ABSTRACT
Introduction.
Clinical researches have shown an increased bone disintegration and lower bone mass in patients with calcium urolithiasis.
Goal.
The goal of our research was to establish the incidence of osteoporosis in adult patients with calcium urolithiasis, on the basis of measuring mineral bone density, using DEXA method, with a special reflection on age subgroups.
Material and methods.
Clinical research was prospective and it was implemented at the University Clinical Center of Banja Luka, at the Clinic for Endocrinology, Diabetes and Metabolic Diseases and at the Urology Clinic. Material in this research consisted of patients divided in two groups, a working and a control group. One hundred and twenty (120) patients were included in both these groups, divided in three age subgroups: 20-40, 40-60 and over 60. The working group consisted of the patients with calcium urolithiasis and the control group consisted of patients without calcium urolithiasis. Establishing of mineral bone density at L2-L4 of lumbal spine vertebrae and hip was done for the patients in both these groups, using DEXA method.
Results.
Analysis of mineral bone density using DEXA method in patients in age groups of working and control groups, as well as in the total sample of working and control groups, have shown that the patients of the working group, over 60, had a decreased mineral bone density (30% of osteopenia and 15% osteoporosis) significantly more expressed when compared to the other two age groups (12.5% in the subgroup 20-40 and 17.5% in the subgroup 40-60), which presents a statistically significant difference (p<0.05). In the control group, when taking into account age groups, osteopenia and osteoporosis were marked in 37.5% and 2.5% in the group of patients over 60, whereas in the youngest population, 5% of osteopenia was found, which presents a statistically significant difference (p<0.05). When observing the total sample of working and control group, there was a statistically significant difference in the working and control group (p<0.01); incidence of osteoporosis in the working group amounted to 7.5% and in the control group it was 0.8%.
Conclusion.
Urolithiasis and osteoporosis are two multifactorial diseases which are evidently reciprocal. This is why we suggest that educating the population about the risk factors for occurrence of these diseases as well as preventive measures that may contribute to their decrease should begin as early as possible.
Keywords: Urolithiasis, calcium kidney stones, osteoporosis, DEXA
1. INTRODUCTION
Urolithiasis and osteoporosis are two multi-factorial diseases, which today, in the epidemiological sense, take an increasingly significant position, with tendency of a constant growth of their incidence rate due to extended life age of people and the factor of unhealthy lifestyle. They are of a social significance – they are an economic and health problem, for an individual as well as for the society on the whole, as the price of treatment is exceptionally expensive (1). Clinical and epidemiological research has shown an increased disintegration and a lower bone mass in patients with urolithiasis. The loss of bone mass and occurrence of osteoporosis was particularly found in idiopathic calcium urolithiasis (2, 3). An additional problem is in women with a negative calcium balance, which is twofold in the period of menopause, a surplus of hormones, such as parathyroid hormone or thyroid gland hormone, as well as a decreased level of estrogen, may cause the loss of calcium, the consequence of which is creation of calcium stones in suffering patients (4, 5, 6, 7). However, pathogenetic mechanisms and factors related to the loss of bone mass in these patients are still being researched (8, 9).
2. RESEARCH GOAL
Having in mind the significance of diseases, the goal of this work was to evaluate correlation between the calcium urolithiasis and osteoporosis qualified using DEXA method.
3. MATERIAL AND METHODS
The clinical research was prospective and it was implemented at the University Clinical Center of Banja Luka, at the Urology Clinic and Clinic for Endocrinology, Diabetes and Metabolic Diseases in the period May 2012 – January 2013.
The material in this project consisted of the patients divided in two groups: working and control groups. One hundred and twenty (120) patients were included in both these groups, divided in three age subgroups: 20-40, 40-60 and over 60. Ultrasonography of the urinary tract was done for all the patients. The patients with ultrasonography findings, which pointed out towards the presence of urolithiasis, were candidates for the working group. They have underwent additional diagnostic procedures of native X-ray scans of the urinary tract, intravenous urography and laboratory checks. Excluding factors were increased value of uric acid (uric stones), infectious (struvite) calculosis, taking of bisphosphonates, hyperparathyroidism, the patients with malign diseases who were receiving hormone therapy (Ca of the prostate, Ca of the breast). The control group consisted of the population of patients, for whom, using initial ultrasound diagnostics, it was established that they do not suffer from upper urinary tract calculosis. After that, the patients of the working and control group underwent densitometry using the instrument LUNAR DPX Product Division Americus GE Healthcare (GENERAL ELECTRIC COMPANY 2006), with the aim to measure mineral bone density, DXA L2-L4 of the lumbal spine and hip – upper part of the femur, including the femur neck.
4. RESULTS
Demographically observed, there were 120 patients in the working and control group: 40 patients in each of the three age subgroups: 20-40, 40-60 and over 60, with an average age of 50,19 years, with a variation interval of 21-86. Basic description of the measure, when observed on the total sample of the working group, are given in Table 1.
Table 1.
Arithmetic mean of the working group patients by age structure
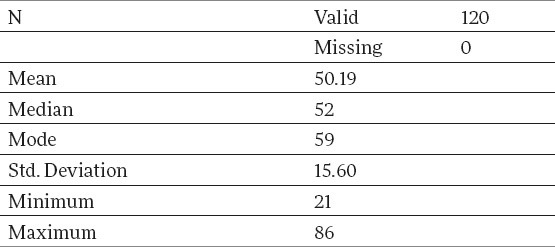
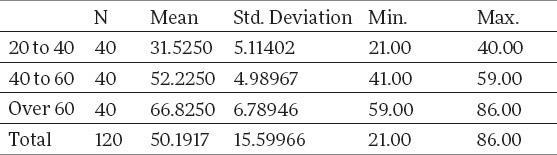
Average age of the patients by age categories of the working group is shown in Table 2.
Table 2.
Arithmetic mean of the con rkign ure n observed on the total sample of the working group, are given in Table 1. Three age subgroutrol group patients by age structure
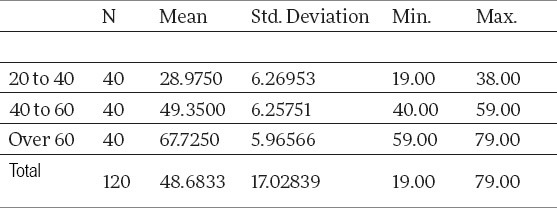
Observed on the total sample of the control group, consisting of the patients without calculosis, from Table 3, it is seen that the average age of the patients amounted to 48.68, with a variation interval of 19-79. Descriptive measures, which describe the age category of the control group sample, are given in Table 4.
Table 3.
Descriptive measures giving the control group age category
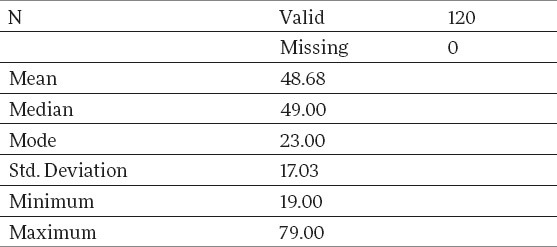
Table 4.
Arithmetic mean by age category of the control group patients
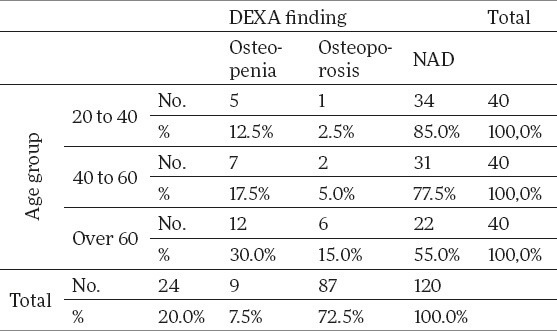
Having analyzed the mineral bone density using DEXA method by age categories of the working group, the research has shown that with the oldest subgroup of patients over 60, the share of osteopenia0%) andest subgroup of patients over 60, the share of osteoporosis ries of the working group, the research has shown that w (30.0%) and osteoporosis (15%) is much higher when compared to the other two age subgroups (12.5% in the subgroup of 20-40 and 17.5% in the subgroup of 40-60), which presents a statistically significant difference (p<0.05). Results of DEXA findings by age categories of the working group have been shown in Table 5.
Table 5.
Results of DEXA findings by the working group age categories
Having analyzed mineral bone density using DEXA method by the control group age categories, the research has shown that a share with osteopenia is a significantly lower than in the subgroup of 20-40 (5%) in comparison to the other two age groups (p<0.05), that is, that the share of those with NAD DEXA finding are statistically higher in the group of 20-40 years when compared to the other two age groups (p<0.05). This is shown in Table 6.
Table 6.
Results of DEXA findings by the control group age categories
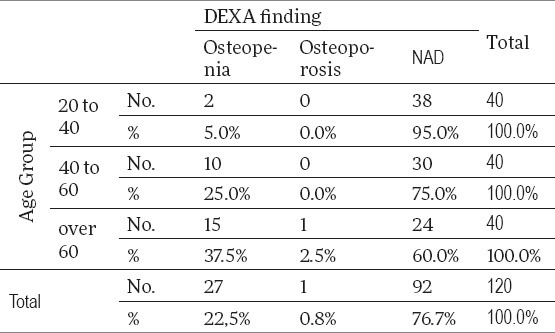
Having applied the hi-square test, we monitored whether the NAD results of DEXA test depended on the age and we have come to the conclusion that there is a huge statistical dependence with this. NAD results of DEXA test drop with age, that is, a share of those not having an NAD test is higher with the older population (Table 6). As for age groups, it can be easily found that, with the youngest population, the share of those with NAD results of DEXA test was 95%, while in the oldest group, this share was significantly lower and it amounted to 60%, which presents a statistically significant difference and confirms the correlation (p<0.01).
During the research, we tested the differences between the incidence of osteoporosis and osteopenia on the total sample of the working and control group (testing of the balance of proportions of two samples, the so-called Z value) and we have come to the result that there was a statistically significant difference in the incidence of osteoporosis in the working and control group (p<0.01); that is, the incidence of osteoporosis in the working group has amounted to 7,5%, whereas in the control group the share of osteoporosis was 0.8%. There was no statistically significant difference of incidence of osteopenia between the working and control groups (Z value was -0.47, that is, p>0.05).
5. DISCUSSION
Many authors have described in their studies a decreased bone mass (osteopenia/osteoporosis) in patients with calcium urolithiasis and hypercalciuria, however, a decreased bone mass is also found with the normocalciuria patients (10, 11, 12). Jeger and associates have established that the bone mass was decreased in all the patients suffering from urolithiasis, that is, that there is an evident reciprocity between urolithiasis and loss of bone mass, regardless of the value of individual parameters (13). The loss of bone mass is especially emphasized with idiopathic calcium urolithiasis, even though no study up to now has managed to identify the mechanisms of reaction and factors which could be used to foresee changes in the mineral bone density (14). A limited amount of calcium, an increased input of salts and animal proteins, polymorphism of receptors for vitamin D may be risk factors in these patients, whereas the role of inflammatory cytokines, osteopontine and prostaglandins, as mediators of bone resorption, are yet to be established (15-18). Newer studies have shown, by analyzing multiple regression, that there is a significant influence of age as well as a daily input of calcium, on the loss of bone mass in the patients with urolithiasis. Bone mass was significantly decreased in patients with urolithiasis compared to the control group (p< 0.001) and it was more conditioned by an older age, particularly in women in menopause (19). Reference data show that clinical and epidemiological research have shown that in older patients with urolithiasis, there is an increased bone disintegration and reduced mineral bone density (MBD) (20, 21).
The results of our study have shown that mineral bone density, on the total working group sample, as per DEXA finding, was in 27.5% cases below expected values: osteopenia was represented in 20% and osteoporosis in 7.5%. Analyzing the results by age subgroups, they have shown that the patients of the oldest subgroups (>60), had a decreased mineral bone density (30% osteopenia and 15% osteoporosis) are significantly more expressed compared to the other two age subgroups (12.5% in the subgroup of 20-40 and 17.5% in the subgroup of 40-60), which presents a statistically significant difference.
In the control group of our study, when observing the total sample of osteopenia and osteoporosis was marked in 23.3% (osteopenia in 22.5% and osteoporosis in 0.8%), whereas, when observed by age groups, 5% of osteopenia was found in the youngest subgroup, that is, 37.5% in the oldest group of patients, which presents a statistically significant difference.
During our research, testing of differences of incidence of osteopenia and osteoporosis in the working and control groups were found, using a statistical method of testing of balance of proportion of the two samples based on the so-called Z value (normal distribution). The results of our research have shown that between the patients in the working and control group there is a statistically significant difference in the incidence of osteoporosis, as follows: in the working group of 7.5% compared to the control group 0.8% (p< 0.01).
Having analyzed the obtained results of our study, by the application of hi-square test, we have shown that there is a correlation of the age group of the patients on one side and DEXA findings on the other; that is, the share of the NAD DEXA findings was significantly lower in age subgroups over 60 (40%) compared to the other two age subgroups (5% and 25%), which presents a statistically highly significant difference.
6. CONCLUSION
The results of the implemented research, when observed on the whole, confirm the correlation between the urolithiasis and osteoporosis, which implies to the need to look at both these diseases simultaneously in a prevention and therapy sense.
Footnotes
CONFLICT OF INTEREST: NONE DECLARED.
REFERENCES
- 1.Caudarella R, Vescini F, Bufa A, La Manna G, Stefoni S. Osteoporosis and urolithiasis. Urol Int. 2004;72(Suppl.1):17–19. doi: 10.1159/000076585. [DOI] [PubMed] [Google Scholar]
- 2.Mente A, Honey RJ, McLaughlin JR, Bull SB, Logan AG. Ethnic differences in relative risk of idiopathic calcium nephrplithiasis in North America. J Urol. 2007;178:1992–1997. doi: 10.1016/j.juro.2007.07.024. [DOI] [PubMed] [Google Scholar]
- 3.Worcester Em, Coe FL. New insights into the pathogenesisof idiopathic hypercalciuria. Semin Nephrol. 2008;28:120–132. doi: 10.1016/j.semnephrol.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sakhaee K, Maalouf NM, Kumar R, et al. Nephrolithiasis-associated bone disease: pathogenesis and treatment options. Kidney Int. 2011;79(4):393–403. doi: 10.1038/ki.2010.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Basiri A, Shakhssalim N, Knoshdel AR, et al. Familiar relations and recurrence pattern in nephrolithiasis: new words about old subjects. Urol. 2010 Jun 10;7(2):81–86. [PubMed] [Google Scholar]
- 6.Gallagher JC. Role of estrogens in the management of postmenopauzal bone loss. Rheum Dis Clin North Am. 2001;27:143–162. doi: 10.1016/s0889-857x(05)70191-5. [DOI] [PubMed] [Google Scholar]
- 7.Joo NS, Hughes BD, Kim YS, Oh K, Yeum KJ. Impact of calcium and vitamin D insufficiencies on serum parathyroid hormon and bone mineral density: Analysis of the fourth and fiften Korea National Health and Nutrition Examination Survey ( KNHANES IV-3, 2009 and KNHANES V-1 2010) J Bone Miner Res. 2013;28(4):764–770. doi: 10.1002/jbmr.1790. [DOI] [PubMed] [Google Scholar]
- 8.Goldfar DS, Fischer ME, Keich Y, et al. A twin study of genetic and dietary influences on nephrolithiasis;a report from the Vietnam Era Twin (VET) Registry. Kidny Int. 2005 Mar;67(3):1053–1561. doi: 10.1111/j.1523-1755.2005.00170.x. [DOI] [PubMed] [Google Scholar]
- 9.Shoback D. Update in Osteoporosis and metabolic bone disorders. J Clin Endokrinol Metab. 2007;92:3–9. doi: 10.1210/jc.2007-0042. [DOI] [PubMed] [Google Scholar]
- 10.Tsuji H, Umekawa T, Kurita T, et al. Analysis of bone mineral density in urolithiasis patients. Int urol. 2005;12:335–339. doi: 10.1111/j.1442-2042.2005.01049.x. [DOI] [PubMed] [Google Scholar]
- 11.Peris P, Martinez- Ferrer A, Monegal A, et al. Aetiology and clinical characteristics of male osteoporosis. Have they changed in the last few years? Clinin Exp Rheumatol. 2008;26:582–588. [PubMed] [Google Scholar]
- 12.Tugcu V, Ozbek E, Aras B, Ozbay B, Islim F, Tasci Al. Bone mineral density measurement in patients with recurrent normocalciuric calcium stone disease. Urol Res. 2007;35:29–34. doi: 10.1007/s00240-006-0074-0. [DOI] [PubMed] [Google Scholar]
- 13.Jaeger P, Lippuner K, Casez JP, Hess B, Ackermann D, Hug C. Low bone mass in idiopathic renal stone formers: magnitude and significance. J bone Miner Res. 1994;9:1525–1532. doi: 10.1002/jbmr.5650091004. [DOI] [PubMed] [Google Scholar]
- 14.Asplin JR, Donahue S, Kinder J, Coe FL. Urine calcium excretion predicts bone loss in idiopathic hypercalciuria. Kidney Int. 2006;70:1463–1467. doi: 10.1038/sj.ki.5001778. [DOI] [PubMed] [Google Scholar]
- 15.Harrington M, Bennett T, Jakobsen J, et al. The effect of a high-protein, high-sodium diet on calcium and bone metabolism in postmenopausal women and its interaction with vitamin D receptor genotype. Br J Nutr. 2004;91:41–51. doi: 10.1079/bjn20031016. [DOI] [PubMed] [Google Scholar]
- 16.Weisinger JR, Alonzo E, Bellorin- Font E, et al. Possible role of cytokines on the bone mineral loss in idiopathic hypercalciuria. Kidney Int. 1996;49:244–250. doi: 10.1038/ki.1996.34. [DOI] [PubMed] [Google Scholar]
- 17.Jackon RD. Calcium plus vitamin D suplementation and the risk of fractures. N Engl J Med. 2006;354(7):669–683. doi: 10.1056/NEJMoa055218. [DOI] [PubMed] [Google Scholar]
- 18.Trinchieri A. Bone mineral content in calcium renal stone formers. Urol Res. 2005;33:247–253. doi: 10.1007/s00240-005-0498-y. [DOI] [PubMed] [Google Scholar]
- 19.Maalouf NM, Sato AH, Welch BJ, et al. Postmenopausal hormone use and the risk of nephrolithiasis: results from the Women's Health Initiative hormone therapy trials. Arch Intern Med. 2010;170(18):1678–1685. doi: 10.1001/archinternmed.2010.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bergsland KJ, Kinder JM, Asplin JR, Coe BJ, Coe FL. Influence of gender and age on calcium oxalate crystal growth inhibition by urine from relatives of stone forming patients. J Urol. 2002;167:2372–2376. [PubMed] [Google Scholar]
- 21.Arrabal- Polo MA, Arrabal-Martin M, de Haro-Munoz T, et al. Mineral density and bone remodeling markers in patients with calcium lithiasis. BJU Int. 2010;108:1903–1908. doi: 10.1111/j.1464-410X.2011.10167.x. [DOI] [PubMed] [Google Scholar]


