SUMMARY
The Salvador-Warts-Hippo (Hippo) pathway is an evolutionarily conserved regulator of organ growth and cell fate. It performs these functions in epithelial and neural tissues of both insects and mammals, and in mammalian organs such as the liver and heart. Despite rapid advances in Hippo pathway research, a definitive role for this pathway in hematopoiesis has remained enigmatic. The hematopoietic compartments of Drosophila melanogaster and mammals possess several conserved features [1, 2]. D. melanogaster possess three types of hematopoietic cells that most closely resemble mammalian myeloid cells: plasmatocytes (macrophage-like cells), crystal cells (involved in wound healing) and lamellocytes (which encapsulate parasites). The proteins that control differentiation of these cells also control important blood lineage decisions in mammals [3–10]. Here, we define the Hippo pathway as a key mediator of hematopoiesis by showing that it controls differentiation and proliferation of the two major types of D. melanogaster blood cells, plasmatocytes and crystal cells. In animals lacking the downstream Hippo pathway kinase Warts, lymph gland cells overproliferated, differentiated prematurely and often adopted a mixed lineage fate. The Hippo pathway regulated crystal cell numbers by both cell autonomous and non-cell autonomous mechanisms. Yorkie and its partner transcription factor Scalloped were found to regulate transcription of the Runx family transcription factor, Lozenge, which is a key regulator of crystal cell fate. Further, Yorkie or Scalloped hyperactivation induced ectopic crystal cells in a non-cell autonomous, and Notch pathway-dependent fashion.
RESULTS AND DISCUSSION
Hippo pathway components are expressed in D. melanogaster hematopoietic cells
We adopted D. melanogaster as a system to investigate a potential role for the Hippo pathway in hematopoiesis since this pathway was first discovered and is best understood in this organism. The best described hematopoietic organ in D. melanogaster is the larval lymph gland, which matures during larval development and ruptures during metamorphosis to give rise to circulating hemocytes in the pupa and adult [1, 2]. The lymph gland is a paired multi-lobed structure: the large primary lymph gland lobes contain differentiating cells in the cortical zone, whilst the medullary zone contains undifferentiated cells and the posterior signalling centre (PSC) acts as a hematopoietic niche which serves to maintain the medullary zone prohemocyte population [1, 2]. The secondary lobes, which vary in number but usually consist of between 2–4 paired lobes, contain undifferentiated hemocyte progenitor cells.
Initially, we studied expression of Hippo pathway components and found that the upstream pathway members Merlin (Mer), Fat (Ft) and four-jointed (fj) were expressed throughout the lymph gland, as was the key transcriptional co-activator protein Yki (Figures S1A–S1E). Some cells exhibited higher Yki expression (Ykihigh), suggesting that Yki activity is non-uniform in lymph glands. 86% of Ykihigh cells were Hindsight positive (Hnt+), which marks terminally differentiated crystal cells [11] (Figures S1D″ and S1E″). Scalloped (Sd), the best-defined of Yki’s partner transcription factors [12–16], was observed throughout the lymph gland, was strongest in the primary lobe medullary zone and was expressed at low levels in 89% of crystal cells (Figures S1F and S1G).
The Hippo pathway kinase, Warts, regulates blood cell differentiation
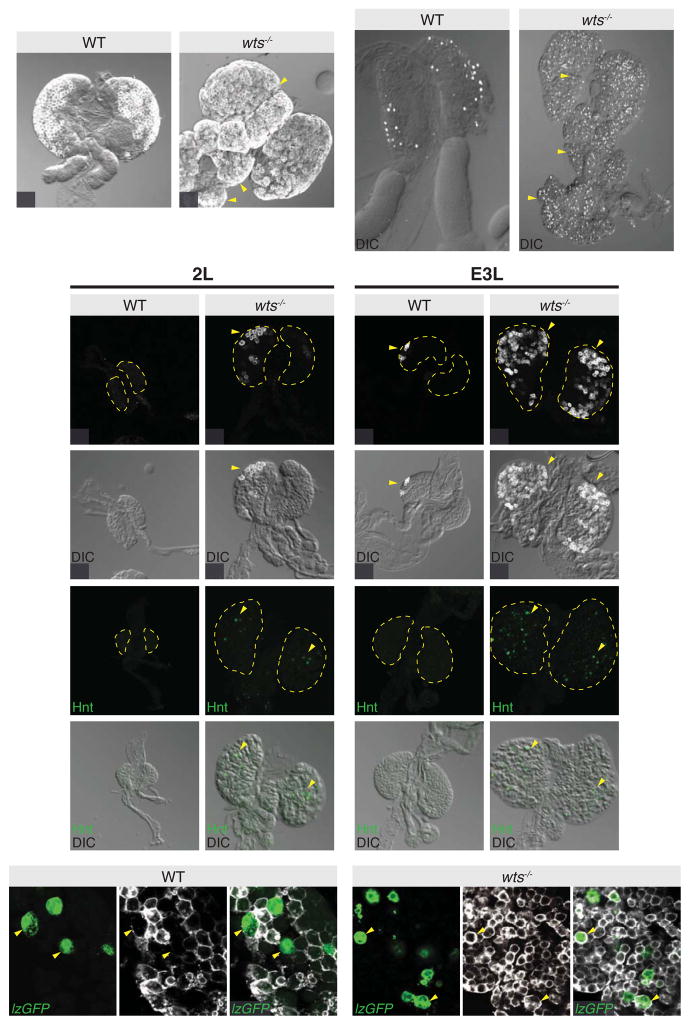
To determine whether the Hippo pathway regulates hematopoietic development, we analysed the number and location of the two predominant differentiated cells, plasmatocytes (P1+) and crystal cells (Hnt+), in lymph glands from wts hypomorphs. Both plasmatocytes and crystal cells were increased in number, and were present throughout the medullary zone of the primary lobe and the secondary lobes where normally only hemocyte progenitors reside, rather than being restricted to the cortical zone of the primary lobe (Figures 1A–1D). To analyse this more closely, we assessed hemocyte differentiation in wild-type and wts lymph glands throughout development in carefully staged animals. Plasmatocytes and crystal cells were absent from wild-type lymph glands at the late second larval instar, however, strikingly, the majority of wts mutant glands displayed strong expression of both Hnt and P1 (Figures 1E–1H′). P1+, but not Hnt+ cells began to appear in wild-type lymph glands in early third larval instar lymph glands, but both cell populations were prevalent in wts lymph glands (Figures 1I–1L′).
Figure 1. Warts regulates blood cell differentiation.
(A–D) Control w1118 (A and C) and wts mutant primary lymph glands (B and D). Plasmatocytes were visualized by anti-P1 staining (greyscale in A and B) and merged with direct interference contrast images. wts mutant lymph glands exhibit P1 expression outside of its normal domain (yellow arrowheads). Crystal cells were visualized by anti-Hnt staining (greyscale in C and D). Primary and secondary lobes are labelled 1° and 2° respectively. wts mutant lymph glands possessed significantly more crystal cells as well as precocious differentiation of secondary gland hemocytes into crystal cells (yellow arrowheads in D).
(E–L) Late second instar control w1118 (E and G) and wts mutant (F and H) lymph glands, and early third instar control w1118 (I and K) and wts mutant (J and L) lymph glands. Plasmatocytes were visualized by anti-P1 staining (greyscale in E, F, I and J) and crystal cells were visualized by anti-Hnt staining (green in G, H, K and L) and merged with direct interference contrast images in (E′–L′). wts mutant lymph glands exhibited differentiation of both plasmatocytes and crystal cells at late second instar larval stages, while control lymph glands did not.
(M–N) Control w1118 (M–M″) and wts mutant primary lymph glands (N–N″). Crystal cells were marked with lz-Gal4 driven expression of UAS-GFP (green in M and N) and plasmatocytes were visualized by anti-P1 staining (greyscale in M′ and N′). Crystal cells did not express P1 in control lymph glands (yellow arrowheads in M–M″), while there was a significant number of crystal cells that also expressed P1 in wts mutant lymph glands (yellow arrowheads in N–N″). GFP and protein expression images are merged in (M″ and N″).
Scale bars = 20μm. See also Figure S1.
Differentiation into either a crystal cell or a plasmatocyte are mutually exclusive fate decisions [17, 18]. To determine whether this fate choice occurred normally in cells with aberrant Hippo pathway activity, we analysed lymph glands from 15 wild-type and 15 wts animals, using P1 antibodies and lz-Gal4 driven expression of UAS-GFP, which marks crystal cells. All wild-type lymph glands displayed either lz+ or P1+ cells but never lz+ P1+ (double positive) cells (Figures 1M–1M″). By contrast, all 15 wts lymph glands displayed multiple cells that were positive for both lz-Gal4 and P1 (Figures 1N–1N″). In three wts lymph glands, the majority of Lz+ cells also expressed P1, whereas12 wts lymph glands displayed more single lz+ cells than lz+ P1+ positive cells. Collectively, these results indicate two key roles for the Hippo pathway in hemocyte differentiation: 1) it maintains the progenitor state of hemocyte progenitors during lymph gland development; and 2) it prevents hemocyte progenitors from differentiating inappropriately to adopt a mixed crystal cell/plasmatoctye fate. The Hippo pathway regulates specific cell fate choices such as the decision between inner cell mass and trophectoderm in the early mouse embryo [19] and between R8 photoreceptor subtypes in the D. melanogaster eye [20, 21]. In these scenarios, the Hippo pathway regulates a binary fate choice, i.e. a decision between one cell type or another. Our data show that the Hippo pathway does not regulate blood cell fate by stimulating binary fate decisions, but rather prevents premature differentiation of both major D. melanogaster blood lineages.
The Hippo pathway kinase, Warts, regulates blood cell proliferation and lymph gland size
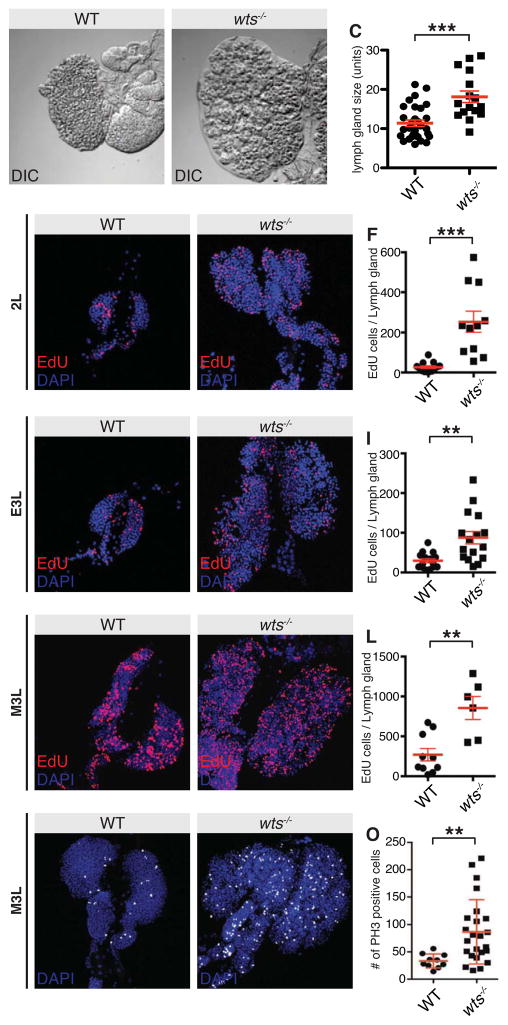
We also noted that wts lymph glands were larger than in control animals. When quantified, wts lymph glands were 58% larger in size than controls, showing that the Hippo pathway also limits lymph gland growth, comparable to its growth-repressive function in other larval tissues such as the imaginal discs and brain (Figures 2A–2C and S2A–S2D) [22–24]. To analyse this more closely we assessed the proliferation profiles of age-matched wts mutant and wild-type lymph glands, throughout development. At each larval stage analysed (late second instar, early third instar and mid-third instar) wts lymph glands were larger and possessed significantly more cells in S phase than did control lymph glands (Figures 2D–2L). Similar results were observed when mitotic cells were analysed using phospho-Histone H3 antibodies (Figures 2M–2O), indicating that Wts normally limits lymph gland hemocyte proliferation. The fact that wts lymph glands were larger and more proliferative cannot account for the observed premature differentiation, as excess proliferation induced by loss of other growth suppressors in the lymph gland such as Tuberous Sclerosis Complex 2 does not cause premature differentiation [25].
Figure 2. Warts regulates lymph gland size and hemocyte proliferation.
(A–C) Direct interference contrast images of control w1118 (A) and wts mutant late third instar lymph glands (B). Lymph gland size was quantified in (C). wts mutant lymph glands were significantly larger than controls (p<0.0001; n=30 for w1118, n=16 for wts-Z; error bars represent SEM).
(D–L) Control w1118 (D, G and J) and wts mutant lymph glands (E, H and K) at late second instar (D–E), early third instar (G–H) and mid third instar (J–K). Cells undergoing DNA replication were visualised using EdU (red in D, E, G, H, J and K) and nuclei were marked with DAPI (blue in D, E, G, H, J and K). The number of EdU positive cells per lymph gland was quantified in (F, I and L).
(M–O) Control w1118 (M) and wts (N) mutant mid third instar larval lymph glands. Mitotic cells were visualised using anti phospho-Histone H3 (white) and nuclei were marked with DAPI (blue). The number of phospho-Histone H3 positive cells per lymph gland was quantified in (O).
Scale bars = 20 μm. See also Figure S2.
The key Hippo pathway transcriptional regulator Yorkie influences crystal cell numbers
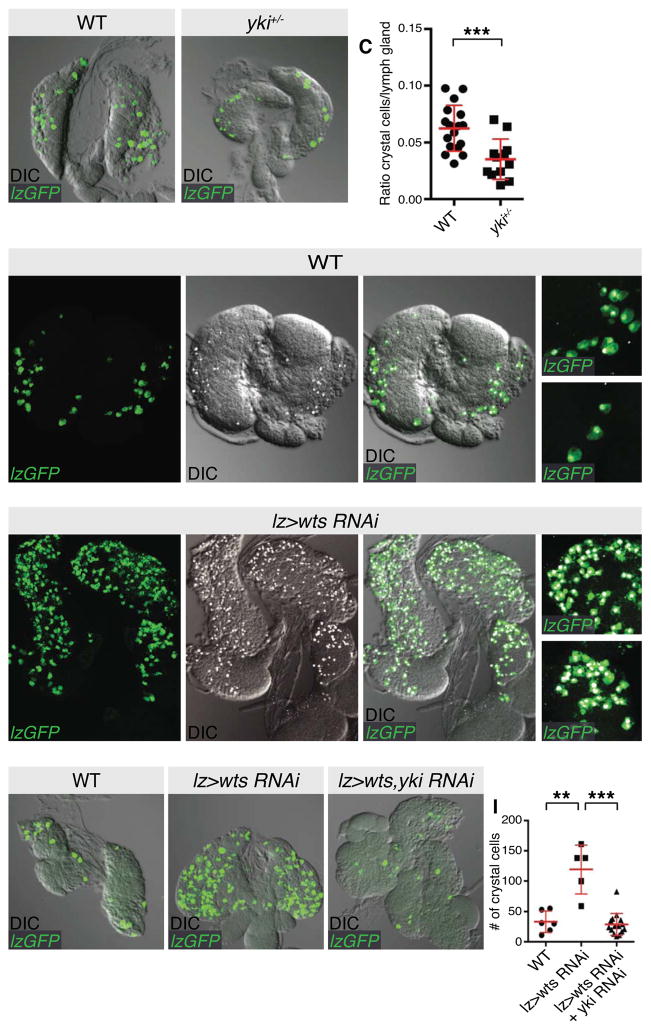
Yki is the key effector of the Hippo pathway and is a direct substrate of the Wts kinase [26]. To explore a role for Yki in hematopoiesis, we employed a null allele, ykiB5. yki mutant animals are lethal soon after embryogenesis [26]. Therefore, we measured the ratio of crystal cells per lymph gland cell (assessed by DAP1 intensity) in third instar larval control animals or yki hemizygotes. As shown in Figures 3A–3C, yki hemizygotes displayed a significant reduction in crystal cells compared to wild-type animals, suggesting that full Yki activity is required for development of the complete complement of crystal cells.
Figure 3. The Hippo pathway transcriptional regulator Yorkie influences crystal cell numbers.
(A–C) A control w1118 lymph gland (A) and a lymph gland that was hemizygous for ykiB5 (B). Crystal cells were visualized by expression of UAS-GFP (green) with the lz-Gal4 driver. The ratio of GFP-positive crystal cells relative to DAPI-positive lymph gland cells from multiple animals were quantified in (C). Lymph glands from ykiB5 hemizygous animals possessed significantly fewer crystal cells (p=0.0008; n=17 for A, n=12 for B; error bars represent SEM).
(D–E) Control lymph glands (D–D‴) or lymph glands expressing the wts RNAi transgene (E–E‴) under the control of the lz-Gal4 driver. Crystal cells were visualized by co-expression of UAS-GFP (green in D–E) and staining for Hnt (greyscale in A′–C′ and merged with direct interference contrast images). GFP and protein expression images are merged with direct interference contrast images in (D″–E″). Magnified images are shown in (D‴–E‴) showing that lz-Gal4 GFP and Hnt expression overlap in both genotypes.
(F–I) Control lymph glands (F), lymph glands expressing the wts RNAi transgene (G) or lymph glands expressing RNAi transgenes for wts and yki (H) under the control of the lz-Gal4 driver. Crystal cells were visualized by expression of UAS-GFP (green in F–H) and merged with direct interference contrast images. GFP-positive crystal cells were quantified in (I). wts RNAi-expressing lymph glands possessed significantly more crystal cells than controls (p=0.001; n=6 for F, n=5 for G; error bars represent SEM), while wts RNAi, yki RNAi-expressing lymph glands significantly suppressed this increase (p<0.0001; n=5 for G, n=15 for H; error bars represent SEM).
Scale bars = 20 μm. See also Figure S2.
To determine whether the Hippo pathway regulates crystal cells in a cell autonomous fashion, we depleted Wts by RNA interference, or overexpressed hyperactive versions of Yki (Yki3SA [27]) or Sd (SdGA [15]) under the control of the crystal cell-specific lz-Gal4 driver. In each case, we observed a significant increase in crystal cells, compared to controls (Figures 3D–3E‴ and Figures S2E–S2H). The increase in crystal cells upon Wts depletion was dependent on Yki as when both proteins were knocked down, the increase in crystal cells was significantly suppressed (Figures 3F–3I). Yki hyperactivation induced ectopic crystal cells in a cell autonomous manner, as lz-driven GFP completely overlapped with Hnt (Figures 3D‴, 3E‴ and S2E‴). To determine whether Sd reduction displayed a similar phenotype to yki hemizygosity, we depleted Sd by RNAi in developing crystal cells using lz-Gal4. Unexpectedly, we observed a two-fold increase in crystal cells (Figures S2I, S2J and S2L). This observation was reminiscent of Yki and Sd’s role in posterior follicle cell fate in the D. melanogaster ovary. At stage 7–9, both loss of function or gain of function Sd induces the same phenotype i.e. increased Cut+ cells [28], because Sd functions with Yki to induce Cut expression in these cells but also acts as a default repressor of Cut when Yki activity is low. However, depletion of the Sd co-repressor Tgi using lz-Gal4 by RNAi did not affect crystal cell number, suggesting that it is not an important regulator of crystal cell number or fate (Figures S2K, L). Collectively, this data suggests that the Hippo pathway regulates proliferation and/or terminal differentiation of Lz+ progenitor cells, which differentiate into crystal cells.
The Hippo pathway regulates expression of the key crystal cell fate determinant, Lozenge
The above data show that total crystal cell numbers in larval lymph glands are sensitive to modulation of Hippo pathway activity during crystal cell maturation. Therefore, we investigated functional links between Lz and the Hippo pathway further. The human homologues of Yki and Lz [YAP and Acute myeloid leukemia 3 (AML3), also known as Polyomavirus enhancer binding protein (PEBP2α)] form a physical complex and YAP activates AML3 in transcription assays [29]. Therefore, we tested whether Yki and Lz formed a physical interaction in S2 cells, but failed to detect such an association (Figure S3A).
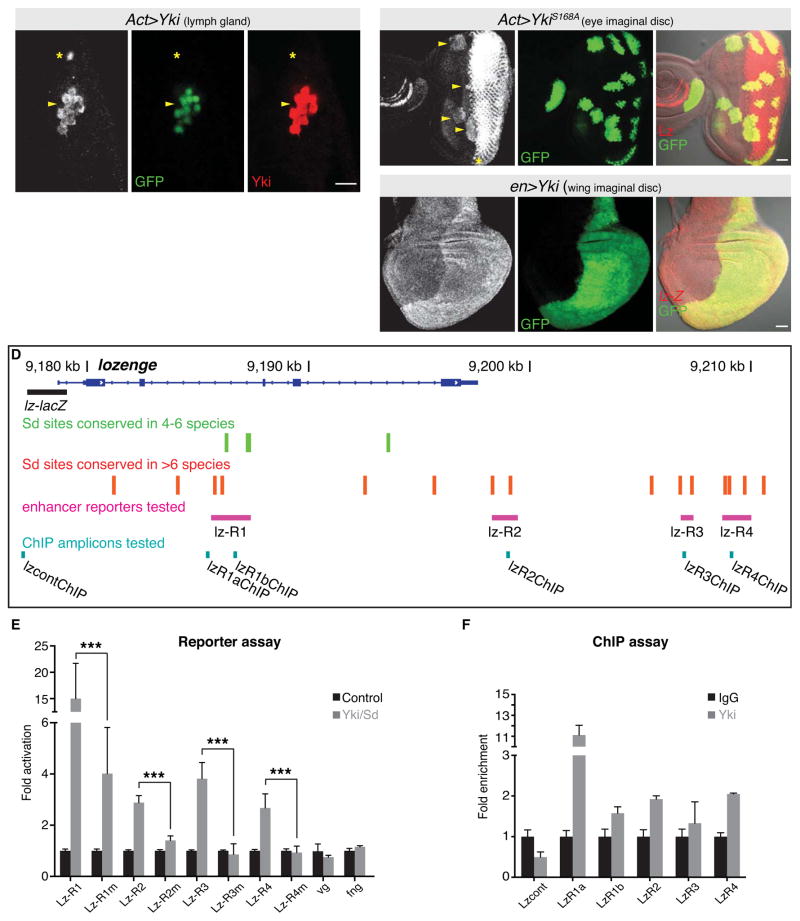
Next, given that wts loss led to premature induction of crystal cells, as well as ectopic crystal cells in lymph gland regions that normally only harbour progenitor cells, we considered the possibility that Yki and Sd regulate Lz expression. To test this, we induced small Yki clones in lymph glands, using the Actin-Gal4 flip out technique. Normally, Lz is expressed in crystal cells of the cortical zone of the primary lymph gland, as well as in the differentiating photoreceptor cells of the third instar larval eye disc [4, 30]. Strikingly, in yki-overexpressing clones, we observed ectopic induction of Lz in both the primary and secondary lymph gland lobes (Figures 4A–4A″, S3B–S3B‴ and not shown). The ability of Yki to induce Lz expression in normally Lz- cells was independent of proliferation, as Lz was induced in single cell yki-expressing clones (Figures S3B–S3B‴). We also observed ectopic expression of Lz in yki-overexpressing clones anterior to the normal Lz expression domain in third instar larval eye-antennal discs (Figures 4B–4B″). This effect was independent of the UAS-yki transgene utilized as we observed the same result with three independent transgenes (Figure S3C–S3E′). Furthermore, we observed ectopic Lz expression in yki-overexpressing clones in larval tissues that do not normally express Lz expression, such as the brain, wing and leg discs (Figure S3F–S3G′ and not shown). To determine whether Yki regulates Lz at the level of transcription, we used a transgenic strain that harboured a 1.5kb fragment of the lz promoter fused to the β-galactosidase gene [31]. When yki was overexpressed with either en-Gal4 or ptc-Gal4, we observed elevation of lz-lacZ showing that Yki indeed can regulate lz at the level of transcription (Figures 4C–4C″ and Figure S3H–S3H″).
Figure 4. The Hippo pathway regulates expression of the key crystal cell fate determinant, Lozenge.
A–B) A primary third instar larval lymph gland (A–A″) and eye-antennal disc (B–B″) harbouring clones of tissue expressing UAS-yki transgenes (marked by GFP in green). Expression of Lz (greyscale in A and B, red in merged image B″) and Yki (red in A″) are shown. Ectopic expression of Lz was induced by yki overexpression in both lymph glands (indicated by arrowhead in A–A″) and eye-antennal discs (indicated by arrowheads in B–B″). Asterisks mark endogenous Lz expression in the lymph gland (A–A″) and the posterior region of the eye-antennal disc (B).
(C) lz expression, as reported by a 1.5kb region of the lz promoter fused to the lacZ gene, in a wing imaginal disc expressing UAS-yki in the posterior domain under the control of en-gal4. lz expression is greyscale in the single channel (C) and red in the merged image (C″), while UAS-yki expression is marked by co-expression of UAS-GFP (green in C′ and C″).
(D) Schematic of the lz gene locus showing conserved Sd sites, the lz-lacZ reporter, the lz reporters (pink) and the ChIP-qPCR amplicons (turquoise).
(E) Reporter assays demonstrate that several regions of the lz locus that bear conserved Sd sites can mediate transcriptional activation by Sd/Yki in Kc167 cells. When these conserved Sd sites were mutated (“m”), this activation was suppressed or abolished. The vg and fng reporters serve as negative controls.
(F) ChIP-qPCR validates binding of endogenous Yki to several of these reporter regions in Kc167 cells, particularly to the R1 region. Yki did not associate with the control region lacking Sd sites.
Scale bars = 20μm. See also Figures S3 and S4.
Scalloped and Yorkie directly activate lozenge transcription in Kc167 cells
Our finding that ectopic Yki cell autonomously induced Lz expression in vivo prompted us to search for direct evidence for regulation of lz transcription by Yki and one of its partner transcription factors Sd. Sd was found to bind the TEAD/TEF family DNA binding motif, CATTCCA, in a Sd/Yki-responsive enhancer of diap1 [14]. A similar but more divergent consensus emerged from genome-wide analysis of Sd binding [32]. We identified 19 candidate Sd motifs, CATTCY and CATTYC, in the entire lz gene that exhibited some evidence for conservation (Figure 4D). Of these, 15 sites were well conserved based on their preservation to D. pseudoobscura or beyond (Figure 4D), suggesting that lz is under strong and extensive evolutionary constraint for regulation by Sd.
We assayed four genomic regions (lz-R1, lz-R2, lz-R3 and lz-R4) containing multiple conserved Sd binding sites for responsiveness to ectopic Sd/Yki in reporter assays in Kc167 cells, which display hemocyte properties [33, 34] and express Lz [18]. When co-transfected with Sd and Yki we observed upregulation of all four lz reporters but no effect on reporters bearing regulatory regions from the vestigial and fringe loci (Figure 4E) [35], neither of which contain Sd sites. All four lz reporters required Sd binding for activation by Yki, as mutation of the Sd sites suppressed or ablated luciferase activity (Figure 4E). These data provide evidence that Sd and Yki can directly activate lz gene expression via multiple cis-regulatory regions in the lz locus.
Since these were ectopic tests, we wished to assess whether endogenous Yki could be detected at the lz locus. We used chromatin immunoprecipitation (ChIP) to query Yki occupancy at the lz enhancers in Kc167 cells. We observed ~2-fold enrichment of Yki to the lz-R1b and lz-R2 ChIP amplicons, but not to a control region upstream of lz that lacked a Sd consensus site (Figure 4F). The conserved sites within the lz-R1 enhancer are towards the 5′ end of this region, but this region lacks desirable amplicon properties. However, we assayed another amplicon (R1a) and found strong (~10-fold) enrichment to Yki at this genomic site (Figure 4F). Taken together, these data support the notion that Yki is endogenously recruited to these Sd sites in hemocytes, and that the Yki/Sd complex activates lz transcription.
The Hippo pathway non-cell autonomously influences crystal cell numbers in a Notch pathway-dependent fashion
When analysing the effects of Yki overexpression on Lz in lymph glands, we noted that expression of hyperactive Yki (Yki3SA) sometimes led to non-cell autonomous induction of Lz (not shown). To investigate this further we expressed either Yki3SA or SdGA with hml-Gal4 [1]. Expression of either transgene induced a significant increase in crystal cell numbers (Figures S4A–S4D). Upon close examination, crystal cells almost never overlapped with hml-expressing cells, indicating that induction of ectopic crystal cells by hyperactive Yki and Sd occurred in a non-cell autonomous fashion (Figures S4E–S4G). A major determinant of crystal cells is the Notch pathway [5, 6, 18], which acts during the third instar larval period of development to promote differentiation of crystal cell progenitors to become mature crystal cells [17]. Consistent with this, a substantial increase in cells displaying Notch activity, observed using a Notch response element-GFP reporter [18], was observed in hml-SdGA lymph glands (Figure S4H–S4I′). Furthermore, the ability of hml-driven Yki3SA to induce ectopic crystal cells was reliant on full Notch pathway activity, as this phenotype was suppressed in animals that were heterozygous for the Notch pathway transcription factor Suppressor of Hairless [Su(H)] (Figure S4J–S4L). Whilst a mechanistic understanding of Notch-Hippo collaboration in the lymph gland requires further study, it is interesting to note that these pathways collaborate to effect numerous development processes in tissues such as the D. melanogaster ovary and brain [36, 37], and the murine embryo and liver [38–40].
CONCLUDING REMARKS
The Hippo pathway is an important regulator of tissue growth and cell-fate decisions in epithelial and neural tissues from both insects and mammals [41]. Here we show that the Hippo pathway controls the overall size of the major hematopoietic organ of D. melanogaster, the larval lymph gland, and promotes the progenitor cell state by repressing Yki activity. Whilst the anti-proliferative activity of the Hippo pathway in the lymph gland mirrors its growth-suppressive role in many D. melanogaster and mammalian tissues, its effects on differentiation do not. For example, wts loss in the larval eye delays or impairs differentiation of pro-neural cells [42, 43], and blocks the transition from neuroepithelium to neuroblasts in the larval brain [36].
In mammals, Runx1, GATA2 and the Notch pathway cooperate to regulate differentiation of megakaryocytes [9]. These same factors also regulate crystal cell fate in D. melanogaster [1–3]. Given that the Notch and Hippo pathways appear to collaborate to regulate crystal cell fate, and that Yki can induce expression of the key Runx family transcription factor, Lz, it is possible that the Hippo pathway also controls mammalian megakaryocyte differentiation. Further, given the well-documented role of the Hippo pathway as a tumour suppressor pathway in human cancers [41, 44], this study provides rationale for an investigation of the Hippo pathway in the aetiology of hematopoietic malignancies.
Supplementary Material
Supplemental Figure S1, related to Figure 1. Hippo pathway components are expressed in D. melanogaster hematopoietic cells.
(A–C) Primary third instar w1118 larval lymph gland lobes. Antibodies detected Mer protein expression (red in A) or Ft protein expression (red in B). fj mRNA expression was reported using a fj-lacZ enhancer trap (red in C). The cortical zone of each primary lymph gland lobe is marked with Cg-Gal4 driven expression of UAS-GFP (green in A′–C′). GFP and protein/gene expression images are merged in (A″–C″). Ft and fj displayed some enrichment in the cortical zone whereas Mer was expressed in a mostly unpatterned fashion.
(D–G) Primary third instar w1118 larval lymph glands. Antibodies were used to detect Yki protein expression (red in D, D″, E and E″) or Sd protein expression (red in F, F″, G and G″). Crystal cells were marked with either a Hnt-specific antibody (green in D′, D″, E′ and E″) or lz-Gal4 driven expression of UAS-GFP (green in F′, F″, G′ and G″). GFP and protein expression images are merged in (D″–G″). In (E–E″) yellow arrowheads indicate crystal cells with high Yki expression, while the asterisk shows a Hnt positive cell with moderate Yki expression. In (G–G″), yellow arrowheads indicate crystal cells that express low levels of Sd.
Scale bars = 20μm
Supplemental Figure S2, related to Figures 2 and 3. Hippo pathway perturbation regulates lymph gland size and crystal cell number.
(A–D) Primary third instar larval lymph glands; the cortical zone (A–B′) is marked with Cg-Gal4 driven expression of UAS-GFP (greyscale in single images and green in merged images), and the medullary zone (C–D′) is marked by expression of 10xSTATGFP, a GFP transgene under transcriptional control of STAT92E binding sites (greyscale in single images and green in merged images). Nuclei are marked with DAPI (blue in merged images). w1118 control lymph glands are shown in (A, A′, C and C′) and wts-lacZ mutant lymph glands are shown in (B, B′, D and D′). wts lymph glands display a proportional increase in size over control lymph glands, without obvious changes in the relative sizes of the cortical and medullary zones.
(E–H) Lymph glands expressing Yki3SA and GFP transgenes (E–E‴), GFP transgene alone (F) or SdGA and GFP transgenes (G) using the lz-Gal4 driver. Crystal cells were visualized by expression of GFP (green) in (E, E″, E‴, F and G) and/or Hnt (white) in (E–E‴) and are merged with direct interference contrast images (DIC) in (E′, E″, F and G). GFP-positive crystal cells from individual primary lymph gland lobes from multiple animals in (F and G) were quantified in (H). SdGA-expressing lymph glands possessed significantly more crystal cells than control lymph glands (p<0.0001; n=14 for F, n=24 for G; error bars represent SEM).
(I–L) A control lymph gland (I) and lymph glands expressing RNAi transgenes for Sd (J) or Tgi (K) under the control of the lz-Gal4 driver. Crystal cells were marked with lz-Gal4 driven expression of UAS-GFP (green in I–K) and anti-Hnt staining (white in I–K) and are merged with direct interference contrast images. Hnt positive crystal cells from primary lymph gland lobes from at least 15 animals were quantified in (L). Sd RNAi-expressing lymph glands possessed significantly more crystal cells than controls, whereas Tgi RNAi-expressing lymph glands did not (p=0.02; n=17 for I, n=25 for J, n=22 for K; error bars represent SEM).
Scale bars = 20μm.
Supplemental Figure S3, related to Figure 4. The Hippo pathway regulates expression of the key crystal cell fate determinant, Lozenge.
(A) S2 cells transfected with epitope-tagged plasmids for Lz, Yki and/or Wts and immunoprecipitated with anti-HA antibodies. Upper immunoblots are of anti-HA immunoprecipitates and lower blots are of input lysates. The positive control protein Wts formed a physical complex with Yki, but Lz did not.
(B–B‴) A third instar larval lymph gland harbouring single cell clones generated using the Actin-Gal4 flip-out technique and expressing a UAS-yki transgene. Expression of Lz (greyscale in B) and Yki (red in B′) are shown, and merged in (B″ and B‴) with a direct interference contrast (DIC) image in (B‴). Ectopic Lz protein expression was observed in single cell yki-expressing lymph gland clones.
(C–H) Third instar larval eye imaginal discs (C–E′, anterior is to the left), wing discs (F, F′ and H–H‴) and a brain lobe (G, G′), harbouring clones of GFP-positive tissue generated using the Actin-Gal4 flip-out technique. (C) Control clones expressed GFP only. (D), (F) and (G) Clones expressed Yki (from a UAS-yki transgene on the second chromosome) and GFP. (E) Clones expressed Yki (from a UAS-yki transgene on the third chromosome) and GFP. In (H) ptc-Gal4 was used to express Yki (from a UAS-yki transgene on the second chromosome) along the anterior/posterior boundary of the wing disc. Lz protein expression (greyscale in C–G and red in C′–G′ was detected using a Lz-specific antibody. lz transcription was reported in (H–H‴) using a lz promoter construct fused to the β-galactosidase gene. Ectopic Lz protein expression (yellow arrowheads in D–G) was observed in yki-expressing eye antennal disc clones anterior to the normal Lz domain, and in wing discs and brain lobes (which do not normally express Lz). lz transcription was detected in the ptc domain of wing discs overexpressing yki in (H–H″).
Scale bars = 20μm.
Supplemental Figure S4, related to Figure 4. The Hippo pathway non-cell autonomously influences crystal cell numbers in a Notch pathway-dependent fashion.
(A–G) hml-Gal4, UAS-GFP x w1118 control (A), hml-Gal4 UAS-GFP, UAS-Yki3SA (B) and hml-Gal4 UAS-GFP, UAS-SdGA (C) primary third instar larval lymph glands stained for Hnt (greyscale in A–C′) and merged with direct interference contrast (DIC) images in (A′–C′). Crystal cells were quantified from individual primary lymph gland lobes from several animals in (D). Yki3SA- expressing lymph glands possessed significantly more crystal cells (p= 0.0017; n=19 for A, n=19 for B, error bars represent SEM), as did SdGA-expressing lymph glands (p< 0.0001; n=12 for C, error bars represent SEM). (E–G) Close-up images of single sections of lymph glands expressing UAS-GFP alone (E) or together with either UAS-Yki3SA (F) or UAS-SdGA (G) under the control of hml-Gal4. Expression of Yki3SA or SdGA induced ectopic crystal cells in a non-cell autonomous fashion.
(H–I) The Notch reporter NRE-GFP (green in H–I′) was combined with either hml-Gal4 x w1118 control (H) or hml-Gal4 x UAS-SdGA (I) and assessed in third larval instar lymph glands. NRE-GFP reporter activity was merged with direct interference contrast (DIC) images in (H′ and I′). hml-Gal4-driven expression of SdGA caused a strong increase in the number of cells that were positive for Notch activity.
(J–L) hml-Gal4, UAS-Yki3SA (J) and hml-Gal4, UAS-Yki3SA x Su(H)Δ47 (K) third larval instar lymph glands stained for Lz (red in J and K, and merged with direct interference contrast images (DIC) in J′ and K′). Lz positive crystal cells from individual primary lymph gland lobes from several animals were quantified in (L). Yki3SA-expressing lymph glands possessed significantly more crystal cells than w1118 (p< 0.001; n=19 for w1118, n=18 for J), while lymph glands that expressed Yki3SA but were heterozygous for Su(H)Δ47 had significantly less crystal cells than those expressing Yki3SA in a wild-type background (p< 0.05; n=20 for K; error bars represent SEM).
Scale bars = 20μm.
Acknowledgments
We thank M. Crozatier for comments on an earlier version of this manuscript and I. Ando, E. Bach, S. Bray, C. Desplan, R. Fehon, K. Guss, I. Hariharan, K. Irvine, J. Jiang, D. Jukam, L. Madden, D. Pan, L. Waltzer, T. Xu, the Developmental Studies Hybridoma Bank, the Australian D. melanogaster Research Support Facility (www.ozdros.com), the Vienna D. melanogaster RNAi Centre and the Bloomington Stock Centre for fly stocks, antibodies and plasmids. K.F.H is a Sylvia and Charles Viertel Senior Medical Research Fellow. F.G was a Cancer Council Victoria Postdoctoral Research Fellow. This work was supported by a Project Grant from the National Health and Medical Research Council of Australia. Q.D. was supported by a fellowship from the Swedish Research Council. Work in E.C.L.’s group was supported by the National Institutes of Health R01NS083833 and R01NS074037.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jung SH, Evans CJ, Uemura C, Banerjee U. The Drosophila lymph gland as a developmental model of hematopoiesis. Development. 2005;132:2521–2533. doi: 10.1242/dev.01837. [DOI] [PubMed] [Google Scholar]
- 2.Krzemien J, Crozatier M, Vincent A. Ontogeny of the Drosophila larval hematopoietic organ, hemocyte homeostasis and the dedicated cellular immune response to parasitism. Int J Dev Biol. 2010;54:1117–1125. doi: 10.1387/ijdb.093053jk. [DOI] [PubMed] [Google Scholar]
- 3.Waltzer L, Gobert V, Osman D, Haenlin M. Transcription factor interplay during Drosophila haematopoiesis. Int J Dev Biol. 2010;54:1107–1115. doi: 10.1387/ijdb.093054lw. [DOI] [PubMed] [Google Scholar]
- 4.Lebestky T, Chang T, Hartenstein V, Banerjee U. Specification of Drosophila hematopoietic lineage by conserved transcription factors. Science. 2000;288:146–149. doi: 10.1126/science.288.5463.146. [DOI] [PubMed] [Google Scholar]
- 5.Lebestky T, Jung SH, Banerjee U. A Serrate-expressing signaling center controls Drosophila hematopoiesis. Genes Dev. 2003;17:348–353. doi: 10.1101/gad.1052803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duvic B, Hoffmann JA, Meister M, Royet J. Notch signaling controls lineage specification during Drosophila larval hematopoiesis. Curr Biol. 2002;12:1923–1927. doi: 10.1016/s0960-9822(02)01297-6. [DOI] [PubMed] [Google Scholar]
- 7.Fossett N, Hyman K, Gajewski K, Orkin SH, Schulz RA. Combinatorial interactions of serpent, lozenge, and U-shaped regulate crystal cell lineage commitment during Drosophila hematopoiesis. Proc Natl Acad Sci U S A. 2003;100:11451–11456. doi: 10.1073/pnas.1635050100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Waltzer L, Ferjoux G, Bataille L, Haenlin M. Cooperation between the GATA and RUNX factors Serpent and Lozenge during Drosophila hematopoiesis. EMBO J. 2003;22:6516–6525. doi: 10.1093/emboj/cdg622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang H, Cantor AB. Common features of megakaryocytes and hematopoietic stem cells: what’s the connection? J Cell Biochem. 2009;107:857–864. doi: 10.1002/jcb.22184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rehorn KP, Thelen H, Michelson AM, Reuter R. A molecular aspect of hematopoiesis and endoderm development common to vertebrates and Drosophila. Development. 1996;122:4023–4031. doi: 10.1242/dev.122.12.4023. [DOI] [PubMed] [Google Scholar]
- 11.Benmimoun B, Polesello C, Waltzer L, Haenlin M. Dual role for Insulin/TOR signaling in the control of hematopoietic progenitor maintenance in Drosophila. Development. 2012;139:1713–1717. doi: 10.1242/dev.080259. [DOI] [PubMed] [Google Scholar]
- 12.Goulev Y, Fauny JD, Gonzalez-Marti B, Flagiello D, Silber J, Zider A. SCALLOPED interacts with YORKIE, the nuclear effector of the hippo tumor-suppressor pathway in Drosophila. Curr Biol. 2008;18:435–441. doi: 10.1016/j.cub.2008.02.034. [DOI] [PubMed] [Google Scholar]
- 13.Ota M, Sasaki H. Mammalian Tead proteins regulate cell proliferation and contact inhibition as transcriptional mediators of Hippo signaling. Development. 2008;135:4059–4069. doi: 10.1242/dev.027151. [DOI] [PubMed] [Google Scholar]
- 14.Wu S, Liu Y, Zheng Y, Dong J, Pan D. The TEAD/TEF family protein Scalloped mediates transcriptional output of the Hippo growth-regulatory pathway. Dev Cell. 2008;14:388–398. doi: 10.1016/j.devcel.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 15.Zhang L, Ren F, Zhang Q, Chen Y, Wang B, Jiang J. The TEAD/TEF family of transcription factor Scalloped mediates Hippo signaling in organ size control. Dev Cell. 2008;14:377–387. doi: 10.1016/j.devcel.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao B, Ye X, Yu J, Li L, Li W, Li S, Lin JD, Wang CY, Chinnaiyan AM, Lai ZC, et al. TEAD mediates YAP-dependent gene induction and growth control. Genes Dev. 2008;22:1962–1971. doi: 10.1101/gad.1664408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krzemien J, Oyallon J, Crozatier M, Vincent A. Hematopoietic progenitors and hemocyte lineages in the Drosophila lymph gland. Dev Biol. 2010;346:310–319. doi: 10.1016/j.ydbio.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 18.Terriente-Felix A, Li J, Collins S, Mulligan A, Reekie I, Bernard F, Krejci A, Bray S. Notch cooperates with Lozenge/Runx to lock haemocytes into a differentiation programme. Development. 2013;140:926–937. doi: 10.1242/dev.086785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nishioka N, Inoue K, Adachi K, Kiyonari H, Ota M, Ralston A, Yabuta N, Hirahara S, Stephenson RO, Ogonuki N, et al. The Hippo signaling pathway components Lats and Yap pattern Tead4 activity to distinguish mouse trophectoderm from inner cell mass. Dev Cell. 2009;16:398–410. doi: 10.1016/j.devcel.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 20.Mikeladze-Dvali T, Wernet MF, Pistillo D, Mazzoni EO, Teleman AA, Chen YW, Cohen S, Desplan C. The growth regulators warts/lats and melted interact in a bistable loop to specify opposite fates in Drosophila R8 photoreceptors. Cell. 2005;122:775–787. doi: 10.1016/j.cell.2005.07.026. [DOI] [PubMed] [Google Scholar]
- 21.Jukam D, Desplan C. Binary regulation of Hippo pathway by Merlin/NF2, Kibra, Lgl, and Melted specifies and maintains postmitotic neuronal fate. Dev Cell. 2011;21:874–887. doi: 10.1016/j.devcel.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Justice RW, Zilian O, Woods DF, Noll M, Bryant PJ. The Drosophila tumor suppressor gene warts encodes a homolog of human myotonic dystrophy kinase and is required for the control of cell shape and proliferation. Genes Dev. 1995;9:534–546. doi: 10.1101/gad.9.5.534. [DOI] [PubMed] [Google Scholar]
- 23.Xu T, Wang W, Zhang S, Stewart RA, Yu W. Identifying tumor suppressors in genetic mosaics: the Drosophila lats gene encodes a putative protein kinase. Development. 1995;121:1053–1063. doi: 10.1242/dev.121.4.1053. [DOI] [PubMed] [Google Scholar]
- 24.Tapon N, Harvey KF, Bell DW, Wahrer DC, Schiripo TA, Haber DA, Hariharan IK. salvador Promotes both cell cycle exit and apoptosis in Drosophila and is mutated in human cancer cell lines. Cell. 2002;110:467–478. doi: 10.1016/s0092-8674(02)00824-3. [DOI] [PubMed] [Google Scholar]
- 25.Dragojlovic-Munther M, Martinez-Agosto JA. Multifaceted roles of PTEN and TSC orchestrate growth and differentiation of Drosophila blood progenitors. Development. 2012;139:3752–3763. doi: 10.1242/dev.074203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang J, Wu S, Barrera J, Matthews K, Pan D. The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila Homolog of YAP. Cell. 2005;122:421–434. doi: 10.1016/j.cell.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 27.Oh H, Irvine KD. In vivo analysis of Yorkie phosphorylation sites. Oncogene. 2009;28:1916–1927. doi: 10.1038/onc.2009.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koontz LM, Liu-Chittenden Y, Yin F, Zheng Y, Yu J, Huang B, Chen Q, Wu S, Pan D. The Hippo effector Yorkie controls normal tissue growth by antagonizing scalloped-mediated default repression. Dev Cell. 2013;25:388–401. doi: 10.1016/j.devcel.2013.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yagi R, Chen LF, Shigesada K, Murakami Y, Ito Y. A WW domain-containing yes-associated protein (YAP) is a novel transcriptional co-activator. Embo J. 1999;18:2551–2562. doi: 10.1093/emboj/18.9.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Flores GV, Daga A, Kalhor HR, Banerjee U. Lozenge is expressed in pluripotent precursor cells and patterns multiple cell types in the Drosophila eye through the control of cell-specific transcription factors. Development. 1998;125:3681–3687. doi: 10.1242/dev.125.18.3681. [DOI] [PubMed] [Google Scholar]
- 31.Bataille L, Auge B, Ferjoux G, Haenlin M, Waltzer L. Resolving embryonic blood cell fate choice in Drosophila: interplay of GCM and RUNX factors. Development. 2005;132:4635–4644. doi: 10.1242/dev.02034. [DOI] [PubMed] [Google Scholar]
- 32.Slattery M, Voutev R, Ma L, Negre N, White KP, Mann RS. Divergent transcriptional regulatory logic at the intersection of tissue growth and developmental patterning. PLoS genetics. 2013;9:e1003753. doi: 10.1371/journal.pgen.1003753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Andres AJ, Cherbas P. Tissue-specific ecdysone responses: regulation of the Drosophila genes Eip28/29 and Eip40 during larval development. Development. 1992;116:865–876. doi: 10.1242/dev.116.4.865. [DOI] [PubMed] [Google Scholar]
- 34.Cherbas L, Willingham A, Zhang D, Yang L, Zou Y, Eads BD, Carlson JW, Landolin JM, Kapranov P, Dumais J, et al. The transcriptional diversity of 25 Drosophila cell lines. Genome research. 2011;21:301–314. doi: 10.1101/gr.112961.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dai Q, Ren A, Westholm JO, Serganov AA, Patel DJ, Lai EC. The BEN domain is a novel sequence-specific DNA-binding domain conserved in neural transcriptional repressors. Genes Dev. 2013;27:602–614. doi: 10.1101/gad.213314.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reddy BV, Rauskolb C, Irvine KD. Influence of fat-hippo and notch signaling on the proliferation and differentiation of Drosophila optic neuroepithelia. Development. 2010;137:2397–2408. doi: 10.1242/dev.050013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Polesello C, Tapon N. Salvador-warts-hippo signaling promotes Drosophila posterior follicle cell maturation downstream of notch. Curr Biol. 2007;17:1864–1870. doi: 10.1016/j.cub.2007.09.049. [DOI] [PubMed] [Google Scholar]
- 38.Tschaharganeh DF, Chen X, Latzko P, Malz M, Gaida MM, Felix K, Ladu S, Singer S, Pinna F, Gretz N, et al. Yes-associated protein up-regulates Jagged-1 and activates the Notch pathway in human hepatocellular carcinoma. Gastroenterology. 2013;144:1530–1542. e1512. doi: 10.1053/j.gastro.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rayon T, Menchero S, Nieto A, Xenopoulos P, Crespo M, Cockburn K, Canon S, Sasaki H, Hadjantonakis AK, de la Pompa JL, et al. Notch and Hippo Converge on Cdx2 to Specify the Trophectoderm Lineage in the Mouse Blastocyst. Dev Cell. 2014 doi: 10.1016/j.devcel.2014.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yimlamai D, Christodoulou C, Galli GG, Yanger K, Pepe-Mooney B, Gurung B, Shrestha K, Cahan P, Stanger BZ, Camargo FD. Hippo pathway activity influences liver cell fate. Cell. 2014;157:1324–1338. doi: 10.1016/j.cell.2014.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harvey KF, Zhang X, Thomas DM. The Hippo pathway and human cancer. Nature reviews. Cancer. 2013;13:246–257. doi: 10.1038/nrc3458. [DOI] [PubMed] [Google Scholar]
- 42.Menut L, Vaccari T, Dionne H, Hill J, Wu G, Bilder D. A mosaic genetic screen for Drosophila neoplastic tumor suppressor genes based on defective pupation. Genetics. 2007;177:1667–1677. doi: 10.1534/genetics.107.078360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang X, Milton CC, Poon CL, Hong W, Harvey KF. Wbp2 cooperates with Yorkie to drive tissue growth downstream of the Salvador-Warts-Hippo pathway. Cell Death Differ. 2011;18:1346–1355. doi: 10.1038/cdd.2011.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harvey K, Tapon N. The Salvador-Warts-Hippo pathway - an emerging tumour-suppressor network. Nature reviews. Cancer. 2007;7:182–191. doi: 10.1038/nrc2070. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure S1, related to Figure 1. Hippo pathway components are expressed in D. melanogaster hematopoietic cells.
(A–C) Primary third instar w1118 larval lymph gland lobes. Antibodies detected Mer protein expression (red in A) or Ft protein expression (red in B). fj mRNA expression was reported using a fj-lacZ enhancer trap (red in C). The cortical zone of each primary lymph gland lobe is marked with Cg-Gal4 driven expression of UAS-GFP (green in A′–C′). GFP and protein/gene expression images are merged in (A″–C″). Ft and fj displayed some enrichment in the cortical zone whereas Mer was expressed in a mostly unpatterned fashion.
(D–G) Primary third instar w1118 larval lymph glands. Antibodies were used to detect Yki protein expression (red in D, D″, E and E″) or Sd protein expression (red in F, F″, G and G″). Crystal cells were marked with either a Hnt-specific antibody (green in D′, D″, E′ and E″) or lz-Gal4 driven expression of UAS-GFP (green in F′, F″, G′ and G″). GFP and protein expression images are merged in (D″–G″). In (E–E″) yellow arrowheads indicate crystal cells with high Yki expression, while the asterisk shows a Hnt positive cell with moderate Yki expression. In (G–G″), yellow arrowheads indicate crystal cells that express low levels of Sd.
Scale bars = 20μm
Supplemental Figure S2, related to Figures 2 and 3. Hippo pathway perturbation regulates lymph gland size and crystal cell number.
(A–D) Primary third instar larval lymph glands; the cortical zone (A–B′) is marked with Cg-Gal4 driven expression of UAS-GFP (greyscale in single images and green in merged images), and the medullary zone (C–D′) is marked by expression of 10xSTATGFP, a GFP transgene under transcriptional control of STAT92E binding sites (greyscale in single images and green in merged images). Nuclei are marked with DAPI (blue in merged images). w1118 control lymph glands are shown in (A, A′, C and C′) and wts-lacZ mutant lymph glands are shown in (B, B′, D and D′). wts lymph glands display a proportional increase in size over control lymph glands, without obvious changes in the relative sizes of the cortical and medullary zones.
(E–H) Lymph glands expressing Yki3SA and GFP transgenes (E–E‴), GFP transgene alone (F) or SdGA and GFP transgenes (G) using the lz-Gal4 driver. Crystal cells were visualized by expression of GFP (green) in (E, E″, E‴, F and G) and/or Hnt (white) in (E–E‴) and are merged with direct interference contrast images (DIC) in (E′, E″, F and G). GFP-positive crystal cells from individual primary lymph gland lobes from multiple animals in (F and G) were quantified in (H). SdGA-expressing lymph glands possessed significantly more crystal cells than control lymph glands (p<0.0001; n=14 for F, n=24 for G; error bars represent SEM).
(I–L) A control lymph gland (I) and lymph glands expressing RNAi transgenes for Sd (J) or Tgi (K) under the control of the lz-Gal4 driver. Crystal cells were marked with lz-Gal4 driven expression of UAS-GFP (green in I–K) and anti-Hnt staining (white in I–K) and are merged with direct interference contrast images. Hnt positive crystal cells from primary lymph gland lobes from at least 15 animals were quantified in (L). Sd RNAi-expressing lymph glands possessed significantly more crystal cells than controls, whereas Tgi RNAi-expressing lymph glands did not (p=0.02; n=17 for I, n=25 for J, n=22 for K; error bars represent SEM).
Scale bars = 20μm.
Supplemental Figure S3, related to Figure 4. The Hippo pathway regulates expression of the key crystal cell fate determinant, Lozenge.
(A) S2 cells transfected with epitope-tagged plasmids for Lz, Yki and/or Wts and immunoprecipitated with anti-HA antibodies. Upper immunoblots are of anti-HA immunoprecipitates and lower blots are of input lysates. The positive control protein Wts formed a physical complex with Yki, but Lz did not.
(B–B‴) A third instar larval lymph gland harbouring single cell clones generated using the Actin-Gal4 flip-out technique and expressing a UAS-yki transgene. Expression of Lz (greyscale in B) and Yki (red in B′) are shown, and merged in (B″ and B‴) with a direct interference contrast (DIC) image in (B‴). Ectopic Lz protein expression was observed in single cell yki-expressing lymph gland clones.
(C–H) Third instar larval eye imaginal discs (C–E′, anterior is to the left), wing discs (F, F′ and H–H‴) and a brain lobe (G, G′), harbouring clones of GFP-positive tissue generated using the Actin-Gal4 flip-out technique. (C) Control clones expressed GFP only. (D), (F) and (G) Clones expressed Yki (from a UAS-yki transgene on the second chromosome) and GFP. (E) Clones expressed Yki (from a UAS-yki transgene on the third chromosome) and GFP. In (H) ptc-Gal4 was used to express Yki (from a UAS-yki transgene on the second chromosome) along the anterior/posterior boundary of the wing disc. Lz protein expression (greyscale in C–G and red in C′–G′ was detected using a Lz-specific antibody. lz transcription was reported in (H–H‴) using a lz promoter construct fused to the β-galactosidase gene. Ectopic Lz protein expression (yellow arrowheads in D–G) was observed in yki-expressing eye antennal disc clones anterior to the normal Lz domain, and in wing discs and brain lobes (which do not normally express Lz). lz transcription was detected in the ptc domain of wing discs overexpressing yki in (H–H″).
Scale bars = 20μm.
Supplemental Figure S4, related to Figure 4. The Hippo pathway non-cell autonomously influences crystal cell numbers in a Notch pathway-dependent fashion.
(A–G) hml-Gal4, UAS-GFP x w1118 control (A), hml-Gal4 UAS-GFP, UAS-Yki3SA (B) and hml-Gal4 UAS-GFP, UAS-SdGA (C) primary third instar larval lymph glands stained for Hnt (greyscale in A–C′) and merged with direct interference contrast (DIC) images in (A′–C′). Crystal cells were quantified from individual primary lymph gland lobes from several animals in (D). Yki3SA- expressing lymph glands possessed significantly more crystal cells (p= 0.0017; n=19 for A, n=19 for B, error bars represent SEM), as did SdGA-expressing lymph glands (p< 0.0001; n=12 for C, error bars represent SEM). (E–G) Close-up images of single sections of lymph glands expressing UAS-GFP alone (E) or together with either UAS-Yki3SA (F) or UAS-SdGA (G) under the control of hml-Gal4. Expression of Yki3SA or SdGA induced ectopic crystal cells in a non-cell autonomous fashion.
(H–I) The Notch reporter NRE-GFP (green in H–I′) was combined with either hml-Gal4 x w1118 control (H) or hml-Gal4 x UAS-SdGA (I) and assessed in third larval instar lymph glands. NRE-GFP reporter activity was merged with direct interference contrast (DIC) images in (H′ and I′). hml-Gal4-driven expression of SdGA caused a strong increase in the number of cells that were positive for Notch activity.
(J–L) hml-Gal4, UAS-Yki3SA (J) and hml-Gal4, UAS-Yki3SA x Su(H)Δ47 (K) third larval instar lymph glands stained for Lz (red in J and K, and merged with direct interference contrast images (DIC) in J′ and K′). Lz positive crystal cells from individual primary lymph gland lobes from several animals were quantified in (L). Yki3SA-expressing lymph glands possessed significantly more crystal cells than w1118 (p< 0.001; n=19 for w1118, n=18 for J), while lymph glands that expressed Yki3SA but were heterozygous for Su(H)Δ47 had significantly less crystal cells than those expressing Yki3SA in a wild-type background (p< 0.05; n=20 for K; error bars represent SEM).
Scale bars = 20μm.