Abstract
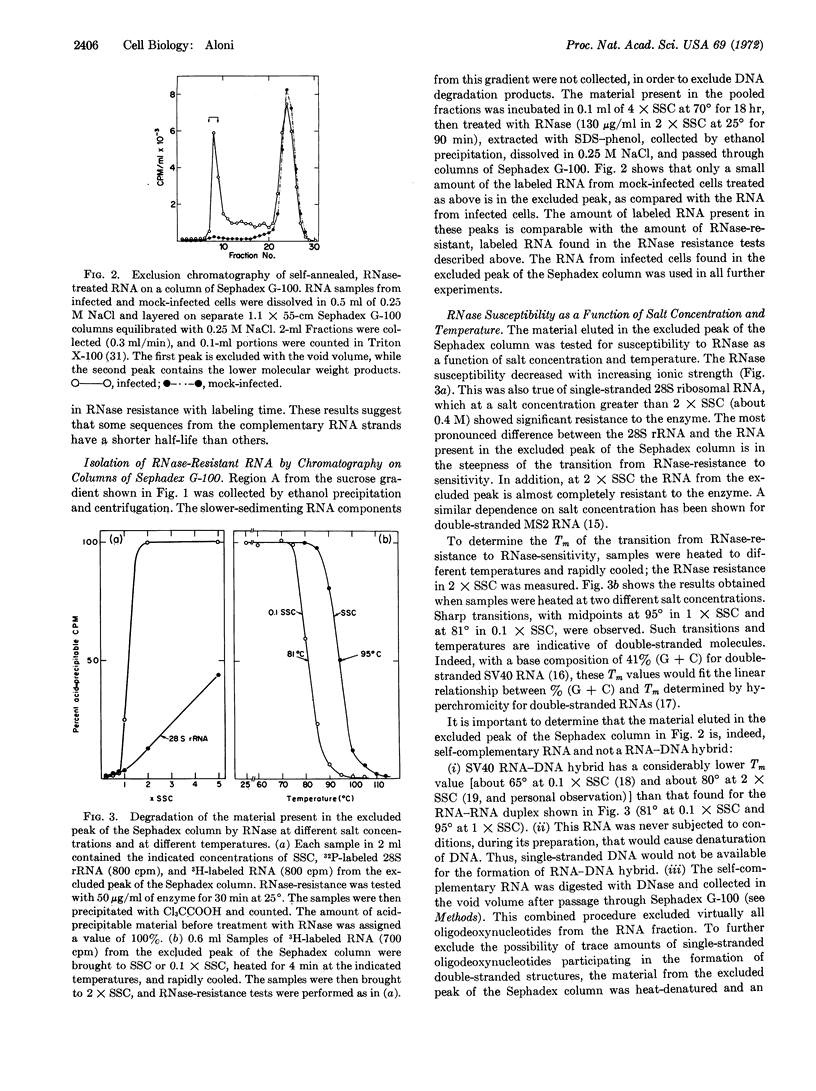
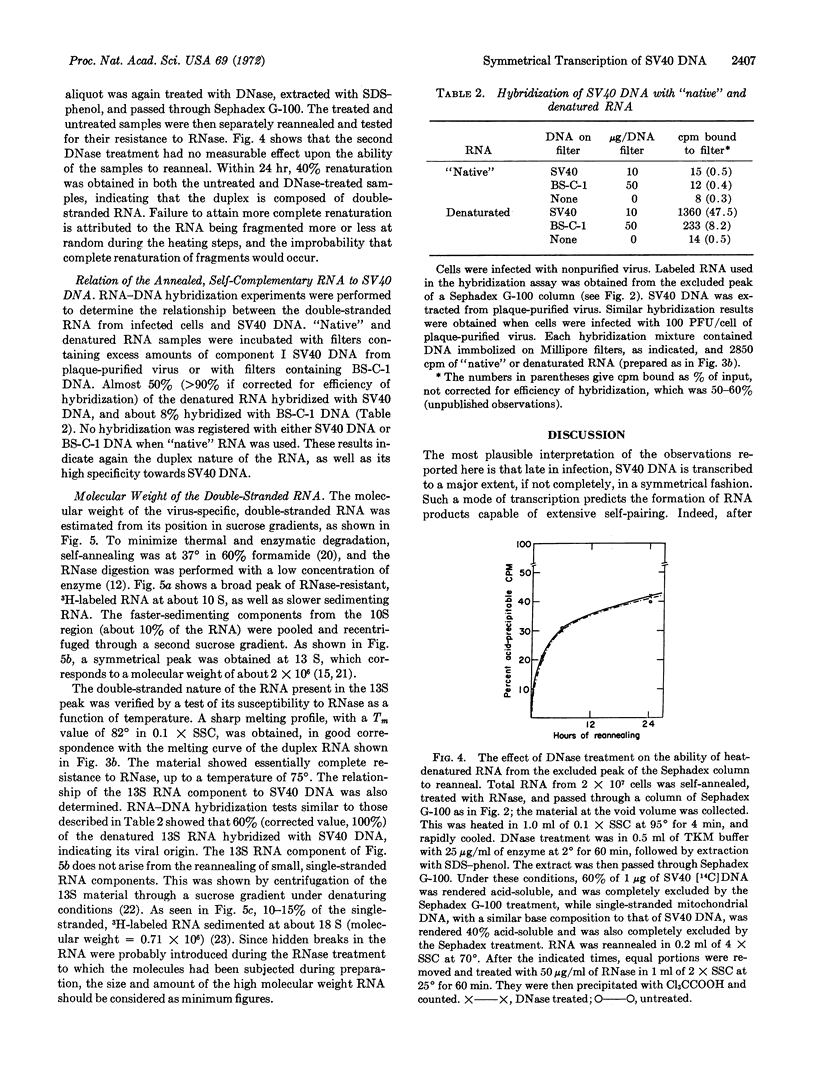
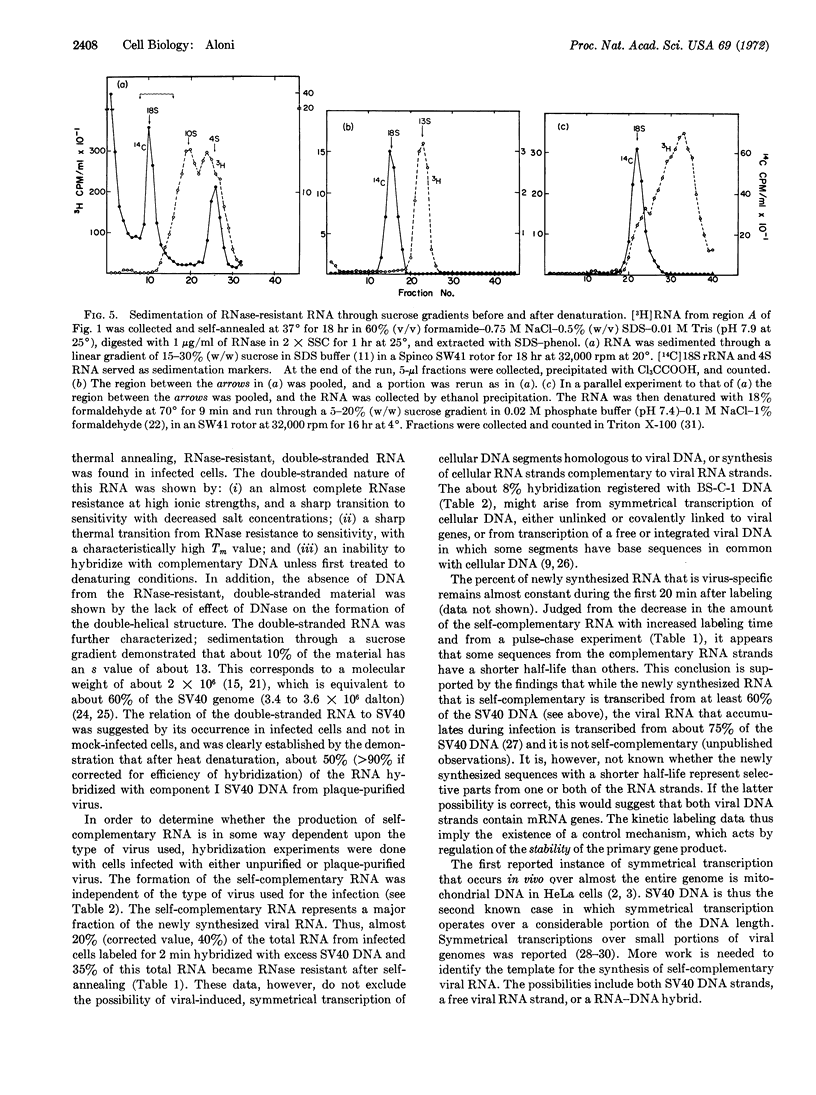
Rapidly labeled RNA was extracted from monkey cells after infection with Simian Virus 40 (SV40) and exposure to short pulses of [5-3H]uridine late in infection. When this RNA was self-annealed, it became resistant to digestion with ribonuclease. The fraction of RNA that resisted the ribonuclease treatment decreased with increased labeling time, or when a short pulse of radioactivity was followed by incubation with unlabeled uridine and actinomycin D. The RNase-resistant RNA was isolated by chromatography on Sephadex G-100 and shown to be double-stranded by its susceptibility to ribonuclease as a function of salt concentration and temperature. This behavior was not due to RNA-DNA hybrid formation, since deoxyribonuclease had no effect upon the double-stranded molecules, even after their denaturation. The relation of the double-stranded RNA to SV40 was demonstrated by the hybridization of about 50% (corrected value, >90%) of the separated RNA strands with component I of SV40 DNA from plaque-purified virus. After self-annealing in formamide at low temperature, about 10% of the rapidly labeled, viral RNA sedimented at 13 S. This value corresponds in size to about 60% of the SV40 DNA.
These observations indicate that late in infection of monkey cells, SV40 DNA is transcribed symmetrically over a considerable portion of its length, and that subsequently some sequences from one or both of the RNA strands are degraded.
Keywords: SV40, monkey cells, actinomycin D, RNase, hybridization
Full text
PDF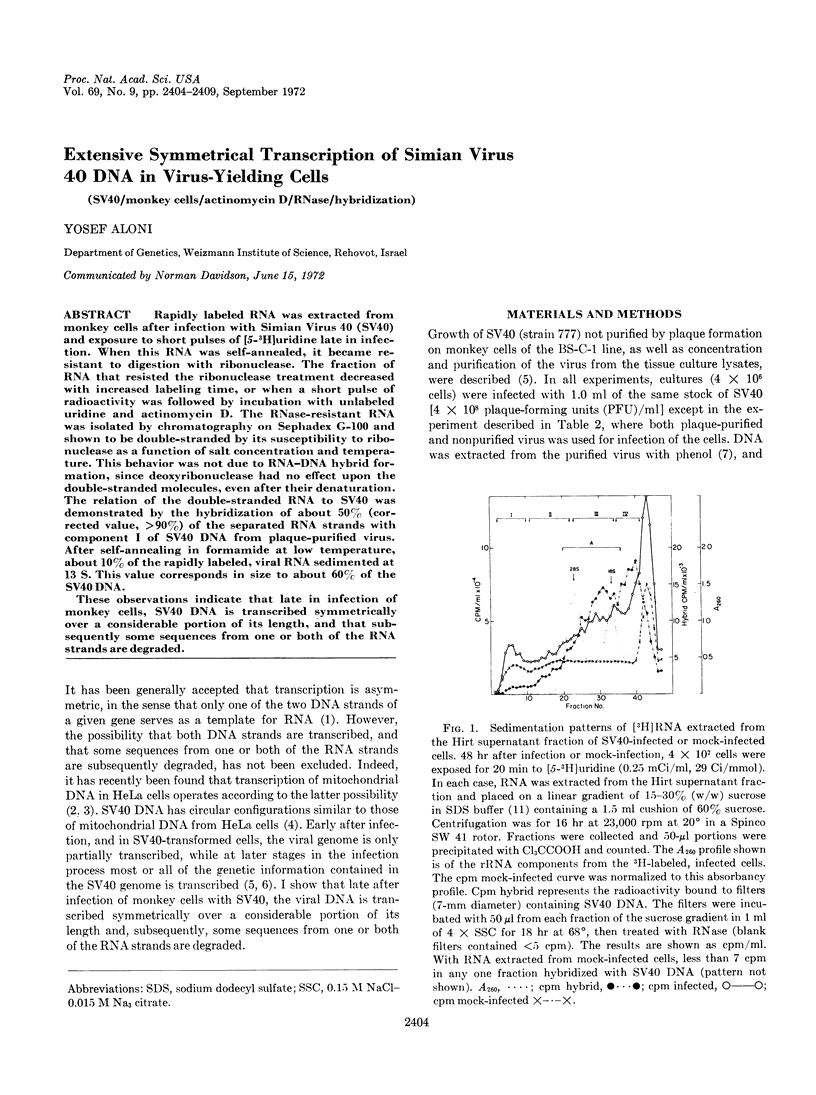


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aloni Y., Attardi G. Symmetrical in vivo transcription of mitochondrial DNA in HeLa cells. Proc Natl Acad Sci U S A. 1971 Aug;68(8):1757–1761. doi: 10.1073/pnas.68.8.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aloni Y., Winocour E., Sachs L. Characterization of the simian virus 40-specific RNA in virus-yielding and transformed cells. J Mol Biol. 1968 Feb 14;31(3):415–429. doi: 10.1016/0022-2836(68)90418-x. [DOI] [PubMed] [Google Scholar]
- Aloni Y., Winocour E., Sachs L., Torten J. Hybridization between SV40 DNA and cellular DNA's. J Mol Biol. 1969 Sep 14;44(2):333–345. doi: 10.1016/0022-2836(69)90179-x. [DOI] [PubMed] [Google Scholar]
- Billeter M. A., Weissmann C., Warner R. C. Replication of viral ribonucleic acid. IX. Properties of double-stranded RNA from Escherichia coli infected with bacteriophage MS2. J Mol Biol. 1966 May;17(1):145–173. doi: 10.1016/s0022-2836(66)80101-8. [DOI] [PubMed] [Google Scholar]
- Blume A., Gilbert F., Wilson S., Farber J., Rosenberg R., Nirenberg M. Regulation of acetylcholinesterase in neuroblastoma cells. Proc Natl Acad Sci U S A. 1970 Oct;67(2):786–792. doi: 10.1073/pnas.67.2.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bovre K., Szybalski W. Patterns of convergent and overlapping transcription within the b2 region of coliphage lambda. Virology. 1969 Aug;38(4):614–626. doi: 10.1016/0042-6822(69)90181-0. [DOI] [PubMed] [Google Scholar]
- Colby C., Duesberg P. H. Double-stranded RNA in vaccinia virus infected cells. Nature. 1969 Jun 7;222(5197):940–944. doi: 10.1038/222940a0. [DOI] [PubMed] [Google Scholar]
- Hirai K., Lehman J., Defendi V. Integration of simian virus 40 deoxyribonucleic acid into the deoxyribonucleic acid of primary infected Chinese hamster cells. J Virol. 1971 Nov;8(5):708–715. doi: 10.1128/jvi.8.5.708-715.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Kallenbach N. R. Theory of thermal transitions in low molecular weight RNA chains. J Mol Biol. 1968 Nov 14;37(3):445–466. doi: 10.1016/0022-2836(68)90114-9. [DOI] [PubMed] [Google Scholar]
- Lavi S., Winocour E. Acquisition of sequences homologous to host deoxyribonucleic acid by closed circular simian virus 40 deoxyribonucleic acid. J Virol. 1972 Feb;9(2):309–316. doi: 10.1128/jvi.9.2.309-316.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConaughy B. L., Laird C. D., McCarthy B. J. Nucleic acid reassociation in formamide. Biochemistry. 1969 Aug;8(8):3289–3295. doi: 10.1021/bi00836a024. [DOI] [PubMed] [Google Scholar]
- McConkey E. H., Hopkins J. W. Molecular weights of some HeLa ribosomal RNA's. J Mol Biol. 1969 Feb 14;39(3):545–550. doi: 10.1016/0022-2836(69)90144-2. [DOI] [PubMed] [Google Scholar]
- Morrison J. M., Keir H. M., Subak-Sharpe H., Crawford L. V. Nearest neighbour base sequence analysis of the deoxyribonucleic acids of a further three mammalian viruses: Simian virus 40, human papilloma virus and adenovirus type 2. J Gen Virol. 1967 Jan;1(1):101–108. doi: 10.1099/0022-1317-1-1-101. [DOI] [PubMed] [Google Scholar]
- Oda K., Dulbecco R. Induction of cellular mRNA synthesis in BSC-1 cells infected by SV40. Virology. 1968 Jul;35(3):439–444. doi: 10.1016/0042-6822(68)90222-5. [DOI] [PubMed] [Google Scholar]
- Oda K., Dulbecco R. Regulation of transcription of the SV40 DNA in productively infected and in transformed cells. Proc Natl Acad Sci U S A. 1968 Jun;60(2):525–532. doi: 10.1073/pnas.60.2.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff R., Bauer W., Vinograd J. A dye-buoyant-density method for the detection and isolation of closed circular duplex DNA: the closed circular DNA in HeLa cells. Proc Natl Acad Sci U S A. 1967 May;57(5):1514–1521. doi: 10.1073/pnas.57.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robberson D., Aloni Y., Attardi G., Davidson N. Expression of the mitochondrial genome in HeLa cells. 8. The relative position of ribosomal RNA genes in mitochondrial DNA. J Mol Biol. 1972 Feb 28;64(1):313–317. doi: 10.1016/0022-2836(72)90340-3. [DOI] [PubMed] [Google Scholar]
- Robberson D., Aloni Y., Attardi G., Davidson N. Expression of the mitochondrial genome in HeLa cells. VI. Size determination of mitochondrial ribosomal RNA by electron microscopy. J Mol Biol. 1971 Sep 28;60(3):473–484. doi: 10.1016/0022-2836(71)90182-3. [DOI] [PubMed] [Google Scholar]
- Robberson D., Aloni Y., Attardi G. Electron microscopic visualization of mitochondrial RNA-DNA hybrids. J Mol Biol. 1971 Jan 28;55(2):267–270. doi: 10.1016/0022-2836(71)90196-3. [DOI] [PubMed] [Google Scholar]
- STUDIER F. W. SEDIMENTATION STUDIES OF THE SIZE AND SHAPE OF DNA. J Mol Biol. 1965 Feb;11:373–390. doi: 10.1016/s0022-2836(65)80064-x. [DOI] [PubMed] [Google Scholar]
- Tai H. T., Smith C. A., Sharp P. A., Vinograd J. Sequence heterogeneity in closed simian virus 40 deoxyribonucleic acid. J Virol. 1972 Feb;9(2):317–325. doi: 10.1128/jvi.9.2.317-325.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinograd J., Lebowitz J., Radloff R., Watson R., Laipis P. The twisted circular form of polyoma viral DNA. Proc Natl Acad Sci U S A. 1965 May;53(5):1104–1111. doi: 10.1073/pnas.53.5.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEIL R. A quantitative assay for a subviral infective agent related to polyoma virus. Virology. 1961 May;14:46–53. doi: 10.1016/0042-6822(61)90130-1. [DOI] [PubMed] [Google Scholar]



