Abstract
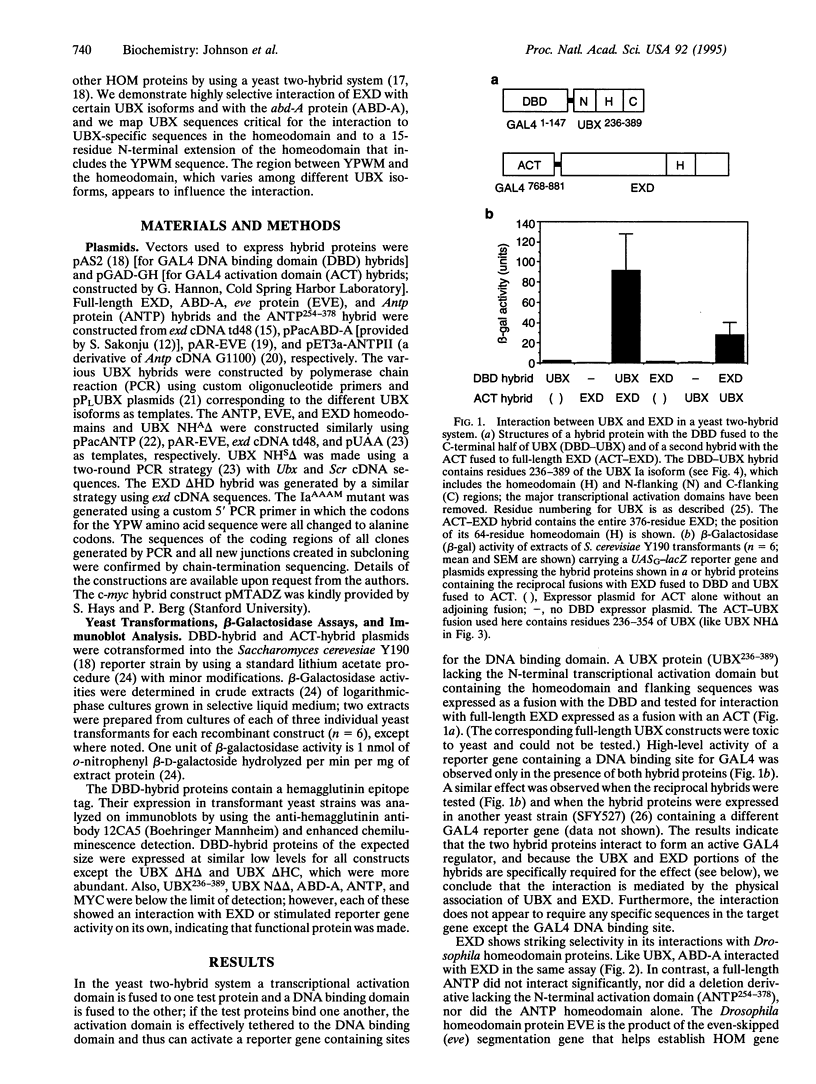
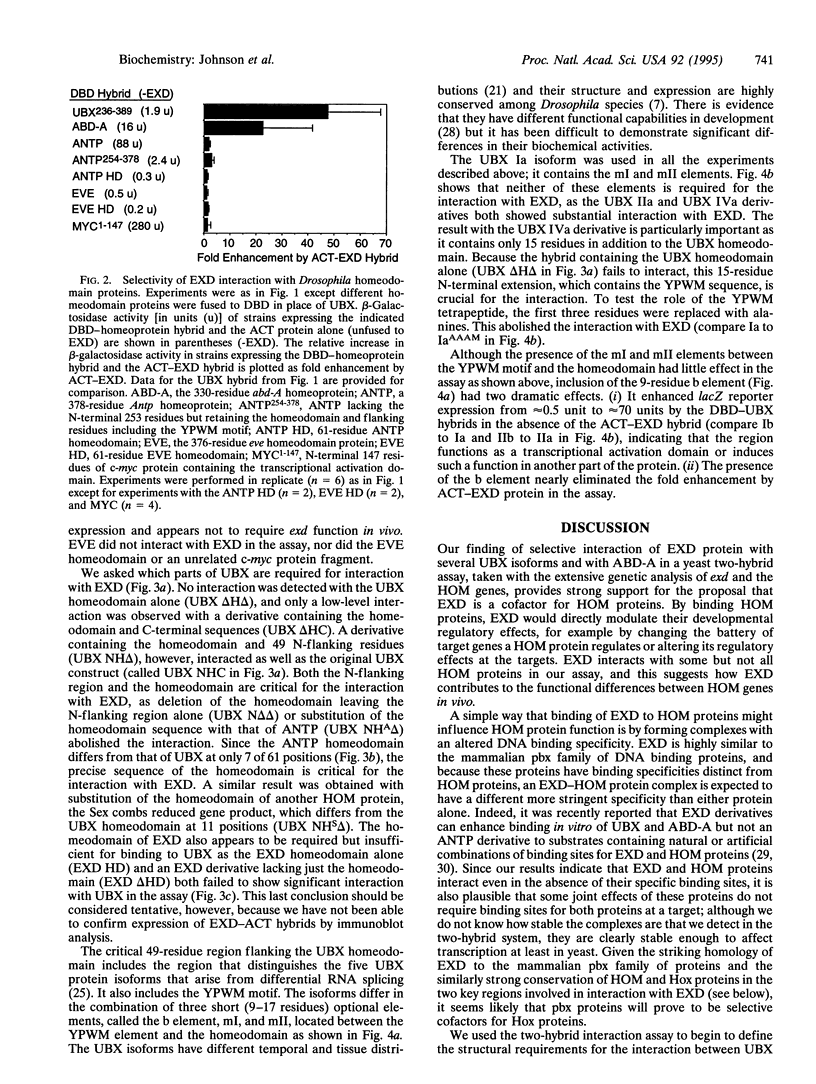
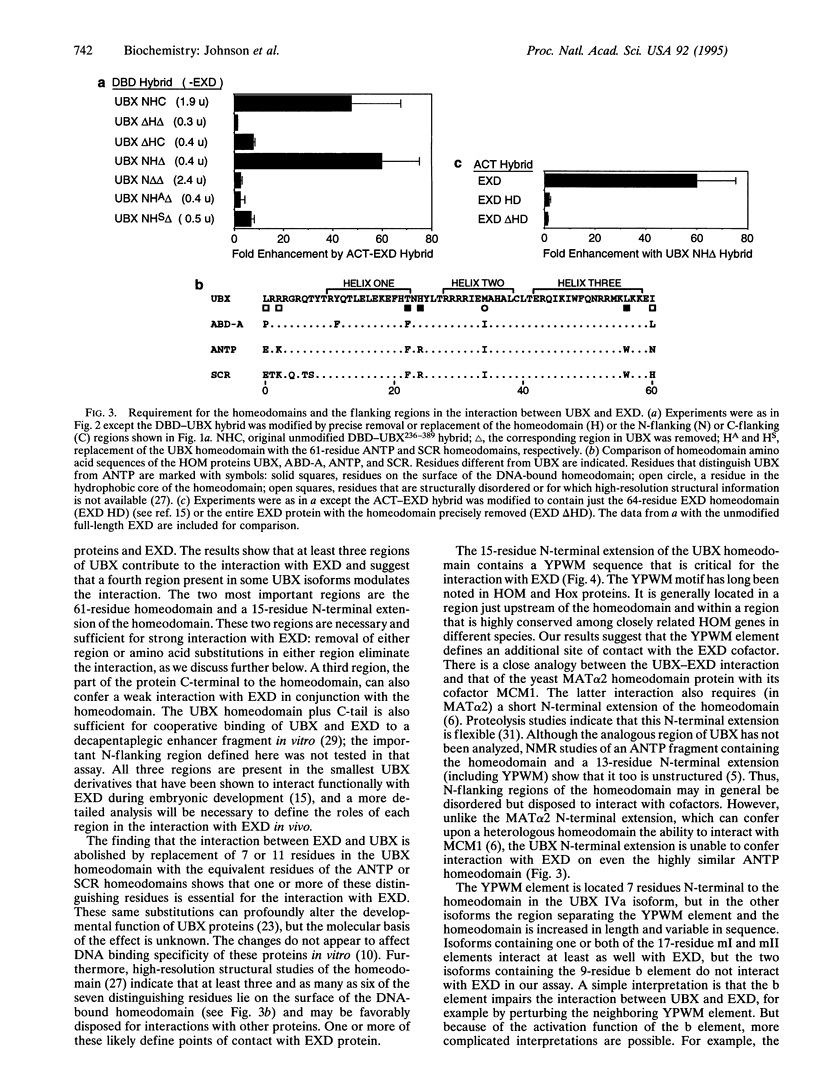
The Drosophila homeotic selector (HOM) genes encode a family of DNA binding transcription factors that specify developmental fates of different body segments by differentially regulating the activity of downstream target genes. A central question is how the HOM proteins achieve their developmental specificity despite the very similar DNA binding specificities of isolated HOM proteins in vitro. Specificity could be achieved by differential interactions with protein cofactors. The extradenticle gene might encode such a cofactor since it interacts genetically in parallel with Ultrabithorax, abdominal-A, and perhaps other HOM genes. By using a yeast two-hybrid system, we demonstrate selective interaction of the extradenticle homeodomain protein with certain Ultrabithorax and abdominal-A proteins but not with an Antennapedia protein or a more distant homeodomain protein. Strong interaction with Ultrabithorax proteins requires only the Ultrabithorax homeodomain and a 15-residue N-terminal extension that includes Tyr-Pro-Trp-Met (YPWM), a tetrapeptide motif found near the homeodomain in most HOM proteins and their mammalian Hox counterparts. The size and sequence of the region between the YPWM element and the homeodomain differ among Ultrabithorax isoforms, and this variable region appears to affect the interaction detected in the assay.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appel B., Sakonju S. Cell-type-specific mechanisms of transcriptional repression by the homeotic gene products UBX and ABD-A in Drosophila embryos. EMBO J. 1993 Mar;12(3):1099–1109. doi: 10.1002/j.1460-2075.1993.tb05751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel P., Chien C. T., Sternglanz R., Fields S. Elimination of false positives that arise in using the two-hybrid system. Biotechniques. 1993 Jun;14(6):920–924. [PubMed] [Google Scholar]
- Bomze H. M., López A. J. Evolutionary conservation of the structure and expression of alternatively spliced Ultrabithorax isoforms from Drosophila. Genetics. 1994 Mar;136(3):965–977. doi: 10.1093/genetics/136.3.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botas J. Control of morphogenesis and differentiation by HOM/Hox genes. Curr Opin Cell Biol. 1993 Dec;5(6):1015–1022. doi: 10.1016/0955-0674(93)90086-6. [DOI] [PubMed] [Google Scholar]
- Chan S. K., Jaffe L., Capovilla M., Botas J., Mann R. S. The DNA binding specificity of Ultrabithorax is modulated by cooperative interactions with extradenticle, another homeoprotein. Cell. 1994 Aug 26;78(4):603–615. doi: 10.1016/0092-8674(94)90525-8. [DOI] [PubMed] [Google Scholar]
- Chan S. K., Mann R. S. The segment identity functions of Ultrabithorax are contained within its homeo domain and carboxy-terminal sequences. Genes Dev. 1993 May;7(5):796–811. doi: 10.1101/gad.7.5.796. [DOI] [PubMed] [Google Scholar]
- Ekker S. C., Jackson D. G., von Kessler D. P., Sun B. I., Young K. E., Beachy P. A. The degree of variation in DNA sequence recognition among four Drosophila homeotic proteins. EMBO J. 1994 Aug 1;13(15):3551–3560. doi: 10.1002/j.1460-2075.1994.tb06662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekker S. C., von Kessler D. P., Beachy P. A. Differential DNA sequence recognition is a determinant of specificity in homeotic gene action. EMBO J. 1992 Nov;11(11):4059–4072. doi: 10.1002/j.1460-2075.1992.tb05499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields S., Song O. A novel genetic system to detect protein-protein interactions. Nature. 1989 Jul 20;340(6230):245–246. doi: 10.1038/340245a0. [DOI] [PubMed] [Google Scholar]
- Gehring W. J., Qian Y. Q., Billeter M., Furukubo-Tokunaga K., Schier A. F., Resendez-Perez D., Affolter M., Otting G., Wüthrich K. Homeodomain-DNA recognition. Cell. 1994 Jul 29;78(2):211–223. doi: 10.1016/0092-8674(94)90292-5. [DOI] [PubMed] [Google Scholar]
- Goutte C., Johnson A. D. Yeast a1 and alpha 2 homeodomain proteins form a DNA-binding activity with properties distinct from those of either protein. J Mol Biol. 1993 Oct 5;233(3):359–371. doi: 10.1006/jmbi.1993.1517. [DOI] [PubMed] [Google Scholar]
- Harper J. W., Adami G. R., Wei N., Keyomarsi K., Elledge S. J. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell. 1993 Nov 19;75(4):805–816. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- Hayashi S., Scott M. P. What determines the specificity of action of Drosophila homeodomain proteins? Cell. 1990 Nov 30;63(5):883–894. doi: 10.1016/0092-8674(90)90492-w. [DOI] [PubMed] [Google Scholar]
- Hoey T., Warrior R., Manak J., Levine M. DNA-binding activities of the Drosophila melanogaster even-skipped protein are mediated by its homeo domain and influenced by protein context. Mol Cell Biol. 1988 Nov;8(11):4598–4607. doi: 10.1128/mcb.8.11.4598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissinger C. R., Liu B. S., Martin-Blanco E., Kornberg T. B., Pabo C. O. Crystal structure of an engrailed homeodomain-DNA complex at 2.8 A resolution: a framework for understanding homeodomain-DNA interactions. Cell. 1990 Nov 2;63(3):579–590. doi: 10.1016/0092-8674(90)90453-l. [DOI] [PubMed] [Google Scholar]
- Kornfeld K., Saint R. B., Beachy P. A., Harte P. J., Peattie D. A., Hogness D. S. Structure and expression of a family of Ultrabithorax mRNAs generated by alternative splicing and polyadenylation in Drosophila. Genes Dev. 1989 Feb;3(2):243–258. doi: 10.1101/gad.3.2.243. [DOI] [PubMed] [Google Scholar]
- Laughon A., Boulet A. M., Bermingham J. R., Jr, Laymon R. A., Scott M. P. Structure of transcripts from the homeotic Antennapedia gene of Drosophila melanogaster: two promoters control the major protein-coding region. Mol Cell Biol. 1986 Dec;6(12):4676–4689. doi: 10.1128/mcb.6.12.4676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez A. J., Hogness D. S. Immunochemical dissection of the Ultrabithorax homeoprotein family in Drosophila melanogaster. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):9924–9928. doi: 10.1073/pnas.88.22.9924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann R. S., Hogness D. S. Functional dissection of Ultrabithorax proteins in D. melanogaster. Cell. 1990 Feb 23;60(4):597–610. doi: 10.1016/0092-8674(90)90663-y. [DOI] [PubMed] [Google Scholar]
- McGinnis W., Krumlauf R. Homeobox genes and axial patterning. Cell. 1992 Jan 24;68(2):283–302. doi: 10.1016/0092-8674(92)90471-n. [DOI] [PubMed] [Google Scholar]
- Peifer M., Wieschaus E. Mutations in the Drosophila gene extradenticle affect the way specific homeo domain proteins regulate segmental identity. Genes Dev. 1990 Jul;4(7):1209–1223. doi: 10.1101/gad.4.7.1209. [DOI] [PubMed] [Google Scholar]
- Qian Y. Q., Otting G., Furukubo-Tokunaga K., Affolter M., Gehring W. J., Wüthrich K. NMR structure determination reveals that the homeodomain is connected through a flexible linker to the main body in the Drosophila Antennapedia protein. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):10738–10742. doi: 10.1073/pnas.89.22.10738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauskolb C., Peifer M., Wieschaus E. extradenticle, a regulator of homeotic gene activity, is a homolog of the homeobox-containing human proto-oncogene pbx1. Cell. 1993 Sep 24;74(6):1101–1112. doi: 10.1016/0092-8674(93)90731-5. [DOI] [PubMed] [Google Scholar]
- Rauskolb C., Wieschaus E. Coordinate regulation of downstream genes by extradenticle and the homeotic selector proteins. EMBO J. 1994 Aug 1;13(15):3561–3569. doi: 10.1002/j.1460-2075.1994.tb06663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saffman E. E., Krasnow M. A. A differential response element for the homeotics at the Antennapedia P1 promoter of Drosophila. Proc Natl Acad Sci U S A. 1994 Aug 2;91(16):7420–7424. doi: 10.1073/pnas.91.16.7420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer R. T., Smith D. L., Johnson A. D. Flexibility of the yeast alpha 2 repressor enables it to occupy the ends of its operator, leaving the center free. Genes Dev. 1988 Jul;2(7):807–816. doi: 10.1101/gad.2.7.807. [DOI] [PubMed] [Google Scholar]
- Vershon A. K., Johnson A. D. A short, disordered protein region mediates interactions between the homeodomain of the yeast alpha 2 protein and the MCM1 protein. Cell. 1993 Jan 15;72(1):105–112. doi: 10.1016/0092-8674(93)90054-t. [DOI] [PubMed] [Google Scholar]
- Winslow G. M., Hayashi S., Krasnow M., Hogness D. S., Scott M. P. Transcriptional activation by the Antennapedia and fushi tarazu proteins in cultured Drosophila cells. Cell. 1989 Jun 16;57(6):1017–1030. doi: 10.1016/0092-8674(89)90340-1. [DOI] [PubMed] [Google Scholar]
- van Dijk M. A., Murre C. extradenticle raises the DNA binding specificity of homeotic selector gene products. Cell. 1994 Aug 26;78(4):617–624. doi: 10.1016/0092-8674(94)90526-6. [DOI] [PubMed] [Google Scholar]



