Summary
One of the biggest questions in learning is how a system can resolve the plasticity and stability dilemma [1–3]. Specifically, the learning system needs to not only have a high capability of learning new items (plasticity), but also have a high stability to retain important items or processing in the system by preventing unimportant or irrelevant information from being learned. This dilemma should hold true for visual perceptual learning (VPL), which is defined as a long-term increase in performance on a visual task as a result of visual experience [4–18]. Although it is well known that aging influences learning [19–24], the effect of aging on the stability and plasticity of the visual system is unclear. To address the question, we asked older and younger adults to perform a task while a task-irrelevant feature was merely exposed. We found that older individuals learn the task-irrelevant features that younger individuals do not learn, both the features that are sufficiently strong for younger individuals to suppress and the features that are too weak for younger individuals to learn. At the same time, there is no plasticity reduction in older individuals within the task tested. These results suggest that the older visual system is less stable to unimportant information than the younger visual system. A learning problem with older individuals may be due to decrease in stability rather than due to a decrease in plasticity, at least in VPL.
Results
To address characteristics of learning with older individuals, we took the advantage of interesting aspects of perceptual learning as a result of mere exposure to a feature. It has been found that at least in some cases mere exposure to a visual feature that is not relevant to a given task with younger individuals does not lead to learning of the feature if it is suprathreshold and/or conspicuous [7–9, 25, 26]. This suggests that if an exposed task-irrelevant feature is detected, the brain of a younger individual should filter out or suppress the feature to avoid replacing existing important information or processing with task-irrelevant and therefore usually insignificant information. That is, the younger brain makes itself stable as well as plastic. If it holds true that older individuals have simply less plasticity than younger individuals, then a smaller magnitude of VPL should occur with older as compared to younger adults, irrespective of whether the learned feature is task-relevant or task-irrelevant.
Note that it has been pointed out that the plasticity and stability dilemma cannot be resolved merely by changes in local circuits, including synaptic weight changes, without changes at a more global system level that include interactions between different types of processes that could include attention [1, 2]. Thus, here we define plasticity as changes resulting from involvement of global processing associated with learning, and discuss the ability to prevent unimportant or irrelevant information from being learned as a result of different types of processing at a global system level as an aspect of stability.
To test the hypothesis that older individuals are simply less plastic at a global system level, two groups of 10 older (ages between 67 and 79 years old) and 10 younger adults (ages between 19 and 30 years old) participated in the experiment with the same procedure (see Supplemental Experimental Procedures for detail). The experiment consisted of 1 day’s pre-test, 8 days of training stage and 1 day’s post-test in their respective order. On each trial of the training stage, subjects were presented with a sequence of 6 letters and 2 digits at the center of the display. After the offset of the sequence, subjects were asked to report the 2 digits as targets in the sequence of otherwise letters (Figure 1). During the presentations of the letters and digits, a motion display was exposed in the background as a task-irrelevant feature. The display consisted of a certain ratio of dots moving coherently from frame to frame and the other dots moving randomly [9, 25, 27, 28]. The coherent motion level (signal strength) was varied in 4 steps (0.3, 0.6, 1.0 and 4.0 x the individual 80% coherent motion detection threshold). The multiplicative values of the individual threshold were used to adjust individual differences on perception of coherent motion, particularly between older and younger subjects [29]. Coherent motion with each coherent level moved in a different direction (see Supplemental Table 1 for detail).
Figure 1.
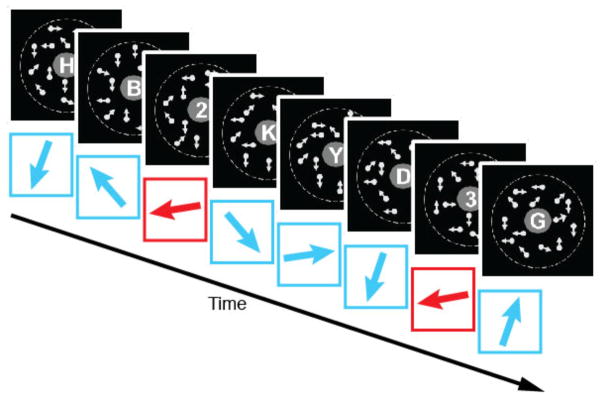
The procedure of a trial in the training stage. Red arrows represent coherent motion direction that was paired with digits as targets (paired direction). Cyan arrows represent coherent motion directions that were not paired with digits (unpaired directions). Arrows are for illustrative purposes and were not presented in the training.
We measured subjects’ performance on a coherent motion discrimination task in the pre- and post-test stages. Since coherent motion was irrelevant to the given task during the training stage, the amount of performance increase in the post-test stages as compared to the pre-test stage, if any, is regarded as the magnitude of task-irrelevant perceptual learning. As shown in Figure 2, for the younger group the amplitude of task-irrelevant learning was the highest around the threshold and no learning was observed when the coherent motion level was 4 x threshold, which is suprathreshold. This result is in accord with the previous study and is regarded as a typical profile of younger adults [28]. However, the results with the older group were different than the results of the younger group. With the increasing coherent motion level, the amplitude of learning did not decline. The results of the following statistical analyses are in accord with this observation.
Figure 2.
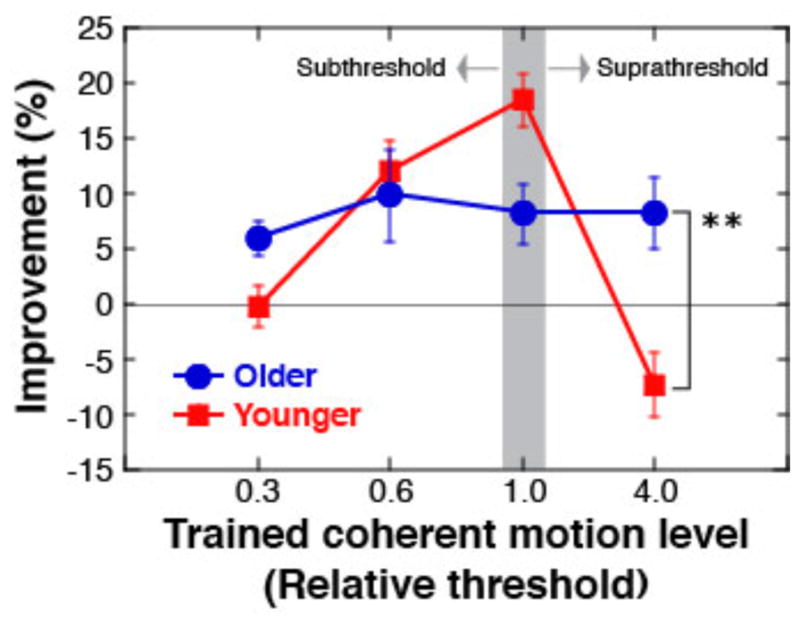
Mean (±s.e.m) performance improvement in motion discrimination. The y-axis represents the mean percent correct across displays with four coherent motion levels (0.3, 0.6, 1.0 and 4.0 x individual threshold) in the pre-test stage subtracted from that in the post-test stage. The x-axis represents the coherent motion level (0.3, 0.6, 1.0 and 4.0 x the individual threshold) exposed in the training stage. The gray area indicates the threshold level. **p<0.01
In order to compare the overall task-irrelevant learning amplitudes between older and younger adults (see Fig. 2), we conducted three-way ANOVA with age (older vs younger groups) and coherent motion level (0.3, 0.6, 1.0, 4.0 x threshold) as between-subject factors and test (pre- vs post- tests) as a within-subject factor. The results showed significant main factors of age [F(1, 32)=5.194, p=0.0295] and test [F(1,32)=18.26, p=0.0002] and also indicated significant interactions between coherent motion level and test [F(3,32)=3.613, p=0.0236]. A t-test applied to performance improvements (performance in the pre-test subtracted from performance in the post-test) for the suprathreshold coherent level (4.0 x threshold) showed that performance improvement in the older group was significantly greater than in the younger group [t(10)=3.566, p=0.0051] as shown in Fig. 2. These results indicate that a greater magnitude of VPL of task-irrelevant coherent motion occurred with the older individuals than with the younger individuals, when a coherent motion level was suprathreshold.
We further conduced two-way ANOVA with coherent motion level (0.3, 0.6, 1.0, 4.0 x threshold) as a between-subject factor and test (pre- vs post- tests) as a within-subject factor for each of the older and younger groups. For the older subjects, only the main factor of test was significant [F (1,16) =17.316, p=0.0007], indicating that performance improvement was constant across the coherent motion levels. For the younger subjects, significant were the main factor of test [F(1,16)=4.952, p=0.0408] and the interaction between test and coherent motion level [F (3,16) =5.033, p=0.0121], indicating that performance improvement was not constant across the coherent motion levels.
Why did task-irrelevant VPL at such a high signal level (4 x the threshold coherent motion level) occur for older subjects whereas it did not occur with younger subjects? A series of studies with younger individuals has suggested the following mechanisms: When a task-irrelevant feature is above threshold or conspicuous, it is detected and suppressed by an attentional system [28, 30, 31]. On the other hand, when it is below threshold, it fails to be detected and therefore to be filtered out by the attentional system [31]. As a result, task-irrelevant VPL of a suprathreshold feature is less likely to occur [28], as shown in the results of the younger group of the current experiment. If this model is true, the occurrence of task-irrelevant VPL of suprathreshold motion with the older group results from the failure of the attentional system to suppress task-irrelevant feature signal. Note that previous studies have found less suppressive control for older as compared to younger adults [32, 33]. Thus, the degree of suppression on a task-irrelevant signal should be smaller with older individuals if the signal is suprathreshold. This may allow for task-irrelevant VPL of a suprathreshold feature to occur. That is, task-irrelevant VPL of a suprathreshold motion may have occurred with older adults because older individuals have a decreased capacity to filter out irrelevant signals. If so, this may make older individuals’ visual system more plastic in a harmful way and therefore less stable. That is, the decreased capacity to filter out irrelevant signals may lead the visual system with older individuals to resolve the plasticity-stability dilemma less effectively as the visual system with younger individuals.
To test the above mentioned hypothesis, we measured the useful field of view (UFOV) tests [34] -- standard tests for attentional processing of older adults [34]-- before and after the training of the current experiment for both age groups. The UFOV tests have three subtests for three different attentional abilities: sub-test 1 for processing speed, sub-test 2 for divided attention and sub-test 3 for selective attention. The selective attention measure in subtest 3 is to assess the ability to filter out task irrelevant information. Note that a lower score in a UFPV test represents higher performance. If the hypothesis that task-irrelevant VPL with the suprathreshold coherent motion level occurred only with the older group because of their lower ability to filter out task-irrelevant signals is true, the score of sub-test 3 should be higher (performance being lower) with the older group than with the younger group.
Three-way ANOVA with age (older vs younger) as a between-subject factor and UFOV subtest (1, 2 vs 3) and test (pre- vs post- tests) as within-subject factors was conducted. The results showed significant main effects of age [F(1,18) = 15.958, p = 0.001] and UFOV subtest [F(2, 36) = 49.48, p < 0.0001] and significant interaction between age and UFOV [F(2,36) = 21.884, p = 0.00001]. However, no significant main effect of test (pre- vs post- tests) was found [F(1,18) = 1.793, p = 0.197], suggesting that none of the tested attention abilities was changed due to the training.
Based on the significant interaction between age and UFOV subtest, we applied two-way ANOVA [age (older vs younger) x test (pre- vs post- tests)] to each of results from three UFOV subtests. Only for UFOV sub-test 3 (selective attention), main effect of age was significant [F(1,18)=30.634 p=0.00003].
These results indicate that older subjects have significantly lower ability for filtering task-irrelevant signals than younger subjects. If task-irrelevant VPL of a suprathreshold motion occurred with older adults because they have lower ability to filter out irrelevant signals, then one can predict that the lower ability should result in a greater amplitude of task-irrelevant VPL of suprathreshold motion. Fig. 3 shows the correlation between the score of the UFOV sub-test 3 (filtering) and the amplitude of task-irrelevant VPL for the older (A) and younger (B) groups. A significant positive correlation was obtained for the older group (r = 0.735, p = 0.048), but not for the younger group (r=−0.46, p=0.179). Almost all the UFOV scores for younger subjects were lower (higher filtering ability) than for older subjects. These results are in accord with the hypothesis that task-irrelevant VPL of a suprathreshold motion occurred with older individuals because older adults have a decreased ability to filter out irrelevant signals, resulting in the undesirable development of task-irrelevant VPL.
Figure 3.
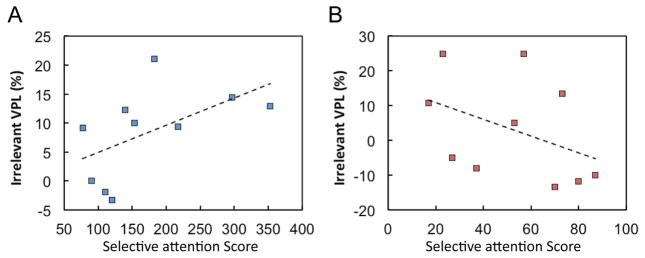
Correlation between Post UFOV sub-test 3 scores (the lower the score, the higher ability to filter out) and performance improvement resulting from exposure to suprathreshold (4.0 x threshold) coherent motion level across older subjects for the older (A) and younger (B) groups.
Discussion
In the present study, we examined how task-irrelevant learning occurs with older and younger individuals. We have found that older individuals learned highly weak and strong task-irrelevant coherent motion directions that younger people did not learn. The amplitude of task-irrelevant VPL with the older individuals was negatively correlated with the degree of ability to filter out task-irrelevant signals.
Task-irrelevant VPL with the 0.3 x threshold coherent motion for older individuals indicates that plasticity of older individuals is not lower than younger individuals. Is this tendency specific for task-irrelevant VPL? To address this question, we analyzed accuracy of the RSVP task during the training stage of the current experiment. No significant difference was found between the performance improvements of the RSVP task for the older and younger groups. Three-way ANOVA (age, training session, coherent motion level) indicates that the main effect of training session was significant [F(7,224)=10.321, p<0.000001], but neither the main effect of age [F(1,32)=1.983, p=0.169] nor coherent motion level [F(3,32)=1.058, p=0.381] was significant. None of the interactions were significant [For age x coherent motion level, F(3,32)=0.941, p=0.432: For age x training day, F(7,224)=2.429, p=0.05: for training session x coherent motion level, F(21,224)=0.584, p=0.855: for age x training session x coherent motion level, F(21,224)=0.738, p=0.715]. These results indicate that older subjects as well as younger subjects showed significant amounts of task-relevant learning. No evidence was obtained that indicate that older individuals have a problem with plasticity. This tendency is in accord with previous studies that showed efficiency of learning visual tasks with older individuals was not significantly different from younger individuals [35–40].
Is there any possibility that the older subjects learned between the two tests (pre- and post-tests) as a result of the repeated testing in the test stage(s) and that this resulted in the rather flat curve for the older group in Fig. 2. To test this possibility, we compared performance (accuracy) for the pre- and post-tests on the motion that was ±60 deg apart from both of the directions paired with targets during training. The mean improvements (performance in the pretest subtracted from performance in the posttest) were −0.047 (± 0.043 SE) for the older group and −0.015 (± 0.056 SE) for the younger group. We applied 2-way ANOVA [age (old vs young) and test (pre- vs post tests)]. None of the main effect of age [F(1, 18)=0.007, p=0.933], main effect of test [F(1, 18)=0.771, p=0.391], or interaction of age x test [F(1,18)=0.206, p=0.655] was significant. These results do not support the possibility that the older subjects learned in the test stage(s).
For the visual system to efficiently adapt to a new environment, the plasticity-stability dilemma needs to be resolved. Our results indicate that older individuals learned strong task-irrelevant signals that younger people did not learn while the results of task-irrelevant and task-relevant VPL showed no evidence that older individuals are less plastic than younger individuals. From this viewpoint, the results of the present study suggest that older individuals have a problem with stability at the global system level to avoid task-irrelevant signals from being learned rather than with plasticity.
Supplementary Material
Highlights.
The older brain is less stable to unimportant visual signal than the younger brain.
No result showed that the older brain is less plastic than the younger brain.
Older subjects learned strong task-irrelevant features younger subjects suppress.
Older subjects learned weak task-irrelevant features younger subjects did not learn.
Acknowledgments
Supported by funds from NIH (R01EY019466, R01AG031941, R01MH091801) and JSPS. We also acknowledge the experiment site support and subjects recruitment from Brookline Senior Center.
Footnotes
Attached Supplemental Information includes Supplemental Experimental Procedures, Supplemental Table 1 and Supplemental References.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Grossberg S. Adaptive Resonance Theory: how a brain learns to consciously attend, learn, and recognize a changing world. Neural networks : the official journal of the International Neural Network Society. 2013;37:1–47. doi: 10.1016/j.neunet.2012.09.017. [DOI] [PubMed] [Google Scholar]
- 2.Abraham WC, Robins A. Memory retention--the synaptic stability versus plasticity dilemma. Trends in Neurosciences. 2005;28:73–78. doi: 10.1016/j.tins.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 3.Sasaki Y, Nanez JE, Watanabe T. Advances in visual perceptual learning and plasticity. Nature Reviews Neuroscience. 2010;11:53–60. doi: 10.1038/nrn2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Szpiro SF, Wright BA, Carrasco M. Learning one task by interleaving practice with another task. Vision Res. 2014;101:118–124. doi: 10.1016/j.visres.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carmel D, Carrasco M. Perceptual learning and dynamic changes in primary visual cortex. Neuron. 2008;57:799–801. doi: 10.1016/j.neuron.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Watanabe T, Sasaki Y. Perceptual learning: toward a conprehensive theory. Ann Rev Psychol. 2015;66 doi: 10.1146/annurev-psych-010814-015214. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seitz AR, Watanabe T. Psychophysics: Is subliminal learning really passive? Nature. 2003;422:36. doi: 10.1038/422036a. [DOI] [PubMed] [Google Scholar]
- 8.Watanabe T, Nanez JE, Koyama S, Mukai I, Liederman J, Sasaki Y. Greater plasticity in lower-level than higher-level visual motion processing in a passive perceptual learning task. Nature neuroscience. 2002;5:1003–1009. doi: 10.1038/nn915. [DOI] [PubMed] [Google Scholar]
- 9.Watanabe T, Nanez JE, Sasaki Y. Perceptual learning without perception. Nature. 2001;413:844–848. doi: 10.1038/35101601. [DOI] [PubMed] [Google Scholar]
- 10.Das A, Demagistris M, Huxlin KR. Different properties of visual relearning after damage to early versus higher-level visual cortical areas. J Neurosci. 2012;32:5414–5425. doi: 10.1523/JNEUROSCI.0316-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li J, Thompson B, Deng D, Chan LY, Yu M, Hess RF. Dichoptic training enables the adult amblyopic brain to learn. Curr Biol. 2013;23:R308–309. doi: 10.1016/j.cub.2013.01.059. [DOI] [PubMed] [Google Scholar]
- 12.Xu JP, He ZJ, Ooi TL. Effectively reducing sensory eye dominance with a push-pull perceptual learning protocol. Curr Biol. 2010;20:1864–1868. doi: 10.1016/j.cub.2010.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu JP, He ZJ, Ooi TL. Perceptual learning to reduce sensory eye dominance beyond the focus of top-down visual attention. Vision Res. 2011;61:39–47. doi: 10.1016/j.visres.2011.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beste C, Wascher E, Gunturkun O, Dinse HR. Improvement and impairment of visually guided behavior through LTP- and LTD-like exposure-based visual learning. Curr Biol. 2011;21:876–882. doi: 10.1016/j.cub.2011.03.065. [DOI] [PubMed] [Google Scholar]
- 15.Dosher BA, Lu ZL. Perceptual learning reflects external noise filtering and internal noise reduction through channel reweighting. Proc Natl Acad Sci U S A. 1998;95:13988–13993. doi: 10.1073/pnas.95.23.13988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gilbert CD, Li W. Adult visual cortical plasticity. Neuron. 2012;75:250–264. doi: 10.1016/j.neuron.2012.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karni A, Sagi D. The time course of learning a visual skill. Nature. 1993;365:250–252. doi: 10.1038/365250a0. [DOI] [PubMed] [Google Scholar]
- 18.Law CT, Gold JI. Neural correlates of perceptual learning in a sensory-motor, but not a sensory, cortical area. Nat Neurosci. 2008;11:505–513. doi: 10.1038/nn2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Freitas C, Farzan F, Pascual-Leone A. Assessing brain plasticity across the lifespan with transcranial magnetic stimulation: why, how, and what is the ultimate goal? Frontiers in neuroscience. 2013;7:42. doi: 10.3389/fnins.2013.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones S, Nyberg L, Sandblom J, Stigsdotter Neely A, Ingvar M, Magnus Petersson K, Backman L. Cognitive and neural plasticity in aging: general and task-specific limitations. Neuroscience and biobehavioral reviews. 2006;30:864–871. doi: 10.1016/j.neubiorev.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 21.Lustig C, Shah P, Seidler R, Reuter-Lorenz PA. Aging, training, and the brain: a review and future directions. Neuropsychology review. 2009;19:504–522. doi: 10.1007/s11065-009-9119-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mahncke HW, Connor BB, Appelman J, Ahsanuddin ON, Hardy JL, Wood RA, Joyce NM, Boniske T, Atkins SM, Merzenich MM. Memory enhancement in healthy older adults using a brain plasticity-based training program: a randomized, controlled study. Proc Natl Acad Sci U S A. 2006;103:12523–12528. doi: 10.1073/pnas.0605194103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Verhaeghen P, Marcoen A, Goossens L. Improving memory performance in the aged through mnemonic training: a meta-analytic study. Psychol Aging. 1992;7:242–251. doi: 10.1037//0882-7974.7.2.242. [DOI] [PubMed] [Google Scholar]
- 24.Yesavage JA, Sheikh JI, Friedman L, Tanke E. Learning mnemonics: roles of aging and subtle cognitive impairment. Psychol Aging. 1990;5:133–137. doi: 10.1037//0882-7974.5.1.133. [DOI] [PubMed] [Google Scholar]
- 25.Seitz A, Kim D, Watanabe T. Rewards evoke learning of unconsciously processed visual stimuli in adult humans. Neuron. 2009;61:700–707. doi: 10.1016/j.neuron.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seitz AR, Dinse HR. A common framework for perceptual learning. Current opinion in neurobiology. 2007;17:148–153. doi: 10.1016/j.conb.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 27.Newsome WT, Pare EB. A selective impairment of motion perception following lesions of the middle temporal visual area (MT) The Journal of neuroscience : the official journal of the Society for Neuroscience. 1988;8:2201–2211. doi: 10.1523/JNEUROSCI.08-06-02201.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsushima Y, Seitz AR, Watanabe T. Task-irrelevant learning occurs only when the irrelevant feature is weak. Current Biology. 2008;18:R516–R517. doi: 10.1016/j.cub.2008.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gilmore GC, Wenk HE, Naylor LA, Stuve TA. Motion perception and aging. Psychology and Aging. 1992;7:654. doi: 10.1037//0882-7974.7.4.654. [DOI] [PubMed] [Google Scholar]
- 30.Meteyard L, Zokaei N, Bahrami B, Vigliocco G. Visual motion interferes with lexical decision on motion words. Current biology : CB. 2008;18:R732–R733. doi: 10.1016/j.cub.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 31.Tsushima Y, Sasaki Y, Watanabe T. Greater Disruption Due to Failure of Inhibitory Control on an Ambiguous Distractor. Science. 2006;314:1786–1788. doi: 10.1126/science.1133197. [DOI] [PubMed] [Google Scholar]
- 32.Betts LR, Taylor CP, Sekuler AB, Bennett PJ. Aging Reduces Center-Surround Antagonism in Visual Motion Processing. Neuron. 2005;45:361–366. doi: 10.1016/j.neuron.2004.12.041. [DOI] [PubMed] [Google Scholar]
- 33.Healey MK, Campbell KL, Hasher L. Cognitive aging and increased distractibility: costs and potential benefits. Progress in brain research. 2008;169:353–363. doi: 10.1016/S0079-6123(07)00022-2. [DOI] [PubMed] [Google Scholar]
- 34.Ball KK, Beard BL, Roenker DL, Miller RL, Griggs DS. Age and visual search: Expanding the useful field of view. The Journal of the Optical Society of America A. 1988;5:2210–2219. doi: 10.1364/josaa.5.002210. [DOI] [PubMed] [Google Scholar]
- 35.Andersen GJ, Ni R, Bower JD, Watanabe T. Perceptual learning, aging, and improved visual performance in early stages of visual processing. Journal of vision. 2010;10:4. doi: 10.1167/10.13.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mishra J, Rolle C, Gazzaley A. Neural plasticity underlying visual perceptual learning in aging. Brain research. 2014 doi: 10.1016/j.brainres.2014.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bower JD, Watanabe T, Andersen GJ. Perceptual learning and aging: improved performance for low-contrast motion discrimination. Frontiers in psychology. 2013;4:66. doi: 10.3389/fpsyg.2013.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McKendrick AM, Battista J. Perceptual learning of contour integration is not compromised in the elderly. Journal of vision. 2013;13:5. doi: 10.1167/13.1.5. [DOI] [PubMed] [Google Scholar]
- 39.Polat U, Schor C, Tong JL, Zomet A, Lev M, Yehezkel O, Sterkin A, Levi DM. Training the brain to overcome the effect of aging on the human eye. Scientific reports. 2012;2:278. doi: 10.1038/srep00278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yotsumoto Y, Chang L, Ni R, Pierce R, Andersen GJ, Watanabe T, Sasaki Y. White matter in the older brain is more plastic than in the younger brain. Nature Comm. 2014 doi: 10.1038/ncomms6504. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


