Abstract
Ancient genomic sequences have started revealing the origin and the demographic impact of Neolithic farmers spreading into Europe1–3. The adoption of farming, stock breeding and sedentary societies during the Neolithic may have resulted in adaptive changes in genes associated with immunity and diet4. However, the limited data available from earlier hunter-gatherers precludes an understanding of the selective processes associated with this crucial transition to agriculture in recent human evolution. By sequencing a ~7,000-year-old Mesolithic skeleton discovered at the La Braña-Arintero site in León (Spain), we retrieved the first complete pre-agricultural European human genome. Analysis of this genome in the context of other ancient samples suggests the existence of a common ancient genomic signature across Western and Central Eurasia from the Upper Paleolithic to the Mesolithic. The La Braña individual carries ancestral alleles in several skin pigmentation genes, suggesting that the light skin of modern Europeans was not yet ubiquitous in Mesolithic times. Moreover, we provide evidence that a significant number of derived, putatively adaptive variants associated with pathogen resistance in modern Europeans were already present in this hunter-gatherer. Hence, these genomic variants cannot represent novel mutations that occurred during the adaptation to the farming lifestyle.
Next-generation sequencing (NGS) technologies are revolutionizing the field of ancient DNA (aDNA), and have allowed the sequencing of complete ancient genomes5,6, such as that of Ötzi, a Neolithic human body found in the Alps1. However, very little is known of the genetic composition of earlier hunter-gatherer populations from the Mesolithic period (ca 10,000–5,000 years before present, BP, that immediately preceded the Neolithic period).
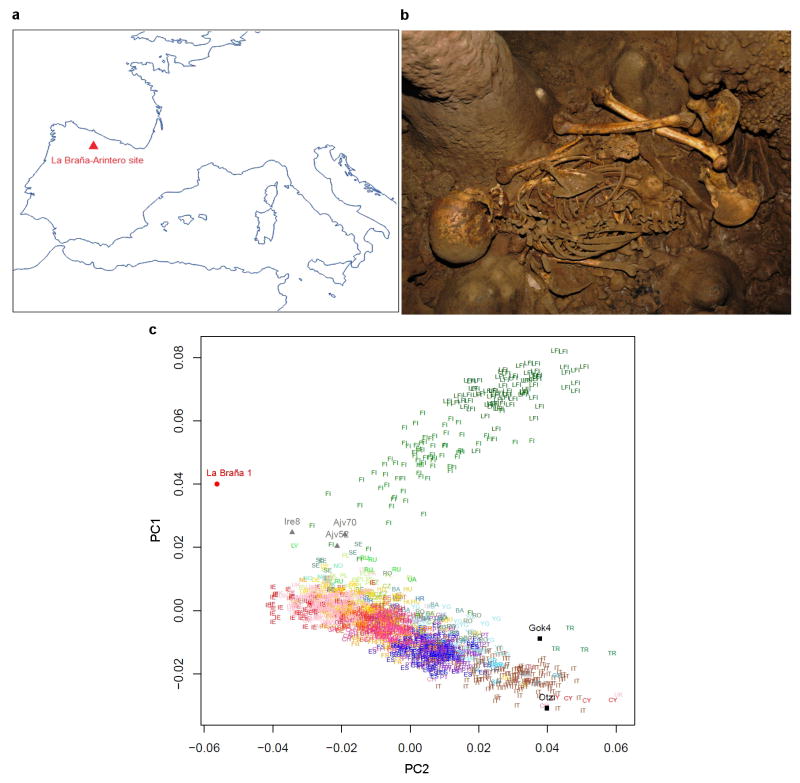
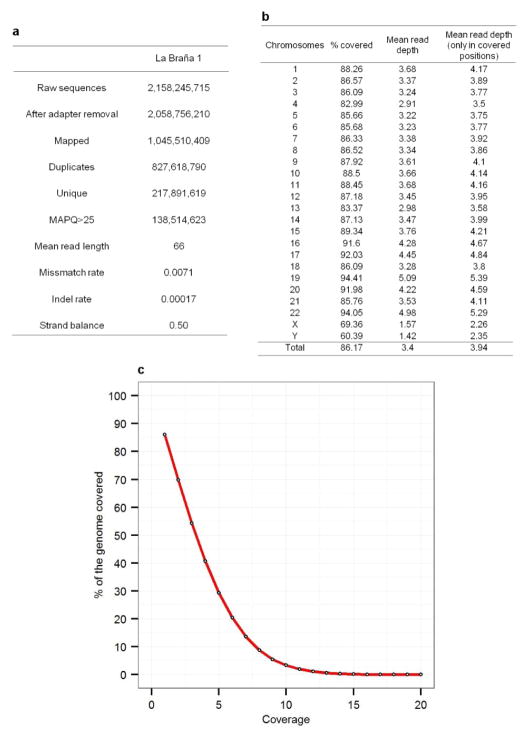
The Iberian site called La Braña-Arintero was discovered in 2006 when two male skeletons were found in a deep cave system, 1,500 meters above sea level in the Cantabrian mountain range (León, Northwestern Spain) (Fig. 1a). The skeletons were dated to ~7,000 years BP (7,940–7,690 calibrated BP)7. Because of the cold environment and stable thermal conditions in the cave, the preservation of these specimens proved to be exceptional (Fig. 1b). We identified a tooth with high human DNA content (48.4%) and sequenced this specimen to a final 3.40X effective genomic depth-of-coverage (Extended Data Fig. 1).
Figure 1. Geographic location and genetic affinities of the La Braña 1 individual.
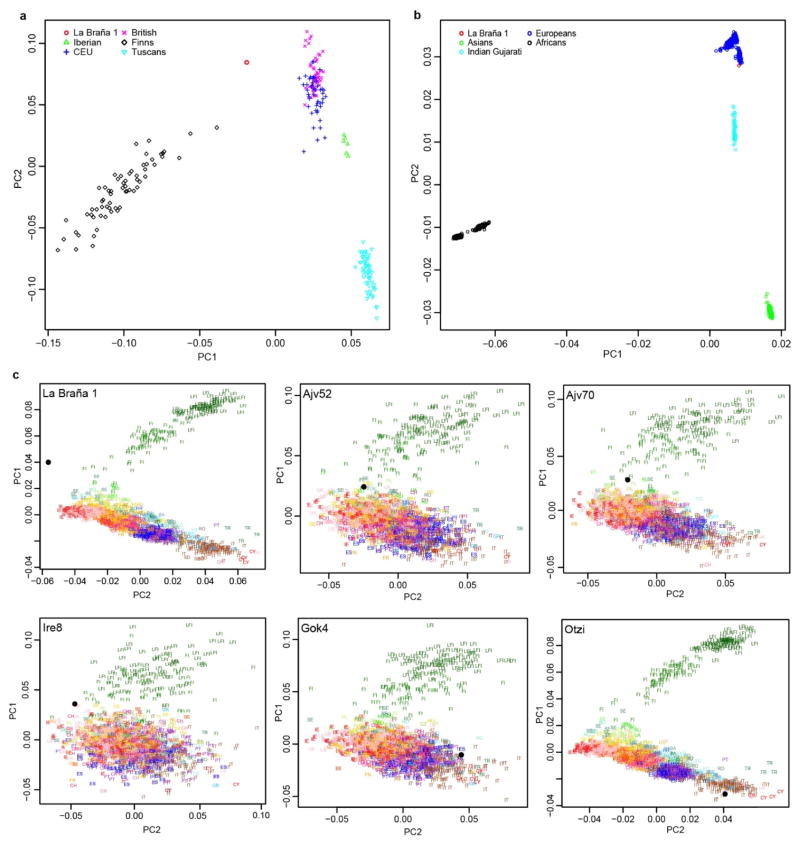
a, Location of the La Braña-Arintero site (Spain). b, The La Braña 1 skeleton as discovered in 2006. c, Principal Component Analysis (PCA) based on the average of the Procustes transformations of individual PCAs with La Barña and each of the five Neolithic samples1,3. The reference populations are the Finnish HapMap, FINHM, and POPRES. Population labels with labeling of 12 with the addition of FI (Finns) or LFI (Late-settlement Finns). Ajv70, Ajv52, Ire8 and Gok4 are Scandinavian Neolithic hunter-gatherers and a farmer, respectively3. Ötzi is the Tyrolean Ice Man1.
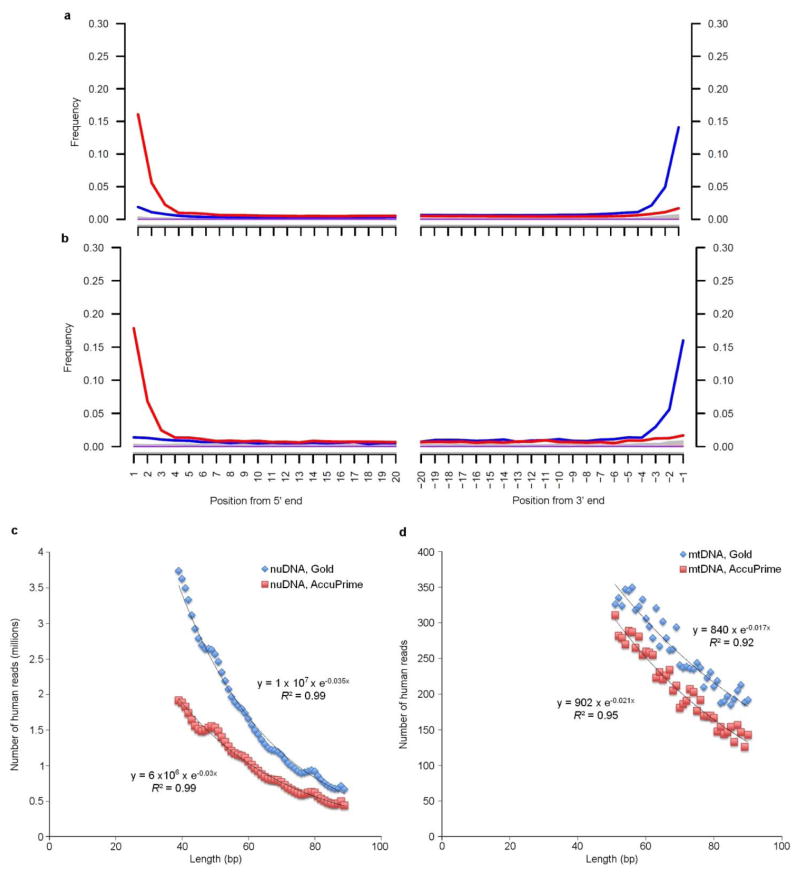
We undertook several tests to assess the authenticity of the genome sequence and to determine the amount of potential modern human contamination. First, we observed that sequence reads from both the mitochondrial DNA (mtDNA) and the nuclear DNA of La Braña 1 showed the typical ancient DNA misincorporation patterns that arise from degradation of DNA over time8 (Extended Data Fig. 2a, b). Second, we showed that the observed number of human DNA fragments was negatively correlated with the fragment length (R2 >0.92), as expected for ancient degraded DNA, and that the estimated rate of DNA decay was low and in agreement with predicted values9 (Extended Data Fig. 2c, d). We then estimated the contamination rate in the mtDNA genome, assembled to a high depth-of-coverage (91X), by checking for positions differing from the mtDNA genome (haplogroup U5b2c1) that was previously retrieved with a capture method2. We obtained an upper contamination limit of 1.69% (0.75%–2.6%, 95% CI) (Supplementary Information). Finally, to generate a direct estimate of nuclear DNA contamination, we screened for heterozygous positions (among reads with >4x coverage) in known polymorphic sites (dbSNP-137) at uniquely mapped sections on the X chromosome6 (Supplementary Information). We found that the proportion of false heterozygous sites was 0.31%. Together these results suggest low levels of contamination in the La Brana 1 sequence data.
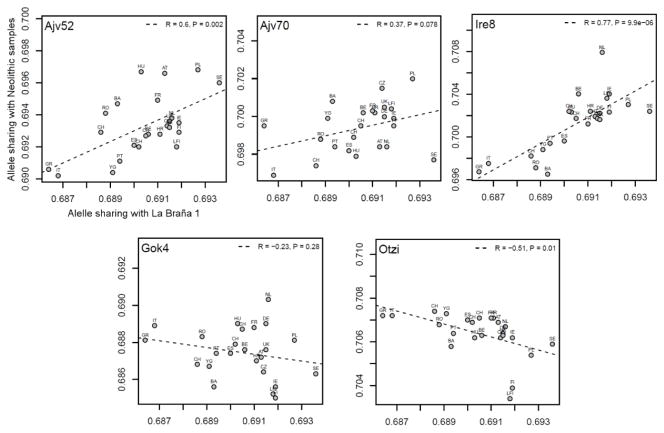
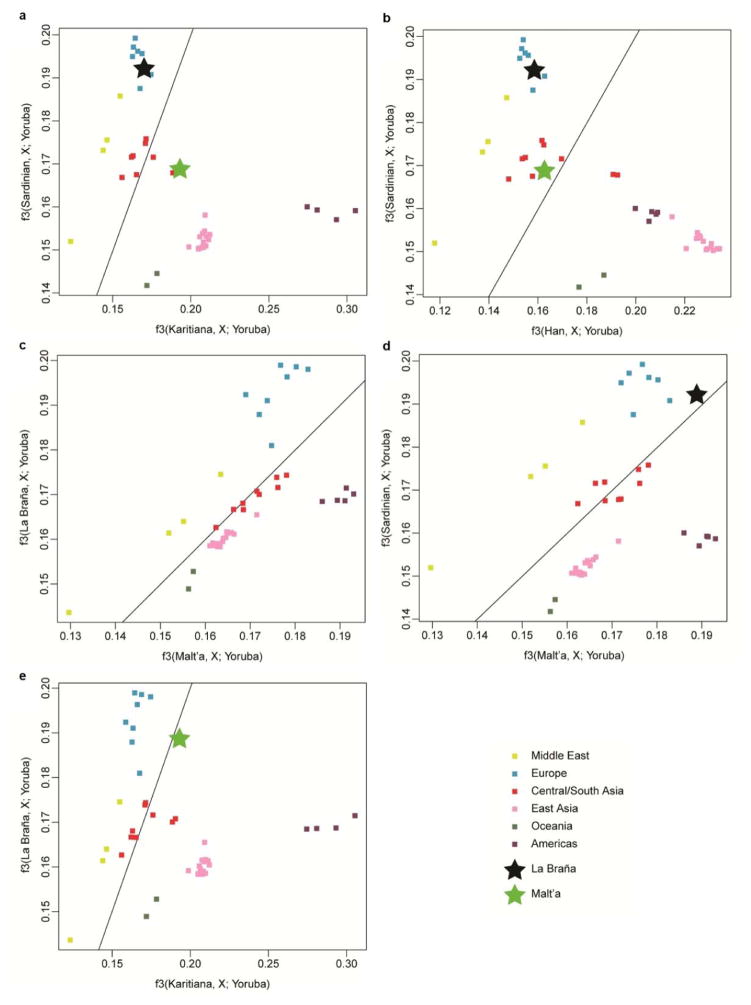
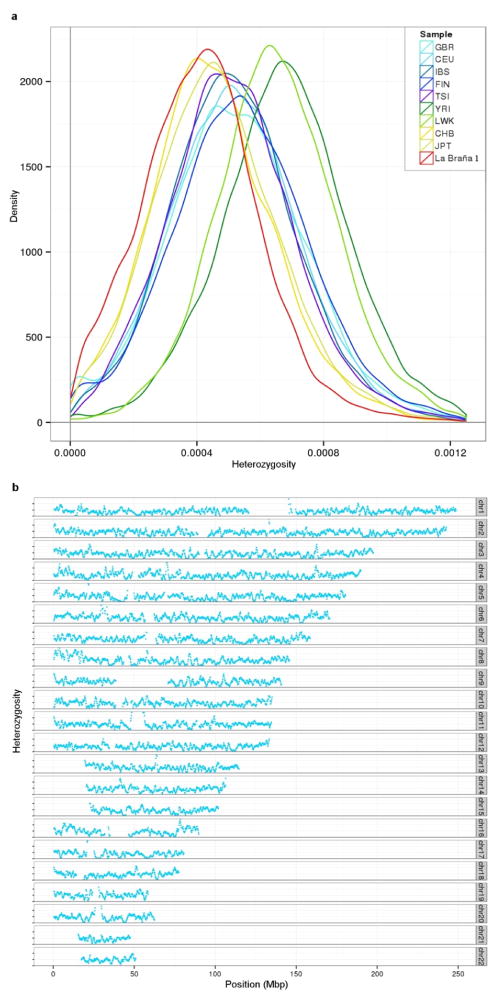
To investigate the relationship to extant European samples, we conducted Principal Component Analysis (PCA)10 and found the ~7,000-year-old Mesolithic sample was divergent from extant European populations (Extended Data Fig. 3a, b), but was placed in proximity to Northern Europeans (e.g. samples from Sweden and Finland)11–14. Additional PCAs and allele sharing analyses with ancient Scandinavian specimens3 supported the genetic similarity of the La Braña 1 genome to Neolithic hunter-gatherers (Ajv70, Ajv52, Ire8) relative to Neolithic farmers (Gok4, Ötzi) (Fig. 1c, Extended Data Fig. 3c and Extended Data Fig. 4). Thus, this Mesolithic individual from Southwestern Europe represents a formerly widespread gene pool that seems to be partially preserved in some modern-day Northern European populations, as suggested previously with limited genetic data2,3. We subsequently explored the La Braña affinities to an ancient Upper Palaeolithic genome from Mal’ta site near Lake Baikal in Siberia15. Outgroup f3 and D statistics16,17, using different modern reference populations, support that Mal’ta is significantly closer to La Braña 1 than to Asians or modern Europeans (Extended Data Fig. 5 and Supplementary Information). These results suggest that despite the vast geographical distance and temporal span, La Braña 1 and Mal’ta share common genetic ancestry, indicating a genetic continuity in ancient Western and Central Eurasia. This observation matches findings of similar cultural artifacts across time and space in Upper Paleolithic Western Eurasia and Siberia, particularly the presence of anthropomorphic “Venus” figurines which have been recovered from several sites in Europe and Russia, including the Mal’ta site15. We also compared the genome-wide heterozygosity of the La Braña 1 genome to a dataset of modern humans with similar coverage (3–4X). The overall genomic heterozygosity was 0.042%, lower than the values observed in present day Asians (0.046–0.047%), Europeans (0.051–0.054%), and Africans (0.066–0.069%) (Extended Data Fig. 6a). The effective population size, estimated from heterozygosity patterns, suggests a global reduction in population size of ~20% relative to extant Europeans (Supplementary Information). Moreover, no evidence of tracts of autozygosity suggestive of inbreeding was observed (Extended Data Fig. 6b).
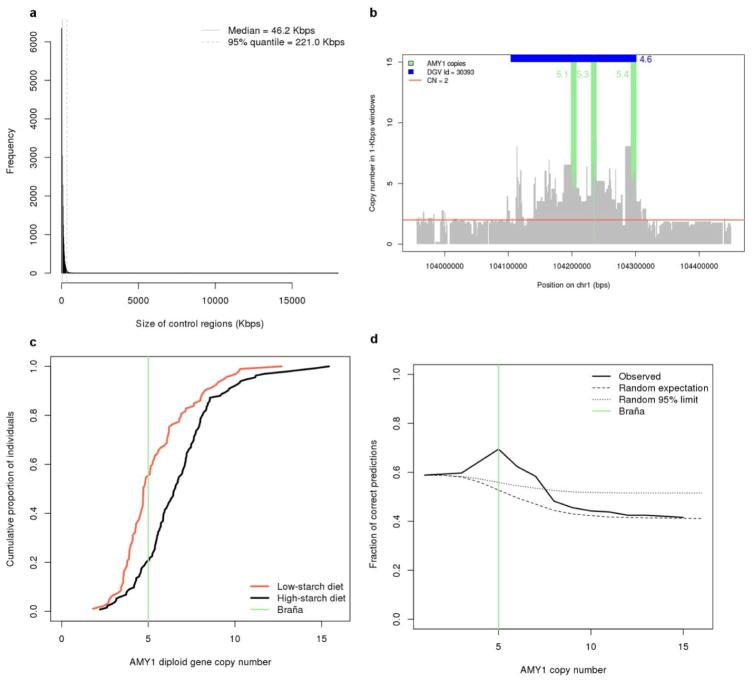
To systematically investigate the timing of selection events in the recent history of modern Europeans, we compared the La Braña genome to modern populations at loci that have been categorized as of interest for their role in recent adaptive evolution. With respect to two recent well-studied adaptations to changes in diet, we found the ancient genome to carry the ancestral allele for lactose intolerance4 and ~5 copies of the salivary amylase (AMY1) gene (Extended Data Fig. 7 and Supplementary Information), a copy number compatible with a low-starch diet18. These results suggest the La Brana hunter-gatherer was poor at digesting milk and starch, supporting the hypotheses that these abilities were selected for during the later transition to agriculture.
To expand the survey, we analyzed a catalog of candidate signals for recent positive selection based on whole-genome sequence variation from the 1000 Genomes Project13, which included 35 candidate non-synonymous variants – ten of which were detected uniquely in the CEU sample (Utah residents with Northern and Western European ancestry)19. For each variant we assessed whether the Mesolithic genome carried the ancestral or derived (putatively adaptive) allele.
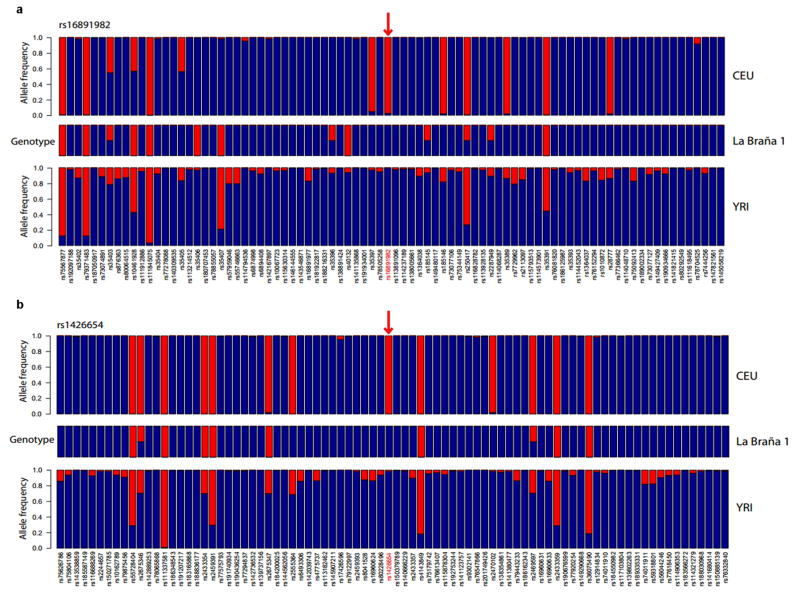
Of the ten variants, the Mesolithic genome carried the ancestral and non-selected allele as a homozygote in three regions: C12orf29 (a gene with unknown function), SLC45A2 (rs16891982) and SLC24A5 (rs1426654) (Table 1). The latter two variants are the two strongest known loci affecting light skin pigmentation in Europeans20–22 and their ancestral alleles and associated haplotypes are either absent or segregate at very low frequencies in extant Europeans (3% and 0% for SLC45A2 and SLC24A5 respectively) (Fig. 2). We subsequently examined all genes known to be associated with pigmentation in Europeans22, finding ancestral alleles in MC1R, TYR and KITLG and derived alleles in TYRP1, ASIP and IRF4 (Supplementary Information). Although the precise phenotypic effects cannot currently be ascertained in a European genetic background, results from functional experiments20 indicate that the allelic combination in this Mesolithic individual is likely to have resulted in dark skin pigmentation and dark/brown hair. Further examination revealed that this individual carried the rs12913832*C single nucleotide polymorphism (SNP) and the associated homozygous haplotype spanning the HERC2/OCA2 locus that is strongly associated with blue eye color23. Moreover, a prediction of eye color based on genotypes at additional loci using HIrisPlex24 yielded a 0.823 maximal and 0.672 minimal probability for being non-brown eyed (Supplementary Information). The genotypic combination leading to a predicted phenotype of dark skin and non-brown eyes is unique and no longer present in contemporary European populations. Our results indicate that the adaptive spread of light skin pigmentation alleles was not complete in some European populations by the Mesolithic, and that the spread of alleles associated with light/blue eye color may have preceded changes in skin pigmentation.
Table 1.
Mesolithic genome allelic state at 10 nonsynonmous variants recently selected in Europeans
| Gene | Name | SNP | Amino acid change | Function | |
|---|---|---|---|---|---|
| La Braña 1 carries the derived allele | PTX4 | Pentraxin 4 | rs2745098 | R281K | May be involved in innate immunity |
| UHRF1BP1 | UHRF1 binding protein 1 | rs11755393 | Q454R | Risk locus for systemic lupus erythematosus | |
| GPATCH1 | G patch domain containing 1 | rs10421769 | L520S | Receptor for OmpA expressed by E. coli | |
| WWOX | WW Domain-Containing Oxidoreductase | rs12918952 | A179T | Acts as a tumor suppressor and plays a role in apoptosis | |
| CCDC14 | Coiled-Coil Domain-Containing Protein 14 | rs17310144 | T365P | Unknown | |
|
| |||||
| La Braña 1 carries both the ancestral and the derived allele | SETX | Senataxin | rs1056899 | V2587I | Involved in Spinocerebellar Araxia and Amyotrophic lateral sclerosis |
| TDRD12 | Tudor domain containing 12 | rs11881633 | E413K | Unknown | |
|
| |||||
| La Braña 1 retains the ancestral allele | C12orf29 | Chromosome 12 open reading frame 29 | rs9262 | V238L | Unknown |
| SLC45A2 | Solute carrier family 45, member 2 | rs16891982 | L374F | Associated to skin pigmentation | |
| SLC24A5 | Solute carrier family 24, member 5 | rs1426654 | A111T | Associated to skin pigmentation | |
Figure 2. Ancestral variants around the SLC45A2 (rs16891982, above) and SLC24A5 (rs1426654, below) pigmentation genes in the Mesolithic genome (blue: ancestral, red: derived).
SNPs around the two diagnostic variants in these two genes were analyzed. The resulting haplotype comprises neighboring SNPs that are also absent in modern Europeans (CEU) but present in Yorubans (YRI). This pattern confirms that the La Braña 1 sample is older than the selective sweep in these regions.
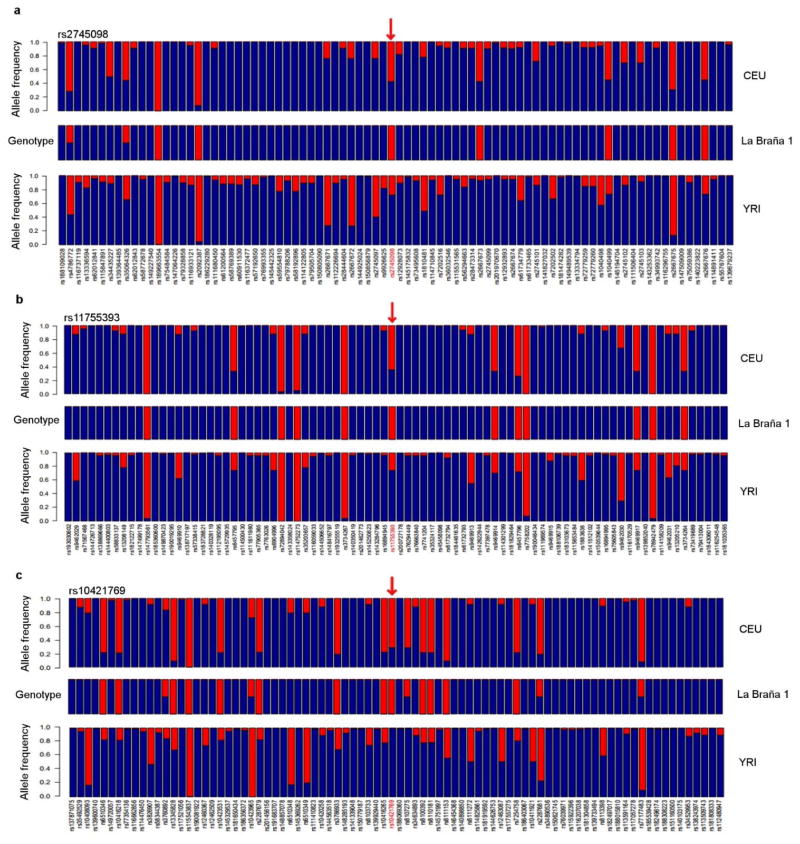
For the remaining loci, La Braña 1 displayed the derived, putatively adaptive variants in five cases, including three genes, PTX4, UHRF1BP1, and GPATCH119, involved in the immune system (Table 1 and Extended Data Fig. 8). The latter is associated with the risk of bacterial infection. We subsequently determined the allelic states in 63 SNPs from 40 immunity genes with previous evidence for positive selection and for carrying polymorphisms shown to influence susceptibility to infections in modern Europeans (Supplementary Information). La Braña 1 carries derived alleles in 24 genes (60%) that have a wide range of functions in the immune system: pattern recognition receptors, intracellular adaptor molecules, intracellular modulators, cytokines and cytokine receptors, chemokines and chemokine receptors and effector molecules. Interestingly, four out of six SNPs from the first category are intracellular receptors of viral nucleic acids (TLR3, TLR8, IFIH1/MDA5 and LGP2)25.
Finally, to explore the functional regulation of the genome, we also assessed the La Braña 1 genotype at all expression quantitative trait loci (eQTL) regions associated to positive selection in Europeans (Supplementary Information). The most interesting finding is arguably the predicted overexpression of eight immunity genes (36% of those with described eQTLs), including three Toll-like receptor genes (TLR1, TLR2 and TLR4) involved in pathogen recognition26.
These observations suggest that the Neolithic transition did not drive all cases of adaptive innovation on immunity genes found in modern Europeans. Several of the derived haplotypes seen at high frequency today in extant Europeans were already present during the Mesolithic, either as neutral standing variation and/or due to selection predating the Neolithic. De novo mutations that increased in frequency rapidly in response to zoonotic infections during the transition to farming should be identified among those genes where La Braña 1 carries ancestral alleles.
To confirm if the genomic traits seen at La Braña can be generalized to other Mesolithic populations, analyses of additional ancient genomes from Central and Northern Europe will be needed. Nevertheless, this genome sequence provides the first insight as to how these hunter-gatherers are related to contemporary Europeans and other ancient peoples in both Europe and Asia, and suggests how ancient DNA can shed light on the timing and nature of recent positive selection.
Methods Summary
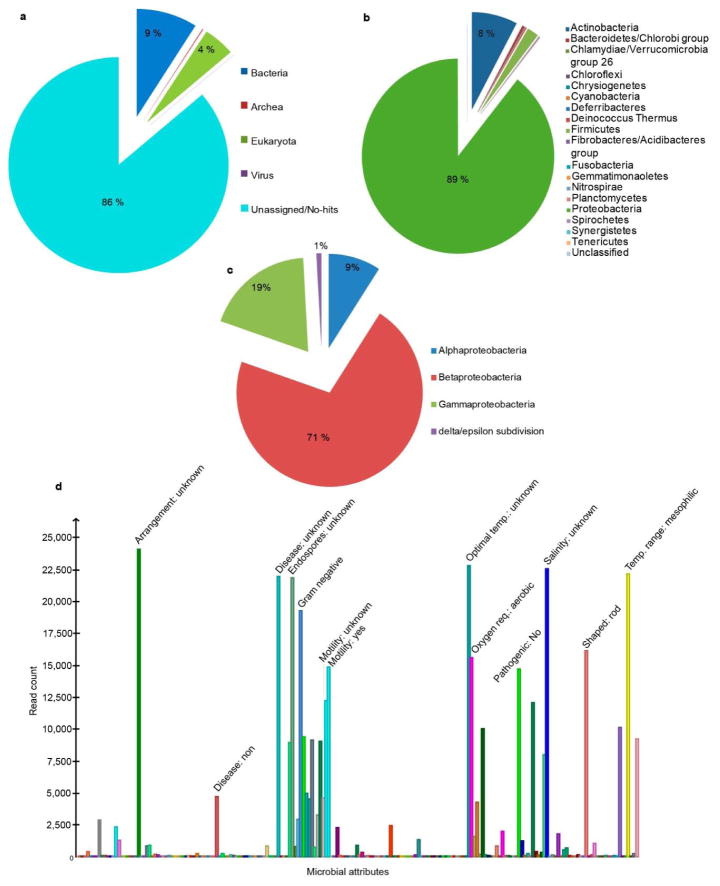
DNA was extracted from the La Braña 1 tooth specimen with a previously published protocol2. Indexed libraries were built from the ancient extract and sequenced on the Illumina HiSeq platform. Reads generated were mapped with BWA27 to the human reference genome (NCBI 37, hg19) after primer trimming. A metagenomic analysis and taxonomic identification was generated with the remaining reads using BLAST 2.2.27+ and MEGAN428 (Extended Data Fig. 9 and Supplementary Information). SNP calling was undertaken using a specific bioinformatic pipeline designed to account for ancient DNA errors. Specifically, the quality of misincorporations likely caused by aDNA damage was rescaled using the mapDamage2.0 software29, and a set of variants with a minimum read depth of 4 was produced with GATK30. Analyses including PCA10, Outgroup f316 and D statistics17 were performed to determine the population affinities of this Mesolithic individual (Supplementary Information).
Extended Data
Extended Data Figure 1. Alignment and coverage statistics of the La Braña 1 genome.
a, Alignment summary of La Braña 1 sequence data to hg19 assembly. b, Coverage statistics per chromosome. The percentage of the chromosome covered by at least one read is shown, as well as the mean read depth of all positions and positions covered by at least one read. c, Percentage of the genome covered at different minimum read depths.
Extended Data Figure 2. Damage pattern of La Braña 1 sequenced reads.
Frequencies of C to T (red) and G to A (blue) misincorporations at the 5′ end (left) and 3′ end (right) are shown for the nuclear DNA (a) and mtDNA (b). Fragment length distribution of reads mapping to the nuclear genome (c) and mtDNA genome (d). Coefficients of determination (R2) for an exponential decline and damage fractions (λ) are provided for the four different datasets.
Extended Data Figure 3. Genetic affinities of the La Braña 1 genome.
a, Principal Component Analysis (PCA) of the La Braña 1 SNP data and the 1000G Project European individuals. b, PCA of La Braña 1 vs Omni world-wide data. Continental terms refer to each Omni population groupings as follows. Africans: Yoruba and Luyha. Asians: Chinese (Beijing, Denver, South, Dai), Japanese and Vietnamese. Europeans: Iberians, Tuscans, British, Finns and CEU; and Indian Gujarati from Texas. c, Each panel shows PC1 and PC2 based on the PCA of one of the ancient samples with the merged POPRES+FINHM sample, prior to Procrustes transformation. The ancient samples include the La Braña 1 sample, four Neolithic samples from Skoglund et al.3 and Keller et al.1.
Extended Data Figure 4. Allele sharing analysis.
Each panel shows the allele sharing of a particular Neolithic sample from Skoglund et al.3 and Keller et al.1 with the La Braña 1 sample. The sample IDs are presented in the upper left of each panel (Ajv52, Ajv70, Ire8, Gok4, and Ötzi). In the upper right of each panel, the Pearson’s correlation coefficient is given with associated p-value.
Extended Data Figure 5. Pairwise outgroup f3 statistics.
a, Sardinian vs. Karitiana. b, Sardinian vs. Han. c, La Braña 1 vs. Mal’ta. d, Sardinian vs Mal’ta. e, La Braña 1 vs. Karitiana. The solid line is the y = x line.
Extended Data Figure 6. Analysis of heterozygosity.
a, Heterozygosity distributions of La Braña 1 and modern individuals with similar coverage from the 1,000 Genomes Project (using 1 Mb-windows with 200 Kb overlap). GBR: Great Britain, CEU: Northern-Western European ancestry, IBS: Iberians, FIN: Finns, TSI: Tuscans, YRI: Yorubans, LWK: Luhya, CHB: Han Chinese, JPT: Japanese. b, Heterozygosity values in 1-Mb windows (with 200 kb overlap) across each chromosome.
Extended Data Figure 7. Amylase copy number analysis.
a, Size distribution of diploid control regions. b, AMY1 gene copy number in La Braña 1. Abbreviations: DGV = Database of Genomic Variation; CN = copy number. c, La Braña 1 AMY1 gene copy number in the context of low-and high-starch diet populations. d, Classification of low-and high-starch diet individuals based on AMY1 copy number. Using data from18, individuals were classified as in low-(less or equal than) or high-starch (higher than) categories and the fraction of correct predictions (g) was calculated. In addition, we calculated the random expectation and 95% limit of low-starch diet individuals classified correctly at each threshold value.
Extended Data Figure 8. Neighboring variants for three diagnostic SNPs related to immunity.
(a) rs2745098 (PTX4 gene), (b) rs11755393 (UHRF1BP1 gene) and (c) rs10421769 (GPATCH1 gene). For PTX4, UHRF1BP1 and GPATCH1, La Braña 1 displays the derived allele and the European-specific haplotype, indicating that the selective sweep was already present in the Mesolithic. Blue: ancestral, red: derived.
Extended Data Figure 9. Metagenomic analysis of the non-human reads.
a, Domain attribution of the non-human reads. b, Proportion of different Bacteria groups. c, Proportion of different types of Proteobacteria. d, Microbial attributes of the microbes present in the La Braña 1 sample.
Supplementary Material
Acknowledgments
Luis Alfredo Grau Lobo (Museo de León), for access to the La Braña specimen and Maanasa Raghavan for early access to Mal’ta genome data. Sequencing was performed at the Danish National High-Throughput DNA-Sequencing Centre, University of Copenhagen. The POPRES data were obtained from dbGaP (accession number # 2038). The Danish National Research Foundation, ERC Starting Grant (260372) to TM-B, and (310372) to MGN, FEDER and Spanish Government Grants BFU2009-13409-C02-02, BFU2012-38236 to AN, BFU2011-28549 to TM-B, BFU2012-34157 to CL-F, ERC (Marie Cure Actions) to MEA, NIH NRSA postdoctoral fellowship (F32GM106656) to CWKC, NIH (R01-HG007089) to JN, NSF postdoctoral fellowship (DBI-1103639) to MDG, the Australian NHMRC to RAS and a predoctoral fellowship from the Basque Government (DEUI) to IO for economic support.
Footnotes
Supplementary Information is linked to the online version of the paper at www.nature.com/nature.
Author Contributions
C.L.-F. and E.W. conceived and lead the project. M.E.P. and J.M.V.E. provided anthropological and archaeological information. O.R. and M.E.A. performed the ancient extractions and library construction, respectively. I.O., M.E.A., F.S.-Q., J.P.-M., S.R., O.R., M.F. and T.M.-B. performed mapping, SNP calling, mtDNA assembly, contamination estimates and different genomic analyses on the ancient genome. I.O., F.S.-Q., G.S., C.W.K.C., M.DG., J.A.R., J.Q., O.R., U.M.M. and A.N. performed functional, ancestry and population genetic analyses. R.N., and J.N. coordinated the ancestry analyses. M.G.N., R.A.S., and P.S. coordinated the immunological, pigmentation and selection analyses, respectively. I.O., M.E.A., T.M.-B., E.W. and C.L.-F. wrote the majority of the manuscript with critical input from R.N., M.G.N., J.N., R.A.S., P.S. and A.N.
Alignment data are available through the Sequence Read Archive (SRA) (PRJNA230689 and SRP033596).
Reprints and permissions information is available at www.nature.com/reprints.
The authors declare no competing financial interests.
References
- 1.Keller A, et al. New insights into the Tyrolean Iceman’s origin and phenotype as inferred by whole-genome sequencing. Nat Commun. 2012;3:698. doi: 10.1038/ncomms1701. [DOI] [PubMed] [Google Scholar]
- 2.Sánchez-Quinto F, et al. Genomic Affinities of Two 7,000-Year-Old Iberian Hunter-Gatherers. Curr Biol. 2012;22:1494–99. doi: 10.1016/j.cub.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 3.Skoglund P, et al. Origins and genetic legacy of Neolithic farmers and hunter-gatherers in Europe. Science. 2012;336:466–9. doi: 10.1126/science.1216304. [DOI] [PubMed] [Google Scholar]
- 4.Laland KN, Odling-Smee J, Myles S. How culture shaped the human genome: bringing genetics and the human sciences together. Nat Rev Genet. 2010;11:137–48. doi: 10.1038/nrg2734. [DOI] [PubMed] [Google Scholar]
- 5.Rasmussen M, et al. Ancient human genome sequence of an extinct Palaeo-Eskimo. Nature. 2010;463:757–762. doi: 10.1038/nature08835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rasmussen M, et al. An Aboriginal Australian genome reveals separate human dispersals into Asia. Science. 2011;334:94–8. doi: 10.1126/science.1211177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vidal Encinas JM, Prada Marcos ME. Los hombres mesolíticos de La Braña-Arintero (Valdelugueros, León) León: Junta de Castilla y León; 2010. [Google Scholar]
- 8.Overballe-Petersen S, Orlando L, Willerslev E. Next-generation sequencing offers new insights into DNA degradation. Trends Biotechnol. 2012;30:364–8. doi: 10.1016/j.tibtech.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 9.Allentoft ME, et al. The half-life of DNA in bone: measuring decay kinetics in 158 dated fossils. Proc R Soc B Biol Sci. 2012;279:4724–4733. doi: 10.1098/rspb.2012.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patterson N, Price AL, Reich D. Population structure and eigenanalysis. PLoS Genet. 2006;2:e190. doi: 10.1371/journal.pgen.0020190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nelson MR, et al. The Population Reference Sample, POPRES: A Resource for Population, Disease, and Pharmacological Genetics Research. Am J Hum Genet. 2008;83:347–358. doi: 10.1016/j.ajhg.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Novembre J, et al. Genes mirror geography within Europe. Nature. 2008;456:98–101. doi: 10.1038/nature07331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.1000 Genomes Project Consortium. An integrated map of genetic variation from 1,092 human genomes. Nature. 2012;491:56–65. doi: 10.1038/nature11632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Surakka I, et al. Founder population-specific HapMap panel increases power in GWA studies through improved imputation accuracy and CNV tagging. Genome Res. 2010;20:1344–51. doi: 10.1101/gr.106534.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raghavan M, et al. Upper Palaeolithic Siberian genome reveals dual ancestry of Native Americans. Nature. 2013 doi: 10.1038/nature12736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reich D, Thangaraj K, Patterson N, Price AL, Singh L. Reconstructing Indian population history. Nature. 2009;461:489–94. doi: 10.1038/nature08365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Green RE, et al. A draft sequence of the Neandertal genome. Science. 2010;328:710–722. doi: 10.1126/science.1188021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perry GH, et al. Diet and the evolution of human amylase gene copy number variation. Nat Genet. 2007;39:1256–60. doi: 10.1038/ng2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grossman SR, et al. Identifying recent adaptations in large-scale genomic data. Cell. 2013;152:703–13. doi: 10.1016/j.cell.2013.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lamason RL, et al. SLC24A5, a putative cation exchanger, affects pigmentation in zebrafish and humans. Science. 2005;310:1782–6. doi: 10.1126/science.1116238. [DOI] [PubMed] [Google Scholar]
- 21.Norton HL, et al. Genetic evidence for the convergent evolution of light skin in Europeans and East Asians. Mol Biol Evol. 2007;24:710–22. doi: 10.1093/molbev/msl203. [DOI] [PubMed] [Google Scholar]
- 22.Sturm RA, Duffy DL. Human pigmentation genes under environmental selection. Genome Biol. 2012;13:248. doi: 10.1186/gb-2012-13-9-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sturm RA, et al. A single SNP in an evolutionary conserved region within intron 86 of the HERC2 gene determines human blue-brown eye color. Am J Hum Genet. 2008;82:424–431. doi: 10.1016/j.ajhg.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walsh S, et al. The HIrisPlex system for simultaneous prediction of hair and eye colour from DNA. Forensic Sci Int Genet. 2013;7:98–115. doi: 10.1016/j.fsigen.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 25.Aoshi T, Koyama S, Kobiyama K, Akira S, Ishii KJ. Innate and adaptive immune responses to viral infection and vaccination. Curr Opin Virol. 2011;1:226–232. doi: 10.1016/j.coviro.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 26.Moresco EMY, LaVine D, Beutler B. Toll-like receptors. Curr Biol. 2011;21:R488–R493. doi: 10.1016/j.cub.2011.05.039. [DOI] [PubMed] [Google Scholar]
- 27.Li H, Durbin R. Fast and accurate short read alignment with Burrows–Wheeler transform. Bioinformatics. 2009;25:1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huson DH, Mitra S, Ruscheweyh HJ, Weber N, Schuster SC. Integrative analysis of environmental sequences using MEGAN4. Genome Res. 2011;21:1552–60. doi: 10.1101/gr.120618.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jónsson H, Ginolhac A, Schubert M, Johnson PLF, Orlando L. mapDamage2.0: fast approximate Bayesian estimates of ancient DNA damage parameters. Bioinformatics. 2013;29:1682–1684. doi: 10.1093/bioinformatics/btt193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McKenna A, et al. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010;20:1297–303. doi: 10.1101/gr.107524.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.