Abstract
Introduction
Acute Respiratory Distress Syndrome (ARDS) is a common cause of organ failure with an associated mortality rate of 40%. The initiating event is disruption of alveolar-capillary interface causing leakage of edema into alveoli. Hypothesis: Electroporation mediated gene delivery of epithelial sodium channel (ENaC) and Na+,K+-ATPase into alveolar cells would improve alveolar clearance of edema and attenuate ARDS.
Methods
Pigs were anesthetized, instrumented, and the superior mesenteric artery was clamped to cause gut ischemia/reperfusion injury (I/R) and peritoneal sepsis (PS) by fecal clot implantation. Animals were ventilated according to ARDSnet protocol. Four hours after injury animals were randomized into groups: 1.Treatment Na+,K+-ATPase/ENaC plasmid (n=5), 2.Control Empty plasmid (n=5). Plasmids were delivered to the lung using bronchoscope. Electroporation was delivered using 8 square wave electric pulses across chest. Following electroporation pigs were monitored 48 hrs.
Results
The PaO2/FiO2 ratio and lung compliance were higher in the treatment group. Lung Wet/Dry Ratio was lower in the treatment group. Relative expression of the Na+,K+-ATPase transgene was higher throughout lungs receiving treatment plasmids. Quantitative histopathology revealed a reduction in intra-alveolar fibrin in the Treatment group. Bronchoalveolar lavage showed increased surfactant protein B in the treatment group. Survival was improved in the treatment group.
Conclusion
Electroporation-mediated transfer of Na+,K+-ATPase/ENaC plasmids improved lung function, reduced fibrin deposits, decreased lung edema, and improved survival in a translational porcine model of ARDS. Gene therapy can attenuate ARDS pathophysiology in a high fidelity animal model suggesting a potential new therapy for patients.
Keywords: Gene therapy, acute lung injury, ARDS, sepsis, pulmonary edema, electroporation
INTRODUCTION
Acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) are life-threatening conditions of respiratory failure induced by direct (aspiration, pneumonia) and indirect (sepsis, trauma) injury to the lung. ARDS has a high mortality rate ranging from 25% to 40% in the United States and ARDS patients utilize 3.6 million hospital days every year (1, 2). Over the past 10 years, mortality has declined from 40% to around 25%, primarily due to improved medical management of patients, including use of protective ventilation strategies, reductions in nosocomial infections, and more conservative fluid management (3, 4). These approaches do not directly address the underlying mechanisms of the disease, but rather reduce secondary lung injury. Although many potential pharmacological and other therapeutic approaches have been developed to control ARDS, these treatments have been unable to decrease the mortality of patients with ARDS (5, 6).
ALI and ARDS are characterized by abnormal accumulation of protein-rich edema fluid in the alveolar spaces, caused by increased movement of fluid from the capillaries into the alveoli, and decreased fluid transport out of alveolar air spaces (1). This is followed by extensive release of inflammatory cytokines, chemokines and neutrophil infiltration, causing impaired gas exchange, systemic inflammation, and multi-organ failure (1). The alveolar epithelium covers 99% of the surface of the lung and is the major site for removal of excess alveolar fluid. Injury to the alveolar epithelium, thus plays an important role in the pathogenesis of increased alveolar fluid. Therefore, treatments to improve alveolar epithelial function represent a key therapeutic strategy to accelerate recovery and decrease the mortality of ARDS patients (7). Studies have shown if the initial pulmonary edema can be reduced by increasing alveolar fluid clearance or alveolar barrier function, inflammation and subsequent injury can also be lessened (8, 9). While a number of pharmacological approaches to modulate alveolar fluid clearance have been proposed in animal and human studies, including the use of β2-adrenergic receptor agonists, the results from several multi-center clinical trials have not shown significant benefit (10, 11). In light of this, we and others have tested whether gene therapy approaches may be alternatives.
Gene therapy is a potentially powerful approach to treat a variety of disease states, including ALI/ARDS. Unfortunately, to date, only limited successes have been made in clinical trials (12). Perhaps the major reason for this is that the majority of viral and non-viral vectors for gene delivery have serious drawbacks limiting efficacy, including inefficiency of gene transfer and immunological and inflammatory responses. Over the past 10 years, we have used moderate electric fields to facilitate gene delivery to the lungs of mice (9, 13, 14) and rats (13, 15), in a technique known as electroporation, that causes no apparent damage and yields high level gene expression. The method is fast, simple, and safe; plasmid DNA in saline is delivered to the airways of anesthetized animals by intubation or aspiration and a series of synchronized pulses (200V/cm at 10 msec each) are delivered across the chest using external pacemaker electrodes placed on the skin. The current study demonstrates that this method is equally productive in a large animal model.
While there is no single gene or mechanism that causes ARDS, we and others have shown that gene transfer of the Na+,K+-ATPase can enhance alveolar fluid clearance in healthy mice and rats as well as provide protection from injury in several rodent models of lung injury (8, 9, 15–17). Our lab also has shown electroporation-mediated gene transfer of the β1 subunit of the Na+,K+-ATPase can be used to treat pre-existing lung injury in a mouse model of endotoxin-induced ALI, reducing lung edema, decreasing the numbers of infiltrating neutrophils, and decreasing protein concentration in the BALF (9). Although intriguing, the major limitations to all of these studies is that they employ small animal models of ALI/ARDS that may not reflect the human situation.
In this study we tested the hypothesis that electroporation-mediated gene transfer encoding Na+,K+-ATPase and epithelial sodium channel (ENaC) would reduce alveolar edema and ARDS pathology by up-regulating mechanisms of pulmonary fluid clearance. We have employed a well-established translational porcine model of ARDS using gut ischemia reperfusion (I/R) injury and peritoneal sepsis (PS) induced by fecal clot implantation in large animals (18) and transferred genes four hours after injury. To our knowledge, this is the first time gene transfer has been used as therapy following injury (PS+I/R) in a clinically applicable large animal model suggesting this approach may have clinical therapeutic potential.
MATERIALS AND METHODS
Plasmid constructs
The “empty” plasmid pcDNA3 was from Promega (Madison, WI). Plasmid expressing a Myc-DDK-tagged α1 subunit of the human epithelial sodium channel from the immediate early promoter/enhancer of CMV (α1-ENaC; pCMV-Myc-DDK-α1ENaC) was obtained from Origene, (Rockville, MD). Plasmid pCMV-Na,K-ATPaseβ1 expresses a GFP-tagged β1 subunit of the rat Na+,K+-ATPase from the CMV promoter as described previously (15). Plasmids were amplified and purified on a multiple gram scale by Aldevron (Fargo, ND), and determined to have less than 100 EU/mg of endotoxin contamination with A260/A280 ratios between 1.90 and 1.92.
Porcine PS+I/R model
All techniques and procedures were reviewed and approved by the Committee for the Human Use of Animals at Upstate Medical University. Healthy female Yorkshire pigs (30–50kg) were maintained NPO the night before surgery. Animals were anesthetized using continuous infusion of ketamine/xylazine to maintain a surgical plane of anesthesia. Animals remained intubated and sedated and were continuously monitored and cared for by the investigators for the full 48 h duration of the experiment. Under sterile conditions, animals underwent tracheostomy, arterial and venous catheterization, and bladder catheterization as previously described (18). Baseline (BL) measurements were taken after surgical preparation and prior to injury.
Following surgical instrumentation and Baseline measurements, secondary lung injury was induced using a “2-hit” model as previously described (18, 19). Briefly: 1) Ischemia/Reperfusion (I/R): The superior mesenteric artery (SMA) was clamped for 30 minutes to induce intestinal ischemia then released. 2) Peritoneal Sepsis (PS): stool was harvested from a cecotomy and mixed with blood to create a fecal clot which was implanted in the abdomen prior to abdominal closure. Time Zero (T0) measurements were taken immediately after the induction of injury (i.e. removal of SMA clamp and placement of fecal clot) upon closure of the abdomen.
Ventilation protocol
Animals were connected to a Dräger™ Evita XL ventilator (Dräger, Lübeck, Germany) with initial settings Tidal Volume (VT) 10 cc/kg, Positive End Expiratory Pressure (PEEP) 5 cmH2O, Respiratory Rate (RR) 12, FiO2 30%, and Inspiratory to Expiratory Ratio 1:2. Animals were transitioned to Low Tidal Volume (LVT) (VT= 6 cc/kg) when they met clinical criteria of PaO2/FiO2 < 300 per ARDSnet protocol (20). Appropriate adjustments in RR were made to maintain adequate minute volume. PEEP and FiO2 were adjusted in response to changes in SaO2 and PaO2 along the “High PEEP, Low FiO2” scale (20). If airway Plateau Pressure (Pplat) rose above 30 cmH2O, VT was further reduced by 1cc/kg increments to 4cc/kg with appropriate adjustments in RR to maintain equivalent minute volume per guidelines. The upper limit for respiratory rate was 35 breaths/min with titrations made in VT if respiratory acidosis was detected (pH < 7.15) according to the protocol.
DNA delivery and electroporation
Four hours after injury (T4), animals were randomized into two groups and gene delivery was carried out in a blinded manner: 1. Treatment Group -Electroporation with plasmids expressing Na,K-ATPase and ENaC (n=5), 2. Control Group -Electroporation with empty plasmid (pcDNA3) (n=5). Fifty ml of plasmid solution containing either (1) 50 mg each of pCMV-Na,K-ATPaseβ1 and pCMV-Myc-DDK-α1ENaC or (2) 50 mg of non-expressing pcDNA3, suspended in 140 mM NaCl/10 mM Tris-Cl, pH 8.0/1 mM EDTA, wasdelivered to the right lower lung lobe and 50 ml was delivered to the left lower lobe using a bronchoscope within a 2–5 minute period. The bronchoscope was removed, a 10 mg bolus of rocuronium bromide was administered, and animals were electroporated immediately using external adult defibrillation/pacing/monitoring electrodes (10 cm by 15 cm; ConMed Corp., Utica, NY) placed axillary on either side of the chest (approximately, 20 cm gap). The electric field was delivered using a BTX ECM830 electroporator (Harvard Apparatus, Holliston, MA) using 8 square wave pulses of 2000V and 150 μsec each (100 V/cm). Voltage and current were measured directly or with an AC22 current probe (Tektronix, Beaverton, OR), respectively, on a digital oscilloscope (Tektronix).
A historical injury only group was referenced from our previously published manuscript comparing ARDSnet ventilation protocol with airway pressure release ventilation (21). Injury Only Group (n=3) underwent the same PS + I/R injury, ventilation protocol, and clinical management as outlined here, but did not undergo bronchoscopy, intratracheal DNA/fluid administration, or electroporation. This group is included to compare the systemic injury in pigs treated with empty plasmid electroporation and pigs that did not undergo electroporation.
Clinical Management
Broad-spectrum antibiotics (Ampicillin 2 grams IV [Bristol Myers Squibb, Princeton, NJ] and Metronidazole 500mg IV [Baxter, Deerfield, IL]) were given following abdominal closure and every 12 hours until the end of the study. Animals were treated with intravenous fluid resuscitation and vasopressors in a protocol adapted from the Early Goal Directed Therapy (EGDT) strategy (22). This strategy is considered standard-of-care for management of hemodynamic collapse in sepsis. Maintenance intravenous fluid requirements were calculated by body weight and given via continuous infusion. IV Ringer’s Lactate was used for maintenance and resuscitation. Hemodynamic parameters were assessed to determine the need for resuscitative fluid bolus according to parameters described by Rivers et al (22). According to EGDT guidelines, continuous infusion of Norepinephrine was started when the animal was no longer responsive to fluid bolus. Vasopressin and epinephrine were added when Norepinephrine was no longer effective.
Physiologic Measurements
Hemodynamic parameters were measured (Agilent, CMS-2001™ System M1176A, with Monitor M1094B Böbingen, Germany) using Edwards transducers (Pressure Monitoring Kit [PXMK1183], Edwards Lifesciences, Irvine, CA). Pulmonary parameters were measured or calculated by the Dräger™ ventilator (Lübeck, Germany). Blood was drawn every 6 hours for ELISA quantification of cytokine levels in systemic circulation. Measurement of blood gases and chemistries were made with a Roche Blood gas analyzer (Cobas b221, Basel, Switzerland). Clinical pathology and blood cultures were performed by the Upstate Medical University pathology laboratory facility.
Necropsy
After 48 hours or time of death, the experimental protocol was terminated, animals were euthanized and necropsy was performed. The end time indicated was either just before death or at 48 hours and the data from animals that died was handled exactly the same as animals that survived the full 48 hours. The lungs, liver, spleen, kidney and small intestine were removed and preserved in formalin. Lungs were inflated to 25 cmH2O using stepwise increases in PEEP to standardized lung volume history and grossly photographed. The left lung was filled with 10% formalin to a height of ~25cm, clamped and immersed in formalin. This technique standardized the inflation pressure necessary for quantitative histologic measurements.
Bronchoalveolar Lavage Fluid (BALF) and Lung Tissue
The right middle lobe of the lung was lavaged with 60mL of normal saline, spun at 3500rpm at 4ºC, and snap frozen for later analysis. Total protein in BALF was determined by the bicinchoninic acid method. Western blot analyses of surfactant protein B (SP-B) expression in the BALF were performed as previously described (23). Expression of the Na+,K+-ATPase transgene protein was determined from the lung tissue (right lower lobe) by Western Blot as previously described (9, 15). β-actin and GAPDH were quantified on the same Western Blots as protein loading controls. Gravimetric analysis of lung tissue was carried out as previously described taking equal number of tissue samples from all lobes of the right lung, except the middle lobe since it was used for BALF, in each animal (9, 15).
Quantitative Histology
Quantitative histological assessment of the lung was based on image analysis of 100 photomicrographs (10 per animal), made at high-dry magnification following a validated, unbiased, systematic sampling protocol (18). Each photomicrograph was scored using a 4-point scale for each of six parameters: atelectasis, fibrinous deposits, blood in air space, vessel congestion, alveolar wall thickness, and leukocytes (18).
Statistics
Data are reported as mean ± SE. Repeated Measures ANOVA with pig number and treatment as random effects were performed to compare differences within and between treatment groups for continuous parameters, probability values <0.05 were considered significant. Post hoc Tukey’s tests were performed on continuous data at specific time points only if significance was found in the group*time effect using RM ANOVA. Quantitative histology data was analyzed using Mann-Whitney U test after testing for normality. Probability values <0.05 were considered significant. All analyses were performed using JMP version 5.1.1 (Cary, NC).
RESULTS
Systemic Injury
As previously established with this model (18), all pigs in both electroporated groups (treatment and control) developed multi-organism bacteremia, as assessed by qualitative blood cultures (E.coli, K. oxytoca, P. aeruginosa, K. pneumonia), and septic shock, which required critical care intervention with fluids, antibiotics, and vasopressors (Table 1). No significant difference was found between groups for mean arterial pressure, bladder pressure,white blood cells, creatinine, tidal volume, plateau pressure or PaCO2 (Table 1). Serum lactate reached higher levels in the empty plasmid group at the final 3 hours of the experiment (Table 1). No significant differences in parameters were detected between animals with injury only (19) or animals receiving empty plasmid, except for INR (Table 1).
Table 1.
Systemic Injury values.
| Treatment Group (n=5) | Control Group (n=5) | Injury Only Group (n=3) | |
|---|---|---|---|
| Heart Rate BL (bpm) | 102.0 ± 9.5 | 105.5 ± 9.2 | 96.3 ± 12.8 |
| Heart Rate End (bpm) | 115.2 ± 3.5* | 98.0 ± 6.8 | 91.6 ± 6.9 |
| MAP BL (mmHg) | 116.6 ± 5.7 | 125.6 ± 8.56 | 125.0 ± 5.9 |
| MAP End (mmHg) | 70.2 ± 3.99 | 57.2 ± 4.3 | 46.0 ± 6.1 |
| Bladder Pressure BL (mmHg) | 1.4 ± 0.7 | 1.8 ± 0.7 | 2.7 ± 1.2 |
| Bladder Pressure End (mmHg) | 11.4 ± 1.1 | 15.4 ± 4.3 | 26.7 ±0.9 |
| Average UOP (mL/kg/hr) | 7.3 ± 0.7 | 8.4 ± 1.1 | 5.17 ± 1.17 |
| Lactate BL (mmol/L) | 2.5 ± 0.2 | 2.1 ± 0.2 | 1.4 ± 0.0 |
| Lactate End (mmol/L) | 2.1 ± 0.4* | 7.8 ± 3.1 | 13.9 ± 2.3 |
| PaCO2 BL (mmHg) | 33.9 ± 1.27 | 36.4 ± 1.4 | 35.0 ± 1.8 |
| PaCO2 End (mmHg) | 55.8 ± 6.4 | 44.6 ± 5.6 | 64.0 ± 8.8 |
| White blood cell count (K/μL) | 14.5 ± 2.2 | 7.7 ± 2.7 | 1.3 ± 0.3 |
| Hemoglobin BL (g/dL) | 9.3 ± 0.4 | 8.9 ± 0.3 | 9.0 ± 0.8 |
| Hemoglobin End (g/dL) | 9.7 ± 0.9 | 9.4 ± 1.2 | 5.9 ± 1.4 |
| Platelets BL (K/μL) | 295.8 ± 53.2 | 329.2 ± 41.1 | 318.0 ± 37.6 |
| Platelets End (K/μL) | 171.8 ± 33.3 | 101.8 ± 43.2 | 40.0 ± 17.0 |
| INR BL | 1.1 ± 0.1 | 1.1 ± 0.0 | 1.2 ± 0.0
|
| INR End | 1.5 ± 0.0 | 2.5 ± 0.6 | 6.7 ± 2.3 |
| Creatinine BL (mg/dL) | 0.7 ± 0.1 | 0.8 ± 0.0 | 0.9 ± 0.1 |
| Creatinine End (mg/dL) | 0.7 ± 0.1 | 0.8 ± 0.1 | 1.0 ± 0.1 |
| Albumin BL (g/dL) | 2.9 ± 0.3 | 3.0 ± 0.1 | 2.8 ± 0.1 |
| Albumin End (g/dL) | 1.8 ± 0.2 | 1.4 ± 0.2 | <1.0 |
BL = baseline, MAP = Mean arterial pressure, UOP = Urine Output, INR = International normalized ratio. Data = mean ± SE,
= p<0.05 Treatment Group vs Control Group. Injury Only Group animals are from historic data using the same methods here but without electroporation (28).
= p<0.05 Control Group vs Injury Only Group.
IV resuscitation
No difference was found in fluid resuscitation between groups (Treatment plasmid 25.19±2.87 vs. Empty plasmid 37.09±7.95 cc/kg/hr, p=0.197). Additionally there was no difference in pressor resuscitation (Treatment plasmid 12.09±3.58 vs. Empty plasmid 15.10±2.93 cc/hr norepinephrine, p=0.533).
Safety of gene delivery and electroporation
Although the transthoracic electroporation procedure has been shown to be safe and effective in healthy mice and rats as well as those with pre-existing LPS-induced lung injury (9, 13–15, 24), the larger size of the pig necessitated larger voltages to be applied across the chest. To obtain a field strength of approximately 100 V/cm, 2000V were applied to the chest in 150 μsec square wave pulses and the measured current was 14.6 Amps, for a total energy of 4.3J, or roughly 0.13 J/kg. These fields were well tolerated by the animals, and no change in cardiac rhythm or electrocardiogram waveforms were detected in any of the animals, either during, immediately after, or at later times after the electroporation procedure in either group.
Lung tissue gene expression
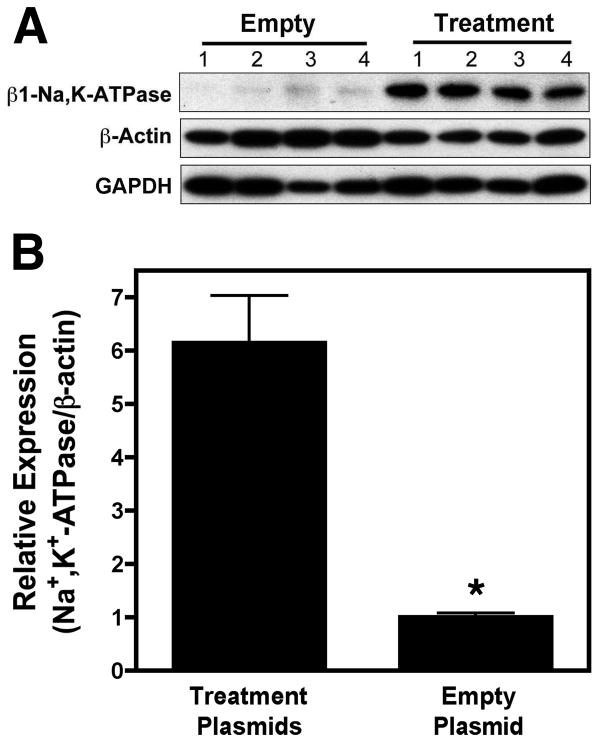
Relative expression of the Na+,K+-ATPase transgene protein compared to that of the endogenous β-actin protein was significantly higher throughout the lungs of animals receiving the treatment plasmids than those receiving the empty control plasmid (Treatment plasmid 6.89±0.34 vs. Empty plasmid 1.00±0.14, p<0.0001) (Figure 1).
Figure 1. Electroporation-mediated gene transfer leads to overexpression of transgenes in Lung tissue.
(A) Expression of the Na+,K+-ATPase β1 subunit transgene was measured by Western blot in 8 to 12 samples per animal from the lower right lobes of animals receiving treatment plasmids or empty plasmid. Expression of endogenous β-actin and GAPDH were used as independent loading controls for each sample. Samples from 4 different animals in each group are shown. (B) Relative expression of the transgene compared to β-actin was determined for all animals in the groups (n=5). *, p<0.001.
Survival
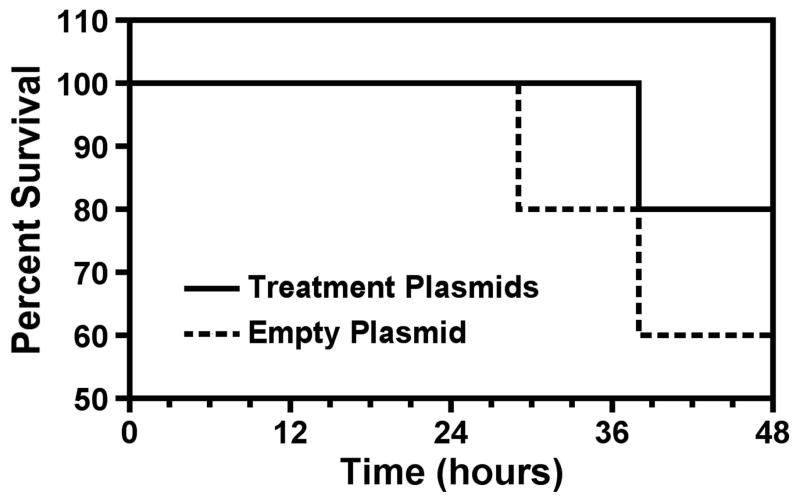
Survival was improved in the treatment group (80% of treatment plasmid animals survived to the 48h endpoint vs. 60% of control animals) (Figure 2). Although the survival data did not reach significance there was a trend toward improvement.
Figure 2. Electroporation-mediated transfer of treatment plasmids improve survival.
Kaplan Meier analysis was performed using treatment group vs control empty plasmid group.
Pulmonary function and edema
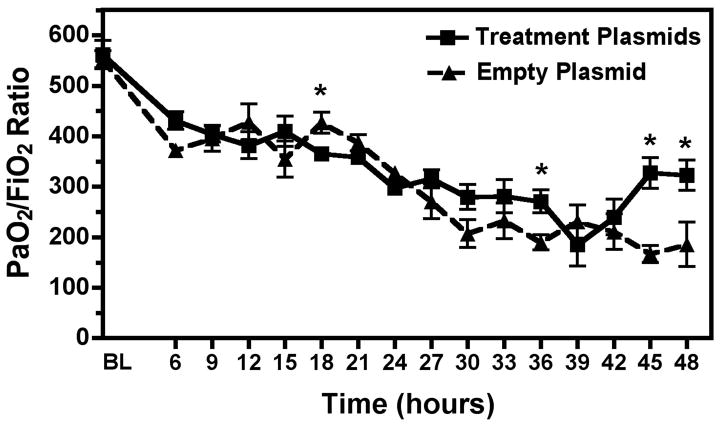
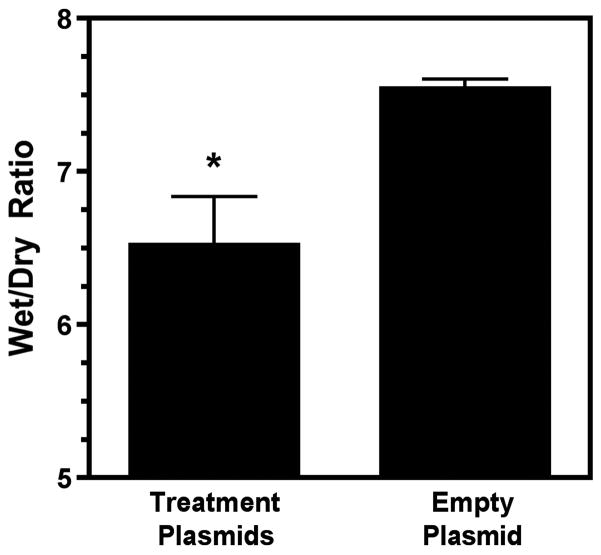
PaO2/FiO2 (P/F) ratio was significantly higher at the end of the experiment in the treatment group (Treatment plasmids 322.63±29.66 vs. Empty plasmid 185.83±43.69, p<0.05) (Figure 3). Lung Compliance was also significantly higher in the Na+,K+-ATPase/ENaC plasmid treated animals (Treatment plasmids 19.54±2.20 vs. Empty plasmid 9.46±1.86 mL/cmH2O, p<0.05) (Figure 4). No significant difference was seen in PaCO2 levels or plateau pressure between groups. The lung wet/dry ratio, a measure of pulmonary edema, was significantly lower in the treatment group (Treatment plasmid 6.52±0.32 vs. Empty plasmid 7.54±0.08, p<0.05) (Figure 5). However, wet/dry ratios in other organs (spleen, liver, kidney, intestine) showed no difference between groups (data not shown).
Figure 3. PaO2/FiO2 ratio over 48 hour experiment was improved in the Treatment group.
Oxygenation reached significantly higher levels in the treatment group vs. the empty plasmid group. P/F ratio < 300 is diagnostic of mild ARDS and P/F < 200 is diagnostic of moderate ARDS. Dashed line = Empty Plasmid Group (n=5), Solid line = Treatment Plasmid Group (n=5). *, p<0.05.
Figure 4. Pulmonary compliance over 48 hour experiment was improved in the Treatment group.
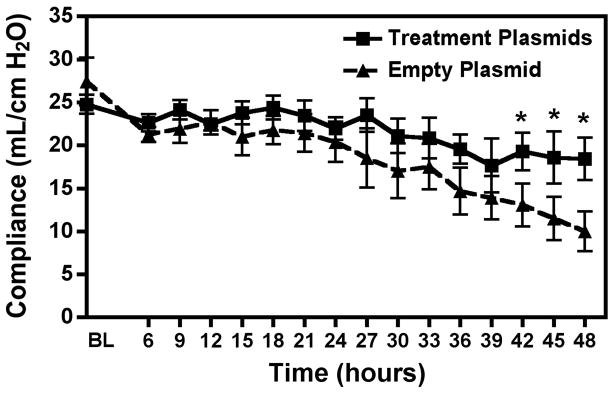
Lung compliance remained stable throughout the experiment in the treatment group. However the empty plasmid group had declining levels of compliance and were significantly lower by the last three hours. Dashed line = Empty Plasmid Group (n=5), Solid line = Treatment Plasmid Group (n=5). *, p<0.05.
Figure 5. Lungs from animals receiving Na+,K+-ATPase and ENaC plasmids showed lower wet to dry ratios, a gravimetric measure of pulmonary edema.
Weights of lung tissue before (wet) and after prolonged heating (dry) were measured and ratios calculated (n=5 per group). *, p<0.01.
Bronchoalveolar lavage fluid (BALF)
Analysis of BALF showed an increase in surfactant protein B in the treatment group (Treatment plasmids 1.14±0.03 vs. Empty plasmid 0.96±0.03, p<0.02). No statistical difference was seen in BALF total protein levels (Treatment plasmid 174.65±55.90 vs. Empty plasmid 144±65.89, p=0.74).
Gross pathology and histopathology
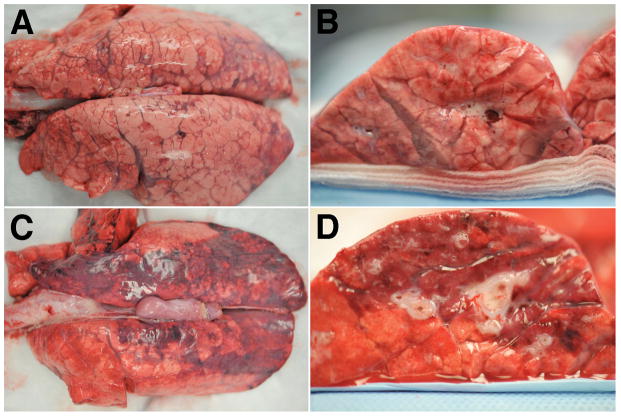
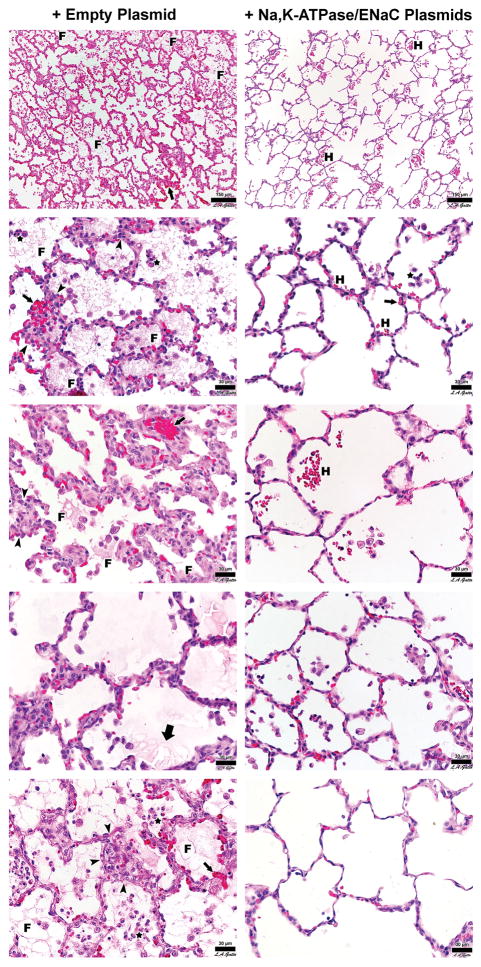
Lungs from animals receiving the empty control plasmid exhibited gross airway edema, posterior lung collapse, and erythema. By contrast, the lungs of animals in the treatment plasmid group had airways that were well inflated, and showed less parenchymal edema and lung collapse (Figure 6). All animals in both groups showed histopathology consistent with sepsis. The histopathology was less severe in the animals receiving treatment plasmids, with some animals exhibiting almost normal histology. All animals receiving the empty plasmid had obvious fibrin deposits in the air compartment whereas animals receiving treatment plasmid had none (Figure 7 and Table 2). Quantitative histopathology affirmed this qualitative observation and revealed significant reduction in intra-alveolar fibrin suggestive of a reduction in edema in the treatment plasmid group (Table 2). Although alveolar wall thickness, numbers of infiltrating cells, and hemorrhage into the airspace appeared less severe in the histological sections qualitatively, quantitative analysis did not reach statistical significance for these variables.
Figure 6. Representative gross lung pathology photographs.
(A, B) Lungs from animals receiving Treatment plasmids. (C, D) Lungs from animals receiving Empty plasmid. (A, C) Posterior Lung surface. (B, D) Cross section view of lower lung lobe.
Figure 7. Representative H&E stained histology sections of lung tissue.
Representative photomicrographs of lungs from treatment plasmid or empty plasmid groups are shown at high magnification. Each panel represents a different animal. Sections of the lungs were chosen at random for each animal and are representative of the degree of injury found throughout the lungs. F = fibrin deposit, H = hemorrhage, Small Arrow = alveolar wall thickening, Thick Arrow = alveolar edema, Arrowhead = leukocyte infiltrates, Star = intra-alveolar cell infiltrates.
Table 2.
Quantitative histopathology lung analysis.
| Empty plasmid | Treatment plasmids | p-value | |
|---|---|---|---|
| Fibrinous Deposits | 1.5±0.79 | 0.32±0.47 | <0.0001 |
| Blood in air space | 0.36±0.53 | 0.20±0.40 | 0.09 |
| Vessel Congestion | 0.94±1.19 | 0.46±0.93 | 0.03 |
| Thick alveolar wall | 0.58±0.81 | 0.76±1.15 | 0.37 |
| Cellular infiltration | 1.98±0.65 | 2.16±0.96 | 0.27 |
Data = mean ± SD, n=50 (10 photos per animal, 5 animals per treatment). Each pathology was scored on a four point scale and compared between groups.
DISCUSSION
In this study we found gene therapy with Na+,K+-ATPase and ENaC plasmids delivered by transthoracic electroporation to the lungs of pigs with sepsis- and ischemia-reperfusion injury-induced ARDS improved lung function, reduced histopathology damage, decreased alveolar edema, increased surfactant, and improved survival when compared to electroporation with empty plasmid or to animals with injury only. These results indicate our gene therapy approach is safe and shows efficacy in a clinically relevant large animal model and may be translatable to human disease. To our knowledge, this is the first study showing that gene therapy is beneficial in any large animal model of ALI/ARDS.
In a meta-analysis of thirty years of clinical trials to treat ARDS, McIntyre et al found only lung protective ventilator strategy is supported by improved outcomes. Other therapies for ARDS could not be recommended, caused more injury, or had insufficient data to be analyzed (5, 6). Obviously, novel treatment options are necessary to reduce the high morbidity and mortality of ARDS in patients. Gene therapy may be one such option.
Gene therapy is a potentially powerful approach to treat ALI/ARDS. Recent advances in the development of more efficient viral and non-viral methods for gene delivery have increased clinical application (12). Over the past two decades, numerous viral and non-viral approaches have been proposed and developed to transfer genes to the lung but all have serious limitations. Inefficiency of gene transfer, immunological, and inflammatory responses are just a few of the problems. For example, cell damage and inflammation are frequently observed when viruses, even those deemed safe such as gutted adenovirus, are used for delivery. In contrast to viral vectors, non-viral vectors generate very little immune response or inflammation in the lung, either as naked plasmid or when complexed with liposomes or other polymers, allowing for multiple vector administrations (25). Unfortunately, the efficiency of gene transfer of many non-viral vectors (including liposomes and nanoparticles) to the airways and alveoli in the lung has been low or even undetectable in human trials (26, 27).
Our lab and others have previously shown electroporation can be used for gene delivery to lung tissue with a high level of gene expression using naked plasmids and electric pulses to transiently open pores in the cell membrane (13, 28, 29). The method is simple, fast, and safe: purified plasmid DNA is administered to the lungs of anesthetized animals via the airways and a series of eight consecutive square wave electric pulses is applied to the lungs using electrodes placed on either side of the chest. At the appropriate field strength (between 100 and 200 V/cm using 0.1 to 10 msec pulses), no tissue damage, inflammation, or mortality has been detected in mice or rats (9, 13, 15, 28, 29). Gene expression has been detected in as little as 12 hours after electroporation delivery in mouse and rat lungs, which is critical for treatment of the acute phase of ARDS (13, 15). Moreover, gene transfer and expression is detected in multiple cell types and layers throughout the lung, including the alveolar epithelium and capillary endothelium, all critical targets for ALI/ARDS therapy (13, 15). The work in this study demonstrates for the first time that this technique is also effective and safe in a large animal model. To achieve a field strength of 100 V/cm across a mouse chest (1 cm), only 100 V are required; using a 35 kg pig with a 20 cm gap between electrodes requires 2000 V. While this may seem high, measurement of the resistance and current accompanying the pulses demonstrated that less than 15J of energy were applied to the chest, which corresponds to a value of about 0.1 J/kg, and is much less than that used for emergent (200–360J) or synchronized (50–360J) cardioversion on an adult, according to Advanced Cardiovascular Life Support guidelines (30). That no changes in cardiac rhythm or electrocardiogram waveform during or following electroporation were detected, suggests that this is a well-tolerated procedure.
The pathology of ARDS is characterized by injurious accumulation of protein rich edema fluid in the alveolar air space (1). The mechanism of Na+,K+-ATPase and ENaC gene therapy is to improve alveolar fluid clearance driven by sodium transport entering the cell by ENaC on the apical surface and then being pumped out by Na+,K+-ATPase on the basolateral surface into the interstitium and the pulmonary circulation. Previous studies demonstrated that overexpression of these sodium pumps can enhance alveolar active transport to clear pulmonary edema (8, 9, 15–17, 31). In this study utilizing a clinically applicable large animal model, we demonstrated that gene transfer of these two ion transporters reduced lung edema as evidenced by both decreased wet/dry ratio and decreased fibrin protein deposition in the alveolar spaces, as seen on histopathology. However, in contrast to our findings in an LPS-induced model of ALI in mice, our data from the pigs did not find a difference in BALF total protein levels. This is perhaps not surprising since we carried out gene transfer only to two lobes of the pig lungs, and not the remaining three lobes. Since BALF distributes throughout the lung, increased fluid clearance in one or two lobes may not be sufficient to manifest itself in overall reduced protein levels in the total BALF, but may be detected in wet/dry ratios in the treated lobes. This may also indicate that the gene therapy did not decrease capillary permeability but did improve clearance of the edema fluid. Taken together, these data suggest that electroporation-mediated gene therapy may prove to be an important treatment and prevention therapy for the pulmonary edema associated with ARDS by enhancing alveolar epithelial function (9, 12).
Our results also demonstrate an increase in surfactant protein B, a key protein in the composition of pulmonary surfactant. The mechanism behind this preservation of surfactant may be decreased pulmonary edema and increased function of alveolar type II cells as seen with the decreased lung edema in our study (32). Previous studies have demonstrated that SP-B is critical for pulmonary function and even survival (23, 33). However, whether and how gene transfer of Na+,K+-ATPase and ENaC subunits regulates surfactant protein B expression or type II pneumocyte activity remains to be determined.
The elevation in serum lactate at the end of the experiment in empty plasmid treatment animals is likely due to their overall poor pulmonary function, decreased oxygenation, and therefore decreased oxygen delivery to peripheral tissues. In a review of lactate and sepsis, Levy et al concluded that increased lactate production is a result of the hypermetabolic state with enhanced glycolysis (34). In a similar review from the Lancet, high serum lactate levels in trauma or sepsis were related to epinephrine stimulation of skeletal muscle aerobic glycolysis and not due to hypoperfusion (35). Whether lactate levels were elevated due to decreased tissue perfusion or elevated septic shock response, we observed minimal difference in other measures of systemic injury including WBC, mean arterial pressure, heart rate, Albumin, platelets, and bladder pressure between groups.
Also interesting to note is the increase in overall lung function, not only decrease in pulmonary edema, after gene therapy with Na+,K+-ATPase and ENaC to only two lobes of the lungs. Pulmonary compliance, a measure of lung and chest wall distensibility, was increased after treatment plasmid delivery whereas those animals that received a non-expressing control plasmid had a decline in compliance. Decreased compliance is typical of ARDS diseased lungs due to increased tissue stiffness and pulmonary edema (1). These findings may indicate a global improvement in alveolar epithelial function and decreased pulmonary edema as opposed to a localized effect in this large animal model. Further, the improvements in histopathology of the lungs receiving the Na+,K+-ATPase and ENaC treatment plasmids were remarkable.
Conclusion
Overall we have shown that electroporation-mediated transfer of Na+,K+-ATPase and ENaC expressing plasmids into the lung parenchyma improved lung function, reduced histopathology, decreased lung edema, and improved survival. Thus, gene therapy can attenuate ARDS pathophysiology in a high fidelity, translational porcine ARDS model suggesting a potentially exciting new therapy for ARDS patients.
Acknowledgments
We would like to thank Drs. Patricia Chess, Steve Georas, Brian Kubiak, and Gokhan Mutlu, for
Funding provided by NIH grant R33 HL092801
Footnotes
The authors have no relevant conflicts of interest.
References
- 1.Matthay MA, Ware LB, Zimmerman GA. The acute respiratory distress syndrome. J Clin Invest. 2012;122(8):2731–40. doi: 10.1172/JCI60331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353(16):1685–93. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 3.Zambon M, Vincent JL. Mortality rates for patients with acute lung injury/ARDS have decreased over time. Chest. 2008;133(5):1120–7. doi: 10.1378/chest.07-2134. [DOI] [PubMed] [Google Scholar]
- 4.Wiedemann HP, Wheeler AP, Bernard GR, Thompson BT, Hayden D, deBoisblanc B, Connors AF, Jr, Hite RD, Harabin AL. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354(24):2564–75. doi: 10.1056/NEJMoa062200. [DOI] [PubMed] [Google Scholar]
- 5.McIntyre RC, Jr, Pulido EJ, Bensard DD, Shames BD, Abraham E. Thirty years of clinical trials in acute respiratory distress syndrome. Crit Care Med. 2000;28(9):3314–31. doi: 10.1097/00003246-200009000-00034. [DOI] [PubMed] [Google Scholar]
- 6.Boyle AJ, Mac Sweeney R, McAuley DF. Pharmacological treatments in ARDS; a state-of-the-art update. BMC Med. 2013;11:166. doi: 10.1186/1741-7015-11-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosenberg AL. Fluid management in patients with acute respiratory distress syndrome. Respir Care Clin N Am. 2003;9(4):481–93. doi: 10.1016/s1078-5337(03)00036-4. [DOI] [PubMed] [Google Scholar]
- 8.Factor P, Dumasius V, Saldias F, Brown LA, Sznajder JI. Adenovirus-mediated transfer of an Na+/K+-ATPase beta1 subunit gene improves alveolar fluid clearance and survival in hyperoxic rats. Hum Gene Ther. 2000;11(16):2231–42. doi: 10.1089/104303400750035753. [DOI] [PubMed] [Google Scholar]
- 9.Mutlu GM, Machado-Aranda D, Norton JE, Bellmeyer A, Urich D, Zhou R, Dean DA. Electroporation-mediated gene transfer of the Na+,K+-ATPase rescues endotoxin-induced lung injury. Am J Respir Crit Care Med. 2007;176(6):582–590. doi: 10.1164/rccm.200608-1246OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gao Smith F, Perkins GD, Gates S, Young D, McAuley DF, Tunnicliffe W, Khan Z, Lamb SE. Effect of intravenous beta-2 agonist treatment on clinical outcomes in acute respiratory distress syndrome (BALTI-2): a multicentre, randomised controlled trial. Lancet. 2012;379(9812):229–35. doi: 10.1016/S0140-6736(11)61623-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matthay MA, Brower RG, Carson S, Douglas IS, Eisner M, Hite D, Holets S, Kallet RH, Liu KD, MacIntyre N, Moss M, Schoenfeld D, Steingrub J, Thompson BT. Randomized, placebo-controlled clinical trial of an aerosolized beta(2)-agonist for treatment of acute lung injury. Am J Respir Crit Care Med. 2011;184(5):561–8. doi: 10.1164/rccm.201012-2090OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin X, Dean DA. Gene therapy for ALI/ARDS. Crit Care Clin. 2011;27(3):705–18. doi: 10.1016/j.ccc.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dean DA, Machado-Aranda D, Blair-Parks K, Yeldandi AV, Young JL. Electroporation as a method for high-level non-viral gene transfer to the lung. Gene Ther. 2003;10(18):1608–1615. doi: 10.1038/sj.gt.3302053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O'Reilly MA, Yee M, Buczynski BW, Vitiello PF, Keng PC, Welle SL, Finkelstein JN, Dean DA, Lawrence BP. Neonatal oxygen increases sensitivity to influenza A virus infection in adult mice by suppressing epithelial expression of Ear1. Am J Pathol. 2012;181(2):441–51. doi: 10.1016/j.ajpath.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Machado-Aranda D, Adir Y, Young JL, Briva A, Budinger GRS, Yeldandi A, Sznajder JI, Dean DA. Gene transfer of the Na+,K+-ATPase β1 subunit using electroporation increases lung liquid clearance in rats. Am J Respir Crit Care Med. 2005;171:204–211. doi: 10.1164/rccm.200403-313OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adir Y, Factor P, Dumasius V, Ridge KM, Sznajder JI. Na,K-ATPase gene transfer increases liquid clearance during ventilation-induced lung injury. Am J Respir Crit Care Med. 2003;168(12):1445–8. doi: 10.1164/rccm.200207-702OC. [DOI] [PubMed] [Google Scholar]
- 17.Factor P, Saldias F, Ridge K, Dumasius V, Zabner J, Jaffe HA, Blanco G, Barnard M, Mercer R, Perrin R, Sznajder JI. Augmentation of lung liquid clearance via adenovirus-mediated transfer of a Na,K-ATPase beta1 subunit gene. J Clin Invest. 1998;102(7):1421–30. doi: 10.1172/JCI3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kubiak BD, Albert SP, Gatto LA, Vieau CJ, Roy SK, Snyder KP, Maier KG, Nieman GF. A clinically applicable porcine model of septic and ischemia/reperfusion-induced shock and multiple organ injury. J Surg Res. 2011;166(1):e59–69. doi: 10.1016/j.jss.2010.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roy S, Sadowitz B, Andrews P, Gatto LA, Marx W, Ge L, Wang G, Lin X, Dean DA, Kuhn M, Ghosh A, Satalin J, Snyder K, Vodovotz Y, Nieman G, Habashi N. Early stabilizing alveolar ventilation prevents acute respiratory distress syndrome: a novel timing-based ventilatory intervention to avert lung injury. J Trauma Acute Care Surg. 2012;73(2):391–400. doi: 10.1097/TA.0b013e31825c7a82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Network A. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342(18):1301–8. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 21.Roy S, Habashi N, Sadowitz B, Andrews P, Ge L, Wang G, Roy P, Ghosh A, Kuhn M, Satalin J, Gatto LA, Lin X, Dean DA, Vodovotz Y, Nieman G. Early airway pressure release ventilation prevents ards-a novel preventive approach to lung injury. Shock. 2013;39(1):28–38. doi: 10.1097/SHK.0b013e31827b47bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, Peterson E, Tomlanovich M. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345(19):1368–77. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 23.Wang G, Taneva S, Keough KM, Floros J. Differential effects of human SP-A1 and SP-A2 variants on phospholipid monolayers containing surfactant protein B. Biochim Biophys Acta. 2007;1768(9):2060–9. doi: 10.1016/j.bbamem.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaufman CD, Geiger RC, Dean DA. Electroporation- and mechanical ventilation-mediated gene transfer to the lung. Gene Ther. 2010;17(9):1098–104. doi: 10.1038/gt.2010.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alton EW, Boyd AC, Cheng SH, Davies JC, Davies LA, Dayan A, Gill DR, Griesenbach U, Higgins T, Hyde SC, Innes JA, McLachlan G, Porteous D, Pringle I, Scheule RK, Sumner-Jones S. Toxicology study assessing efficacy and safety of repeated administration of lipid/DNA complexes to mouse lung. Gene Ther. 2014;21(1):89–95. doi: 10.1038/gt.2013.61. [DOI] [PubMed] [Google Scholar]
- 26.Alton EW, Stern M, Farley R, Jaffe A, Chadwick SL, Phillips J, Davies J, Smith SN, Browning J, Davies MG, Hodson ME, Durham SR, Li D, Jeffery PK, Scallan M, Balfour R, Eastman SJ, Cheng SH, Smith AE, Meeker D, Geddes DM. Cationic lipid-mediated CFTR gene transfer to the lungs and nose of patients with cystic fibrosis: a double-blind placebo-controlled trial. Lancet. 1999;353(9157):947–54. doi: 10.1016/s0140-6736(98)06532-5. [DOI] [PubMed] [Google Scholar]
- 27.Konstan MW, Davis PB, Wagener JS, Hilliard KA, Stern RC, Milgram LJ, Kowalczyk TH, Hyatt SL, Fink TL, Gedeon CR, Oette SM, Payne JM, Muhammad O, Ziady AG, Moen RC, Cooper MJ. Compacted DNA nanoparticles administered to the nasal mucosa of cystic fibrosis subjects are safe and demonstrate partial to complete cystic fibrosis transmembrane regulator reconstitution. Hum Gene Ther. 2004;15(12):1255–69. doi: 10.1089/hum.2004.15.1255. [DOI] [PubMed] [Google Scholar]
- 28.Gazdhar A, Bilici M, Pierog J, Ayuni EL, Gugger M, Wetterwald A, Cecchini M, Schmid RA. In vivo electroporation and ubiquitin promoter--a protocol for sustained gene expression in the lung. J Gene Med. 2006;8(7):910–8. doi: 10.1002/jgm.911. [DOI] [PubMed] [Google Scholar]
- 29.Pringle IA, McLachlan G, Collie DD, Sumner-Jones SG, Lawton AE, Tennant P, Baker A, Gordon C, Blundell R, Varathalingam A, Davies LA, Schmid RA, Cheng SH, Porteous DJ, Gill DR, Hyde SC. Electroporation enhances reporter gene expression following delivery of naked plasmid DNA to the lung. J Gene Med. 2007;9(5):369–80. doi: 10.1002/jgm.1026. [DOI] [PubMed] [Google Scholar]
- 30.Link MS, Atkins DL, Passman RS, Halperin HR, Samson RA, White RD, Cudnik MT, Berg MD, Kudenchuk PJ, Kerber RE. Part 6: electrical therapies: automated external defibrillators, defibrillation, cardioversion, and pacing: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122(18 Suppl 3):S706–19. doi: 10.1161/CIRCULATIONAHA.110.970954. [DOI] [PubMed] [Google Scholar]
- 31.Machado-Aranda DA, Suresh MV, Yu B, Raghavendran K. Electroporation-mediated in vivo gene delivery of the Na+/K+-ATPase pump reduced lung injury in a mouse model of lung contusion. J Trauma Acute Care Surg. 2012;72(1):32–9. doi: 10.1097/TA.0b013e31823f0606. discussion 39–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bhattacharya J, Matthay MA. Regulation and repair of the alveolar-capillary barrier in acute lung injury. Annu Rev Physiol. 2013;75:593–615. doi: 10.1146/annurev-physiol-030212-183756. [DOI] [PubMed] [Google Scholar]
- 33.Wang G, Guo X, Diangelo S, Thomas NJ, Floros J. Humanized SFTPA1 and SFTPA2 transgenic mice reveal functional divergence of SP-A1 and SP-A2: formation of tubular myelin in vivo requires both gene products. J Biol Chem. 2010;285(16):11998–2010. doi: 10.1074/jbc.M109.046243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Levy B. Lactate and shock state: the metabolic view. Curr Opin Crit Care. 2006;12(4):315–21. doi: 10.1097/01.ccx.0000235208.77450.15. [DOI] [PubMed] [Google Scholar]
- 35.James JH, Luchette FA, McCarter FD, Fischer JE. Lactate is an unreliable indicator of tissue hypoxia in injury or sepsis. Lancet. 1999;354(9177):505–8. doi: 10.1016/S0140-6736(98)91132-1. [DOI] [PubMed] [Google Scholar]