Abstract
♦ Introduction: Residual renal function (RRF) plays an important role in outcome of peritoneal dialysis (PD) including mortality. It is, therefore, important to provide a strategy for the preservation of RRF. The objective of this study was to evaluate relative protective effects of new glucose-based multicompartmental PD solution (PDS), which is well known to be more biocompatible than glucose-based conventional PDS, on RRF compared to conventional PDS by performing a systematic review (SR) of randomized controlled trials.
♦ Methods: We searched studies presented up to January 2014 in MEDLINE, EMBASE, the COCHRANE library, and local databases. Three independent reviewers reviewed and extracted prespecified data from each study. The random effects model, a more conservative analysis model, was used to combine trials and to perform stratified analyses based on the duration of follow-up. Study quality was assessed using the Cochrane Handbook for risk of bias. Eleven articles with 1,034 patients were identified for the SR.
♦ Results: The heterogeneity of the studies under 12 months was very high, and the heterogeneity decreased substantially when we stratified studies by the duration of follow-up. The mean difference of the studies after 12 months was 0.46 mL/min/1.73 m2 (95% confidence interval = 0.25 to + 0.67).
♦ Conclusion: New PDS showed the effect to preserve and improve RRF for long-term use compared to conventional PDS, even though it did not show a significant difference to preserve RRF for short-term use.
Keywords: Residual renal function, peritoneal dialysis solutions, randomized controlled trials, systematic review
Residual renal function (RRF) has a major impact on the outcome of peritoneal dialysis (PD) (1,2), and is directly correlated with patient survival (3,4). The declining rate of RRF was also positively associated with all-cause mortality and technique failure in patients on long-term PD (5-7). It is, therefore, important to search a strategy for preserving RRF in PD.
Glucose degradation products (GDP) generated during heat sterilization of glucose-based conventional PD solutions (PDS) have been suggested to impair not only local peritoneal membrane function but also systemic remnant kidney function directly and indirectly via the formation of advanced glycation end product (AGE). Both GDP and AGE cause apoptosis, inflammation, and fibrosis in a context-dependent manner (8-10). In this connection, 3-deoxyglucosone (3-DG) was detected in the blood during dialysis with conventional PDS (11), suggesting that GDP in the PDS can be diffused into systemic circulation. Consistent with cytotoxic effect of GDP in various cells and tissues (12-15), renal tubular epithelial cells underwent apoptosis in response to 3,4-dideoxyglucosone-3-ene (3,4-DGE) (16), the most cytotoxic GDP in PDS (17).
With this background and the advance of technology, a new glucose-based biocompatible PDS, which contains a lower level of GDP and neutral pH than glucose-based conventional PDS, has been developed. Three major products, Balance (Fresenius Medical Care North America, Waltham, MA, USA), Physioneal (Baxter Healthcare Corporation, Deerfield, IL, USA), and Gambrosol trio (Gambro Lundia AB, Lund, Sweden) of new biocompatible PDS are available commercially.
The application of new PDS with less GDP results in a significantly lower peritoneal thickness in vivo (18) and may help to preserve peritoneal and vascular function compared to the conventional PDS (19). Consistent with this, the use of a new PDS increases cancer antigen 125 (CA125) and procollagen I peptide (PICP) and decreases hyaluronic acid (HA) in peritoneal effluent compared to a conventional PDS, confirming the protective effect on the peritoneal membrane as a dialyzing organ (20-22).
The systemic effect of the new PDS on RRF, cardiovascular effect, and survival has been also actively studied by comparing the preservative effect to conventional PDS (21-32). Consequently, it is important to synthesize relevant results of clinical studies involving chronic PD patients. We aimed to evaluate the preservative effect of the new biocompatible PDS on RRF compared to conventional PDS by conducting a systematic review (SR) of randomized controlled trials (RCTs) which will give us rationale for the selection of PDS.
Methods
Studies Eligible for Review
Studies were eligible if they were RCTs comparing the effect of new PDS on RRF with that of conventional PDS in chronic PD patients. Studies with PD patients using mainly amino acid and icodextrin solutions were excluded. Eligible patients included continuous ambulatory PD and automated PD patients.
Finding Relevant Studies
We searched the relevant studies presented up to January 2014 (last search: January 9th) in the international and local databases. For searching MEDLINE, EMBASE, and the COCHRANE databases, the search terms, ‘peritoneal dialysis and (residual renal function or RRF) and (RCT or randomized controlled trial* or randomised controlled trial*)’ were utilized. We searched local databases of KMBase and KoreaMed with the search terms, ‘peritoneal dialysis and residual renal function.’ The search was restricted to the English language.
Assessment of Study Quality
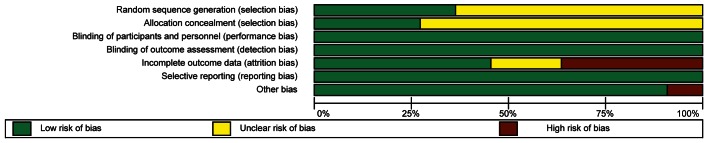
Three reviewers (E-Y Seo, SH An, and JH Cho) independently assessed methodological qualities of the final 16 RCTs. One reviewer assessed 12 studies and each study was assessed by 2 reviewers. If 2 reviewers had disagreements even after a thorough discussion, another reviewer joined the discussion to break the deadlock. The studies were assessed for validity using the Cochrane Handbook for risk of bias on RCT (33). The Cochrane’s tool has 7 domains which include the following: random sequence generation for selection bias, allocation concealment for selection bias, blinding of participants and personnel for performance bias, blinding of outcome assessment for detection bias, incomplete outcome data for attrition bias, selective reporting for reporting bias, and others. The risk level of each study in regard to its bias was graded as ‘low risk of bias,’ ‘unclear risk of bias,’ or ‘high risk of bias.’
Collecting Data
Data were extracted in duplicate using a data extraction form. The form was developed by the 3 reviewers and was supplemented and revised by YL Kim. Two studies (25,34) were seen to employ the same patients. The study by Kim et al. (25) was published after reinterpreting the previous results (34) with adjustments for age, gender, and the Davies score. We included the study by Kim et al. (25) for conducting a SR based on statistical rationale. Most studies evaluated the RRF with a mean value of creatinine clearance and urea clearance and standard deviation (SD) as described (35). The study by le Poole et al. (36) was excluded because the RRF was evaluated only by creatinine clearance. The Euro-Balance Trial (22) showed the result with median value and interquartile range (IQR). We, thus, converted the median value and IQR to mean and SD value. The study by Haag-Weber et al. (24) expressed a result as monthly change percentage and confidence interval (CI) instead of each value at the time point. We, therefore, converted them to mean and SD value of final value using the Cochrane’s tool (33). As the RRF unit, mL/min, mL/min/1.73 m2, L/week/1.73 m2, and L/day were used and we standardized the RRF unit of all studies to mL/min/1.73 m2. After standardizing the method of measurement and unit, we applied all values to Review Manager. Data standardization was performed by E-Y Seo and HS Suh in duplicate. The potential for publication bias was addressed by drawing a funnel plot and visual assessment.
Results
The combined search identified a total of 238 citations, 218 of which were judged ineligible after the title and abstract review (Figure 1). The major reasons for exclusion were study population, duplicates, and PDS used not meeting the inclusion criteria. Full text analysis of the remaining 20 articles led to 12 studies which met the inclusion criteria (i.e. glucose-based multi compartmental PDS). Among 20 articles, we found 2 protocols (37,38) without results and excluded them. We additionally excluded 2 correspondence articles (39,40) because they were not the original articles. We excluded 1 study in which the biocompatible solutions did not divide into icodextrin, amino acid, and low GDP-lactate solution (41). One study showed mixed results of cross-over design, so we could not include the data (42). At the stage of collecting data, we excluded 2 studies due to duplication (34) or inconsistent measurement method of the RRF (36). Two among the final 12 studies were performed by Kim et al. One showed the result at 12 months and the more recent study showed the result at 24 months with the same patients (28). Therefore, we applied the result only at 24 months, and the SR was conducted on the final 11 studies (1,034 patients) (21-30,43,44) (Figure 1).
Figure 1 —
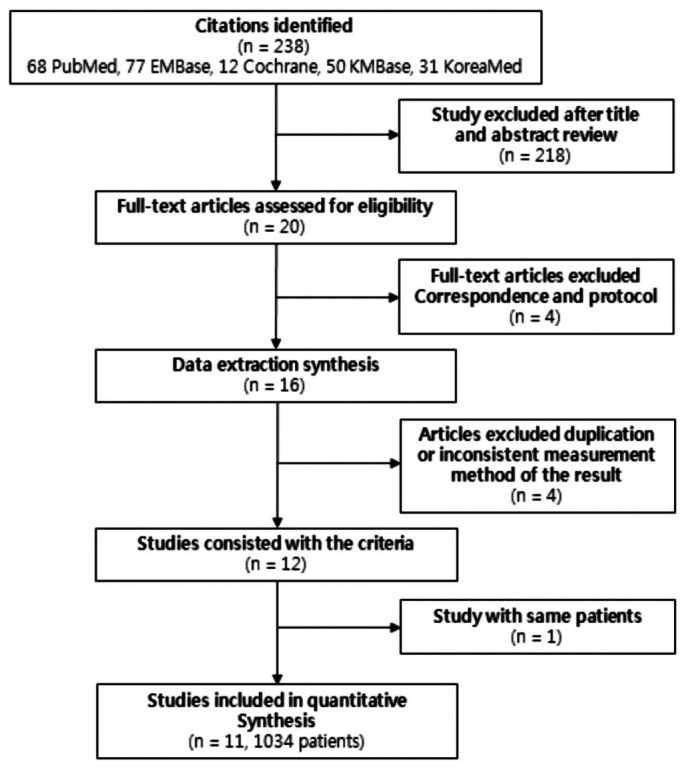
Selection of studies.
The study quality was assessed by the Cochrane Handbook for risk of bias on RCTs and scored (33). The risk of bias graphs are presented in Figure 2 and the risk of bias summary is shown in Supplementary Figure 1.
Figure 2 —
Risk of bias graph of included trials.
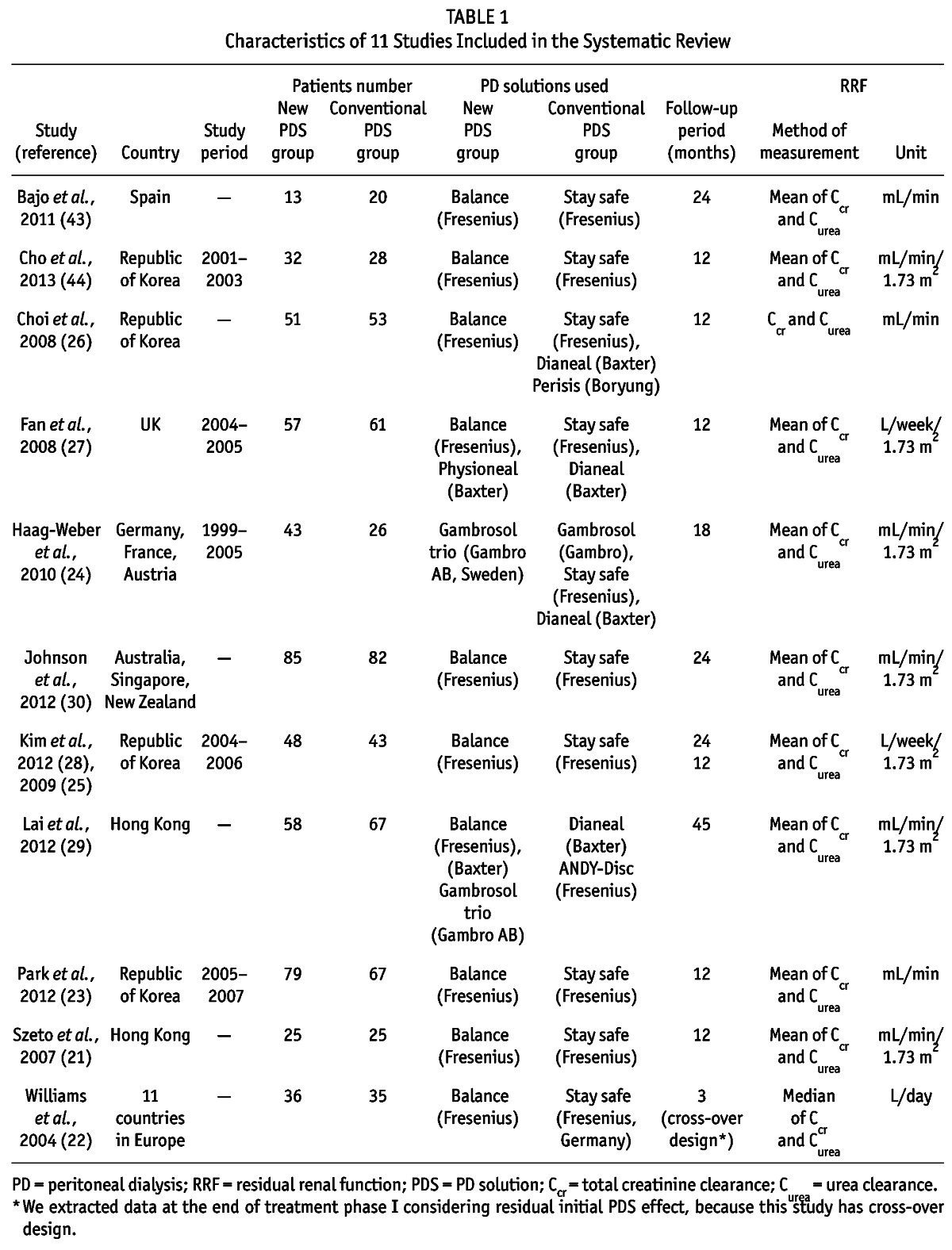
The characteristics of studies included in the final SR are listed in Table 1. The detailed characteristics of each study are presented in Supplementary Table 1. Data were extracted using an internally developed data extraction form, and the Review Manager software (Revman 2011; Version 5.1, The Nordic Cochrane Centre, Copenhagen, Denmark) was used for statistical analyses after standardizing the method of measurement and the unit of the RRF.
TABLE 1.
Characteristics of 11 Studies Included in the Systematic Review
We found no major asymmetrical appearance in the funnel plot to address a publication bias (Figure 3).
Figure 3 —
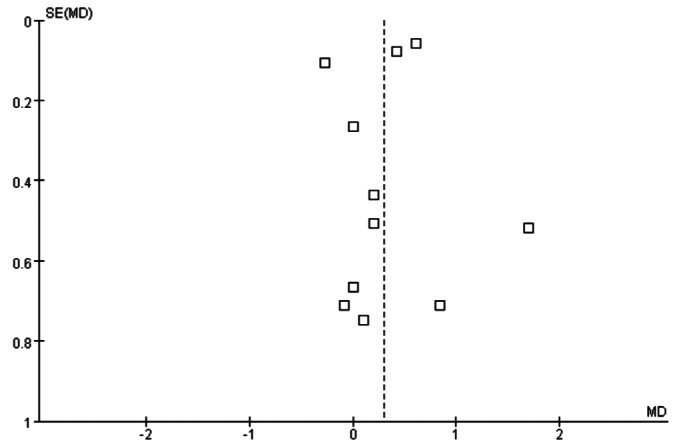
Funnel plot analysis to detect publication bias.
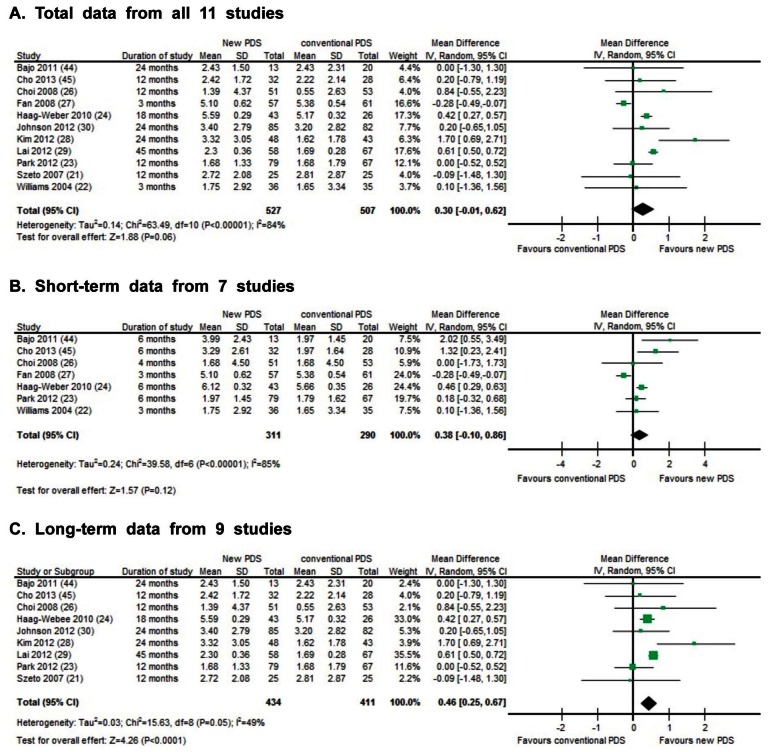
The final value of the RRF in each of 11 studies was compared for evaluating preservation of the RRF. Heterogeneity and overall effect was evaluated using random effects model. Since heterogeneity was very high (84%) from all studies (Figure 4A), we categorized studies depending on the duration of observation: short-term studies (3 months to 6 months, Figure 4B) vs long-term studies (12 months to around 45 months, Figure 4C) in order to find the cause of high heterogeneity. Where a study showed both short-term and long-term results, we analyzed both results. While heterogeneity of the short-term studies remained very high (85%), that of the long-term studies was relatively low (49%). We conducted analyses with the long-term studies accordingly. The overall preservative effect on RRF in patients who were treated by the new PDS for more than 12 months was more effective compared to patients on conventional PDS. The mean difference of final RRF value between the conventional PDS and the new PDS groups of long-term study was 0.46 mL/min/1.73 m2 (95% confidence interval = +0.25 to +0.67) showing improvement of RRF. The p-value for the overall effect was below 0.0001.
Figure 4 —
Effect of biocompatible and conventional peritoneal dialysis solution on residual renal function: A) total data, B) short-term data, and C) long-term data.
Discussion
This SR utilizing random effects model suggests that long-term use of the new glucose-based biocompatible PDS may have a preservative effect on the RRF compared to the glucose-based conventional PDS.
The 11 RCTs which were included in this SR were estimated to be of a fairly good methodological quality. We classified 7 domains, which are either random sequence generation or allocation concealment for selection bias, blinding of participants and personnel for performance bias, blinding of outcome assessment for detection bias, incomplete outcome data for attrition bias, selective reporting for reporting bias, and other bias according to the Cochrane Handbook (33). Among these 7, 3 domains including performance bias, detection bias, and reporting bias, were assessed as “low risk of bias” in all studies. There are, however, some biases in the other 4 domains. The most common bias was “attrition bias.” Four of the 11 studies had “high risk of bias” because they each showed only the per-protocol analysis without intention-to-treat analysis. Two of 11 studies had “unclear risk of bias” as they did not state the method of analysis. The other 5 studies had “low risk of bias”. The second common bias was “selection bias”. Seven or 8 studies among 11 had “unclear risk of bias”. They did not state the method for random sequence generation and allocation concealment.
Two common measurements were used to assess the outcome of continuous data: one is to use final value of experimental groups and the other is to use change score. Nine among 11 studies in this SR reported the final value and 2 studies showed the changes from baseline of the RRF as the primary outcome. We got the raw data of mean value and SD from the author of 1 study (30). For the other study, we converted the changes in that study to the mean and SD of final value and compared the final values of the conventional PDS group with those of the new PDS group. In fact, in this respect, the Cochrane guideline (33) advises not to focus on the changes from baseline because most studies have different numbers of participants between the baseline and the final measurement due to missed visits and study withdrawals, and RCT has the assumption that patients in each group at baseline have the same conditions through randomization.
The random effects model for the SR is more conservative, which allows for heterogeneity by assuming that underlying effects follow a normal distribution. Utilizing the random effects model for this SR, we found profound heterogeneity from all 11 studies and the heterogeneity of short-term studies was very high. Accordingly, the sensitivity analyses were performed to examine whether our overall findings are robust to individual studies. The studies by Fan et al. (27), Haag-Weber et al. (24), and Lai et al. (29) had a major impact on these analyses. The common features of these 3 studies were the fact that SDs were smaller than other studies. When the study of Fan et al. (27) was excluded from the analysis of all the studies, the result was very different from the original analysis of all studies showing the positive preservative effect (p < 0.0001, I2 = 44%) of the new PDS. The other 2 studies did not show any major impact on the original analysis when each study was excluded.
Systematyic review is very sensitive to the time frame for inclusion of RCTs. In this respect, our SR included RCTs published until January 2014 compared to September 2012 in the SR by Cho et al. (45). Our results agree with those of Cho et al. with updated RCTs, supporting the conclusion that new glucose-based biocompatible PDS preserve and improve the RRF for long-term use better than glucose-based conventional PDS. In addition, we have included RCTs using ‘mean value of both creatinine clearance and urea clearance’ and standardized the method of measurement and unit of RRF to get more precise results. In fact, the diverse RCTs showed a mixed unit of RRF like mL/min, mL/min/1.73 m2, L/week/1.73 m2, and L/day.
This SR is a novel evaluation which can show that the new PDS containing low GDP better protects the RRF than conventional bioincompatible PDS. However, 1 major limitation of our SR is that the RRF conditions at the baseline of the patients were diverse in all 11 studies. It has been suggested that any beneficial effect of a biocompatible PDS on RRF is expected if a PDS is introduced at an earlier stage of chronic kidney disease, that is, in patients with a relatively well-preserved RRF, rather than at a later stage when RRF is poor (46). A subgroup analysis with patients who had the RRF of 2 mL/min/1.73 m2 or more showed that the RRF of the new PDS group was significantly higher than that of the conventional PDS group after 12 months of PD (p = 0.004), while intention-to-treat analysis showed that the beneficial effect of the new PDS on RRF had statistical significance (p = 0.048) (25). It is conceivable that any PDS has little effect on RRF at a later stage of chronic kidney disease when the RRF is extremely poor at baseline, and that the exact comparison between the conventional PDS and new PDS is difficult. The second limitation is the small sample size and relatively short duration of follow-up in studies included in the present SR, which cannot give an exact conclusion.
The overall preservative effect on RRF of the new PDS in the long-term study by random effects model showed improvement of RRF compared to the conventional PDS.
There is controversy in the literature (47,48) regarding the mechanism by which biocompatible PDS preserves the RRF. One is a direct beneficial effect of biocompatible PDS with lower level of GDP inducing apoptosis of renal tubular cell (16). The other is an indirect effect which comes from less effective ultrafiltration (UF) and consequent hypervolemia. Ten studies from 11 RCTs in this SR have showed the results of UF change. However, the results are very diverse. Among them, 2 studies (23,29) showed less effective UF with biocompatible PDS. Other studies (21,22,24,26,28,30,43,44) showed equivocal or increasing UF volume with biocompatible PDS group. The better preservation of RRF is less likely related to hypervolemia.
In conclusion, the present SR shows that the new biocompatible PDS preserves and improves RRF for long-term use compared to conventional PDS, and suggests these beneficial effects of the new PDS could be translated into a better long-term clinical outcome.
Disclosures
The authors have no conflicts of interest to disclose.
Supplementary Material
Acknowledgments
This work was supported by the MEST through the National Research Foundation of Korea (2012 R1A2A1A03006092 to H Ha) and Korea Healthcare Technology R&D Project, Ministry of Health and Welfare, Republic of Korea (A102065 to Y-L Kim).
We thank Ms. Su-Hyun Kim, a reference librarian in the central library, Ewha Womans University, for her advice on searching the terms in this systematic review.
The results presented in this paper have not been published previously in whole or in part, except in abstract form for scientific meetings.
Footnotes
Supplemental material available at www.pdiconnect.com
References
- 1. Wang AY, Woo J, Wang M, Sea MM, Sanderson JE, Lui SF, et al. Important differentiation of factors that predict outcome in peritoneal dialysis patients with different degrees of residual renal function. Nephrol Dial Transplant 2005; 20:396–403. [DOI] [PubMed] [Google Scholar]
- 2. Bargman JM, Thorpe KE, Churchill DN. Relative contribution of residual renal function and peritoneal clearance to adequacy of dialysis: a reanalysis of the CANUSA study. J Am Soc Nephrol 2001; 12:2158–62. [DOI] [PubMed] [Google Scholar]
- 3. Termorshuizen F, Korevaar JC, Dekker FW, van Manen JG, Boeschoten EW, Krediet RT. The relative importance of residual renal function compared with peritoneal clearance for patient survival and quality of life: an analysis of the Netherlands Cooperative Study on the Adequacy of Dialysis (NECOSAD)-2. Am J Kidney Dis 2003; 41:1293–302. [DOI] [PubMed] [Google Scholar]
- 4. Diaz-Buxo JA, Lowrie EG, Lew NL, Zhang SM, Zhu X, Lazarus JM. Associates of mortality among peritoneal dialysis patients with special reference to peritoneal transport rates and solute clearance. Am J Kidney Dis 1999; 33:523–34. [DOI] [PubMed] [Google Scholar]
- 5. Han SH, Ahn SV, Yun JY, Tranaeus A, Han DS. Mortality and technique failure in peritoneal dialysis patients using advanced peritoneal dialysis solutions. Am J Kidney Dis 2009; 54:711–20. [DOI] [PubMed] [Google Scholar]
- 6. Rocco M, Soucie JM, Pastan S, McClellan WM. Peritoneal dialysis adequacy and risk of death. Kidney Int 2000; 58:446–57. [DOI] [PubMed] [Google Scholar]
- 7. Liao CT, Chen YM, Shiao CC, Hu FC, Huang JW, Kao TW, et al. Rate of decline of residual renal function is associated with all-cause mortality and technique failure in patients on long-term peritoneal dialysis. Nephrol Dial Transplant 2009; 24:2909–14. [DOI] [PubMed] [Google Scholar]
- 8. Müller-krebs S, Kihm LP, Zeier B, Gross ML, Wieslander A, Haug U, et al. Glucose degradation products result in cardiovascular toxicity in a rat model of renal failure. Perit Dial Int 2010; 30:35–40. [DOI] [PubMed] [Google Scholar]
- 9. Schwenger V, Morath C, Salava A, Amann K, Seregin Y, Deppisch R, et al. Damage to the peritoneal membrane by glucose degradation products is mediated by the receptor for advanced glycation end-products. J Am Soc Nephrol 2006; 17:199–207. [DOI] [PubMed] [Google Scholar]
- 10. Welten AG, Schalkwijk CG, ter Wee PM, Meijer S, van den Born J, Beelen RJ. Single exposure of mesothelial cells to glucose degradation products (GDPs) yields early advanced glycation end-products (AGEs) and a proinflammatory response. Perit Dial Int 2003; 23:213–21. [PubMed] [Google Scholar]
- 11. Erixon M, Wieslander A, Lindén T, Carlsson O, Jönsson JA, Simonsen O, et al. 3,4-dideoxyglucosone-3-ene in peritoneal dialysis fluids infused into the peritoneal cavity cannot be found in plasma. Perit Dial Int 2009; 29(Suppl 2):28–31. [PubMed] [Google Scholar]
- 12. Witowski J, Korybalska K, Wisniewska J, Breborowicz A, Gahl GM, Frei U, et al. Effect of glucose degradation products on human peritoneal mesothelial cell function. J Am Soc Nephrol 2000; 11:729–39. [DOI] [PubMed] [Google Scholar]
- 13. Chetyrkin SV, Zhang W, Hudson BG, Serianni AS, Voziyan PA. Pyridoxamine protects proteins from functional damage by 3-deoxyglucosone: mechanism of action of pyridoxamine. Biochemistry 2008; 47:997–1006. [DOI] [PubMed] [Google Scholar]
- 14. Lee HK, Seo IA, Suh DJ, Lee HJ, Park HT. A novel mechanism of methylglyoxal cytotoxicity in neuroglial cells. J Neurochem 2009; 108:273–84. [DOI] [PubMed] [Google Scholar]
- 15. Catalan MP, Santamaría B, Reyero A, Ortiz A, Egido J, Ortiz A. 3,4-di-deoxyglucosone-3-ene promotes leukocyte apoptosis. Kidney Int 2005; 68:1303–11. [DOI] [PubMed] [Google Scholar]
- 16. Justo P, Sanz AB, Egido J, Ortiz A. 3,4-dideoxyglucosone-3-ene induces apoptosis in renal tubular epithelial cells. Diabetes 2005; 54:2424–9. [DOI] [PubMed] [Google Scholar]
- 17. Linden T, Cohen A, Deppisch R, Kjellstrand P, Wieslander A. 3,4-Dideoxyglucosone-3-ene (3,4-DGE): a cytotoxic glucose degradation product in fluids for peritoneal dialysis. Kidney Int 2002; 62:697–703. [DOI] [PubMed] [Google Scholar]
- 18. Ikehara O, Nishimura H, Naito T, Higuchi C, Sanaka T. Effects of neutral pH and reduced glucose degradation products in a new peritoneal dialysis solution on morphology of peritoneal membrane in rats. Nephron Exp Nephrol 2005; 100:e30–9. [DOI] [PubMed] [Google Scholar]
- 19. Tomo T. Peritoneal dialysis solutions low in glucose degradation products-evidence for clinical benefits. Perit Dial Int 2008; 28(Suppl 3):123–7. [PubMed] [Google Scholar]
- 20. Jones S, Holmes CJ, Krediet RT, Mackenzie R, Faict D, Tranaeus A, et al. Bicarbonate/lactate-based peritoneal dialysis solution increases cancer antigen 125 and decreases hyaluronic acid levels. Kidney Int 2001; 59:1529–38. [DOI] [PubMed] [Google Scholar]
- 21. Szeto CC, Chow KM, Lam CW, Leung CB, Kwan BC, Chung KY, et al. Clinical biocompatibility of a neutral peritoneal dialysis solution with minimal glucose-degradation products—A 1-year randomized control trial. Nephrol Dial Transplant 2007; 22:552–9. [DOI] [PubMed] [Google Scholar]
- 22. Williams JD, Topley N, Craig KJ, Mackenzie RK, Pischetsrieder M, Lage C, et al. The Euro-Balance Trial: the effect of a new biocompatible peritoneal dialysis fluid (balance) on the peritoneal membrane. Kidney Int 2004; 66:408–18. [DOI] [PubMed] [Google Scholar]
- 23. Park SH, Do JY, Kim YH, Lee HY, Kim BS, Shin SK, et al. Effects of neutral pH and low-glucose degradation product-containing peritoneal dialysis fluid on systemic markers of inflammation and endothelial dysfunction: a randomized controlled 1-year follow-up study. Nephrol Dial Transplant 2012; 27:1191–9. [DOI] [PubMed] [Google Scholar]
- 24. Haag-Weber M, Krämer R, Haake R, Islam MS, Prischl F, Haug U, et al. Low-GDP fluid (Gambrosol trio®) attenuates decline of residual renal function in PD patients: a prospective randomized study. Nephrol Dial Transplant 2010; 25:2288–96. [DOI] [PubMed] [Google Scholar]
- 25. Kim S, Oh J, Kim S, Chung W, Ahn C, Kim SG, et al. Benefits of biocompatible PD fluid for preservation of residual renal function in incident CAPD patients: a 1-year study. Nephrol Dial Transplant 2009; 24:2899–908. [DOI] [PubMed] [Google Scholar]
- 26. Choi HY, Kim DK, Lee TH, Moon SJ, Han SH, Lee JE, et al. The clinical usefulness of peritoneal dialysis fluids with neutral pH and low glucose degradation product concentration: an open randomized prospective trial. Perit Dial Int 2008; 28:174–82. [PubMed] [Google Scholar]
- 27. Fan SL, Pile T, Punzalan S, Raftery MJ, Yaqoob MM. Randomized controlled study of biocompatible peritoneal dialysis solutions: effect on residual renal function. Kidney Int 2008; 73:200–6. [DOI] [PubMed] [Google Scholar]
- 28. Kim S, Oh KH, Oh J, Kim SJ, Chung W, Song YR, et al. Biocompatible peritoneal dialysis solution preserves residual renal function. American Journal of Nephrology 2012; 36:305–16. [DOI] [PubMed] [Google Scholar]
- 29. Lai KN, Lam MF, Leung JC, Chan LY, Lam CW, Chan IH, et al. A study of the clinical and biochemical profile of peritoneal dialysis fluid low in glucose degradation products. Perit Dial Int 2012; 32:280–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Johnson DW, Brown FG, Clarke M, Boudville N, Elias TJ, Foo MW, et al. Effects of biocompatible versus standard fluid on peritoneal dialysis outcomes. J Am Soc Nephrol 2012; 23:1097–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Theodoridis M, Tziakas D, Passadakis P, Kantartzi K, Roumeliotis A, Thodis E, et al. The effect of bicarbonate peritoneal dialysis solutions on cardiac structural and functional alterations. Nephrol Dial Transplant 2011; 26:4061–7. [DOI] [PubMed] [Google Scholar]
- 32. Lee HY, Park HC, Seo BJ, Do JY, Yun SR, Song HY, et al. Superior patient survival for continuous ambulatory peritoneal dialysis patients treated with a peritoneal dialysis fluid with neutral pH and low glucose degradation product concentration (Balance). Perit Dial Int 2005; 25:248–55. [PubMed] [Google Scholar]
- 33. Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0., The Cochrane Collaboration, 2011. [Available at: http://www.cochrane-handbook.org]
- 34. Kim SG, Kim S, Hwang YH, Kim K, Oh JE, Chung W, et al. Could solutions low in glucose degradation products preserve residual renal function in incident peritoneal dialysis patients? A 1-year multicenter prospective randomized controlled trial (Balnet Study). Perit Dial Int 2008; 28(Suppl 3):117–22. [PubMed] [Google Scholar]
- 35. van Olden RW, Krediet RT, Struijk DG, Arisz L. Measurement of residual renal function in patients treated with continuous ambulatory peritoneal dialysis. J Am Soc Nephrol 1996; 7:745–8. [DOI] [PubMed] [Google Scholar]
- 36. le Poole CY, van Ittersum FJ, Weijmer MC, Valentijn RM, ter Wee PM. Clinical effects of a peritoneal dialysis regimen low in glucose in new peritoneal dialysis patients: a randomized crossover study. Adv Perit Dial 2004; 20:170–6. [PubMed] [Google Scholar]
- 37. Johnson DW, Clarke M, Wilson V, Woods F, Brown FG. Rationale and design of the balANZ trial: a randomised controlled trial of low GDP, neutral pH versus standard peritoneal dialysis solution for the preservation of residual renal function. BMC Nephrol 2010; 11:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tom P. Effect of low glucose degradation product peritoneal dialysis solution Gambrosol-Trio on residual renal function in patients receiving peritoneal dialysis - a randomized controlled trial. Cochrane Database Syst Rev. [Available at: http://www.thecochranelibrary.com]
- 39. Brown F, Johnson DW. A randomized controlled trial to determine whether treatment with a neutral pH, low glucose degradation product dialysate (balance) prolongs residual renal function in peritoneal dialysis patients. Perit Dial Int 2006; 26:112–3. [PubMed] [Google Scholar]
- 40. Woodrow G. A randomized controlled trial to determine whether treatment with a neutral pH, low glucose degradation product dialysate (balance) prolongs residual renal function in peritoneal dialysis patients. Perit Dial Int 2006; 26:113–4. [PubMed] [Google Scholar]
- 41. Lui SL, Yung S, Yim A, Wong KM, Tong KL, Wong KS, et al. A combination of biocompatible peritoneal dialysis solutions and residual renal function, peritoneal transport, and inflammation markers: a randomized clinical trial. Am J Kidney Dis 2012; 60:966–75. [DOI] [PubMed] [Google Scholar]
- 42. Weiss L, Stegmayr B, Malmsten G, Tejde M, Hadimeri H, Siegert CE, et al. Biocompatibility and tolerability of a purely bicarbonate-buffered peritoneal dialysis solution. Perit Dial Int 2009; 29:647–55. [PubMed] [Google Scholar]
- 43. Bajo MA, Pérez-Lozano ML, Albar-Vizcaino P, Peso G, Castro MJ, Gonzalez-Mateo G, et al. Low-GDP peritoneal dialysis fluid (’balance’) has less impact in vitro and ex vivo on epithelial-to-mesenchymal transition (EMT) of mesothelial cells than a standard fluid. Nephrol Dial Transplant 2011; 26:282–91. [DOI] [PubMed] [Google Scholar]
- 44. Cho KH, Do JY, Park JW, Yoon KW, Kim YL. The effect of low-GDP solution on ultrafiltration and solute transport in continuous ambulatory peritoneal dialysis patients. Perit Dial Int 2013; 33:382–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cho Y, Johnson DW, Badve SV, Craig JC, Strippoli GF, Wiggins KJ. The impact of neutral-pH peritoneal dialysates with reduced glucose degradation products on clinical outcomes in peritoneal dialysis patients. Kidney Int 2013; 84:969–79. [DOI] [PubMed] [Google Scholar]
- 46. Locatelli F, La Milia V. Preservation of residual renal function in peritoneal dialysis patients: still a dream? Kidney Int 2008; 73:143–5. [DOI] [PubMed] [Google Scholar]
- 47. Davies SJ. Preserving residual renal function in peritoneal dialysis: volume or biocompatibility? Nephrol Dial Transplant 2009; 24:2620–2. [DOI] [PubMed] [Google Scholar]
- 48. Blake PG, Jain AK, Yohann S. Biocompatible peritoneal dialysis solutions: many questions but few answers. Kidney Int 2013; 84:864–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.