ABSTRACT
Introduction:
Patient-oriented therapy represents a modern approach in the treatment of patients with diabetes, an approach which is supported in the most recent guidelines by the ADA and the European Association for the Study of Diabetes (EASD). The progressive nature of diabetes demands the introduction of insulin therapy much earlier in order to prevent the development of late complications of the disease.
Material and methods:
The study included 30 patients who had been treated with long-acting insulin analogue and metformin in doses of 3 x 850 mg at least 6 months prior to study entry and in which a good glycaemic control had not been achieved, or with HbA1c > 7%. Patients who had a BMI > 28 kg /m2 were included in the study.
Results and discussion:
At the beginning of the study the patients were switched to combined therapy with long-acting basal analog, metformin and liraglutide in a dosage of 0.6 mg of 1x1. After 12 weeks of the new therapeutic regimen we recorded a significant reduction in the parameter levels that we monitored in the study. BMI value after the test was 28.2±1.39 kg/m2, p=0.025, HbA1c 7.24±0.47%, p=0.030, fasting blood glucose level 7.04±0.32 mmol/l, p=0.023, postprandial glucose level 7.6±0.46 mmol/l, p=0.012, systolic blood pressure level 123±5.75 mmHg, p=0.015, diastolic blood pressure level 79.1±2.91 mmHg, p=0.03. During research that we have conducted over 12 weeks, a reduction of body weight was achieved while improving the value of parameters significant for the study.
Conclusion:
There was a significant lowering of HbA1c, fasting blood glucose levels, postprandial glucose levels and better blood pressure control by which we have proved that GLP1 analogues in combination with basal insulin and metformin provide a good glycaemic control with a cardio protective effect, and reduce the risk of late complications.
Keywords: diabetes mellitus, GLP 1, basal insulin analogues, obese patients
1. INTRODUCTION
Patient-oriented therapy represents a modern approach in the treatment of patients with diabetes, an approach which is supported in the most recent guidelines by the ADA and the European Association for the Study of Diabetes (EASD) (1). The progressive nature of diabetes demands the introduction of insulin therapy much earlier in order to prevent the development of late complications of the disease. Though often this is not enough, it is necessary to combine other anti-diabetic agents in order to achieve target blood glucose values. It is necessary to consider multiple parameters for each individual during the introduction of anti-diabetic agents which will allow minimizing hypoglycemia and weight gain. Results of major world studies clearly indicate that efforts for better glucose control often lead to the risk of hypoglycemia which is a significant risk factor for development of cardiovascular complications. It is necessary to consider the duration of the disease for each and every individual, as well as the presence of late complications and comorbidities with consideration of potential adverse effects of the treatment (2). The current therapeutic algorithms are more and more the subject of a debate, which is why there is a constant need for seeking to improve the therapeutic approach by the introduction of new anti-diabetic agents.
There is a connection between obesity and type 2 diabetes. The prevalence of diabetes in the world is caused by the increasing number of obese people, and at the same time, the treatment of patients with type 2 diabetes leads to weight gain. Evidence provided from the world studies show that patients who were treated with insulin during the twelve-year period, on average gained about 8 kg in weight. Similar effects are shown by the treatment with sulfonylurea and thiazolidinedione derivatives which leads to hypoglycemia and weight gain. All these facts clearly indicate that we must pay special attention to the treatment of obese patients with type 2 diabetes.
Figure 1.
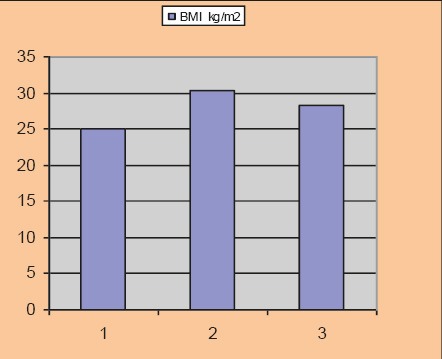
BMI before and after the test
Figure 2.
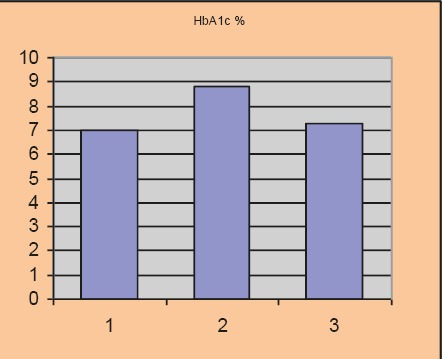
HbA1c before and after the test
Figure 3.
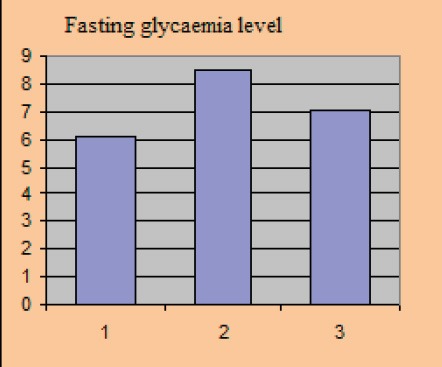
Fasting glycaemia level
Figure 4.
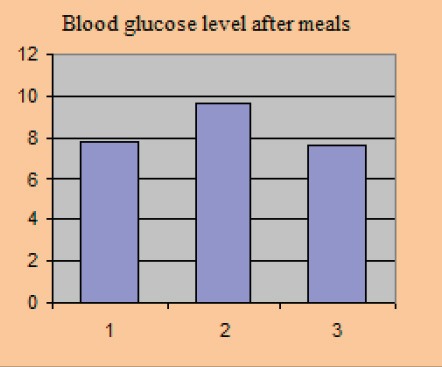
Blood glucose level after meals
Ideal treatment of type 2 diabetes involves a treatment of glycemia, blood pressure, dyslipidemia, and body weight. Increntin therapy that includes agonists of the GLP 1 receptors, acts on lowering the level of HbA1c and body weight, together with the reduction of cardiovascular risk factors such as blood pressure and dyslipidemia (3, 4).
2. GOAL
To compare the effects of therapy with basal insulin analogues combined with a GLP 1 analogue and metformin with therapy of basal insulin analogues in combination with metformin.
3. MATERIAL AND METHODS
The study included 30 patients who had been treated with long-acting insulin analogue and metformin in doses of 3 x 850 mg at least 6 months prior to study entry and in which a good glycaemic control had not been achieved, or with HbA1c > 7%. Patients who had a BMI> 28 kg /m2 were included in the study. The parameters monitored prior and after the study were: BMI, HbA1c, blood pressure, mean blood glucose - fasting and postprandial. The patients were switched with new therapy using basal insulin analogs combined with GLP1 analogues and metformin for 12 weeks. We compared the parameters prior and after the study.
4. RESULTS
The study included 30 patients who were treated in the Counseling Clinic of the Endocrinology Clinic in Sarajevo. For all patients included in the study we calculated the average standard deviation.
The average age of the involved population group amounted to 54.1±5.06 years; duration of diabetes was 4.06±1.73 years. The study included 18 women and 12 men. The average BMI at inclusion in the study was 30.4±1.42 kg/m2.
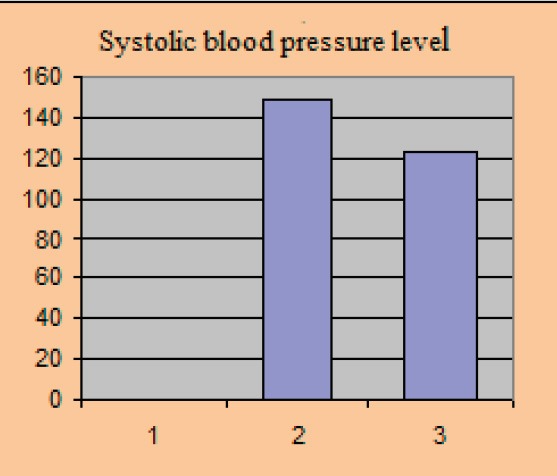
Average baseline parameter values covered in the study amounted to: HbA1c 8.81±0.77%, the average value of fasting blood glucose level was 8.4±0.61 mmol/l, postprandial glycaemic level was 9.61±0.84 mmol/l, systolic blood pressure level with included stable dose of antihypertensive 149.0±8.15 and diastolic blood pressure level 90.3±3.2 mmHg.
Figure 5.
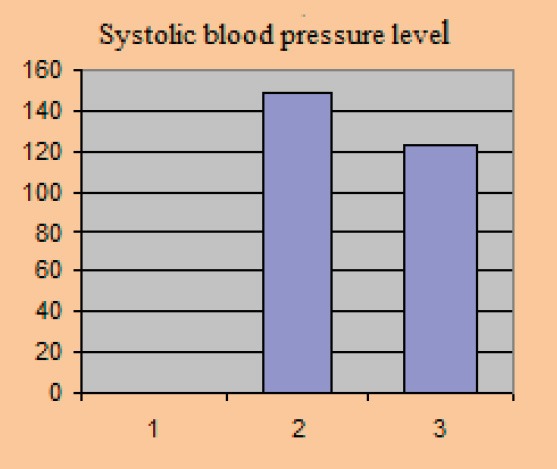
Systolic blood pressure level
Figure 6.
Diastolic blood pressure level.
At the beginning of the study the patients were switched to combined therapy with long-acting basal analog, metformin and liraglutide in a dosage of 0.6 mg of 1x1.
After 12 weeks of the new therapeutic regimen we recorded a significant reduction in the parameter levels that we monitored in the study. BMI value after the test was 28.2±1.39 kg/m2, p=0.025, HbA1c 7.24±0.47%, p=0.030, fasting blood glucose level 7.04±0.32 mmol/l, p=0.023, postprandial glucose level 7.6±0.46 mmol/l, p=0.012, systolic blood pressure level 123±5.75 mmHg, p=0.015, diastolic blood pressure level 79.1±2.91 mmHg, p=0.03.
5. DISCUSSION
Diabetes mellitus type 2 has a progressive course which requires patients’constant monitoring of glycemia together with supervision of attending physicians in order to act in a timely manner and include additional antidiabetic drug. It is clear that hyperglycemia leads to further destruction of pancreatic beta cells because of their gluco-toxicity and further progression of diabetes disease and complications afterwards. In recent decades, attempts are made to find the optimal therapeutic solution that will enable action on complete pathophysiology mechanism of type 2 diabetes and also at the same time, reduce the risk of weight gain and the occurrence of hypoglycemia.
Long-acting basal insulin analogues provide 24- hour supply of insulin, which compensates for the lack of endogenous insulin that has arisen because of the destruction of beta cells. Exogenous insulin also suppresses hyperinsulinemia that develops due to insulin resistance which is one of the characteristics of type 2 diabetes. This explains the significance of including long-acting insulin analogues in the treatment of type 2 diabetes. ORIGIN trial clearly represents the significance of the basal insulin analogues in the treatment of diabetes and impaired glucose tolerance. During the six years of treatment with basal long-acting insulin analogues, number of newly diagnosed patients with type 2 diabetes was reduced in the treated population with impaired glucose tolerance. Exogenous insulin prevents and delays at the same time further destruction of the beta cells of the pancreas, thus reducing glucotoxicity (5).
Incretin hormone glucagon-like-peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) are released into the small intestine during the food intake and increase in blood glucose in the body. Incretin hormones stimulate glucose-dependent insulin secretion, whereas GLP 1 also suppresses glucagon secretion thus delaying the gastrointestinal emptying and lowering postprandial glycemia. Type 2 diabetes is associated with reduced increntin activity. Therefore, the introduction of exogenous GLP 1 analogue provides for pharmacological increntin effect. The pharmacological effect of GLP1 involves stimulation of endogenous insulin secretion, suppressing the secretion of glucagon, which provides good glycaemic control. At the same time, this is the basic pathophysiological disorder in type 2 diabetes. It is important to be pointed out that the effect is observed only at elevated blood glucose, thereby reducing the risk of hypoglycemia. Delayed emptying of the stomach and GLP1 action in the central nervous system on appetite decrease enables the reduction of body weight.
It is demonstrated through study that after 8 years of insulin therapy application leads to increase in body weight by 5 kg. This justifies the importance of the use of GLP1 analogues in combination with long-acting insulin analogue (6,7,8).
Metformin, which is a mild insulin senziter but has a significant effect on suppression of gluconeogenesis, prevents destruction of the beta cells and has an anti-atherogenic effect. With all this being said, metformin is a significant addition to the regulation of postprandial glucose.
This therapeutic approach enables action on different pathophysiological processes in type 2 diabetes reducing the risk of weight gain and hypoglycemia which were leading therapeutic challenges over the last decade in the treatment of type 2 diabetes.
During research that we have conducted over 12 weeks, a reduction of body weight was achieved while improving the value of parameters significant for the study. There was a significant lowering of HbA1c, fasting blood glucose levels, postprandial glucose levels and better blood pressure control by which we have proved that GLP1 analogues in combination with basal insulin and metformin provide a good glycaemic control with a cardio protective effect, and reduce the risk of late complications (9,10,11).
6. CONCLUSION
Intensification of treatment of type 2 diabetes by adding liraglutide in addition to existing basal insulin analogue and metformin therapy provides improvements in HbA1c levels together with better glycaemic control, fasting/postprandial glucose levels and body weight. Significant reductions in blood pressure poster an additional vasodilatory effect and reduce the risk of cardiovascular complications.
Footnotes
CONFLICT OF INTEREST: NONE DECLARED.
REFERENCES
- 1.Inzucchi SE, Bergenstal RM, Buse JB, et al. American Diabetes Association (ADA);European Association for the Study of Diabetes (EASD). Management of hyperglycemia in type 2 diabetes: a patient-centered approach. Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes Care. 2012;35:1364–1379. doi: 10.2337/dc12-0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bennett WL, Maruthur NM, Singh S, et al. Comparative effectiveness and safety of medications for type 2 diabetes: an update including new drugs and 2-drug combinations. Ann Intern Med. 2011;154:602–613. doi: 10.7326/0003-4819-154-9-201105030-00336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hollander P, Raslova K, Skjoth TV, Rastam J, Liutkus JF. Efficacy and safety of insulin detemir once daily in combination with sitagliptin and metformin: the transition randomized controlled trial. Diabetes, Obesity & Metabolism. 2011;13:268–275. doi: 10.1111/j.1463-1326.2010.01351.x. [DOI] [PubMed] [Google Scholar]
- 4.Bain SC, Seufert J, Thomsen AB, Furber S, Alessio D. Liraglutide +metformin in type 2 diabetes: clinical benefits associated with switch or use early in the disease process. Diabetes. 2010;61(Suppl 1):A301. abstract 1168-P. [Google Scholar]
- 5.The Origin Trial Investigators Basal Insulin and Cardiovascular and Other Outcomes in Dysglycemia. N Engl J Med. 2012;367:319–328. doi: 10.1056/NEJMoa1203858. [DOI] [PubMed] [Google Scholar]
- 6.Clinical Trials.gov. Liraglutide effect and action in diabetes: evaluation of cardiovascular outcome results – a long term evaluation (LEADER®) ( NCT01179048) 2012a. [accessed December 18 2012]. Available from: http://www.clinicaltrials.gov/ct2/show/NCT01179048 .
- 7.ClinicalTrials.gov. The effect of liraglutide versus placebo when added to basal insulin analogues with or without metformin in subjects with type 2 diabetes ( NCT01617434) 2012c. [accessed December 18 2012]. Available from: http://clinicaltrials.gov/ct2/show/NCT01617434 .
- 8.DeVries JH, Bain SC, Rodbard HW, Seufert J, D’Alessio D, Thomsen AB, et al. Sequential intensification of metformin treatment in type 2 diabetes with liraglutide followed by randomized addition of basal insulin prompted by A1C targets. Diabetes Care. 2012;35:1446–1454. doi: 10.2337/dc11-1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hsu PF, Sung SH, Cheng HM, Yeh JS, Liu WL, Chan WL, et al. Association of clinical symptomatic hypoglycemia with cardiovascular events and total mortality in type 2 diabetes mellitus: a nationwide population-based study. Diabetes Care. 2012;36:894–900. doi: 10.2337/dc12-0916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, et al. Management of hyperglycaemia in type 2 diabetes: a patient-centered approach. Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetologia. 2012;55:1577–1596. doi: 10.1007/s00125-012-2534-0. [DOI] [PubMed] [Google Scholar]
- 11.Pratley R, Nauck M, Bailey T, Montanya E, Cuddihy R, Filetti S, et al. One year of liraglutide treatment offers sustained and more effective glycaemic control and weight reduction compared with sitagliptin, both in combination with metformin, in patients with type 2 diabetes: a randomised, parallel-group, open-label trial. International Journal of Clinical Practice. 2011;65:397–407. doi: 10.1111/j.1742-1241.2011.02656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


