ABSTRACT
Objective:
Children and adolescents are considered as the best target groups for preventing and controlling the cardiovascular diseases risk factors and reducing mortality in adulthood. Alternative medicine and herbal drugs have been taken into account for managing dyslipidemia in this population. The beneficial effects of Sumac (Rhus coriaria L.) on lipid profile have been confirmed in some laboratory and animal studies. This study was designed to investigate the clinical effects of sumac fruits on dyslipidemia in 12-18 years-old adolescents.
Methods:
This randomized triple-blinded clinical trial was conducted on 72 obese adolescents with dyslipidemia from August 2011 to June 2012 in Isfahan Cardiovascular Research Center, Isfahan, Iran. Eligible adolescents were randomly assigned to two case and control groups. The control group received placebo capsules and the case group received capsules containing 500 mg of powdered sumac fruits, each three times a day for one month. Biochemical parameters including 12-hrs fasting serum levels of total cholesterol (Total-C), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), and triglycerides (TG) were measured before the initiation and after the completion of the study protocol. Statistical analysis was performed using the SPSS software, version 16.0, using Independent Samples T-test, or Paired Samples T-test, for between-group and within-group analysis, respectively.
Findings:
The plasma levels of Total-C, LDL-C, and TG changed significantly over-time in the case group. Furthermore, between-group analysis showed a statistically significant difference between case and control groups with this regard (P < 0.05, for all statistical comparisons). However, HDL-C levels have not been changed significantly over-time within the case or control group, neither between the two study groups.
Conclusion:
In this study, the considerable effects of Rhus coriaria (sumac) on reducing serum levels of Total-C, LDL-C, and TG have been noticed during one-month trial. However, probably due to the concise period of sumac consumption, its effect on HDL-C was not statistically significant.
Keywords: Rhus coriaria, Sumac, dyslipidemia, adolescent
1. INTRODUCTION
Cardiovascular diseases (CVD) are the leading cause of death in many advanced and developing countries, of which coronary heart disease (CHD) accounts for approximately half of mortalities (1, 2). The most common cause of CHD is narrowing of the coronary arteries due to the presence of atherosclerotic plaques (3-5). The risk of atherosclerosis is directly related to increasing levels of serum cholesterols (6). During the past decades, epidemiologic studies have established a direct relationship between blood low-density lipoprotein cholesterol (LDL-C) concentrations in a population and the incidence of CHD events (7-9); while high-density lipoprotein cholesterol (HDL-C) has an inverse relationship with CHD (10).
Obesity, as an important cause of CVDs, is accompanied by several cardiovascular risk factors namely hypertension, dyslipidemia, glucose intolerance and finally an increased risk of CVD morbidity and mortality (11, 12). Patients with central obesity have an increased mobilization of fatty acids from adipose cells to the systemic circulation, which leads to increased triglyceride (TG) synthesis by the liver. Although these patients are often being managed with weight reduction and increased physical activity (13-15), the results of numerous major clinical trials have conclusively demonstrated the value of lipid-modifying therapy to protect them from CHD events (15-18).
Successful management of dyslipidemia alters the natural course of atherosclerosis and reduces CHD; however, the challenge for the clinician is to implement treatments that meet and maintain the desired treatment goals for some special populations such as children and adolescents. In this population, an adequate trial of therapeutic lifestyle changes (TLC) should be used in all patients, but pharmacotherapy, being concerned about potential side effects, should be instituted concurrently only in high-risk patients, such as those with the metabolic syndrome (19, 20). In such medical conditions, complementary medicine can be regarded as an adjunct to conventional medical care (21-23).
Several studies have been directed to evaluate the effects of alternative medicine namely herbal drugs in managing hyperlipidemia in vulnerable populations such as children (24, 25). Of medicinal herbs, those with antioxidant properties have been well noticed with this regard, which may originated from the finding that native LDL per se does not contribute to the development of atherosclerosis; instead, LDL must be oxidized before it becomes a causing factor for atherosclerosis (26). Rhus coriaria Linn. (Anacardiacea), commonly known as sumac, is a well-known spice widely consumed in the world which has also been utilized extensively for medicinal purposes (27). Different parts of the plant have been used in diverse preparations in traditional medicine (28). The antioxidant components of this plant made it a favorable target for laboratory and animal studies in different conditions such as oxidative stress cytotoxicity (29), diabetes (30), diabetic nephropathy (31), and cerebral ischemia (32). The protective effects of Rhus coriaria extract against lipid peroxidation (29, 31, 33) as well as its lipid lowering properties (34, 35) have been examined on extracted cells or animals; however, no human study yet evaluated the hypocholesterolemic effects of sumac in a clinical setting. Since children and adolescents have been granted as the best target group for implementing the interventions with the aim of improving lifestyle and preventing chronic diseases of adulthood (36), and considering potential concerns on hypocholesterolemic drugs side effects in this susceptible population, we designed this study to investigate the role of Rhus coriaria fruits, as an alternative herbal medicine, in controlling serum lipid indices of dyslipidemic obese adolescents.
2. MATERIALS AND METHODS
This triple-blinded randomized placebo-controlled clinical trial was conducted from August 2011 to June 2012 in Isfahan Cardiovascular Research Center, affiliated with Isfahan University of Medical Sciences, Isfahan, Iran. The study protocol was ethically designed in accordance with the Declaration of Helsinki (37) and approved by the local board of human studies (Registration code: 388595).
Adolescents (12 to 18 years old) who met the following criteria were included to the study: 1) not having the present and past history of smoking behavior, 2)with dyslipidemia defining as serum Total-C or LDL-C or TG equal or more than the age- and gender-specific 95th percentile, or HDL-C lower than 5th percentile, 3)and having a body mass index (BMI) equal or more than the age- and gender-specific 95th percentile, proportionate for Iranian children and adolescents (38). The exclusion criteria were considered as following: 1) getting used to smoke during the study, 2) suffering a disease effective on blood lipids, 3) using any other drugs effective on blood lipids and blood sugar levels, 4) and not following the study protocol for more than one week.
Eligible patients were selected by convenient sampling method. The study protocol is figured in CONSORT diagram of the study (Figure 1). After providing detailed description to the participant and their parents, all the qualified people were asked to sign the written consent. At the first referring visit, biochemical parameters including fasting serum levels of Total-C, LDL-C, HDL-C, and TG, as well as laboratory tests related to thyroid hormones and blood sugar were taken from patients, for the later in order to exclude the samples having exclusion criteria regarding diseases which may affect the blood lipids.
Figure 1.
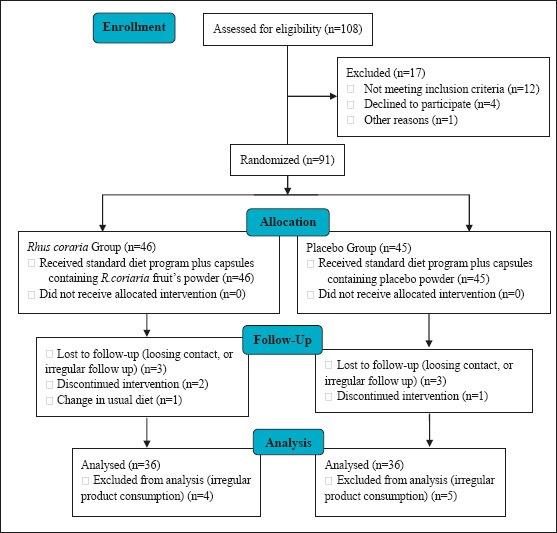
CONSORT diagram of the study
Each participant received detailed oral information about the study objectives and protocol, as well as standard recommendations for healthy eating habits and regular physical activity (39). Then, they were assigned to two study groups by simple randomization method. The case group was asked to take a capsule containing 500 mg powdered sumac fruit, three times a day (preferably after each meal) for one month. The participants in placebo group were provided with the identical packages of capsules containing equal amounts of a placebo powder (lactose) and were asked to consume one capsule, three times a day for the same time period.
2.1. Sample Size Calculation
In order to have a 90% chance of detecting the difference (at least 10 mg/dl) in mean cholesterol change at 5% level of significance, and considering the standard deviation of total cholesterol as 15.47 mg/dl (based on the achieved data from a previous similar study [40]), the sample size using t-student formula was determined to be 35 for case and control group.
2.2. Dosage Form Preparation of the Herbal Product
Rhus coriaria fruits were prepared from a medicinal herbs market in Isfahan province, Iran, and were confirmed by two herbal botanists regarding taxonomical aspects. Considering that the most indicative feature and effective gradients of sumac are polyphenolic components, Folin-Ciocalteu method was used for standardization of the plant based on determining its phenolic amount (41). Accordingly, the appropriate amount of powdered fruits for each dose to be included in one capsule was determined as 500 mg. Placebo capsules were filled with the same amount of lactose which is confirmed to have no effects on lipid profile.
2.3. Follow-up
Children's compliances were followed the by regular phone calls. Also, in the cases of non-compliance or emerging any related side effects, parents were asked to contact our clinic. The participants who did not regularly take the medication were excluded from final analysis. Figure 1 shows the recruitment process of the study. After one month of the study initiation for each participant, samples referred for final laboratory tests of lipid profile. These steps were the same for participants in both the case and control groups.
2.4. Statistical Analysis
Collected data were analyzed by SPSS software (Chicago, IL, USA) version 18.0. Normal distribution of quantitative variables was evaluated using Kolmogorov-Smirnov test. Chi-square and Student's t-test analysis was performed to compare the baseline demographic (sex, age, BMI) and biochemical parameters between study subjects in both groups. The changes of the biochemical parameters in each study group during the study period were analyzed using paired-samples t-test. Multivariate general linear model was used to compare the over-time changes in the measured parameters between case and control groups. P-value of less than 0.05 was considered statistically significant.
3. RESULTS
Seventy two volunteers completed the study. Demographic characteristics of participants in two study groups are presented in table 1. Kolmogorov-Smirnov analysis demonstrated normally distribution of all quantitative parameters in the two study groups (p > 0.05). The groups did not differ statistically regarding the mean age and baseline body mass index (BMI), as well as baseline lipid profile measures (as indicated in table 2).
Table 1.
Demographic data and baseline BMI in two study groups. Data presented as Number, or Mean ± SD, where applicable. BMI: body mass index, calculated by dividing weight by the height squared (kg/m2); SD: standard deviation.
* Between-group analysis using Chi-square or Independent-samples t-test.
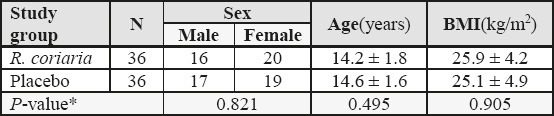
Table 2.
Biochemical parameters before initiation and after completion of the study protocol in two study groups. Data presented as Mean ± SD, HDL-C: high-density lipoprotein cholesterol; LDL-C: low-density lipoprotein cholesterol; TG: triglyceride; Total-C: total cholesterol; SD: standard deviation. * Within-group analysis using Paired-samples t-test; **Between-group analysis using General linear model (multivariate) test.
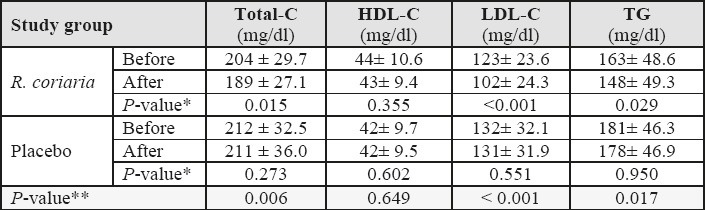
Laboratory data of serum lipid profile are presented in table 2. As shown, the amount of Total-C, LDL-C, and TG changed significantly over time (during one-month study period) in the R. coriaria group; while there are no significant changes in all investigated parameters in control group. Between-group analysis shows that the differences in the biochemical measures between two study groups are statistically significant for Total-C, LDL-C and TG, while HDL-C did not differ significantly. Participants of both groups did not report any significant drug side.
4. DISCUSSION
Knowing the fact that the existing risk factors in childhood and adolescence are the same as that found in adulthood, different scientific societies have announced children and adolescents as the best target group for implementing the interventions toward improving lifestyle and preventing chronic diseases of adulthood (36). The results of two previous studies presented a high prevalence of dyslipidemia and rapid emergence of CVD predisposing factors in 12-18 years old adolescents in Iran (42, 43).
Several chemical drugs have been used for decreasing serum lipids and consequently atherosclerosis. Concerns of side effects make their use unjustifiable for the primary stages of hyperlipidemia especially for children and adolescents. In recent years, there is an ongoing effort to integrate complementary and alternative medicine into conventional medical practice for treating many health conditions including cardiovascular diseases (21). Being most widely used, medicinal plants are favorable alternatives to chemical drugs, especially in more susceptible populations such as pediatrics and adolescents.
Some previous clinical trials addressed positive effects of some widespread medicinal plants on dyslipidemia in adolescents; of these, three double-blinded placebo-controlled studies evaluated the clinical effects of Roselle (Hibiscus sabdariffa), Common Jujube (Zizyphus jujuba), and Purslane (Portulaca oleraceae) on lipid indices of obese children and adolescents (44-46). The promising results were concluded to be yielded due to the medically active ingredients of these noteworthy medicinal herbs. Of all, antioxidant and anti-inflammatory characteristics are taken for granted. Oxidation is needed for LDL particles to become the initiator of atherosclerosis process within vessel cell walls (26); so, being rich with antioxidant ingredients, such plants may be considered as a therapeutic approach in halting this destructive process.
Considering the protective effects of these compounds, sumac has also been introduced as a vascular protective and CVD preventive medicinal plant in traditional medicine (27, 47). As evidenced by numerous basic researches, a broad-spectrum of medicinally significant phytochemical components has been identified from various parts of sumac (27, 32, 48-53), supporting its traditional uses. Polyphenols can effectively reduce the lipid absorbance from gastrointestinal tract, due to their high resin binding capacities. Also, relatively high amounts of water-soluble tannins in sumac fruits have documented to play an evident antioxidant role (52); the effect which has been confirmed for some other plants like grape and pomegranate, used to improve vascular reactivity in pediatrics with metabolic syndrome (54). Some other investigations are indicative of high inhibitory activity of sumac on xanthine oxidase which justifies its serum cholesterol reducing effects (55).
Given the importance of antioxidant and free radical scavenging activities against lipid peroxidation as the initial stage of atherosclerosis (31, 34), considerable effects of sumac on hyperlipidemia can be elucidated (33). One study demonstrated the protective effect of consuming sumac with food on some risk factors of atherosclerosis (serum lipid indices such as Total-C and LDL-C) and oxidative stress in rabbits (35).
The present study as the initiative one in the adolescent age group revealed that sumac fruits could be effective in decreasing serum Total-C, TG and LDL-C of dyslipidemic patients. The study was designed following the previous findings of sumac anti-hyperlipidemic effects in animal models (31, 34, 35). In one previous research assessing the sumac effect on preventing the vessels smooth muscular cells immigration (with the aim of evaluating its effects on preventing atherosclerosis), sumac extract was tested on mice carotid cultured cells for 10 days. The results were indicative of a 62 parentage reduction of these cells immigration, a considerable finding on its effect in halting the progression of atherosclerosis (51).
In the present study, despite considerable effects of sumac on reducing serum levels of Total-C, LDL-C, and TG, the amount of HDL-C has not been noticeably changed during one-month trial. According to above-mentioned medicinally-active characteristics and mechanisms of this plant, the obtained findings on serum lipid indices were expected. Although the identical results were concluded from previous similar studies (44-46), the ineffectiveness of sumac fruits on HDL-C may be attributed to the low dose or concise period of its consumption, which is also evident in the three previous similar studies conducted with the same objectives on the same population as the present study.
5. CONCLUSION
Our study demonstrated the advantages of sumac -a medicinal herb with the favorable safety profile which is routinely consumed with fatty meals since long time-on reducing the damaging lipid particles in obese adolescents. Although being triple-blinded and placebo-controlled, make our investigation to be unique as the first human study with this regard, but the existing limitation on study period would encourage the future well-established long-term clinical trials. This approach may reveal the therapeutic as well as adverse effects of consuming this traditionally favorite medicinal plant for the purpose of improving lipid profile and consequently reducing the CVD risks.
Acknowledgments
This study was a Pharmacy Doctoral thesis project financially supported by the Vice-chancellery for research and technology at Isfahan University of Medical Sciences. Authors would like to thank Dr. Iraj Mehregan for his scientific assistance in taxonomic confirmation of the Rhus coriaria fruits and also the staff members of laboratory department of Isfahan Cardiovascular Research Institute for their kind help and assistance.
Footnotes
CONFLICT OF INTEREST: NONE DECLARED.
REFERENCES
- 1.Morrow DA, Gersch BJ. Chronic coronary artery disease. In: Mann DL, Zipes DP, Libby P, Bonow RO, editors. Braunwald's Heart Disease: A Textbook of Cardiovascular Medicine. 10th ed. Philadelphia, PA: WB Saunders; 2014. p. 1353. [Google Scholar]
- 2.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, et al. Heart disease and stroke statistics-2013 update: a report from the American Heart Association. Circulation. 2013;127(1):e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Antman E, creager M, Braunwald E, William G, Challender P, Edelman E, et al. Philadelphia: Lippincott Williams and Wilkins; 2003. Pathophysiology of Heart Disease; pp. 111–130. [Google Scholar]
- 4.Reddy KS. Cardiovascular diseases in the developing countries: dimensions,determinants, dynamics and directions for public health action. Public Health Nutrition. 2002;5(1A):231–237. doi: 10.1079/phn2001298. [DOI] [PubMed] [Google Scholar]
- 5.Masic I, Rahimic M, Dilic M, Kadribasic R, Toromanovic S. Socio-medical characteristics of coronary disease in Bosnia and Herzegovina and the world. Mater Sociomed. 2011;23(3):171–183. doi: 10.5455/msm.2011.23.171-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tabas I, Williams KJ, Borén J. Sub-endothelial lipoprotein retention as the initiating process in atherosclerosis: update and therapeutic implications. Circulation. 2007;116(16):1832–1844. doi: 10.1161/CIRCULATIONAHA.106.676890. [DOI] [PubMed] [Google Scholar]
- 7.Neaton JD, Blackburn H, Jacobs D, Kuller L, Lee DJ, Sherwin R, et al. Serum cholesterol level and mortality findings for men screened in the Multiple Risk Factor Intervention Trial. Multiple Risk Factor Intervention Trial Research Group. Arch Intern Med. 1992;152(7):1490–500. [PubMed] [Google Scholar]
- 8.Jacobs D, Blackburn H, Higgins M, Reed D, Iso H, McMillan G, et al. Report of the Conference on Low Blood Cholesterol: mortality associations. Circulation. 1992;86(3):1046–1060. doi: 10.1161/01.cir.86.3.1046. [DOI] [PubMed] [Google Scholar]
- 9.Castelli WP. Epidemiology of coronary heart disease: the Framingham study. Am J Med. 1984;76(2A):4–12. doi: 10.1016/0002-9343(84)90952-5. [DOI] [PubMed] [Google Scholar]
- 10.Gordon DJ, Probstfield JL, Garrison RJ, Neaton JD, Castelli WP, Knoke JD, et al. High-density lipoprotein cholesterol and cardiovascular disease: four prospective American studies. Circulation. 1989;79(1):8–15. doi: 10.1161/01.cir.79.1.8. [DOI] [PubMed] [Google Scholar]
- 11.Padwal RS, Sharma AM. Prevention of cardiovascular disease: Obesity, diabetes and the metabolic syndrome. Cardiol. 2010;26:18–20. doi: 10.1016/s0828-282x(10)71077-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raj M. Obesity and cardiovascular risks in children and adolescents. Indian journal of endocrinology and metabolism. 2012;16(1):13–19. doi: 10.4103/2230-8210.91176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fujioka S, Matsuzawa Y, Tokunaga K, Tarui S. Contribution of intra-abdominal fat accumulation to the impairment of glucose and lipid metabolism in human obesity. Metabolism. 1987;36(1):54–59. doi: 10.1016/0026-0495(87)90063-1. [DOI] [PubMed] [Google Scholar]
- 14.Barakat HA, Carpenter JW, McLendon VD, Khazanie P, Leggett N, Heath J, et al. Influence of obesity, impaired glucose tolerance, and NIDDM on LDL structure and composition. Possible link between hyperinsulinemia and atherosclerosis. Diabetes. 1990;39(12):1527–1533. doi: 10.2337/diab.39.12.1527. [DOI] [PubMed] [Google Scholar]
- 15.Sever PS, Dahlöf B, Poulter NR, Wedel H, Beevers G, Caulfield M, et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcome Trial-Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet. 2003;361:1149–1458. doi: 10.1016/S0140-6736(03)12948-0. [DOI] [PubMed] [Google Scholar]
- 16.Colhoun HM, Betteridge DJ, Durrington PN, Hitman GA, Neil HA, Livingstone SJ, et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicenter randomised placebo-controlled trial. Lancet. 2004;364:685–696. doi: 10.1016/S0140-6736(04)16895-5. [DOI] [PubMed] [Google Scholar]
- 17.Cannon CP, Braunwald E, McCabe CH, Rader DJ, Rouleau JL, Belder R, et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350(15):1495–1504. doi: 10.1056/NEJMoa040583. [DOI] [PubMed] [Google Scholar]
- 18.LaRosa JC, Grundy SM, Waters DD, Shear C, Barter P, Fruchart JC, et al. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med. 2005;352(14):1425–1435. doi: 10.1056/NEJMoa050461. [DOI] [PubMed] [Google Scholar]
- 19.Wood PD, Stefanick ML, Dreon DM, Frey-Hewitt B, Garay SC, Williams PT, et al. Changes in plasma lipids and lipoproteins in overweight men during weight loss through dieting as compared with exercise. N Engl J Med. 1988;319(18):1173–1179. doi: 10.1056/NEJM198811033191801. [DOI] [PubMed] [Google Scholar]
- 20.Wood PD, Stefanick ML, Williams PT, Haskell WL. The effects of plasma lipoproteins of a prudent weight- reducing diet, with or without exercise, in overweight men and women. N Engl J Med. 1991;325(7):461–466. doi: 10.1056/NEJM199108153250703. [DOI] [PubMed] [Google Scholar]
- 21.Frishman WH, Beravol P, Carosella C. Alternative and Complementary Medicine for Preventing and Treating Cardiovascular Disease. Dis Mon. 2009;55:121–192. doi: 10.1016/j.disamonth.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 22.Faridi P, Zarshenas MM. Ibn Sina's book on drugs for cardiovascular diseases. Int J Cardiol. 2010;145:223. doi: 10.1016/j.ijcard.2010.04.064. [DOI] [PubMed] [Google Scholar]
- 23.Masic I. Thousand year anniversary of the historical book: “Kitab al Qanun fit Tibb”-The Canon of Medicine, written by Abdullah ibn Sina. J Res Med Sci. 2012;17:993–1000. [PMC free article] [PubMed] [Google Scholar]
- 24.Kalra S, Gandhi A, Kalra B, Agrawal N. Management of dyslipidemia in children. Diabetol Metab Syndr. 2009;1(1):26–30. doi: 10.1186/1758-5996-1-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O’Gorman CS, O’Neill MB, Conwell LS. Considering statins for cholesterol reduction in children if lifestyle and diet changes do not improve their health: a review of the risks and benefits. Vasc Health Risk Manag. 2010;7:1–14. doi: 10.2147/VHRM.S7356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steinberg D, Parthasarathy S, Carew TE, Khoo JC, Witztum JL. Beyond cholesterol: modifications of low density lipoprotein that increases its atherogenicity. N Engl J Med. 1989;320(14):915–924. doi: 10.1056/NEJM198904063201407. [DOI] [PubMed] [Google Scholar]
- 27.Mazza SRG. Biological activities of extracts from sumac (Rhus spp.). a review. Plant Foods Hum Nutr. 2007;62(4):165–175. doi: 10.1007/s11130-007-0058-4. [DOI] [PubMed] [Google Scholar]
- 28.Shabbir A. Rhus coriaria Linn, a plant of medicinal, nutritional and industrial importance: A review. The Journal of Animal & Plant Sciences. 2012;22(2):505–512. [Google Scholar]
- 29.Pourahmad J, Eskandari MR, Shakibaei R, Kamalinejad M. A search for hepatoprotective activity of aqueous extract of Rhus coriaria L. against oxidative stress cytotoxicity. Food and Chemical Toxicology. 2010;48:854–858. doi: 10.1016/j.fct.2009.12.021. [DOI] [PubMed] [Google Scholar]
- 30.Mohammadi S, Montasser Kouhsari S, MonavarFeshani A. Antidiabetic properties of the ethanolic extract of Rhus coriaria fruits in rats. DARU. 2010;18(4):270–275. [PMC free article] [PubMed] [Google Scholar]
- 31.Salimi Z, Heidari R, Nejati V, Eskandary A, Ghasemi Z. Effect of Sumac (Rhus coriaria L.) extract on lipid peroxidation and diabetic nephropathy in diabetic rats. Journal of Birjand University of Medical Sciences. 2012;18(4):275–284. [Google Scholar]
- 32.Lee K, Kim J, Lee BJ, Park JW, Leem KH, Youngmin Bu. Protective effects of Galla Rhois, the excrescence produced by the sumac aphid, Schlechtendalia chinensis, on transient focal cerebral ischemia in the rat. Journal of Insect Science. 2012;12 doi: 10.1673/031.012.0110. Article 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Candan F, Sökmen A. Effects of Rhus coriaria L. (Anacardiaceae) on Lipid Peroxidation and Free Radical Scavenging Activity. Phytother Res. 2004;18:84–86. doi: 10.1002/ptr.1228. [DOI] [PubMed] [Google Scholar]
- 34.Shafiei M, Nobakht M, Moazzam AA. Lipid-lowering effect of Rhus coriaria L. (sumac) fruit extract in hypercholesterolemic rats. Pharmazie. 2011;66:988–992. [PubMed] [Google Scholar]
- 35.Madihi Y, Merrikhi A, Baradaran A, Rafieian-kopaei M, Shahinfard N, Ansari R, et al. Impact of Sumac on postprandial high-fat oxidative stress. Pak J Med Sci. 2013;29(1 Suppl):340–345. [Google Scholar]
- 36.Magarey AM, Daniels LA, Boulton TJ, Cockington RA. Predicting obesity in early adulthood from childhood and parental obesity. Int J Obes Relat Metab Disord. 2003;27(4):505–513. doi: 10.1038/sj.ijo.0802251. [DOI] [PubMed] [Google Scholar]
- 37.WMA Declaration of Helsinki – Ethical Principles for Medical Research Involving Human Subjects. [accessed: Augt 2014]. Available at: http://www.wma.net/en/30publications/10policies/b3/
- 38.Kelishadi R, Hashemipour M, Sarraf-Zadegan N, Amiri M. Trend of atherosclerosis risk factors in children of Isfahan. Asian Cardiovasc Thorac. 2001;9:36–40. [Google Scholar]
- 39.Behrman R, Kliegman R, Jenson H. 19th ed. Philadelphia: Elsevier Saunders; 2011. Nelson textbook of pediatrics. [Google Scholar]
- 40.Kelishadi R, Hashemi M, Mohammadifard N, Khavarian N. Association of changes in oxidative and proinflammatory states with changes in vascular function after a lifestyle modification trial among obese children. Clinical chemistry. 2008;54(1):147–153. doi: 10.1373/clinchem.2007.089953. [DOI] [PubMed] [Google Scholar]
- 41.Waterman PG, Mole S. Oxford: Blackwell Scientific Publication; 1994. Analysis of phenolic plant metabolites. [Google Scholar]
- 42.Kelishadi R, Sadri G, Tavasoli AA, Kahbazi M, Roohafza HR, Sadeghi M, et al. Cumulative prevalence of risk factors for atherosclerotic cardiovascular diseases in Iranian adolescents: IHHP-HHPC. J Pediatr (Rio J) 2005;81(6):447–453. doi: 10.2223/JPED.1418. [DOI] [PubMed] [Google Scholar]
- 43.Kelishadi R, Razaghi EM, Gouya MM, Ardalan G, Gheiratmand R, Delavari A, et al. Association of physical activity and the metabolic syndrome in children and adolescents: CASPIAN Study. Horm Res. 2007;67(1):46–52. doi: 10.1159/000096121. [DOI] [PubMed] [Google Scholar]
- 44.Sabzghabaee AM, Ataei E, Kelishadi R, Ghannadi A, Soltani R, Badri S, et al. Effect of Hibiscus sabdariffa Calices on Dyslipidemia in Obese Adolescents: A Triple-masked Randomized Controlled Trial. Mater Sociomed. 2013;25(2):76–79. doi: 10.5455/msm.2013.25.76-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sabzghabaee AM, Khayam I, Kelishadi R, Ghannadi A, Soltani R, Badri S, et al. Effect of Zizyphus Jujuba Fruits on Dyslipidemia in Obese Adolescents: a Triple-masked Randomized Controlled Clinical Trial. Med Arh. 2013;67(3):156–159. [PubMed] [Google Scholar]
- 46.Sabzghabaee AM, Kelishadi R, Jelokhanian H, Asgary S, Ghannadi A, Badri S. Clinical Effects of Portulaca Oleracea Seeds on Dyslipidemia in Obese Adolescents: a Triple-blinded Randomized Controlled Trial. Med Arh. 2014;68(3):195–199. [PubMed] [Google Scholar]
- 47.Shabbir A. Rhus coriaria Linn, a plant of medicinal, nutritional and industrial importance. The Journal of Animal and Plant Sciences. 2012;22(2):505–512. [Google Scholar]
- 48.Kosar M, Bozan B, Temelli F, Baser KHC. Antioxidant activity and phenolic composition of sumac (Rhus coriaria L.). extracts. Food Chemistry. 2007;103:952–959. [Google Scholar]
- 49.Kossah R, Nsabimana C, Zhang H, Chen W. Optimization of Extraction of Polyphenols from Syrian Sumac (Rhus coriaria L.). and Chinese Sumac (Rhustyphina L.) Fruits. Research Journal of Phytochemistry. 2010:1–8. [Google Scholar]
- 50.Bashash M, Bolandi M, Zamindar N. Phenolic Content of Selected Sumac Fruits from Iran, Extracted With Different Solvents. Journal of Chemical Health Risks. 2012;2(4):17–20. [Google Scholar]
- 51.Zargham H, Zargham R. Tannin extracted from Sumac inhibits vascular smooth muscle cell migration. McGill Journal of Medicine. 2008;11(2):119–123. [PMC free article] [PubMed] [Google Scholar]
- 52.Beretta G, Rossoni G, Santagati NA, Facino RM. Anti-ischemic activity and endothelium-dependent vasorelaxant effect of hydrolysable tannins from the leaves of Rhus coriaria (Sumac) in isolated rabbit heart and thoracic aorta. Planta Med. 2009;75(14):1482–1488. doi: 10.1055/s-0029-1185797. [DOI] [PubMed] [Google Scholar]
- 53.Pourahmad J, Eskandari MR, Shakibaei R, Kamalinejad M. A search for hepatoprotective activity of aqueous extract of Rhus coriaria L. against oxidative stress cytotoxicity. Food and Chemical Toxicology. 2010;48:854–858. doi: 10.1016/j.fct.2009.12.021. [DOI] [PubMed] [Google Scholar]
- 54.Hashemi M, Kelishadi R, Hashemipour M, Zakerameli A, Khavarian N, Ghatrehsamani S, et al. Acute and long-term effects of grape and pomegranate juice consumption on vascular reactivity in paediatric metabolic syndrome. Cardiol Young. 2010;20(1):73–77. doi: 10.1017/S1047951109990850. [DOI] [PubMed] [Google Scholar]
- 55.Canadan F. Effect of Rhus coriaria L. (Anacardiaceae) on superoxide, free radical scavenging and xanthine oxidase activity. J of Enzyme Inhibitor and Medical Chemistry. 2003;18(1):59–62. doi: 10.1080/1475636031000069273. [DOI] [PubMed] [Google Scholar]


