Abstract
For over two decades, immunologists and biomaterials scientists have co-existed in parallel world with the rationale of understanding the molecular profile of immune responses to vaccination, implantation, and treating incurable diseases. Much of the field of biomaterials-based immunotherapy has relied on evaluating model antigens such as chicken egg ovalbumin in mouse models but their relevance to humans has been point of much discussion. Nevertheless, such model antigens have provided important insights about the mechanisms of immune regulation and served as a proof-of-concept for plethora of biomaterials-based vaccines. After years of extensive development of numerous biomaterials for immunomodulation, it is only recently that an experimental scaffold vaccine implanted beneath the skin has begun to use the human model to study the immune responses to cancer vaccination by co-delivering patient-derived tumor lysates and immunomodulatory proteins. If successful, this scaffold vaccine will change the way we approached untreatable cancers, but more importantly, will allow a faster and more rational translation of therapeutic regimes to other cancers, chronic infections, and autoimmune diseases. Most materials reviews have focused on immunomodulatory adjuvants and micro-nano-particles. Here we provide an insight into emerging hydrogel and scaffold based immunomodulatory approaches that continue to demonstrate efficacy against immune associated diseases.
Keywords: immunology, hydrogels, biomaterials, scaffolds, immunomodulation, polymers
1. Introduction
Immunomodulatory biologics such as antigenic proteins and peptides, nucleic acids, natural and synthetic adjuvants, drugs, and extracellular matrix components can be utilized to treat a broad spectrum of immune-related diseases. The ultimate goal of immunomodulation is (i) to either reduce immune cell activation in hyperactive immune conditions such as transplant rejection, autoimmune and inflammatory diseases; or (ii) to induce immune cell activation to reverse hypoactive immune responses in cancer and chronic infections [1]. The immunomodulatory agents engage and modulate the intracellular or cell surface receptors on host immune cells such as toll like receptors on dendritic cells (DCs), antigen-specific T-cell receptor (TCR), and B-cell receptor (BCR). Together, modulation of these signaling cascades determines the direction, magnitude, and persistence of B and T-cell responses.
Prophylactic vaccines have become one of human medicine’s most potent weapons and in the past century, research has contributed tremendously to the significant decrease in mortality and morbidity from infectious diseases [2]. Yet we are far from treating established or unexpected chronic diseases and demonstrTating global success. Seasonal diseases such as influenza imposes substantial risk due to the transient nature of immune response and the adaptability of viruses. With incurable cancers like melanoma [3] and lymphoma [4], the recurring outbreaks of emerging infectious diseases such as the 2009 H1N1 influenza A [5] and 2014 Ebola hemorrhagic fever [6], and drug resistance infections like tuberculosis [7], there is a strong need for new translational strategies to achieve active immunomodulation. Similarly, either alone or by combining with existing therapies to treat autoimmune diseases [8] like rheumatoid arthritis and type 1 diabetes mellitus, immunomodulation using ex vivo programmed immune cells has the potential to improve our ability to control tolerogenic immune response.
A rapidly growing field of research is the design of scaffolds and hydrogels made of synthetic and natural materials, with ability to modulate the immune response. Greater understanding of the molecular basis of cellular phenomenon such as apoptosis, receptor-ligand interactions, and immune cell activation has aided the development of materials-based therapeutic strategies for disease treatment. Biomedical researchers and clinicians have been continuously shifting focus towards engineered approaches at multiple length-scales (macro-to-nanoscale) that could target organs like lymph nodes, lymphoid residing cells and their intracellular compartments, function as surrogate lymphoid-like tissues, and deliver multiple biomolecules that could modulate the extent of the humoral and cellular immune response. Pioneering work has been done in encapsulating antigens, immunomodulatory agents, and drugs inside micro-and-nanoparticles. Several of these have been extensively discussed by others and us elsewhere [9],[10],[11],[12]. Here we discuss emerging hydrogel and scaffold-based strategies for fighting against cancer, infections, and autoimmune diseases as well creating immune cell microenvironments.
2. Hydrogels and scaffolds as biological delivery systems
Polymeric scaffolds and hydrogels are three-dimensional polymeric networks swollen in water, aqueous media or biological/physiological fluids, which have been widely used for cell encapsulation and controlled release of therapeutic proteins, peptides, drugs and nucleic acids [13],[14],[15],[16],[17],[18],[19],[20],[21],[22],[23],[24]. The choice of polymer could be dictated by the end use such as the site of implantation and expected performance including release kinetics, biocompatibility, and immunogenicity. Synthetic polymers provide structural and mechanical design flexibility that is less achievable with natural occurring polymers, however the advantage of biocompatibility, cell adhesion, enzymatic and hydrolytic degradability, minimal inflammatory response, and ability to stimulate a specific cellular response with natural polymers can also be critical in polymer choice. Functional design of macroscopic scaffolds and their release kinetics has been discussed earlier in excellent reviews by Schoichet [25] and Kearney and Mooney [26].
Hydrogels have become immensely popular in regenerative medicine, due to their biocompatibility, design flexibility, and a broad spectrum of choice of base material. The hydrophilic nature and high swelling ratio also make hydrogels permeable to oxygen, nutrients, metabolites, and waste products. A number of materials and crosslinking techniques have been explored to design hydrogels with varying properties such as pore size, mechanical strength, and degradation rate as reviewed by us and others elsewhere [13],[27],[28],[29],[30],[31],[27],[32],[33],[34],[35]. For example, photoinitiated polymerization of polymers that are end-functionalized with acrylates and vinyl sulfone groups provide a unique ability to spatially and temporally control the development of implantable materials for cell and protein delivery, as well as for the fabrication of scaffolding with complex structures. Choice of material for forming these hydrogels range from entirely synthetic polymers such as poly(ethylene glycol) (PEG) to purely natural polymers such as dextran, gelatin, and hyaluronic acid, with degradation based on the hydrolytic or enzymatic degradation of bonds in the polymer backbone or crosslinks. The mechanical properties of these hydrogels are controlled by network crosslinking density, polymer functionalization and reaction mixture concentrations.
Polymeric solutions that are capable of forming an in situ three dimensional crosslinked network have gained recent attention because of the mild crosslinking conditions required to make these hydrogels thus allowing for the encapsulation of biomolecules like DNA, RNA, proteins as well as cells. Although it is possible to create in situ crosslinked networks through UV radiation mediated chemical crosslinking of acrylate functionalized polymers [34],[35], the process requires long exposure to UV rays and could get hampered by the limited penetration depth of UV rays through skin. Of particular importance is the Michael-type conjugate addition reaction based on in situ crosslinkable hydrogels. Michael-type addition is a conjugate addition with a carbanion or enolate-type nucleophile to α,β-unsaturated carbonyl-containing groups in presence of a base-catalyzed or nucleophile-catalyzed condition [36],[37],[30]. These hydrogels have been extensively used in cell and protein delivery applications [38],[39],[40],[41],[42],[43]. We have used these hydrogels for combinatorial delivery of antigens and immunomodulatory agents [23],[24] as discussed later in the review.
Despite the long standing history of hydrogels and scaffolds for drug delivery and tissue engineering, it is only recently that researchers have begun to explore the potential of macroscopic gels, thin films, scaffolds, microscopic needles, or hydrogel-nanoparticles in a wide range of immunological disease applications, with significant clinical impulse observed in the past decade itself [44],[45],[46],[47],[11],[48],[23].
3. Dendritic cell trafficking and primary mechanism of immunomodulation using scaffolds
Landmark studies by Sir James Gowans and colleagues in 1964 demonstrated for the first time that lymphocytes, during homeostasis, sample the blood stream for foreign antigen as they recirculate from blood, through secondary lymphoid organ such as lymph nodes, into lymphatics and return to the blood stream. This immune cell trafficking is essential for maintaining the robust immunity in the body and the lymphocytes migrate as often as 1–2 times per day. Humans have nearly 450 lymph nodes scattered all over the body. Since their first identification almost 40 years ago [49], DCs have emerged as one of the most important professional antigen presenting cells (APCs) bridging the two indispensable arms of the immune system i.e. innate and adaptive immunity [50].
The innate immunity establishes the first line of immunological response during infection with early recognition of pathogenic signals and subsequent initiation of proinflammatory response. The adaptive immunity, on the other hand, eliminates infected cells and pathogens in the later stages of infection and constitutes immunological memory against invaded pathogens. DCs play a central role in a series of immunological events during infection, immunization, and immunotherapies that eventually lead to adaptive T and B-cell-mediated immunity.
DCs can be directly activated in vivo by injecting immunogenic antigens or primed ex vivo with antigens followed by injecting back into the body. For cancer immunotherapy, DC-based vaccines are frequently developed by deriving naïve DCs from peripheral blood of patients and successfully loaded with tumor-associated antigens, ex vivo [51],[52]. These ex vivo primed DCs, when infused back into the cancer patient at the peripheral tissue sites, are expected to function normally and provide anti-tumor T-cell response. It is expected that the infused, primed DCs will respond to the homing signals mediated by chemokine and cytokines, migrate to the draining lymph nodes and present antigens to the residing T-cells via major histocompatibility complexes (MHC). Promising studies in animal models has shown robust cluster of differentiation (CD)4 and CD8 T-cell responses, however the ex vivo DC vaccine strategy (direct cell delivery) has only provided marginal survival rate in patients compared to chemotherapeutic regimens despite showing an antigen-specific T-cell expansion. It is speculated that antigen-loaded DCs in draining lymph nodes and other lymphoid organs have limited ability to support the effector phase of the immune response following T-cell priming therefore failing to generate the necessary magnitude and duration of functional CD8+ Cytotoxic T lymphocyte (CTL) response to induce regression of tumors. Such partially effective tumor immunotherapy strategies are detrimental as they can amplify defective CTLs under the influence of tumor microenvironment that are rich in immunosuppressive cytokines such as interleukin (IL)-10 and transforming growth factor-β (TGF-β) [53],[54],[55],[56].
Engineering hydrogels and scaffolds to synchronize with the migration, activation, and target specificity of our immune system requires complex designs and properties that are important for cell modulation. Several recent reports provide evidence that delivery of biological therapeutics using hydrogels and scaffolds could actively regulate the kinetics of multiple steps in the immune response (e.g. vaccines) or as materials that evade immune response and scar formation [45],[23],[24],[57],[58],[59],[60],[61],[62],[63],[64],[65],[66],[67],[66],[68],[69],[70],[71],[44], [72]. Using scaffolds, DCs can be exploited for immunotherapy through two primary mechanism as illustrated in Figure 1, built upon the core concepts of conventional vaccines: (1) ex vivo priming and implantation of activated DCs, or (2) delivery of antigens and adjuvants captured by DCs in vivo.
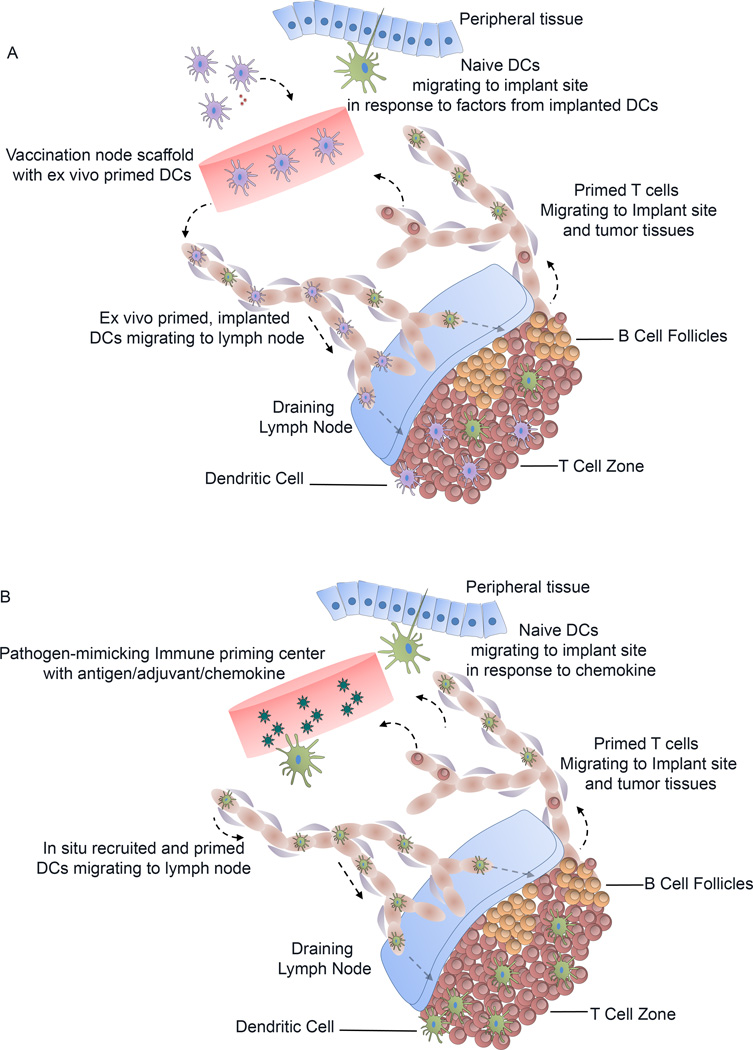
Figure 1. Dendritic cell trafficking and modulation using scaffolds.
A) Cell-loaded vaccination nodes: Ex vivo primed DC (purple) are delivered through injections or implantation of pre-fabricated scaffolds, subcutaneously into mice. A few of the programmed DCs migrate to the draining lymph nodes and present antigen to the T-cells in T-cell zone. Cytokines and chemokines secreted by programmed DCs at the implantation site recruits host’s own naïve DCs (green) and programmed T-cells to induce a robust immune response. B) Pathogen-mimicking immune centers with no cells: In situ crosslinked hydrogels or implanted scaffolds release chemokines and growth factors in the tissue to attract naïve DCs. Recruited DCs engulf antigenic vaccines and return to draining lymph node to present antigen to naïve T helper cells (CD4+ T-cells). Programmed T-cells can then migrate to the implant site or nearby tumor regions to destroy malignant cells.
4. Three-dimensional scaffolds as immunological microenvironments and delivery of ex vivo programmed immune cells
Creation of an artificial ex vivo site for culturing primary T-cells and DCs and allowing them to interact could be a powerful strategy to manipulate immune cell behavior prior to adoptive immunotherapy. However, very few studies have been done in this area. Current design rationale behind 3D scaffolds as secondary lymphoid organ is based on the mechanically soft nature of the lymphoid microenvironment, structural complexity, and the presence of key cell signaling molecules, as well as the organogenesis and plasticity of which has been discussed in an elegant review by Irvine et al [73]. Studies by Suematsu and Watanabe [74] used collagen sponge scaffolds carrying a thymus-derived Lymphotoxin (LT)-β receptor and vascular cell adhesion molecule-1 (VCAM-1) expressing stromal cell line transduced to express murine lymphotoxin LT-α and used as an implantable scaffold for synthetic lymph node formation. When implanted in mice, the scaffold based organoid formed an organized secondary lymphoid–like with compartmentalized zones of B-cell and T-cell clusters, high endothelial venule-like vessels (HEVs), germinal centers and networks of follicular DC (Figure 2A). Interestingly, implantation with activated DCs promoted the cluster formation for B and T-cells in the organoids in presence of LT-α expressing stromal cells. Using a composite macroporous PEG hydrogel scaffolds infused with collagen, Stachowiak et al. engineered an inverse opal hydrogel mimicking a lymphoid organ to study immune cell migration. Using colloidal crystal templating, ordered, interconnected arrays of porous hydrogel (Figure 2B) was formed with pores on the order of tens of microns length scale [73],[62]. These PEG-gels, infused with collagen, promoted intra-scaffold migration of encapsulated T-cells and DCs, with T-cell migration dependent on the connecting pore size. Both these systems demonstrate the promise of tissue-engineered approaches to generate lymphoid-like microenvironment.
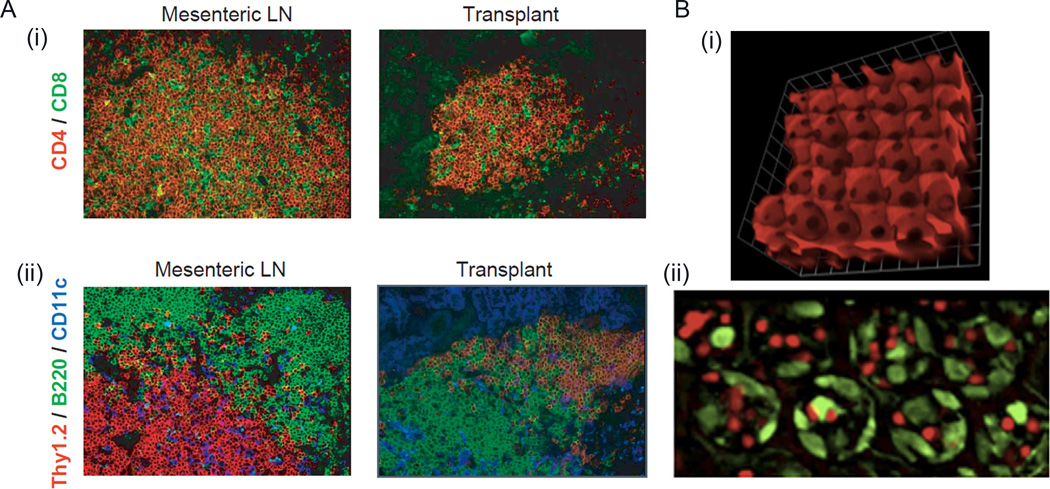
Figure 2. Lymphoid tissue engineering using scaffolds.
A) Naturally occurring collagen-based lymphoid organoids. When transplanted in mouse, tissue-engineered organoids are structure similar to secondary lymphoid organs such as mesenteric lymph node (LN). (i) CD4+ T-cells in stained red and CD8+ T-cells are stained green. (ii) Mesenteric LN and transplant were stained for Thy1.2+ T-cells in red, B220+ B-cells in green and CD11c+ DCs in blue. B) Composite PEG-based inverse opal scaffolds: (i) Image represents an oblique view of fluorescently-labeled inverse opal PEG scaffolds with macroscale pores. The interior of these scaffolds could be decorated with cell supportive ECM proteins such as fibronectin or laminin. (ii) Plan view of inverse opal scaffolds with naive CD4+ T-cells (red) interacting with DCs (green) that are spread on the interior side of the ECM protein-conjugated PEG scaffold. Reproduced with permission [73],[74].
Interesting studies by Lee and Kotov [75] also demonstrate the feasibility of re-engineering thymic microenvironment using notch signaling protein functionalized 3D inverted colloidal crystal (ICC) scaffolds for ex vivo T-cell development of human hematopoietic stem cells (HSC). The thymic microenvironment is a complex milieu of progenitors, supportive stromal cells, signaling interactions like Delta and Notch, cells undergoing clonal selection through MHC–TCR interactions, as well as abundant adhesion, expansion and homing molecules present as extracellular matrix. These polyacrylamide hydrogel ICC scaffolds were formed using layer-by-layer (LBL) molecular assembly technique and had 110 µm uniform pore size with ~30 µm large interconnecting channels. The ICC hydrogels have LBL coating of clay and poly(diallyl dimethylammonium chloride) (PDDA) that promotes stromal cell adhesion and immobilization of Notch ligands through electrostatic interactions. The repeated proliferation and notch induction resulted in differentiation of HSCs into T-cell lineage with expression of CD3, CD4, TCRα, and CD117. The study shows the possibility of generating a 3D system for high throughput regeneration of T-cells from stem cells, ex vivo and could potentially be applied to DCs as well.
In situ gelling hydrogels offer the unique ability to serve as depot or vaccination nodes to deliver either ex vivo primed DCs or recruit host’s DCs in situ and program them for inducing cellular immune response. The encapsulation process being mild, does not alter the bioactivity of the molecules or functionality of the cells. Pioneering work by Hori et al. [46] in Irvine’s group has resulted in an alginate based injectable hydrogels which can be loaded with ex vivo primed immune cells along with immunomodulatory proteins (Figure 3A). These self-gelling depots are composed of polysaccharide alginate mixed with calcium ion containing microspheres whereby upon injection, calcium (Ca2+) ions diffuses into the alginate solution and ionically cross-links the polymer chains in <60 min. Chemokines reversibly bind to these gels and encapsulated DCs exhibited survival and spreading in vivo (Figure 3A). Subcutaneous injection of soft alginate gels carrying immunomodulatory cytokines and/or DCs have shown promising in vivo responses with priming of naive CD8+ T-cells in the local draining lymph nodes followed by migration and infiltration of primed antigen-specific T-cells to the gels [46],[47]. Hori et al showed that the presence of CpG oligonucleotides in conjunction with injected crosslinkable alginate solution was important in generating activated MHC-II+ CD40+ DCs, which points out the need for danger signal in DC activation and secretion of inflammatory cytokines like IL-6 and IL-12p70 during immunotherapy.
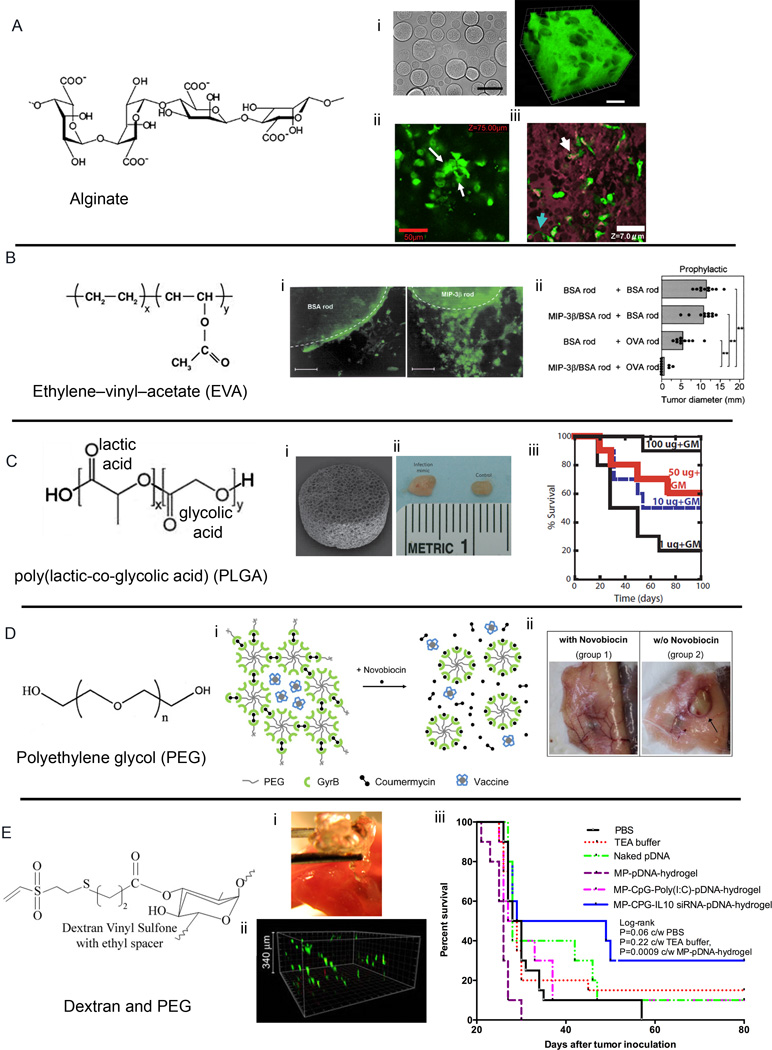
Figure 3. Injectable and implantable strategies for immunomodulation.
A) In situ crosslinkable alginate gel co-delivering immunomodulatory factors. (i) 3D injectable alginate gel with calcium-crosslinked alginate microspheres. Alginate microspheres (non-fluorescent voids) were distributed throughout the fluorescent alginte matrix. (ii) GFP+ DCs in alginate hydrogels, explanted 22 h after s.c. injection in C57Bl/6 mice. White arrows indicate spread DCs. (iii) Infiltrating GFP+ cells infiltrate and occupy void spaces in porous alginate matrix (purple) explanted from mice 48 h after injection Scale bars: 50 µm. B) Implantable EVA rods with chemokines and antigens. (i) Accumulation of Langerhans cells around BSA rod (left) and MIP-3β rod (right) implanted in mice and examined after 24 h. Original magnification, 200; scale bars, 100 µm. (ii) Co-implantation of MIP-3β rods and OVA rods initiates protective immunity in mice challenged with E.G7-OVA tumor cells in the scapular region five days after rod implantation (C) Engineered PLGA scaffold vaccine against tumor (i) SEM of PLGA scaffold. (ii) Photograph of lymph nodes from control mice and infection mimic mice after 10 days of implantation of matrices incorporating 10 µg CpG-ODN+3,000 ng GM-CSF. (iii) Survival times of mice vaccinated with PLGA vaccines 14 days before B16-F10 melanoma tumor challenge. (D) Drug-responsive hydrogel vaccine depot. (i) Gyrase B (Gyr B) functionalized 8-arm PEG hydrogel providing a molecular switch based on the interaction of the GyrB to the aminocoumarin antibiotics coumermycin and novobiocin. (ii) Mice were sacrificed at day 98 after treatment with or without Novobiocin. Non-dissolved hydrogel of the group 2 mouse is indicated by an arrow. (E) An injectable synthetic immune-priming center made of Dextran Vinyl Sulfone and 4-arm PEG-SH mediates efficient T-cell class switching and T-helper 1 response against B-cell lymphoma (i) Formation of in situ crosslinkable hydrogels in mouse quad muscle, retrieved after 8 h post injection. (ii) Primary DC infiltration through 3D hydrogels (iii) Kaplan–Meier survival curve indicating protection against A20 B-lymphoma in Balb/C immunized mice with various indicated formulations. Reproduced with permission [64],[46],[47],[48],[23],[24]
5. Macroscale implantable Scaffolds to induce and program immune cells, in situ
Unrelated studies have begun to reveal the importance of simultaneous delivery of multiple biomolecules such as antigens and adjuvants that could modulate the extent of the humoral and cellular immune response. Prior studies also emphasize the importance of the delivery kinetics of immunomodulatory molecules in induction and persistence of a robust immune response. For in vivo immunomodulatory purposes, there are several design considerations for hydrogels and scaffolds, such as mild fabrication or gelation conditions, delivery kinetics, and recruitment/infiltration of immune cells. These design considerations are important both in the context of immunization and in natural responses to transient versus persistent infections. For example, it is critical that the vaccination material is responsive enough under physiological conditions for timely delivery of cargo that may require dissolution of the hydrogel and the release of the incorporated vaccine load. We and others have shown that the magnitude, functionality, and phenotype of antigen-specific CD4+ and CD8+ T-cell responses can be shaped by controlled release of antigen and immunomodulatory biologics over multi-week periods. The prolonged retention and persistent immunomodulatory signals elicit stronger immune responses against persistent pathogens which cannot be eradicated by bolus immunizations.
In an early attempt to program immature DCs in vivo, Takashima and colleagues recruited Langerhans cells using ethylene–vinyl–acetate (EVA) polymer rods (Figure 3B) [76]. Langerhans cells, the immature DCs residing in the epidermis, were recruited and entrapped using chemokine MIP-3β incorporated into the 10 mm EVA rods. Implantation of MIP-3β rods caused local accumulation of LCs without affecting their surface densities. This work demonstrated the feasibility of DC-based vaccination against tumor in vivo by co-implanting a xenogeneic antigen ovalbumin (OVA)-incorporated EVA rods in abdominal skin and the immunized animals developed robust CTL response against the OVA-transduced EL4 tumor line, E.G7-OVA [76].
Gubeli et al. [64] recently reported a strategy to reduce repetitive booster doses of hepatitis B vaccine by controlling the release of the vaccine from an oral drug-responsive hydrogel depot. PEG chains end-functionalized with protein Gyrase B (GyrB) were used to form a hydrogel with addition of coumermycin that would dimerize GyrB and physically entrap Hepatitis vaccine. Interestingly, addition of aminocoumarin antibiotic novobiocin competitively replaced coumermycin resulting in GyrB monomerization and hydrogel dissolution within 2 h and the release of the vaccine (Figure 3D). Mice immunized with hydrogels and receiving the novobiocin stimulus demonstrated significantly higher anti-HBs titers compared to control groups including the classical 2-dose injection regime, therefore clearly demonstrating that advantage of controlled, prolonged release of the HBsAg vaccine in a stimulus-inducible manner.
The breakthrough in scaffold-based cancer immunotherapy came in a seminal report where Mooney and collaborators [44],[45] engineered tablet-shaped macroporous PLGA scaffold (~85% of total volume represents pores) to deliver tumor lystaes with other immune stimulatory proteins. Implantable scaffold, made of polylactide-co-glycolide (PLGA) consisted of DC attracting cytokine granulocyte-macrophage colony-stimulating factor (GM-CSF), immunostimulatory CpG oligonucleotide, and tumor lysate as antigen. These scaffold recruited naïve CD11c+ DCs and programmed them to induce robust prophylactic immunity against murine B16-F10 melanoma tumor [45]. Importantly, the group investigated the ability of these scaffolds to provide therapeutic vaccination against established melanoma [44]. These studies showed that under the influence of GM-CSF, DCs expressing CD11c and CD11b accumulated the implantation site with nearly 87% CD11b+ DCs residing inside the implant. Scaffolds loaded with CpG oligonucleotides (complexed to cationic polymer polyethyleneimine) alone recruited CD11c+PDCA-1+ plasmatoid DCs that increased with GM-CSF and with increasing dose of CpG oligonucleotides. Taken together these studies showed that by using polymeric scaffolds delivering multiple immunomodulatory molecules could stimulate CD4+ and CD8+ cellular response, increase the production of tumor-suppressive Interferon (IFN)-γ, IL-12, attenuated FoxP3+ TReg cells and immunosuppressive cytokines, and caused regression of established melanoma which can be attributed to the high CD8+ T-cells to FoxP3+ TReg cells that has been linked to therapeutic tumor immunity in murine and human systems (Figure 3C) [77]. The vaccine implant, called WDVAX, has since moved into clinical trials for treatment of melanoma [78].
6. Macroscale injectable Scaffolds to induce and program immune cells, in situ
Vaccine implants discussed so far require minor surgery, often subcutaneous, and therefore there is a significant need to make scaffold based strategies that are less invasive. Roy et al. have previously reported in situ injectable, biodegradable polyethylene oxide (PEO) based hydrogels for nucleic acid-mediated gene therapy against peanut allergy where the polymeric solution remained liquid outside the body and gelled once injected [79]. Specifically, tetrafunctional polyethylene oxide amine (P4Am) and tetrafunctional polyethylene oxide succinimidyl glutarate (P4SG) entrapped the DNA vaccine by varying the density of the polymer network and concentration of the PEG components, the release kinetics of entrapped DNA and gelling ability could be controlled between a branched viscous polymer and a cross-linked hydrogel. These hydrogels gelled in approximately 18 minutes and slowly degraded over a period of 28 days. These injectable hydrogels successfully delivered plasmid DNA encoding human secreted embryonic alkaline phosphatase (pSEAP) into skeletal muscles of immunocompetent animals resulting in high serum levels of the protein for a significantly longer period of time relative to that achieved with unformulated DNA injections. The technique offers a minimally invasive application of polymeric solution thus making it an ideal candidate for vaccine delivery applications.
The alginate gels developed by Hori et al. released encapsulated cytokines over a period of 1–2 weeks and remained intact for 21 days (Figure 3A) [46],[47]. This raises an important question on how the release rate of encapsulated cytokines/chemokines and degradation of depot could potentially affect the recruitment, infiltration, phagocytosis, and homing of immune cells in response to hydrogels and implanted scaffolds. To answer these questions, hydrogels with modular design are needed with preferable injectable characteristics. As discussed earlier, Michael type addition hydrogels offer a unique ability to encapsulate cells and deliver proteins at physiological conditions, 37 °C and pH ranging from 7.1–7.8. In particular, the conjugate addition of thiols onto unsaturated esters offers several benefits including high speed, easy preparation or availability of the unsaturated polymer, and, most importantly, it provides the least influence of competing nucleophiles (for example, at physiological pH, the rate of reaction of amines is slower than thiols). Michael type addition between thiol-modified polymers and acrylate or vinylsulfone modified polymers have been explored by several research groups to form hydrogels [80],[30],[42],[40],[81].
We have engineered dextran [82],[83] and PEG-based in situ crosslinkable hydrogels for immunomodulation against lymphoid malignanicies (Figure 3E), specifically B-cell lymphoma. In these studies the goal was to encapsulate antigen/adjuvant carrying vaccine along with DC chemo-attractants within an in situ crosslinking hydrogel and deliver them as a single injectable formulation. To effectively synchronize with the timeline of DC trafficking, hydrogel design required that the polymer network to degrade in a timely fashion (i.e. within a 3–4 days) such that (a) the chemo-attractants are released slowly over a 2–3 day period thereby attracting large number of immature DCs to the site of injection and (b) at the same time vaccine is released from the gel (due to gel degradation) and is available for uptake by the attracted naïve DCs. For injectability and controlled degradability (slow and fast-), we engineered Michael-type addition gels by combining vinyl sulfone functionalized dextran with 4-arm-thiolated PEG. Immunomodulation was achieved by simultaneous delivery of small interfering RNA (inhibiting T-cell suppressive cytokines) and tumor-gene encoding DNA to same DC, using a single particle carrier [24]. When compared with conventional PEG diacrylate based Michael-type addition hydrogels [30], dextran based gels swelled significantly less making them more suitable for injections as vaccine centers. This approach resulted in robust anti-tumor biased cellular immune response in a prophylactic, mouse tumor model of lymphoma as a function of degradation rate of the hydrogel with those degrading within 2 days inducing the maximum cellular immune response [23].
Using the same dextran vinyl sulfone immune priming centers, in a more recent study, Pradhan et al. [48] co-delivered three immunomodulatory molecules: Toll like receptor 9 stimulating and Th1-biasing CpG oligonucleotides, Th1 biasing IL-10 siRNA, and weakly immunogenic lymphoma idiotype DNA antigen. Hydrogels were used to chemoattract naïve DCs, in vivo. In mice challenged with lethal dose of lymphoma tumor cells, hydrogel based immunomodulation outperformed non-hydrogel groups by extending the median survival of mice to 39 days (Figure 3E) Using these hydrogels and co-delivering IL-10 siRNA and DNA vaccine, we have shown ~ 40% animal survival over 60 days and in studies by Pallab et al, 30% mice were tumor free in CpG/IL-10siRNA/DNA hydrogel group and alive post 80 days as compared to only less than 15% surviving with naked DNA vaccine. Immunomodulatory hydrogels with the combination of CpG, IL-10 siRNA and DNA vaccine outperformed another combination of CpG with poly I:C adjuvant and DNA vaccine. It is noteworthy that the combinatorial hydrogel immunotherapy developed by Singh et al. [23] and Pradhan et al. [48] report robust induction of cellular immunity, yet only moderate 30–40% animal survival is observed against lethal lymphoma challenge. This anti-lymphoma response is still superior compared to other tested vaccine delivery platforms. Certainly, at the moment, the in vivo protection is below the levels needed to effectively translate these hydrogel vaccine into clinic against B-cell Llymphoma.We speculate that future direction should focus on overcoming the immunosuppressive tumor microenvironment, understanding the role of immune tolerance with very high immune response, and taking into consideration that lymphoma originates in lymph nodes, the primary site of immune reaction, which could play a key role in further suppressing the immune response.
7. Micro and Nanoengineered Hydrogel Vaccines
Numerous materials and strategies have been explored to engineer micro- and nanoscale particles for immunomodulation application. A detailed discussion on such micro or nano-scale approaches can be found in some recent reviews [10],[11],[84],[9]. Nanoengineered hydrogels represent an innovative platform for the protection and delivery of proteins and peptides to the sampling DCs leading to T-cell stimulation and B-cell mediated antibody response.
Sexton et al. [70] developed LBL-assembled disulfide poly(methacrylic acid) (PMASH) hydrogel capsules as immunogenic vaccines with ability to encapsulate model antigen ovalbumin (OVA) as well as multiple OVA peptides. LBL assembly has proven to be a robust and versatile technique for synthesizing polymer capsules, largely due to the ability to fine-tune the physicochemical properties and functionality of the obtained capsules. LBL-assembled hydrogel capsules provide a fine control over size and material composition, allowing for tunable loading of the vaccine cargo that can be intracellularly processed by DCs and other APCs. Therapeutic molecules were immobilized onto the surface of a template particle and localized within polymer hydrogel capsules. Once the polymer thin film was fabricated, the core particles were removed.
It is important to note that in these hydrogels, therapeutic molecule encapsulation relied on both size exclusion and electrostatic repulsion provided by the negatively charged capsule. This protective hydrogel vaccine induced OVA-specific CD4+ and CD8+ T-cell response in transgenic mouse model of OVA tumor. The particulate nature of the capsule makes them easy target to APCs that phagocytose them. Such vaccines not only provide high payload ability but also a protective environment to degradable antigens. Interestingly, conversion of thiol moieties on PMASH make them stable under oxidative extracellular microenvironment however allows for degradation under reducing intracellular conditions, allowing for intracellular delivery of vaccine components. The kinetics of intracellular delivery and T-cell stimulation was dependent on several competing factors, such as the degree of substitution (thiolation) and number of deposited polymer layers.
Another interesting strategy is the use of fibrillized peptides, peptidomimetics, and peptide derivatives for immunomodulation purposes. Injectable self-assembling peptide nanofibers have been studied to create intramyocardial microenvironments and prevention of glial scar formation and axon elongation after spinal cord injury [85],[86]. Rudra et al. reported self-assembling β-sheet nanofibers fused with short peptide epitopes from Plasmodium falciparum circumsporozoite (CS) protein, NANP(3). C57BL/6 mice primed with nanoflibers-NANP(3) induced T-cell- and MyD88-dependent antibody responses that lasted up to 40 weeks, making these self-assembling biomaterials a robust self-adjuvanting multi-antigenic immunotherapeutic platform [87]. Park et al. called engineered Nanolipogel (NLG) that encapsulate TGF-βinhibitor SB505 and IL-2 for stimulating T-cells [88]. The nanoparticles consisted of a core-shell structure of PEGylated liposomal coating. Since the goal was to deliver multiple immunomodulators altogether, the bioactive molecules were encapsulated inside the core surrounded by a hydrogel that consisted of water-soluble PEG, lactide groups, and terminal acrylate groups. Nanolipogel delivering SB505 and IL-2 not only activated CD8+ T-cells but also the natural killer (NK) cells, causing marked reduction in tumor mass and higher animal survival. Taken together, nanoscale gels are still in preliminary development stages but hold tremendous promise to provide a unique and attractive vaccine strategy for the control of several chronic diseases ranging from cancers to infectious diseases.
8. Scaffolds and hydrogels to induce systemic tolerance and reduce inflammation
Immunomodulation by suppressing immune cell activation plays a critical role in treating autoimmune diseases and inflammation. Adoptive transfer of ex vivo antigen primed tolerogenic DCs generated from myeloid precursors could suppress autoimmune response by inducing activation-induced cell death, anergy (a tolerance mechanism), and interaction with regulatory T-cells. Compelling preclinical results indicate that adoptive transfer of regulatory T-cells (TReg cells) can prevent or cure autoimmune diseases and allograft rejection (transplants), by restoring immune tolerance to self-antigens or alloantigens [89]. TReg cell immunotherapy has been employed to modulate the immune response in preventing graft rejection after hematopoietic stem-cell transplantation (HSCT) after bone marrow transplantation. TReg cells function by suppressing effector T-cells (CD4 and CD8) as well as regulate the activation of DCs by creating a microenvironment rich in immunosuppressive cytokine IL-10 and TGF-β1, and often through cell-cell interactions. Various CD4+ regulatory T-cell subsets have been extensively reviewed elsewhere [89] and are beyond the scope of this review. Here we specifically review a subset of CD4+ T helper cells phenotypically characterized as forkhead box P3 (FoxP3)+ CD4+CD25+ cells, commonly called TReg cells.
Studies in preclinical models indicate that polyclonal TReg cells can prevent autoimmune diseases, whereas only self-antigen-specific TReg cells can cure active autoimmunity. Similar to ex vivo priming of DCs, antigen-specific TReg cells can be generated ex vivo by exposing CD4+ T helper cells to TGF-β1 in the presence of DCs and target self-antigen such as insulin and GAD65 for type 1 diabetes mellitus myelin basic protein in multiple sclerosis, and type II collagen in rheumatoid arthritis [90],[91].
Type 1 diabetes, a disease in which the insulin producing β-cells are destroyed due to autoimmunity (Figure 4), is currently being treated in clinics by infusion of cadaver derived allogeneic pancreatic islet cells. Islet transplantation requires administration of immunosuppressive drugs to nonspecifically suppress hyperactive immune system in patient’s body which makes them prone to infections and increased chances of neoplasia. In Type 1 diabetes, several preclinical models have shown that the transfer of ex vivo primed TReg cells can block disease development. Shea and colleagues have explored the possibility to establish islet antigen (BDC2.5 mimotope)-specific immune tolerance using ex vivo primed TReg cells in macroporous PLG scaffolds [72]. The study demonstrated that co-localized TReg cells could protect islet grafts in an extra-hepatic and extra-renal transplant site with robust insulin production and co-localized Foxp3+ TReg cells around islets. The functional design of macroporous scaffolds permitted efficient seeding of islets and TReg cells and shielded the transplanted islets from transplant associated inflammation and diabetogenic immunosuppression therapy. This effect, however, could also be attributed to the immunomodulatory properties of antigen-specific TReg cells along with direct cell-cell interactions. In summary, this study highlights the importance of scaffold-based immunomodulation using ex vivo primed TReg cells for enhanced islet survival and engraftment and addresses several shortcomings of current islet transplant-based clinical approaches to treat type 1 diabetes mellitus (T1DM).
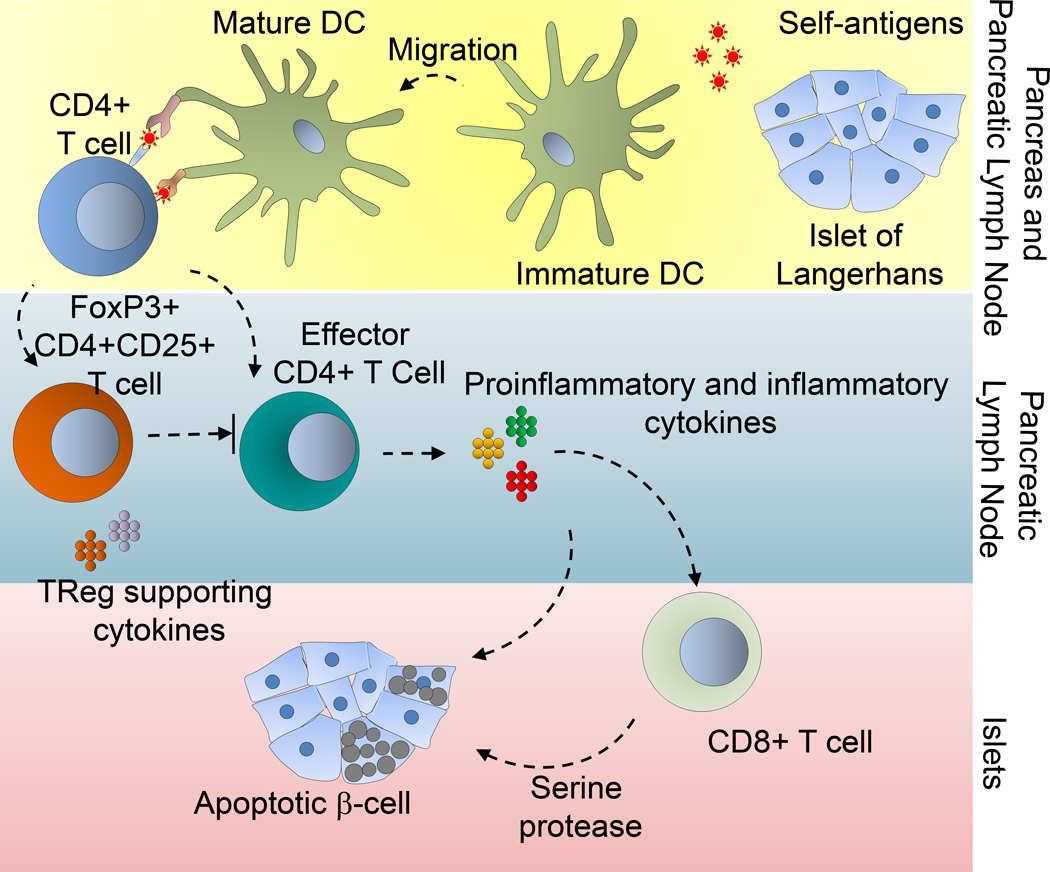
Figure 4. Scaffold based immunomodulation of TReg cells in type 1 diabetes mellitus (T1DM).
Schematic represents immunological mechanism associated with T1DM leading to apoptosis of pancreatic β-cell islets. Islets can be encapsulated in scaffolds made of polymeric materials such as PLGA. Protection of scaffold transplanted islets by TReg cells can be associated with insulin production and Foxp3+ TReg co-localization around islets.
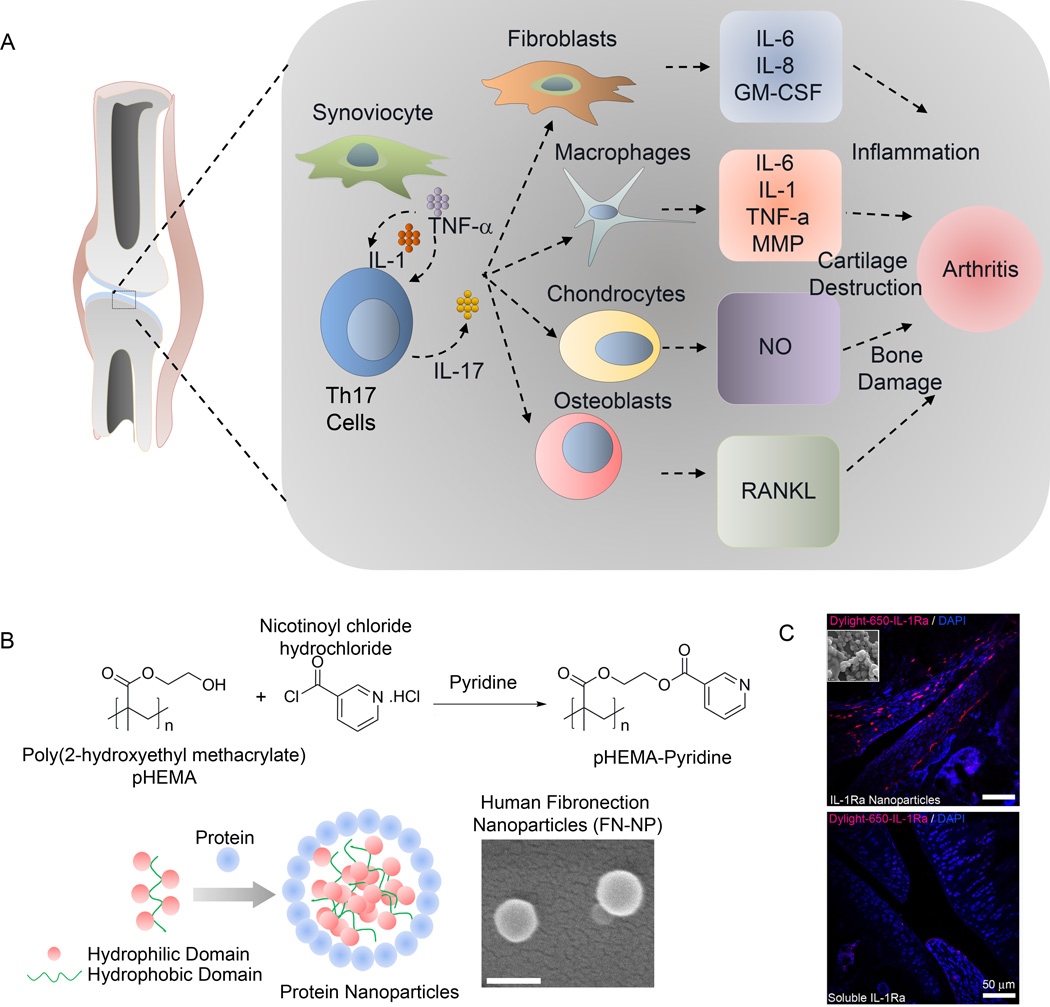
Another important area of hyperactive immune condition is rheumatoid arthritis (RA). Similar to T1DM, DCs and T-cells are central to immune tolerance in rheumatoid arthritis in their role as professional APCs and TReg cells, respectively. Immunomodulatory therapies for RA are aimed towards exploiting the tolerogenic capacity of DCs, behavior of TReg cells, and targeted suppression of specific cytokines such as IL-1, 6, and 17 [92]. Several of these aspects are illustrated in Figure 5A and have been discussed elegantly in reviews by others. Similar to RA, inflammatory response is prominent in osteoarthritis (OA) (Figure 5A). Although the OA levels of pro-inflammatory cytokines are lower than those observed in rheumatoid arthritis, IL-1 and tumor necrosis factor-α (TNF-α) have been implicated in OA pathogenesis by promoting synovial inflammation and activating chondrocytes and synovial fibroblasts [93],[94],[95]. These cytokines stimulate their own production and induce synovial cells and chondrocytes to express IL-6, IL-8, and other inflammatory mediators as well as proteases and prostaglandins [93],[94],[95].
Figure 5. Immunomodulation in arthritis.
A) Schematic of immune regulation in arthritis; B) hydrogel nanoparticle-based for enhanced knee joint retention to reduce inflammation in osteoarthritis. Schematic of nanoparticle self-assembly based on protein/polymer complexation. PHEMA–pyridine was synthesized by reacting PHEMA with nicotinoyl chloride hydrochloride in tetrahydrofuran and pyridine. SEM images of Fibronectin nanoparticles (FN-NP, Scale: 200 nm); C) IL-1Ra-tethered particles are distributed throughout the intra-articular joint space. IL-1Ra was tagged with a Dylight-IR-650 dye prior to tethering IL-1Ra to particles. Tagged IL-1Ra-tethered particles or soluble IL-1Ra was injected into the right stifle joint of 8–10 wk old rats while the left stifle joints received saline. Cryosectioned samples were counterstained with DAPI to localize dye tagged protein. Scale bar = 50 µm. Reproduced with permission [97],[96].
We recently reported self-assembling nanoparticles presenting IL-1 receptor antagonist (IL-1Ra) for enhanced delivery, retention, and bioactivity in the OA joint [96]. We demonstrated a RAFT-chemistry based self-assembly nanoparticles (300 nm) that efficiently bound IL-1Ra, targeted fibroblast-like synoviocytes (FLS) that are located inside joints in the synovium and inhibited IL-1β mediated signaling [96]. These 300 nm nanoparticles demonstrated significantly longer retention time of IL-1Ra in the rat stifle joint compared to that of soluble IL-1Ra and no adverse effect on the cartilage structure in the joint.
We next engineered nanoparticles made of hydrogel materials PHEMA, modified with hydrophobic pyridine (Figure 5B). The unique hydrophilic/hydrophobic balance of properties of the polymer allowed us to precisely control the size of nanoparticles in the 300–900 nm range and demonstrated a size dependent retention of protein-nanoparticle in rat knee joint with 900 nm protein particles remained localized to the knee for at least 14 days compared to bolus protein that was depleted within hours of injection. These nanoparticles made from hydrogel forming biomaterials can have a wide range of immunomodulatory applications for protein, peptides, and other therapeutic delivery to treat inflammation and can be expanded to treat diseases like infections and cancer.
9. Outlook and Future Perspective
The advancement and rapid translation of recent experimental scaffold vaccines into human clinical trials have emphasized the need for engineered strategies to quantitatively manipulate, in situ, immune cell recruitment, activation, and homing to the draining lymph nodes. Novel engineered macro- to nanoscale hydrogels and scaffolds continue to show promise as immunomodulatory platforms against cancer, infection, and autoimmunity. Clinically, tumors like melanoma, multiple myeloma, and lymphoma are poorly immunogenic because the target-antigens are often non-mutated self-antigens, making it difficult to induce or enhance an anti-tumor immune response. Therapeutic vaccines for B-cell non-Hodgkin lymphoma using the clonal tumor immunoglobulin idiotype, have been under development for more than three decades. Results from some of these Phase III trials have recently been released and in essence most of them failed to achieve their primary endpoints. Failure to eliminate poorly immunogenic cancers is often because of inadequate priming, low cell numbers and suboptimal phenotype of effector T-cells. These barriers could be overcome by biomaterials-based immune-engineering approaches that lie at the interface between material science and immunology. We recently showed that the above barriers in lymphoma could be “partially” overcome by applying bioengineering approaches to precisely bias the type of immune response. There is also a need to engineer systems that could manipulate diseased tissue microenvironment. Likewise, lymphoid tissue engineering is another promising arena for personalized immunotherapy however several aspects of the DC, B, and T-cell development like positive selection, antigen specificity, graft versus host rejection upon transplantation needs to be addressed. Although, initial studies in adoptive DC therapy have shown promising immune response, there exist several major limitations, including morbidity associated with patient cell isolation, high cost of ex vivo cell manipulation, time lag in “training” the immune cells, regulatory concerns, as well as the fact that ~90% of transplanted DCs die before they home to lymph nodes [45]. Nevertheless, with such provocative possibilities of generating secondary lymphoid tissues, thymus, and immune cells ex vivo from stem cells, new possibilities towards more effective personalized adoptive immunotherapy remain opened.
Acknowledgements
The authors would like to acknowledge financial support by grants from the National Institutes of Health (1R21 CA185236-01 (A.S.), EB 1R01-000346-21 (N.A.P)), the National Science Foundation (N.A.P.), and the Melinda and Bill Gates Foundation (N.A.P). The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
Contributor Information
Ankur Singh, Email: as2833@cornell.edu, Sibley School of Mechanical and Aerospace Engineering, Cornell University, Ithaca, NY 14853, USA.
Nicholas A. Peppas, Email: peppas@che.utexas.edu, Department of Chemical Engineering, Department of Biomedical Engineering and College of Pharmacy, The University of Texas at Austin, Austin, TX 78712, USA.
References
- 1.Yao S, Zhu Y, Chen L. Nature reviews. Drug discovery. 2013;12:130. doi: 10.1038/nrd3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Finn OJ. Nat Rev Immunol. 2003;3:630. doi: 10.1038/nri1150. [DOI] [PubMed] [Google Scholar]
- 3.Flaherty KT, Hodi FS, Fisher DE. Nat Rev Cancer. 2012;12:349. doi: 10.1038/nrc3218. [DOI] [PubMed] [Google Scholar]
- 4.Chiarle R, Voena C, Ambrogio C, Piva R, Inghirami G. Nat Rev Cancer. 2008;8:11. doi: 10.1038/nrc2291. [DOI] [PubMed] [Google Scholar]
- 5.Monsalvo AC, Batalle JP, Lopez MF, Krause JC, Klemenc J, Hernandez JZ, Maskin B, Bugna J, Rubinstein C, Aguilar L, Dalurzo L, Libster R, Savy V, Baumeister E, Cabral G, Font J, Solari L, Weller KP, Johnson J, Echavarria M, Edwards KM, Chappell JD, Crowe JE, Jr., Williams JV, Melendi GA, Polack FP. Nature medicine. 2011;17:195. doi: 10.1038/nm.2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.CDC. in http://www.cdc.gov/vhf/ebola/resources/outbreaks.html.
- 7.Dorman SE, Chaisson RE. Nature medicine. 2007;13:295. doi: 10.1038/nm0307-295. [DOI] [PubMed] [Google Scholar]
- 8.Roep BO, Buckner J, Sawcer S, Toes R, Zipp F. Nature medicine. 2012;18:48. doi: 10.1038/nm.2626. [DOI] [PubMed] [Google Scholar]; Steinman L, Merrill JT, McInnes IB, Peakman M. Nature medicine. 2012;18:59. doi: 10.1038/nm.2625. [DOI] [PubMed] [Google Scholar]
- 9.Purwada A, Roy K, Singh A. Acta Biomaterialia. 2014;10:1728. doi: 10.1016/j.actbio.2013.12.036. [DOI] [PubMed] [Google Scholar]
- 10.Moon JJ, Huang B, Irvine DJ. Advanced materials. 2012;24:3724. doi: 10.1002/adma.201200446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hubbell JA, Thomas SN, Swartz MA. Nature. 2009;462:449. doi: 10.1038/nature08604. [DOI] [PubMed] [Google Scholar]
- 12.Boehler RM, Graham JG, Shea LD. Biotechniques. 2011;51:239. doi: 10.2144/000113754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peppas NA, Bures P, Leobandung W, Ichikawa H. Eur J Pharm Biopharm. 2000;50:27. doi: 10.1016/s0939-6411(00)00090-4. [DOI] [PubMed] [Google Scholar]
- 14.Kim JJ, Park K. J Control Release. 2001;77:39. doi: 10.1016/s0168-3659(01)00447-3. [DOI] [PubMed] [Google Scholar]
- 15.St’astny M, Plocova D, Etrych T, Ulbrich K, Rihova B. Eur J Cancer. 2002;38:602. doi: 10.1016/s0959-8049(01)00421-x. [DOI] [PubMed] [Google Scholar]
- 16.Anseth KS, Metters AT, Bryant SJ, Martens PJ, Elisseeff JH, Bowman CN. J Control Release. 2002;78:199. doi: 10.1016/s0168-3659(01)00500-4. [DOI] [PubMed] [Google Scholar]
- 17.Orienti I, Trere R, Luppi B, Bigucci F, Cerchiara T, Zuccari G, Zecchi V. Arch Pharm (Weinheim) 2002;335:89. doi: 10.1002/1521-4184(200203)335:2/3<89::AID-ARDP89>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 18.Tiller JC. Angew Chem Int Ed Engl. 2003;42:3072. doi: 10.1002/anie.200301647. [DOI] [PubMed] [Google Scholar]
- 19.Peppas NA, Wood KM, Blanchette JO. Expert Opin Biol Ther. 2004;4:881. doi: 10.1517/14712598.4.6.881. [DOI] [PubMed] [Google Scholar]
- 20.Phelps EA, Enemchukwu NO, Fiore VF, Sy JC, Murthy N, Sulchek TA, Barker TH, Garcia AJ. Advanced materials. 2012;24:64. doi: 10.1002/adma.201103574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suri S, Schmidt CE. Tissue Eng Part A. 2010;16:1703. doi: 10.1089/ten.tea.2009.0381. [DOI] [PubMed] [Google Scholar]
- 22.Suri S, Han LH, Zhang W, Singh A, Chen S, Schmidt CE. Biomed Microdevices. 2011;13:983. doi: 10.1007/s10544-011-9568-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singh A, Qin H, Fernandez I, Wei J, Lin J, Kwak LW, Roy K. Journal of controlled release : official journal of the Controlled Release Society. 2011;155:184. doi: 10.1016/j.jconrel.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 24.Singh A, Suri S, Roy K. Biomaterials. 2009 doi: 10.1016/j.biomaterials.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schoichet MS. Macromolecules. 2010;43:581. [Google Scholar]
- 26.Kearney CJ, Mooney DJ. Nature materials. 2013;12:1004. doi: 10.1038/nmat3758. [DOI] [PubMed] [Google Scholar]
- 27.Peppas NA, Huang Y, Torres-Lugo M, Ward JH, Zhang J. Annu Rev Biomed Eng. 2000;2:9. doi: 10.1146/annurev.bioeng.2.1.9. [DOI] [PubMed] [Google Scholar]
- 28.Kopecek J, Yang J. Angewandte Chemie. 2012;51:7396. doi: 10.1002/anie.201201040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin CC, Anseth KS. Pharmaceutical Research. 2009;26:631. doi: 10.1007/s11095-008-9801-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Metters A, Hubbell J. Biomacromolecules. 2005;6:290. doi: 10.1021/bm049607o. [DOI] [PubMed] [Google Scholar]
- 31.Lin CC, Metters AT. Advanced drug delivery reviews. 2006;58:1379. doi: 10.1016/j.addr.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 32.Seliktar D. Science. 2012;336:1124. doi: 10.1126/science.1214804. [DOI] [PubMed] [Google Scholar]
- 33.Garcia AJ. Ann Biomed Eng. 2014;42:312. doi: 10.1007/s10439-013-0870-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Park YD, Tirelli N, Hubbell JA. Biomaterials. 2003;24:893. doi: 10.1016/s0142-9612(02)00420-9. [DOI] [PubMed] [Google Scholar]
- 35.Davis KA, Burdick JA, Anseth KS. Biomaterials. 2003;24:2485. doi: 10.1016/s0142-9612(02)00582-3. [DOI] [PubMed] [Google Scholar]
- 36.Nair DP, Podgorski M, Chatani S, Gong T, Xi W, Fenoli CR, Bowman CN. Chemistry of Materials. 2014;26:724. [Google Scholar]
- 37.van de Wetering P, Metters AT, Schoenmakers RG, Hubbell JA. J Control Release. 2005;102:619. doi: 10.1016/j.jconrel.2004.10.029. [DOI] [PubMed] [Google Scholar]
- 38.Chung IM, Enemchukwu NO, Khaja SD, Murthy N, Mantalaris A, Garcia AJ. Biomaterials. 2008;29:2637. doi: 10.1016/j.biomaterials.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Elbert DL, Hubbell JA. Biomacromolecules. 2001;2:430. doi: 10.1021/bm0056299. [DOI] [PubMed] [Google Scholar]
- 40.Hiemstra C, Aa LJ, Zhong Z, Dijkstra PJ, Feijen J. Biomacromolecules. 2007;8:1548. doi: 10.1021/bm061191m. [DOI] [PubMed] [Google Scholar]
- 41.Hiemstra C, van der Aa LJ, Zhong Z, Dijkstra PJ, Feijen J. Macromolecules. 2007;40:1165. [Google Scholar]
- 42.Hiemstra C, Zhong Z, van Steenbergen MJ, Hennink WE, Feijen J. J Control Release. 2007;122:71. doi: 10.1016/j.jconrel.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 43.Kim J, Wacker BK, Elbert DL. Biomacromolecules. 2007;8:3682. doi: 10.1021/bm700756z. [DOI] [PubMed] [Google Scholar]
- 44.Ali OA, Emerich D, Dranoff G, Mooney DJ. Sci Transl Med. 2009;1:8ra19. doi: 10.1126/scitranslmed.3000359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ali OA, Huebsch N, Cao L, Dranoff G, Mooney DJ. Nat Mater. 2009;8:151. doi: 10.1038/nmat2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hori Y, Winans AM, Huang CC, Horrigan EM, Irvine DJ. Biomaterials. 2008;29:3671. doi: 10.1016/j.biomaterials.2008.05.033. [DOI] [PubMed] [Google Scholar]
- 47.Hori Y, Winans AM, Irvine DJ. Acta Biomater. 2008 doi: 10.1016/j.actbio.2008.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pradhan P, Qin H, Leleux JA, Gwak D, Sakamaki I, Kwak LW, Roy K. Biomaterials. 2014 doi: 10.1016/j.biomaterials.2014.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Steinman RM, Cohn ZA. The Journal of experimental medicine. 1973;137:1142. doi: 10.1084/jem.137.5.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Steinman RM, Hemmi H. Current topics in microbiology and immunology. 2006;311:17. doi: 10.1007/3-540-32636-7_2. [DOI] [PubMed] [Google Scholar]
- 51.Steinman RM, Banchereau J. Nature. 2007;449:419. doi: 10.1038/nature06175. [DOI] [PubMed] [Google Scholar]
- 52.Schuler G, Schuler-Thurner B, Steinman RM. Curr Opin Immunol. 2003;15:138. doi: 10.1016/s0952-7915(03)00015-3. [DOI] [PubMed] [Google Scholar]
- 53.Nagaraj S, Gupta K, Pisarev V, Kinarsky L, Sherman S, Kang L, Herber DL, Schneck J, Gabrilovich DI. Nature medicine. 2007;13:828. doi: 10.1038/nm1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rosenberg SA, Yang JC, Restifo NP. Nature medicine. 2004;10:909. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Piersma SJ. Cancer Microenviron. 2011;4:361. doi: 10.1007/s12307-011-0066-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tong CC, Kao J, Sikora AG. Immunol Res. 2012;54:266. doi: 10.1007/s12026-012-8306-6. [DOI] [PubMed] [Google Scholar]
- 57.Jewell CM, Lopez SC, Irvine DJ. Proc Natl Acad Sci U S A. 2011;108:15745. doi: 10.1073/pnas.1105200108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nochi T, Yuki Y, Takahashi H, Sawada S, Mejima M, Kohda T, Harada N, Kong IG, Sato A, Kataoka N, Tokuhara D, Kurokawa S, Takahashi Y, Tsukada H, Kozaki S, Akiyoshi K, Kiyono H. Nature materials. 2010;9:572. doi: 10.1038/nmat2784. [DOI] [PubMed] [Google Scholar]
- 59.Hori Y, Winans AM, Irvine DJ. Acta Biomaterialia. 2009;5:969. doi: 10.1016/j.actbio.2008.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wu Y, Wu S, Hou L, Wei W, Zhou M, Su Z, Wu J, Chen W, Ma G. European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V. 2012;81:486. doi: 10.1016/j.ejpb.2012.03.021. [DOI] [PubMed] [Google Scholar]
- 61.Matsuo K, Ishii Y, Quan YS, Kamiyama F, Mukai Y, Yoshioka Y, Okada N, Nakagawa S. Journal of controlled release : official journal of the Controlled Release Society. 2011;149:15. doi: 10.1016/j.jconrel.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 62.Stachowiak AN, Irvine DJ. J Biomed Mater Res A. 2008;85:815. doi: 10.1002/jbm.a.31661. [DOI] [PubMed] [Google Scholar]
- 63.Kageyama S, Kitano S, Hirayama M, Nagata Y, Imai H, Shiraishi T, Akiyoshi K, Scott AM, Murphy R, Hoffman EW, Old LJ, Katayama N, Shiku H. Cancer science. 2008;99:601. doi: 10.1111/j.1349-7006.2007.00705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gubeli RJ, Schoneweis K, Huzly D, Ehrbar M, Charpin-El Hamri G, El-Baba MD, Urban S, Weber W. Sci Rep. 2013;3:2610. doi: 10.1038/srep02610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li X, Galliher-Beckley A, Huang H, Sun X, Shi J. Vaccine. 2013;31:4508. doi: 10.1016/j.vaccine.2013.07.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wu Y, Wei W, Zhou M, Wang Y, Wu J, Ma G, Su Z. Biomaterials. 2012;33:2351. doi: 10.1016/j.biomaterials.2011.11.068. [DOI] [PubMed] [Google Scholar]
- 67.Liechty WB, Caldorera-Moore M, Phillips MA, Schoener C, Peppas NA. Journal of controlled release : official journal of the Controlled Release Society. 2011;155:119. doi: 10.1016/j.jconrel.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Agarwal R, Singh V, Jurney P, Shi L, Sreenivasan SV, Roy K. P Natl Acad Sci USA. 2013 doi: 10.1073/pnas.1305000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chou HY, Lin XZ, Pan WY, Wu PY, Chang CM, Lin TY, Shen HH, Tao MH. Journal of immunology. 2010;185:5468. doi: 10.4049/jimmunol.1001875. [DOI] [PubMed] [Google Scholar]
- 70.Sexton A, Whitney PG, Chong SF, Zelikin AN, Johnston AP, De Rose R, Brooks AG, Caruso F, Kent SJ. ACS Nano. 2009;3:3391. doi: 10.1021/nn900715g. [DOI] [PubMed] [Google Scholar]
- 71.Chong SF, Sexton A, De Rose R, Kent SJ, Zelikin AN, Caruso F. Biomaterials. 2009;30:5178. doi: 10.1016/j.biomaterials.2009.05.078. [DOI] [PubMed] [Google Scholar]
- 72.Graham JG, Zhang X, Goodman A, Pothoven K, Houlihan J, Wang S, Gower RM, Luo X, Shea LD. Tissue Eng Part A. 2013;19:1465. doi: 10.1089/ten.tea.2012.0643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Irvine DJ, Stachowiak AN, Hori Y. Seminars in immunology. 2008;20:137. doi: 10.1016/j.smim.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 74.Suematsu S, Watanabe T. Nature biotechnology. 2004;22:1539. doi: 10.1038/nbt1039. [DOI] [PubMed] [Google Scholar]
- 75.Lee J, Kotov NA. Small. 2009;5:1008. doi: 10.1002/smll.200801242. [DOI] [PubMed] [Google Scholar]
- 76.Kumamoto T, Huang EK, Paek HJ, Morita A, Matsue H, Valentini RF, Takashima A. Nat Biotechnol. 2002;20:64. doi: 10.1038/nbt0102-64. [DOI] [PubMed] [Google Scholar]
- 77.Hodi FS, Butler M, Oble DA, Seiden MV, Haluska FG, Kruse A, Macrae S, Nelson M, Canning C, Lowy I, Korman A, Lautz D, Russell S, Jaklitsch MT, Ramaiya N, Chen TC, Neuberg D, Allison JP, Mihm MC, Dranoff G. P Natl Acad Sci USA. 2008;105:3005. doi: 10.1073/pnas.0712237105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dolgin E. Nature. 2013;504:S16. doi: 10.1038/504S16a. [DOI] [PubMed] [Google Scholar]
- 79.Roy K, Wang D, Hedley ML, Barman SP. Mol Ther. 2003;7:401. doi: 10.1016/s1525-0016(03)00008-x. [DOI] [PubMed] [Google Scholar]
- 80.Tortora M, Cavalieri F, Chiessi E, Paradossi G. Biomacromolecules. 2007;8:209. doi: 10.1021/bm0607269. [DOI] [PubMed] [Google Scholar]
- 81.Hahn SK, Park JK, Tomimatsu T, Shimoboji T. Int J Biol Macromol. 2007;40:374. doi: 10.1016/j.ijbiomac.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 82.Franssen O, Vandervennet L, Roders P, Hennink WE. J Control Release. 1999;60:211. doi: 10.1016/s0168-3659(99)00074-7. [DOI] [PubMed] [Google Scholar]
- 83.Franssen O, Stenekes RJ, Hennink WE. J Control Release. 1999;59:219. doi: 10.1016/s0168-3659(98)00193-x. [DOI] [PubMed] [Google Scholar]
- 84.Irvine DJ, Swartz MA, Szeto GL. Nature materials. 2013;12:978. doi: 10.1038/nmat3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Davis ME, Motion JP, Narmoneva DA, Takahashi T, Hakuno D, Kamm RD, Zhang S, Lee RT. Circulation. 2005;111:442. doi: 10.1161/01.CIR.0000153847.47301.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tysseling-Mattiace VM, Sahni V, Niece KL, Birch D, Czeisler C, Fehlings MG, Stupp SI, Kessler JA. J Neurosci. 2008;28:3814. doi: 10.1523/JNEUROSCI.0143-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rudra JS, Mishra S, Chong AS, Mitchell RA, Nardin EH, Nussenzweig V, Collier JH. Biomaterials. 2012;33:6476. doi: 10.1016/j.biomaterials.2012.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Park J, Wrzesinski SH, Stern E, Look M, Criscione J, Ragheb R, Jay SM, Demento SL, Agawu A, Licona Limon P, Ferrandino AF, Gonzalez D, Habermann A, Flavell RA, Fahmy TM. Nat Mater. 2012;11:895. doi: 10.1038/nmat3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Esensten JH, Wofsy D, Bluestone JA. Nat Rev Rheumatol. 2009;5:560. doi: 10.1038/nrrheum.2009.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tarbell KV, Petit L, Zuo X, Toy P, Luo X, Mqadmi A, Yang H, Suthanthiran M, Mojsov S, Steinman RM. The Journal of experimental medicine. 2007;204:191. doi: 10.1084/jem.20061631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Roncarolo MG, Battaglia M. Nature reviews. Immunology. 2007;7:585. doi: 10.1038/nri2138. [DOI] [PubMed] [Google Scholar]
- 92.van den Berg WB, Miossec P. Nat Rev Rheumatol. 2009;5:549. doi: 10.1038/nrrheum.2009.179. [DOI] [PubMed] [Google Scholar]
- 93.Malemud CJ. Drugs Aging. 2010;27:95. doi: 10.2165/11319950-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 94.Goldring MB, Otero M, Tsuchimochi K, Ijiri K, Li Y. Ann Rheum Dis. 2008;67(Suppl 3):ii75. doi: 10.1136/ard.2008.098764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bondeson J, Blom AB, Wainwright S, Hughes C, Caterson B, van den Berg WB. Arthritis Rheum. 2010;62:647. doi: 10.1002/art.27290. [DOI] [PubMed] [Google Scholar]
- 96.Whitmire RE, Wilson DS, Singh A, Levenston ME, Murthy N, Garcia AJ. Biomaterials. 2012;33:7665. doi: 10.1016/j.biomaterials.2012.06.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Singh A, Agarwal R, Diaz-Ruiz CA, Willett NJ, Wang P, Lee LA, Wang Q, Guldberg RE, Garcia AJ. Adv Healthc Mater. 2014 doi: 10.1002/adhm.201400051. [DOI] [PMC free article] [PubMed] [Google Scholar]