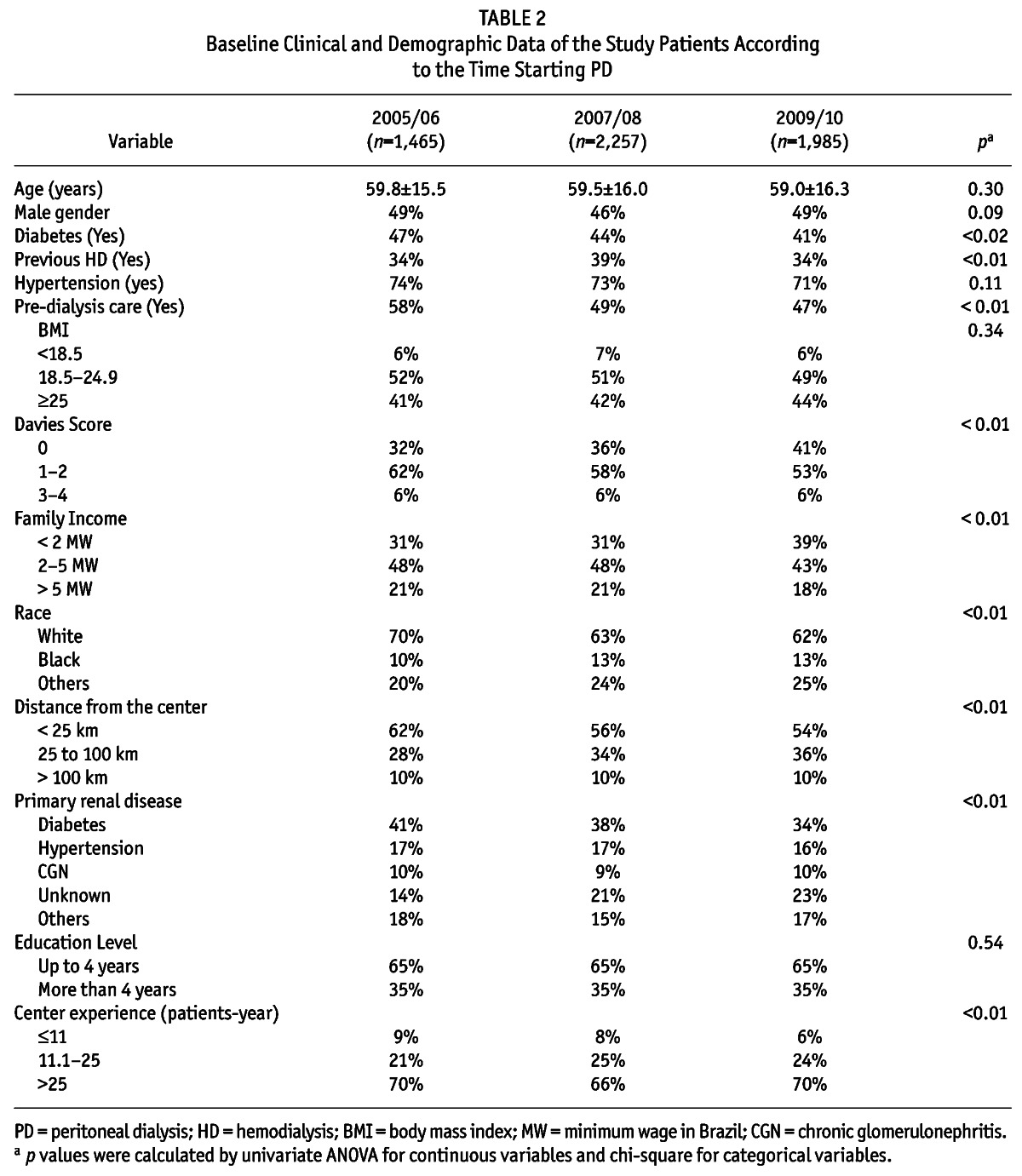
Table 2 shows the characteristics of the study population divided into different time periods of PD initiation. The prevalence of diabetic patients starting PD decreased 5% over the years, and there was an increase of 9% in the prevalence of patients with low comorbidity index as measured by Davies Score and a reduction of 8.1% of patients of white race starting PD over the observation time period. The use of APD as the initial therapy in incident patients increased substantially from 37% to 53%, a 16% increase in 5 years. The full comparisons of clinical and demographic characteristics over the vintages are presented in Table 2.
TABLE 2.
Baseline Clinical and Demographic Data of the Study Patients According to the Time Starting PD
Technique Survival
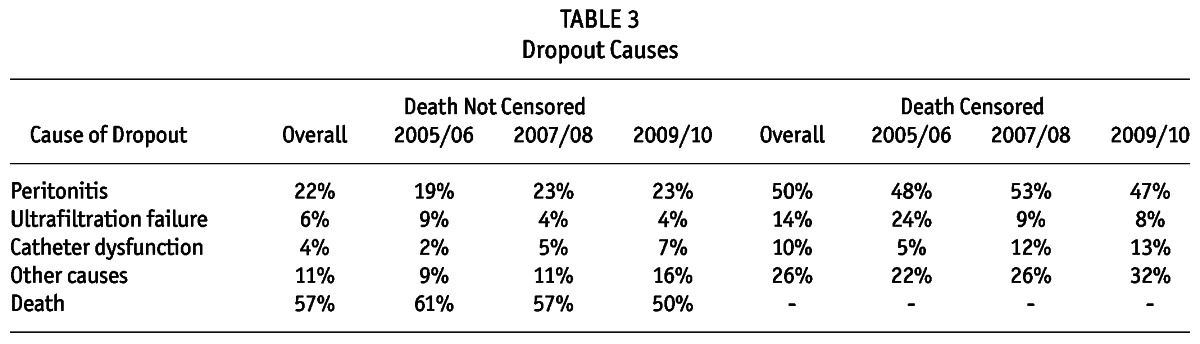
Out of the 5,707 incident patients, 607 (11%) were definitively transferred to HD during the study period. The main cause of technique failure was peritonitis (65%) followed by ultrafiltration failure (19%), catheter dysfunction (13%), refractory exit-site infection (3%) and other causes (2%). The technique survival at 1, 2, 3, and 4 years was respectively: 94%, 87%, 81%, and 72%. The percentages of patients remaining on the therapy after combining death with technique failure at 1, 2, 3, and 4 years were respectively 84%, 68%, 54%, and 41%. The dropout rate (and not technique failure) at 1, 2, 3, 4, and 5 years was respectively 22%, 37%, 50%, 63%, and 72%. Death was responsible for more than 50% of the study dropout with peritonitis coming next. Table 3 summarizes the causes of dropout. Importantly, our technique and patient survival were similar to previous reports from different cohorts (2,9-12). Peritonitis was the most important cause of technique failure followed by ultrafiltration failure.
TABLE 3.
Dropout Causes
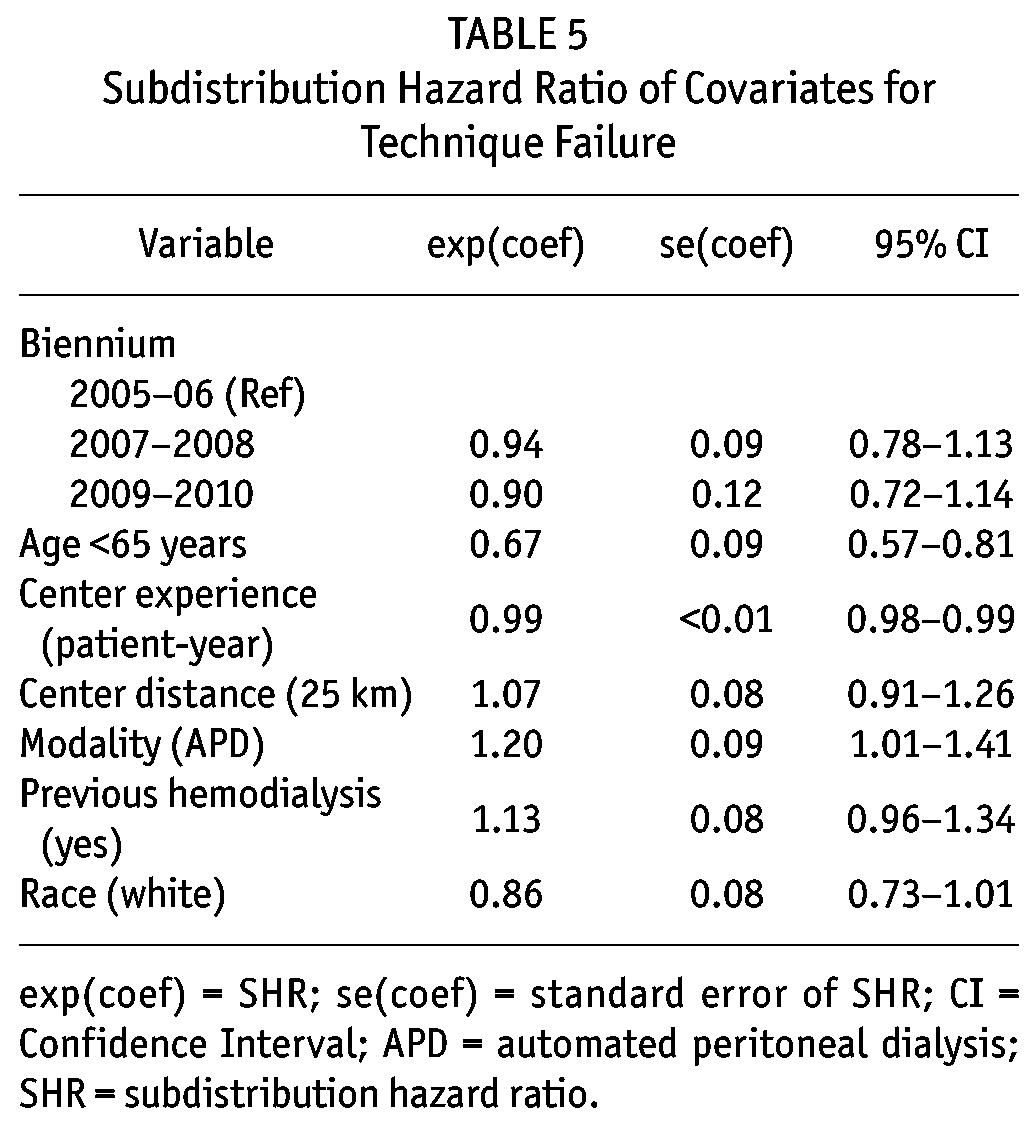
Six covariates presented a p value lower than 0.20 in the univariate analysis and were included in the multivariate model: age ≥ 65 years, race, distance from the dialysis center, center experience, initial PD modality, and previous HD. There was no improvement in technique survival over the years. There were however, 3 independent predictors of technique failure: center experience in patient-years (relative risk reduction [SHR] 0.99, 95% confidence interval [CI] 0.98 - 0.99), age < 65 years (SHR: 0.67, 95% CI, 0.57 - 0.81), and APD as initial therapy (SHR: 1.20, 95% CI, 1.01 - 1.41) (Table 4).
TABLE 4.
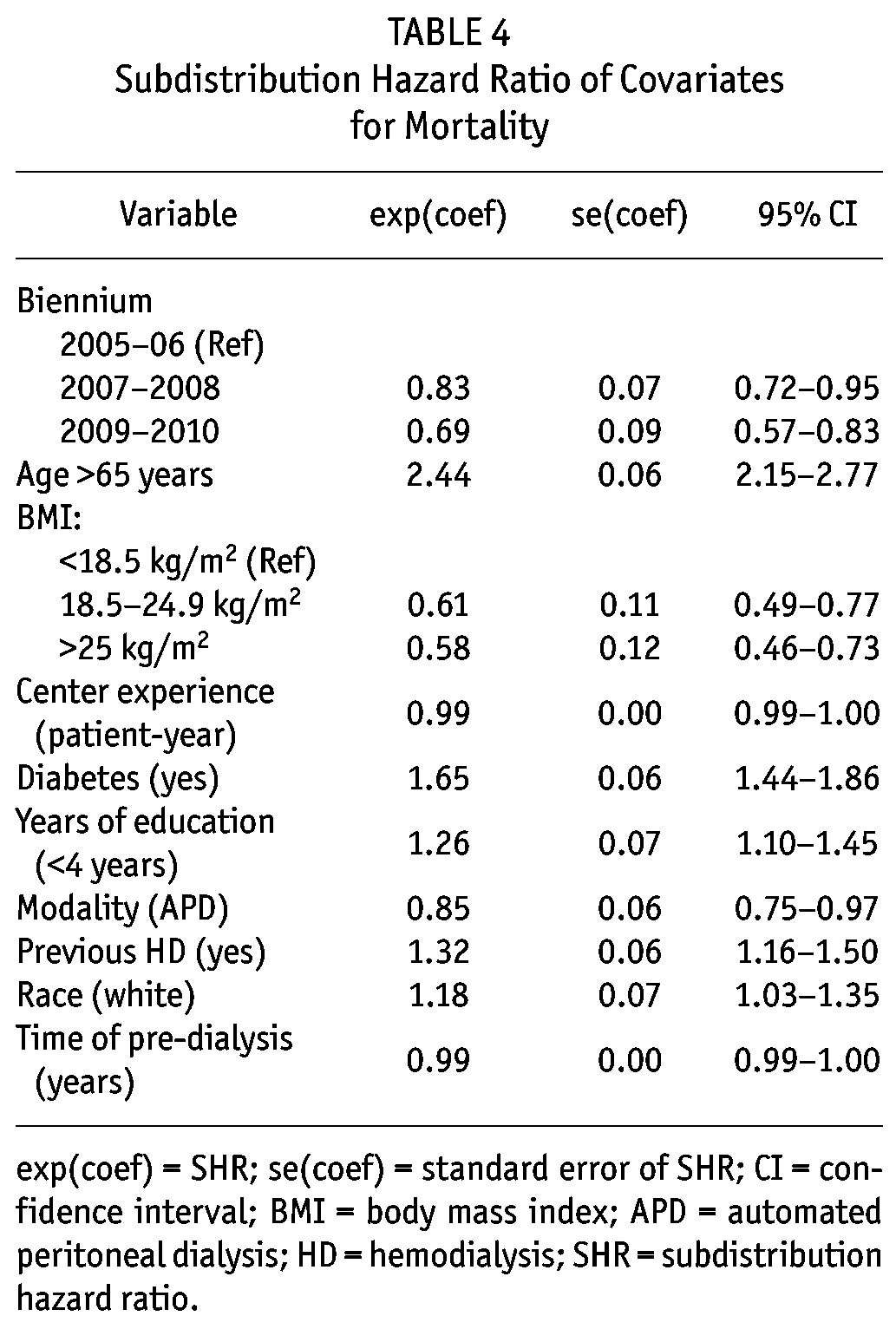
Subdistribution Hazard Ratio of Covariates for Mortality
Patient Survival
There were 1,057 deaths (19%) between incident patients during the study. In fact, death was the leading cause of dropout (54% of all cases) mainly by cardiovascular disease (36%), followed by infection not related to PD (35%) and peritonitis (9%) (Table 3). Overall non-adjusted patient survival at 85%, 74%, 64%, 54%, and 48% at 1, 2, 3, 4, and 5 years, respectively.
Mortality rates for all causes improved over the years: compared to 2005/2006 patients, those starting in 2007/2008 had a SHR of 0.83, 95% CI 0.72 - 0.95; and from 2009/2010 a SHR of 0.69, 95% CI 0.57 - 0.83). After the inclusion of all variables with a p value lower than 0.20 at the univariate analysis (age ≥ 65 years, BMI, diabetes, previous HD, white race, literacy, PD modality, center experience, time of pre-dialysis care, and the year the patient started PD), we ended up with 10 independent predictors of mortality: diabetes (SHR: 1.65, 95% CI, 1.44 - 1.86), previous HD (SHR: 1.32, 95% CI, 1.16 - 1.50), APD as initial therapy (SHR: 0.85, 95% CI, 0.75 - 0.97), age ≥ 65 (SHR: 2.44, 95% CI, 2.15 - 2.77), white race (SHR: 1.18, 95% CI, 1.03 - 1.35), education level (< 4 years) (SHR: 1.26, 95% CI, 1.10 - 1.45), BMI (normal BMI compared to low BMI: SHR: 0.61, 95% CI, 0.49 - 0.77; high BMI compared to low BMI: SHR: 0.58, 95% CI, 0.46 - 0.73), center experience (SHR: 0.99, 95% CI, 0.99 - 1.00), time of pre-dialysis care (SHR: 0.99, 95% CI, 0.99 - 1.00) and the year the patient started PD (2007/2008 compared to 2005/2006: SHR: 0.83, 95% CI, 0.72 - 0.95; 2009/2010 compared to 2007/2008: SHR: 0.69, 95% CI, 0.57 - 0.83) (Figure 2). A full description of patient survival according different subsets of patients can be seen in Table 5.
Figure 2 —
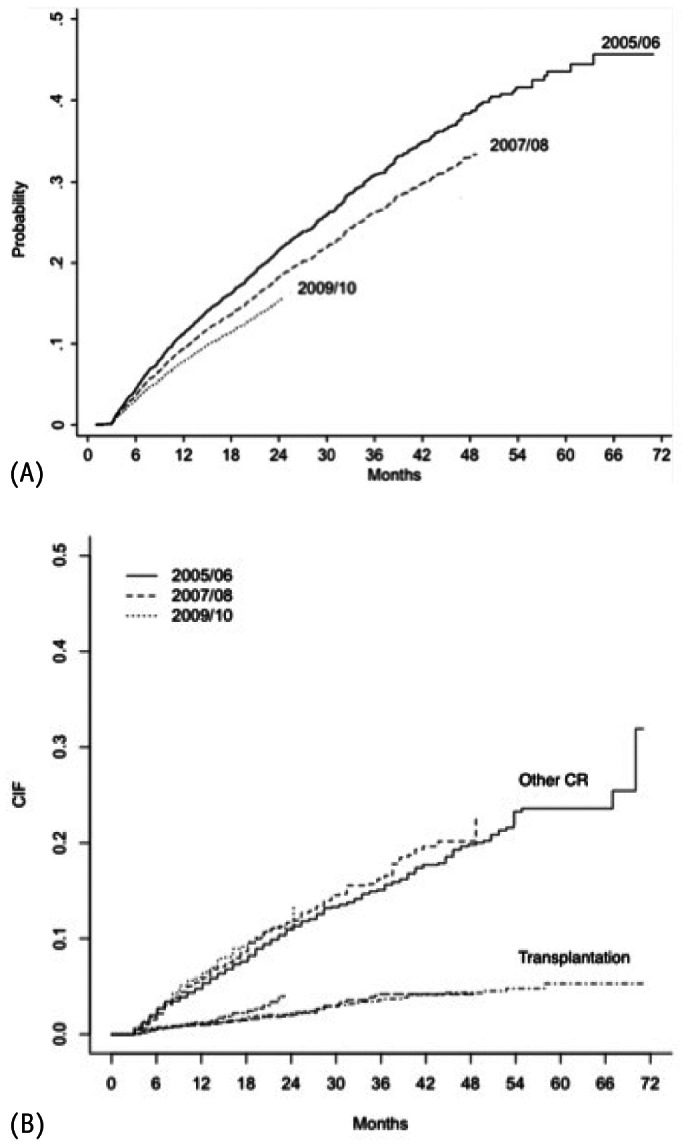
Cumulative incidence failure for both: Event of Interest (A) and Competing Risks (B).
TABLE 5.
Subdistribution Hazard Ratio of Covariates for Technique Failure
Discussion
Observational studies from different regions of the world provide valuable information in patient selection, clinical practice, and their relationship to patient and technique outcome. The present study is the first large cohort from a developing country to confirm the improvement in patient survival in recent years, previously observed only in developed countries (2,9).
The demographic characteristics of our PD patients changed over time and we noted a significant reduction in diabetic patients starting PD, a slight reduction in age at the beginning of therapy and an increase in patients without comorbidities according to the Davies score. Comparing our population with other large cohorts, we have a higher percentage of diabetic patients starting PD when compared to the European and Asiatic cohorts (15 - 27%) but similar to the USRDS (44%) (2-5), and the mean age of our cohort was in average 5 to 7 years older than most others large PD cohorts. These changes in our patient profile likely reflect a change in clinical practice probably based on several emerging data pointing to better outcomes of younger non-diabetic patients in PD (2,9,13). In terms of PD modality, there was a huge increase in the percentage of patients starting PD on APD (30% in 5 years) and our prevalence is nowadays similar to most developed countries (14). Since its introduction during the 1990s, the use of APD increased strongly worldwide and currently is the main PD modality in many developed countries despite its higher cost (14). Such temporal change is not only related to formal clinical indications (e.g. high transporters) but also to a common belief, although not confirmed in some studies, of a better quality of life (15-17).
Technique Survival: Main Findings and Predictors
Traditionally, peritonitis is the leading cause of technique failure, which was not different in the present study. In fact, almost 2 out of 3 patients who were definitively transferred to HD did it as a consequence of a peritonitis episode. Technique failure at 1, 2, and 3 years was similar to previous studies from different cohorts (10,11). There were 3 independent predictors of technique failure in our cohort, namely: age, center experience, and APD as initial PD modality. The impact of age in technique failure is conflicting in the literature: while a higher risk in elderly patients was found in a study from North America (18), Lim et al. reported a significant advantage of elderly over younger PD patients in the ANZDATA cohort (11). Less controversial is the impact of center experience on outcomes (10,18-20). Center size has been reported to have a direct impact on outcomes. Apparently, the higher experience acquired treating a larger number of patients directly influences outcomes (19,20). Exploring the data using categorization of the variable center experience, we found a threshold for better results when a center treated at least 29 patient-years. Last, APD is usually the modality of choice for the treatment of high transporters in PD. It is known that these patients are at high risk for ultrafiltration failure and present significantly more comorbidities that may influence outcomes, particularly when glucose-sparing solutions are not available, as was our case at the time (21,22).
Mortality
Despite all efforts, mortality rates remain extremely high in dialysis patients (23). Not surprisingly, death was responsible for more than 50% of the study dropout and, in line with previous reports, cardiovascular mortality was the most frequent cause of death, followed by non-related PD infections, and peritonitis (24). Several known risk factors associated with poor outcomes in previous reports contributed to these results. Age was the most important risk factor followed by diabetes. Patients with a BMI below the normal value of 18.5 were at high risk of mortality, most likely reflecting malnourishment. Patients originating from HD present with end-stage renal disease more often and have a lower, if any, residual renal function than those starting dialysis in PD and this is probably the explanation for previous HD being a risk factor for mortality. White race was another risk factor for mortality in line with our previous findings and also results from developed countries (25-27). The better outcome of black patients is probably linked to genetic reasons but further studies are needed to clarify such findings. Regarding PD modality, this is the first time that, in a large cohort, CAPD patients presented with worse outcomes. The reasons are not clear to us, but may have occurred by chance (especially due the high number of variables in our database). The association of education with mortality has been described previously in patients with and without kidney disease and these patients should be followed more carefully (28,29). Finally, the benefit of pre-dialysis care in patient mortality demonstrated by others was confirmed in our study (30,31).
Importantly, we observed for the first time in a developing country an improvement in mortality rates over the years. This improvement occurred progressively with patients starting PD at 2007 and 2008 presenting better survival than those from 2005/2006, and with patients commencing PD in 2009/2010 compared with patients from both 2005/2006 and 2007/2008 (Figure 3). At first glance the better clinical profile of patients starting dialysis could be an explanation, but the difference persisted even after adjustment for several covariates. The factors responsible for this improvement were not clear, but are probably related to an improvement in clinical practice. The increase in the prevalence of APD, not only as the primary PD modality but also after switching during the study could have influenced outcomes through different mechanisms including better fluid control and/or improvement of uremic toxin removal. Another possibility is related to a better management of PD-related infections that has been massively tackled by medical societies through development of campaigns and development and diffusion of clinical guidelines. Furthermore, we should take into account that other risk factors not captured in the present study could also have influenced survival rates, including a better selection of patients to start PD. Importantly, our results are in line with previous data from large cohorts that looked into secular trends: Mehrotra et al. in a large study with almost 65,000 PD patients from the USRDS reported a significant improvement ranging from 3 to 5% in 2 to 5 years patient survival (9).
Figure 3 —
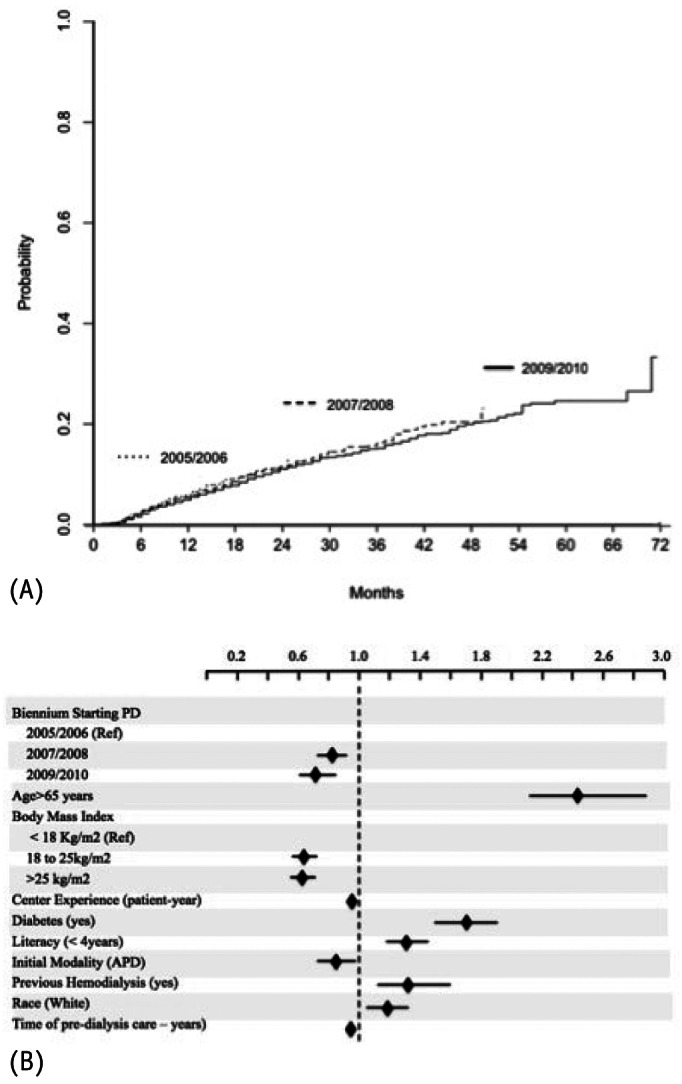
Independent determinants of patient survival using competing risks analysis. A shows improvements in patient survival along the years (see details in Table 4) while B demonstrates that the competing risks were similar between groups and did not interfere in the main event of interest. The black diamonds represent the subdistribution hazard ratio for covariates using the Gray’s method and the horizontal lines the 95% confidence interval. PD = peritoneal dialysis; APD = automated PD.
This study presents several limitations. First, this is an observational study and, as such, all significant associations found should be interpreted with caution. Second, residual renal function was not available for the majority of patients and was not included in our analysis. Nevertheless, our study has some very important strengths: it was a prospective, nationwide, cohort with outcomes adjusted for several clinical and demographic covariates using a competing risks analysis. Its characteristics share several similarities with other cohorts from different parts of the world supporting the quality of our database.
In conclusion, we described the largest cohort of PD patients in Latin America, a region responsible for 25% of PD patients in the world. Determinants of outcome do not differ significantly from other regions of the world. Even with no changes in technique survival, this is the first study reporting a significant trend in patient survival improvement throughout the vintages in a developing country and after adjusting for multiple covariates.
Disclosures
RPF is a recipient of a scholarship from the Brazilian Council for Research (CNPq). RPF received research grants, consulting fees and speaker honorarium from Baxter Healthcare. AF received consulting fees and speaker honorarium from Baxter Healthcare. PB received consulting fees and speaker honorarium from Baxter Healthcare. TPM received consulting fees and speaker honorarium from Baxter Healthcare. The other authors declare that they have no other relevant financial interests. This study (design, implementation, software development, data collection) was funded by Baxter Healthcare, Brazil. The current data extraction and analysis was supported by an investigator driven study grant provided to PUCPR, as part of the Clinical Evidence Council Program from Baxter Healthcare.
Supplementary Material
Footnotes
Supplemental material available at www.pdiconnect.com
References
- 1. Canada-USA (CANUSA) multicentre study of peritoneal dialysis adequacy: description of the study population and preliminary results. CANUSA Peritoneal Dialysis Study Group. Adv Perit Dial 1992; 8:88–92. [PubMed] [Google Scholar]
- 2. Mujais S, Story K. Peritoneal dialysis in the US: evaluation of outcomes in contemporary cohorts. Kidney Int Suppl 2006. November; (103):S21–6. [DOI] [PubMed] [Google Scholar]
- 3. Jager KJ, Merkus MP, Boeschoten EW, Dekker FW, Stevens P, Krediet RT. Dialysis in The Netherlands: the clinical condition of new patients put into a European perspective. NECOSAD Study Group. Netherlands Cooperative Study on the Adequacy of Dialysis phase 1. Nephrol Dial Transplant 1999. October; 14(10):2438–44. [DOI] [PubMed] [Google Scholar]
- 4. Brown EA, Davies SJ, Rutherford P, Meeus F, Borras M, Riegel W, et al. Survival of functionally anuric patients on automated peritoneal dialysis: the European APD Outcome Study. J Am Soc Nephrol 2003. November; 14(11):2948–57. [DOI] [PubMed] [Google Scholar]
- 5. Lam MF, Tang C, Wong AK, Tong KL, Yu AW, Li CS, et al. ASPD: A prospective study of adequacy in Asian patients on long term, small volume, continuous ambulatory peritoneal dialysis. Perit Dial Int 2006. Jul-Aug; 26(4):466–74. [PubMed] [Google Scholar]
- 6. McDonald SP, Russ GR, Kerr PG, Collins JF, Australia and New Zealand Dialysis and Transplant Registry. ESRD in Australia and New Zealand at the end of the millennium: a report from the ANZDATA registry. Am J Kidney Dis 2002. December; 40(6):1122–31. [DOI] [PubMed] [Google Scholar]
- 7. Fernandes N, Bastos MG, Cassi HV, Machado NL, Ribeiro JA, Martins G, et al. The Brazilian Peritoneal Dialysis Multicenter Study (BRAZPD): characterization of the cohort. Kidney Int Suppl 2008. April(108):S145–51. [DOI] [PubMed] [Google Scholar]
- 8. McDonald SP, Marshall MR, Johnson DW, Polkinghorne KR. Relationship between dialysis modality and mortality. J Am Soc Nephrol 2009. January; 20(1):155–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mehrotra R, Chiu YW, Kalantar-Zadeh K, Bargman J, Vonesh E. Similar outcomes with hemodialysis and peritoneal dialysis in patients with end-stage renal disease. Arch Intern Med 2011. January 24; 171(2):110–8. [DOI] [PubMed] [Google Scholar]
- 10. Afolalu B, Troidle L, Osayimwen O, Bhargava J, Kitsen J, Finkelstein FO. Technique failure and center size in a large cohort of peritoneal dialysis patients in a defined geographic area. Perit Dial Int 2009. May-Jun; 29(3):292–6. [PubMed] [Google Scholar]
- 11. Lim WH, Dogra GK, McDonald SP, Brown FG, Johnson DW. Compared with younger peritoneal dialysis patients, elderly patients have similar peritonitis-free survival and lower risk of technique failure, but higher risk of peritonitis-related mortality. Perit Dial Int 2011. Nov-Dec; 31(6):663–71. [DOI] [PubMed] [Google Scholar]
- 12. Shen JI, Mitani AA, Saxena AB, Goldstein BA, Winkelmayer WC. Determinants of peritoneal dialysis technique failure in incident US patients. Perit Dial Int 2013. Mar-Apr; 33(2):155–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. United States Renal Data System. Available at: http://www.usrds.org.
- 14. Jain AK, Blake P, Cordy P, Garg AX. Global trends in rates of peritoneal dialysis. J Am Soc Nephrol 2012. March; 23(3):533–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. de Wit GA, Merkus MP, Krediet RT, de Charro FT. A comparison of quality of life of patients on automated and continuous ambulatory peritoneal dialysis. Perit Dial Int 2001. May-Jun; 21(3):306–12. [PubMed] [Google Scholar]
- 16. Guney I, Solak Y, Atalay H, Yazici R, Altintepe L, Kara F, et al. Comparison of effects of automated peritoneal dialysis and continuous ambulatory peritoneal dialysis on health-related quality of life, sleep quality, and depression. Hemodial Int 2010. October; 14(4):515–22. [DOI] [PubMed] [Google Scholar]
- 17. Michels WM, van Dijk S, Verduijn M, le Cessie S, Boeschoten EW, Dekker FW, et al. Quality of life in automated and continuous ambulatory peritoneal dialysis. Perit Dial Int 2011. Mar-Apr; 31(2):138–47. [DOI] [PubMed] [Google Scholar]
- 18. Chidambaram M, Bargman JM, Quinn RR, Austin PC, Hux JE, Laupacis A. Patient and physician predictors of peritoneal dialysis technique failure: a population based, retrospective cohort study. Perit Dial Int 2011. Sep-Oct; 31(5):565–73. [DOI] [PubMed] [Google Scholar]
- 19. Guo A, Mujais S. Patient and technique survival on peritoneal dialysis in the United States: evaluation in large incident cohorts. Kidney Int Suppl 2003. December(88):S3–12. [DOI] [PubMed] [Google Scholar]
- 20. Huisman RM, Nieuwenhuizen MG, Th de Charro F. Patient-related and centre-related factors influencing technique survival of peritoneal dialysis in The Netherlands. Nephrol Dial Transplant 2002. September; 17(9):1655–60. [DOI] [PubMed] [Google Scholar]
- 21. Fernandez-Reyes MJ, Bajo MA, Del Peso G, Ossorio M, Diaz R, Carretero B, et al. The influence of initial peritoneal transport characteristics, inflammation, and high glucose exposure on prognosis for peritoneal membrane function. Perit Dial Int 2012. Nov-Dec; 32(6):636–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Reyes MJ, Bajo MA, Hevia C, Del Peso G, Ros S, de Miguel AG, et al. Inherent high peritoneal transport and ultrafiltration deficiency: their mid-term clinical relevance. Nephrol Dial Transplant 2007. January; 22(1):218–23. [DOI] [PubMed] [Google Scholar]
- 23. U.S. Renal Data System, USRDS 2012 Annual Data Report: Atlas of End-Stage Renal Disease in the United States. [Google Scholar]
- 24. Lee CC, Sun CY, Wu MS. Long-term modality-related mortality analysis in incident dialysis patients. Perit Dial Int 2009. Mar-Apr; 29(2):182-90. [PubMed] [Google Scholar]
- 25. Fernandes NM, Hoekstra T, van den Beukel TO, Tirapani L, Bastos K, Pecoits-Filho R, et al. Association of ethnicity and survival in peritoneal dialysis: a cohort study of incident patients in Brazil. Am J Kidney Dis 2013. July; 62(1):89–96. [DOI] [PubMed] [Google Scholar]
- 26. Robinson BM, Joffe MM, Pisoni RL, Port FK, Feldman HI. Revisiting survival differences by race and ethnicity among hemodialysis patients: the Dialysis Outcomes and Practice Patterns Study. J Am Soc Nephrol 2006. October; 17(10):2910–8. [DOI] [PubMed] [Google Scholar]
- 27. Tanna MM, Vonesh EF, Korbet SM. Patient survival among incident peritoneal dialysis and hemodialysis patients in an urban setting. Am J Kidney Dis 2000. December; 36(6):1175–82. [DOI] [PubMed] [Google Scholar]
- 28. Goulart AC, Fernandes TG, Santos IS, Alencar AP, Bensenor IM, Lotufo PA. Predictors of long-term survival among first-ever ischemic and hemorrhagic stroke in a Brazilian stroke cohort. BMC Neurol 2013. May 24; 13(1):51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Khattak M, Sandhu GS, Desilva R, Goldfarb-Rumyantzev AS. Association of education level with dialysis outcome. Hemodial Int 2012. January; 16(1):82–8. [DOI] [PubMed] [Google Scholar]
- 30. Kazmi WH, Obrador GT, Khan SS, Pereira BJ, Kausz AT. Late nephrology referral and mortality among patients with end-stage renal disease: a propensity score analysis. Nephrol Dial Transplant 2004. July; 19(7):1808–14. [DOI] [PubMed] [Google Scholar]
- 31. Kim do H, Kim M, Kim H, Kim YL, Kang SW, Yang CW, et al. Early referral to a nephrologist improved patient survival: prospective cohort study for end-stage renal disease in Korea. PloS One 2013; 8(1):e55323. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


