Abstract
Sepsis is a major cause of acute kidney injury (AKI) with high rates of morbidity and mortality. Surfactant proteins A and D (SP-A, SP-D) play a critical role in host defense and regulate inflammation during infection. Recent studies indicate SP-A and SP-D are expressed in the kidney. The current study examines the role of SP-A and SP-D in the pathogenesis of sepsis-induced AKI. Wild-type (WT) and SP-A/SP-D double knockout (KO) C57BL/6 mice were treated by cecal ligation and puncture (CLP) or sham surgery. Histological, cellular and molecular indices of kidney injury were investigated in septic mice 6 and 24 h after CLP. 24 h post-CLP, kidney injury was more severe, renal function was decreased, blood creatinine and BUN were higher in septic SP-A/SP-D KO mice (p<0.05, vs septic WT mice). Kidney edema and vascular permeability were increased in septic SP-A/SP-D KO mice (p<0.01, vs septic WT mice). Apoptotic cells increased significantly (p<0.01) in the kidney of septic SP-A/SP-D KO mice compared to septic WT mice. Molecular analysis revealed levels of Bcl-2 (an inhibitor of apoptosis) were lower and levels of caspase-3 (a biomarker of apoptosis) were higher in the kidney of septic SP-A/SP-D KO mice (p<0.01, vs septic WT mice). Furthermore, levels of NF-κB and phosphorylated IκB-α increased significantly in the kidney of septic SP-A/SP-D KO mice than septic WT mice, suggesting SP-A/SP-D KO mice have a more pronounced inflammatory response to sepsis. We conclude SP-A and SP-D attenuate kidney injury by modulating inflammation and apoptosis in sepsis-induced AKI.
Keywords: Innate immunity, Sepsis, Surfactant protein A, Surfactant protein D, Acute Kidney Injury
Introduction
Acute kidney injury (AKI) is a serious problem that affects more than 20% of critically ill patients (1). The presence of AKI is an independent risk factor for mortality in sepsis. Although AKI can develop from a variety of clinical disorders, sepsis, a major cause, accounts for more than 50% of AKI cases in critically ill patients (2). Despite significant advances in the support of kidney function, the mortality rate of septic patients is still high (about 15–30%) in part due to our poor understanding for the mechanisms of AKI (3). Septic animal models have been developed, which demonstrate the pathogenesis of AKI is caused by renal cell apoptosis, renal endothelial cell dysfunction, intrarenal hemodynamic change, and infiltration of inflammatory cells in the kidney (3–4). Of these factors, cell apoptosis and tissue significant inflammation in the kidney appear to be critical mediators of sepsis-induced AKI (5). Furthermore, recent studies indicate innate immunity and inflammatory signaling pathways are involved in the pathogenesis of septic AKI (5).
Surfactant protein A and D (SP-A and SP-D) are members of C-type lectin family, which play a critical role in host defense and the regulation of inflammation in a variety of infections (6). Although SP-A and SP-D are predominantly expressed in the lung, they are also expressed in other tissues/organs including kidney (7). Like other members of C-type lectin family, SP-A and SP-D proteins consist of four functional domains: N-terminus, triple-helical collagen-like domain, neck region, and carbohydrate recognition domain (CRD). The CRD domain of SP-A and SP-D can directly bind to the carbohydrate molecules on the surface of microorganisms, which facilitates the clearance and uptake of pathogens by macrophages and neutrophils (8). As pattern recognition proteins, SP-A and SP-D interact with different receptors on the surface of inflammatory and epithelial cells in innate immunity. They modulate inflammatory processes by regulating signaling pathways involved in inflammation, including nuclear factor-kappa B (NF-κB) pathway (9–10). Based on these observations, we felt SP-A and SP-D proteins maybe involve in the pathogenesis of sepsis-induced AKI.
To avoid the complication of potential overlapping functions of SP-A and SP-D in single gene knockout mice, SP-A and SP-D double knockout (SP-A/SP-D KO) mice were recently generated to study pulmonary infections (6, 11–12). These studies indicate SP-A and SP-D proteins have protective effects in microbial infections because mice lacking SP-A and SP-D expression are more susceptible to viral and bacterial pathogens (6). SP-A and SP-D are also involved in the regulation of noninfection-induced tissue injury. For instance, SP-A and SP-D demonstrate protective effects on indirect lung injury induced by bleomycin and lipopolysaccharide (LPS) (13–14). SP-A can attenuate vascular permeability and apoptosis in bleomycin-induced acute lung injury (ALI) (13). SP-D deficiency results in increased macrophage recruitment and pulmonary inflammation in LPS-induced lung injury (14).
In the present study, we hypothesize SP-A and SP-D demonstrate protective effects in sepsis-induced AKI. To test this hypothesis, we had performed polymicrobial abdominal sepsis by cecal ligation and puncture (CLP) operation in wild type (WT) and SP-A/SP-D KO mice and investigated the mechanisms of sepsis-induced AKI. We found that septic SP-A/SP-D KO mice showed decreased kidney function, increased kidney cell apoptosis and vascular permeability compared to septic WT mice, suggesting SP-A and SP-D play a protective role in sepsis-induced AKI.
Materials and Methods
Animals
SP-A and SP-D double knockout (SP-A/SP-D KO) mice with a C57BL/6 background were originally generated by Dr. Hawgood’s laboratory of The University of California, San Francisco. The SP-A/SP-D KO strain was backcrossed at least 10 generations against a C57BL/6 background (11). No significant difference of phenotype exists between age- and sex-matched WT and SP-A/SP-D KO mice. Age, sex and background-matched C57BL/6 WT mice were obtained from Jackson Laboratories (Bar Harbor, ME). In addition, SP-A single gene knockout (SP-A KO) mice were used in some experiments in the study. Mice were housed in the pathogen-free conditions and the protocol for this study has approved by Institutional Animal Care and Use Committee at SUNY Upstate Medical University and meets the National Institutes of Health and ARRIVE guidelines on the use of laboratory animals.
Sepsis-induced AKI mouse model and specimen collection
In the present study, sepsis-induced AKI was induced by subjecting mice to cecal ligation and puncture (CLP) (4, 15). Male and female mice between 8 – 12 weeks old were anesthetized using intraperitoneal ketamine/xylazine (90 mg/kg ketamine, 10 mg/kg xylazine) injection. For CLP mice, after laparotomy, at 1.3 cm position of the cecum from distal pole to the base of the cecum, a 5-0 silk ligature was made, without interrupting bowel continuity. The cecum was punctured twice with a 22-gauge needle and gently squeezed to extrude a 1-mm3 column of fecal materials. The cecum was returned to the abdominal cavity. For sham mice, neither ligation nor puncture was made. The abdominal incision was closed with 5-0 silk sutures. After operation, mice were given the fluid resuscitation with 1 ml warmed saline immediately. Buprenorphine (0.05 mg/kg body weight s.c) was injected for postoperative analgesia every 12 hrs. At 6 h and 24 h post-CLP, mice were sacrificed under anesthesia. Blood was collected from the inferior vena cava using 1-ml syringe. Kidneys were frozen in liquid nitrogen or fixed in 10% formalin for further study. In some experiments, mice were subjected to peritoneal lavage with 4 ml of saline supplemented with 20% fetal bovine serum, and 2 ml of peritoneal lavage fluid was collected for further analysis.
Evans, blue dye leakage assay
At 6 h and 24 h post-CLP, mice were anesthetized as described above and 100 µl/mouse of Evans, blue (EB) dye solution (50 mg/kg, Sigma, St. Louis, MO) was injected via the tail vein as described previously (16). Thirty min after injection, mice were perfused through left ventricle with 30 ml PBS to eliminate all the blood. Kidneys were collected for analysis. To determine EB dye leakage in the kidney, kidneys were homogenized with 0.4 ml of formamide per kidney. The solution was incubated at 55°C for 24 h. The resulting samples were centrifuged at 21,130×g for 25 min. The supernatant containing EB dye was removed into new tube, and then Evans, blue dye was detected spectrophotometrically by the absorbance at 620 nm. The standard curve was prepared using different concentrations of EB dye.
Blood chemistry assay
After blood collection, the concentrations of blood urea nitrogen (BUN) and lactate, as well as blood pH value were analyzed immediately using a Roche Diagnostic analyzer (Roche, Indianapolis, IN). The content of serum creatinine was determined using a creatinine (serum) colorimetric assay kit (Cayman Chem, Ann Arbor, MI).
Histological analysis
Kidneys were fixed in 10% formalin for at least 24 h and embedded in paraffin. About 4-µm sections from six mice for each condition were stained with Haematoxylin and Eosin (H/E) or Periodic acid-Schiff (PAS) and then assessed qualitatively for kidney injury histopathology in a blinded fashion by two experienced investigators. Histological kidney injury scores were assessed and calculated as previously described (17).
Apoptotic cell determination by TUNEL assay
About 4-µm sections from six mice for each condition were incubated at 60°C for 20min. The sections were deparaffinized in xylene twice, treated with graded series of alcohol [100%, 90%, 80% and 70% ethanol/ddH2O] and rinsed in PBS (pH 7.5). Apoptotic cells were detected using deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) kit (Roche, Indianapolis, IN) following the manufacturer’s instructions. Apoptotic (TUNEL-positive) cells were quantified in 20 randomly chosen fields at ×400 magnification.
Quantification of aerobic bacterial CFUs and inflammatory cells in peritoneal lavage fluid
Peritoneal lavage fluid (PLF) was collected as described above for quantification of colony-forming units (CFUs) and inflammatory cells (macrophages/monocytes and neutrophils). One to ten µl of each PLF sample were diluted into 1 ml of sterile PBS, then 100 µl of the diluted sample was aseptically plated on LB agar plate and the plate was incubated at 37°C for 16 h. The CFUs were determined using the Quantity One colony-counting software (Bio-Rad, Hercules, CA). To quantify inflammatory cells in PLF samples, total cells in 1 ml of the PLF samples were collected by centrifugation at 250×g for 10 min. The cell pellets were resuspended with 1 ml of sterile saline (4°C) and then diluted 10–1000 times. All the cells in 200 µl diluted cell solution were mounted on a slide by cytospin centrifugation. Cells were stained with HEMA staining kit (Fisher, Pittsburg, PA). Polymorphonuclear neutrophils (PMNs) and monocytes/macrophages in the PLF were analyzed by microscope at ×400 magnification.
Western blot analysis
Frozen kidney tissues were homogenized in RIPA buffer containing cocktail of protein inhibitors (Roche, Indianapolis, IN). The samples were centrifuged at 13,000×g for 10 min and supernatant was recovered for the Western blot analysis as described previously (18). In brief, protein concentrations were determined using the micro BCA protein assay kit (Thermo Scientific, Rockford, IL). Protein samples (80 µg/sample) were subjected to separate by reducing 12% SDS-polyacrylamide gel electrophoresis, and then transferred onto PVDF membrane at 4°C overnight (Bio-Rad, Hercules, USA). The membrane was blocked in 3% non-fat milk in Tris-buffered saline, and probed using a primary antibody against Bcl-2 (Santa Cruz Biotechnology, Dallas, Texas), or caspase-3 (Santa Cruz Biotechnology, Dallas, Texas), or NF-κB p65 (Santa Cruz Biotechnology, Dallas, Texas), or p-IkB-α (Santa Cruz Biotechnology, Dallas, Texas), and subsequently incubated with a secondary HRP-conjugated antibody (Bio-Rad, Hercules, CA). Bands were detected using Pierce ECL Western Blotting detection reagent (Thermo Scientific, Rockford, IL) and the blots were exposed to X-film. The membrane was stripped and reprobed with β-actin antibody (Santa Cruz Biotechnology, Dallas, Texas). The bands on the films were quantified by the Quantity One software (Bio-Rad, Hercules, CA).
Cytokine determination in kidney tissue
The concentrations of IL-6 and TNF-α in kidney tissue were assayed using commercially available murine enzyme linked immunosorbent kits following the manufacturer’s instructions (SuperArray Bioscience, Frederick, MD). All samples were analyzed in triplicates.
Statistical analysis
In the present study, all the data are expressed as mean ± SEM. Statistic analysis was performed using the Sigmastat software version 3.5 (Jandel Scientific, CA). Statistic differences were assessed using one-way ANOVA and Tukey’s post-hoc tests. p <0.05 was considered statistically significant.
Results
Kidney function
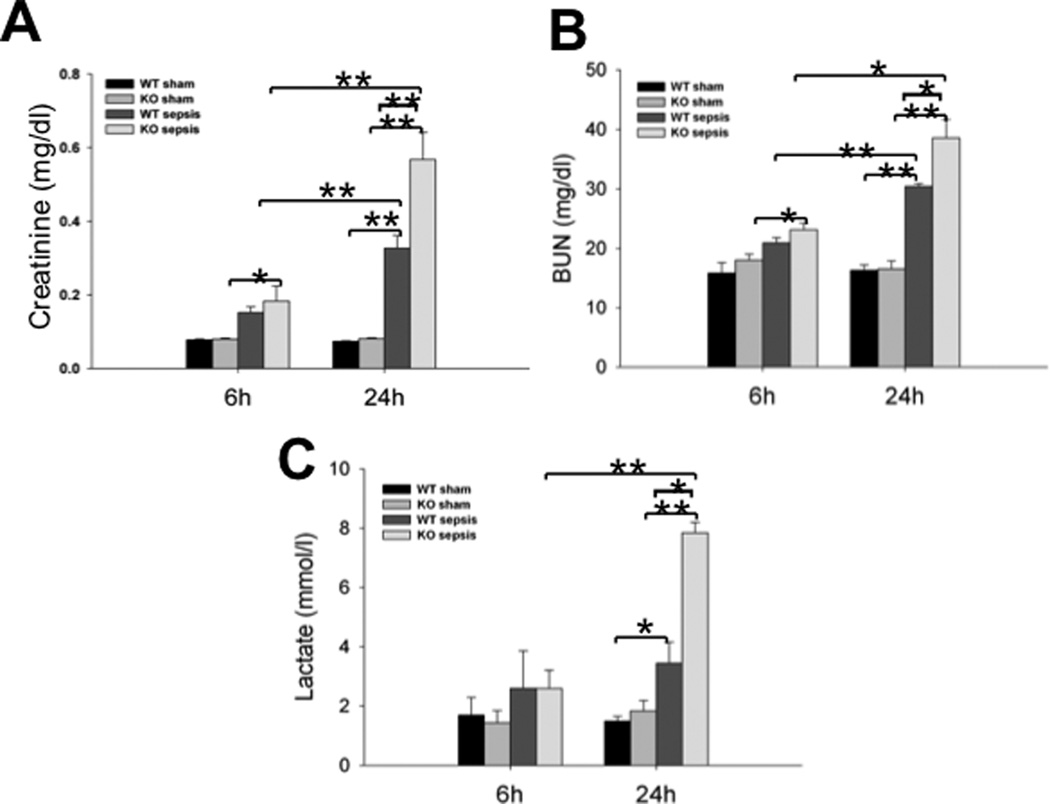
To study the role of SP-A and SP-D in sepsis-induced AKI, we measured the serum creatinine, blood urea nitrogen (BUN), lactate and pH levels in CLP-induced septic WT and SP-A/SP-D KO mice and sham mice 6 h and 24 h after CLP. The levels of serum creatinine and BUN were usually used as biomarkers of kidney injury (27). The results indicate the levels of creatinine and BUN were significantly higher in the septic mice (Fig. 1A, p<0.01, vs sham mice) 24 h post-CLP. Septic SP-A/SP-D KO mice had higher levels of creatinine and BUN than septic WT mice 24 h after CLP (Fig. 1A, p<0.05). There was no difference in BUN observed between septic and sham mice 6 h after CLP. Similarly, the blood lactate level was increased in septic mice compared to sham mice 24 h after CLP, and septic SP-A/SP-D KO mice had higher level of blood lactate than septic WT mice 24 h after CLP (Fig. 1B, p<0.05). However, no change was found in the level of blood pH value between septic and sham mice (data not shown). Additionally, we also analyzed serum creatinine in septic SP-A KO mice (n= 5) and sham SP-A KO mice (n=5) 24 h after CLP. The results showed that the level of serum creatinine in septic SP-A KO mice was significantly higher than sham SP-A KO mice (septic vs sham SP-A KO mice = 0.41± 0.05 mg/dl vs 0.10 ± 0.01 mg/dl, p<0.01). Furthermore, the level of serum creatinine in septic SP-A KO mice was lower compared to septic SP-A/SP-D KO mice (0.56 ± 0.07 mg/dl, p<0.05) but higher compared to septic WT mice (0.32 ± 0.03 mg/dl, p<0.05).
Fig. 1. The levels of creatinine, BUN, and Lactate in the blood of septic WT and SP-A/SP-D KO mice.
Blood samples were collected from WT and SP-A/SP-D KO mice 6 h and 24 h after CLP or sham operation. Septic WT and SP-A/SP-D KO mice had higher levels of serum creatinine (A), BUN (B) and lactate (C) compared with sham group 24 h after CLP. Increased levels of creatinine (A), BUN (B) and lactate (C) were found in septic SP-A/SP-D KO mice compared with septic WT mice 24 h after CLP. Graphs represent the mean ± SEM. *p<0.05, **p<0.01. (n = 6 mice/group)
Kidney histology
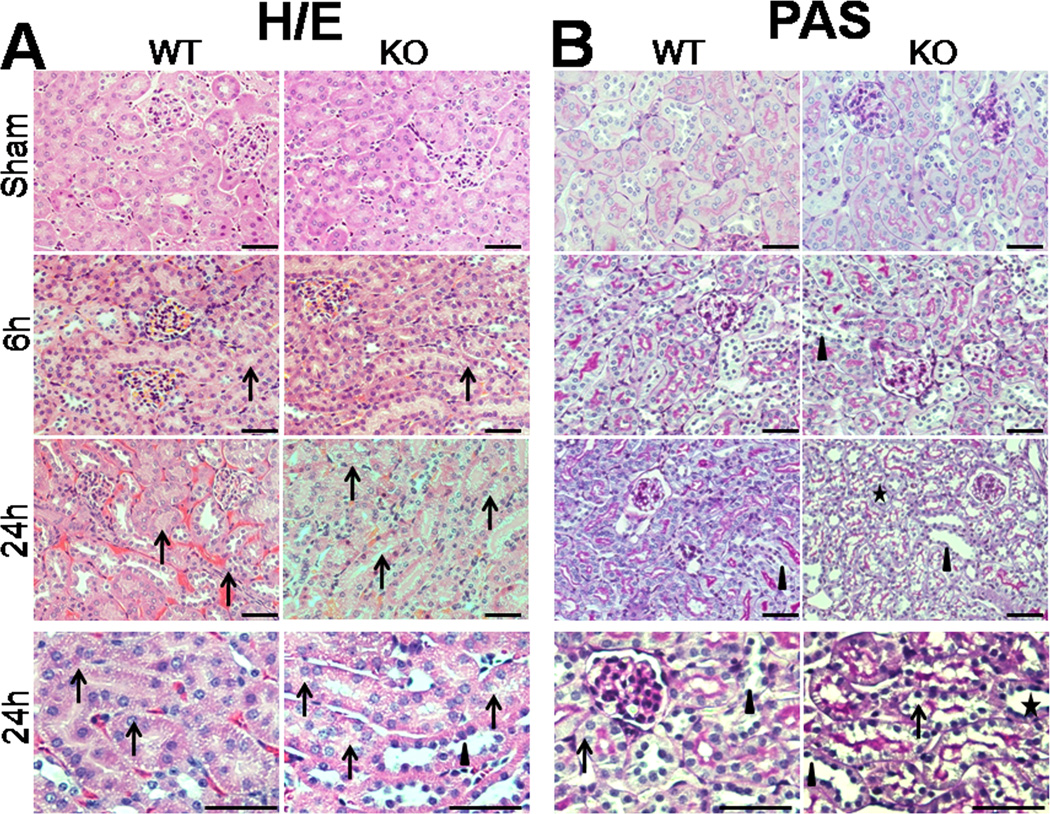
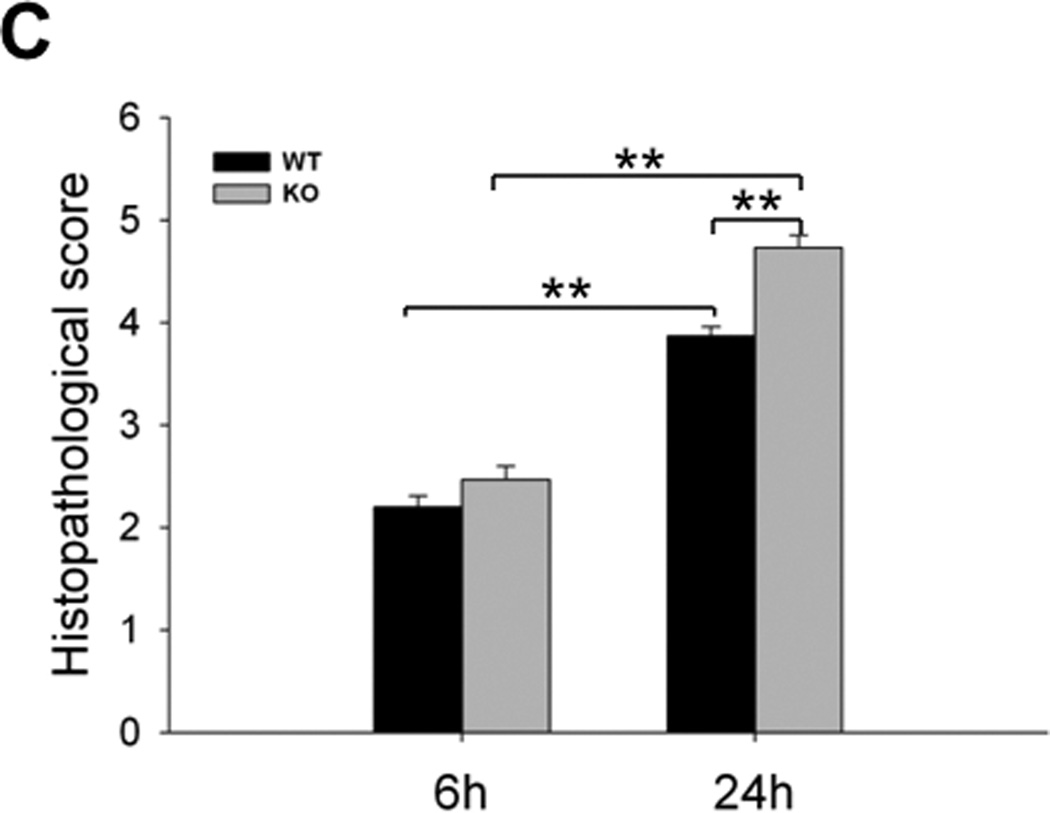
To assess the effects of SP-A and SP-D on kidney injury in CLP-induced sepsis, we examined kidney histology with H/E staining and PAS staining at 6 h and 24 h after CLP. There were obvious pathological changes in kidneys from septic but not sham mice (Fig. 2). Kidney injury in septic mice was prominent in the renal cortex. Microscopic analysis with H/E staining found hemorrhage, vacuolization of tubular epithelial cells in the kidney from septic mice 24 h post-CLP, but septic SP-A/SP-D KO mice showed more severe kidney injury 24 h post-CLP (Fig. 2A). With PAS staining, septic SP-A/SP-D KO mice exhibited increased loss of brush border and flattened tubular cells in the kidney when compared with septic WT mice 24 h post-CLP (Fig. 2B). Furthermore, the histopathological score of kidney injury increased in both septic WT and SP-A/SP-D KO mice from 6 h to 24 h post-CLP (Fig. 2C, p<0.01) but septic SP-A/SP-D KO mice had higher kidney injury score compared with septic WT mice 24 h post-CLP (Fig. 2C, p<0.01). These results indicate mice lacking SP-A and SP-D had more severe sepsis-induced AKI. In addition, we also examine single gene SP-A efefct in the sepsis-induced AKI using SP-A KO mice. Histological analysis showed kidney injury (hemorrhage and vacuolization of tubular epithelial cells, loss of brush border) in septic SP-A KO mice (n = 5 mice) 24 h after CLP but not sham SP-A KO mice (n = 5 mice). Qualitative assessment of kidney histopathological injury indicated that the score of septic SP-A KO mice (the score = 4.4 ± 0.1) was lower (p<0.05) compared to septic SP-A/SP-D KO mice (the score = 4.8 ± 0.1) but higher (p<0.05) compared to septic WT mice (the score = 3.8 ± 0.1).
Fig. 2. Histology of the kidney in septic WT and SP-A/SP-D KO mice.
The histopathology of kidney tissues was analyzed in septic WT and SP-A/SP-D KO mice, as well as control (sham). Histological sections of kidney tissues were stained with H/E (A) or PAS (B) method. The histological score of kidney tissue injury was assessed (C). Kidney histology shows the presence of vacuolar degeneration of tubular cells (arrows), flattened tubular cells with tubular lumen dilatation (stars) and lack of brush border (triangles) in septic but not sham mice. Compared with septic WT mice, the kidney injury score is higher in septic SP-A/SP-D KO mice 24 h after CLP. Magnification ×200 or ×400. Graphs represent the mean ± SEM. **p<0.01. Bars = 100 µm. (n = 6 mice/group)
Kidney vascular permeability
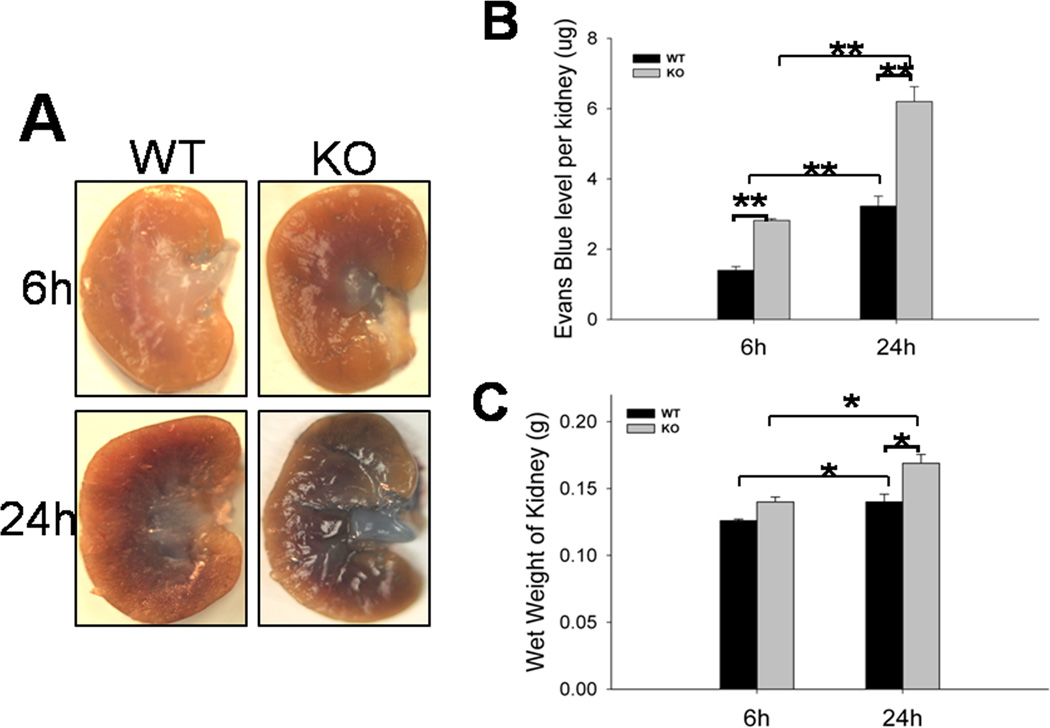
Endothelial cells play an integral role in the vascular response to acute inflammation in the kidney and result in increased vascular permeability (3, 19). Since increased hemorrhage was observed in the kidney tissues from septic mice, we examined whether SP-A and SP-D had a role in maintaining cell integrity and regulating vascular permeability during sepsis-induced AKI. As described in methods, the level of EB dye in the kidney was determined and used as index of kidney vascular permeability. The level of the EB dye leakage increased in septic mice 6 h and 24 h after CLP surgery. The septic SP-A/SP-D KO mice showed more blue dye leakage in the kidney than septic WT mice (Fig. 3A). Quantitative analysis of the EB dye indicates the level of EB dye was higher in the kidneys of septic SP-A/SP-D KO mice compared to septic WT mice 6 h and 24 h post-CLP(Fig. 3B, p<0.01). In addition, with septic WT mice, the wet weight of the kidney from septic SP-A/SP-D KO mice was higher than septic WT mice 24 h post-CLP (Fig. 3C, p<0.05). These data suggest SP-A and SP-D participate in regulating cell integrity and vascular permeability during sepsis-induced AKI.
Fig. 3. Kidney vascular permeability and wet weight of the kidneys in septic WT and SP-A/SP-D KO mice.
Evans Blue (EB) dye (50 mg/kg) was injected via the mouse tail vein and EB leakage as described in the methods. Representative photos of the kidneys from septic WT and SP-A/SP-D KO mice 6 h or 24 h after CLP are shown in the panel (A). The levels of EB dye (B) recovered in kidney tissues and the wet weights of the kidneys (C) were higher in septic SP-A/SP-D KO mice compared with septic WT mice. Values represent the mean ± SEM, *p<0.05, **p<0.01. (n = 6 mice/group)
Kidney cell apoptosis
Previous studies have observed increased apoptosis in the kidneys from septic animals and patients (20–21). To study the role of SP-A and SP-D in renal cell apoptosis in sepsis-induced AKI, we examined apoptotic cells and apoptosis-related protein expression in the kidney tissues of septic WT and SP-A/SP-D KO mice. First, renal apoptotic cells were analyzed by TUNEL assay. As shown in the Figure 4, those cells with the brown nucleus were apoptotic. The results demonstrate obvious apoptosis of tubular cells occurred in the kidneys of septic mice 6 h and 24 h after CLP treatment, but not in sham mice (Fig. 4A). After quantifying analysis of apoptotic cells, we found that the number of apoptotic cells was larger in the kidney of septic SP-A/SP-D mice than that of septic WT mice 6 h and 24 h after CLP (Fig. 4B, p<0.01).
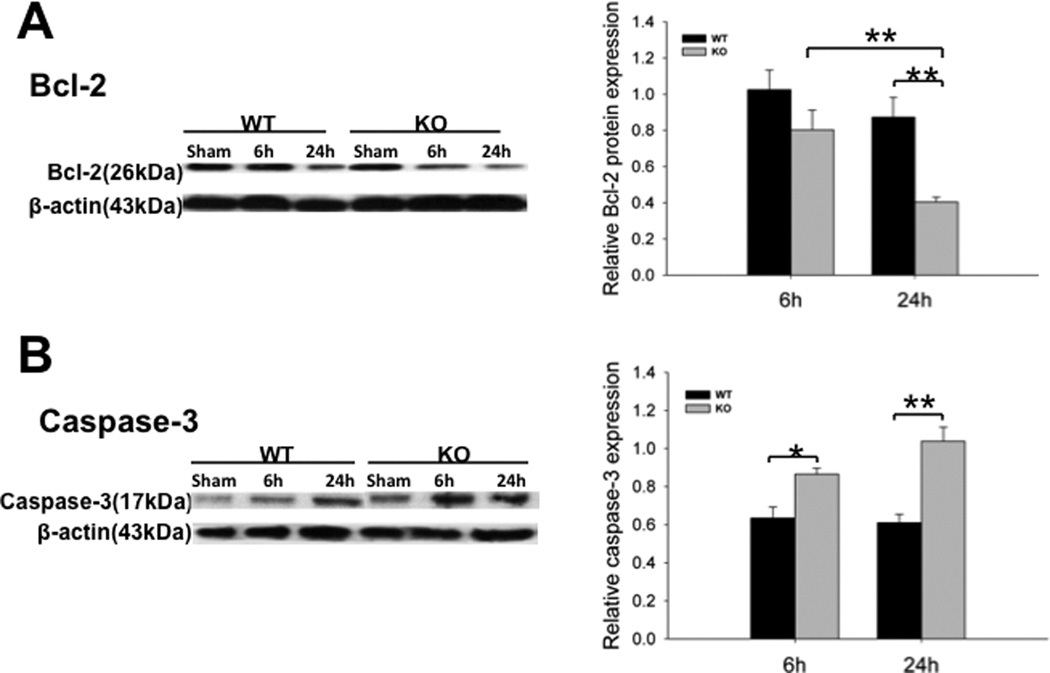
Fig. 4. Apoptotic cells in the kidney of septic WT and SP-A/SP-D KO mice.
Cell apoptosis was examined by TUNEL assay in the kidney of septic WT and SP-A/SP-D KO mice 6 h and 24 h after CLP or sham (A).The cells with brown nucleus are apoptotic (arrows). Apoptotic cells were quantified by per high power field (HPF) at ×400 magnification. The number of apoptotic cells was larger in septic SP-A/SP-D KO mice than septic WT mice 6 h and 24 h post-CLP (B). Values represent the mean ± SEM, *p<0.05, **p<0.01. Bars=100 µm. (n = 6 mice/group)
We also examined the expression of apoptosis-related proteins in the kidney by Western blot analysis. Bcl-2, an inhibitor of apoptosis, was used as a negative biomarker of cell apoptosis. The higher Bcl-2 protein expression means the less cell apoptosis. Septic mice showed decreased level of Bcl-2 expression in the kidney compared with sham mice (p<0.01). The level of Bcl-2 of septic SP-A/SP-D KO mice was lower than that of septic WT mice 24 h after CLP (Fig. 5A, p<0.01). Another hallmark of ongoing cell apoptosis, caspase-3, was also examined. The caspase-3 expression was increased in the kidney of septic mice (p<0.01, vs sham mice). Septic SP-A/SP-D KO mice expressed higher level of caspase-3 than septic WT mice at both 6 h (p<0.05) and 24 h (p<0.01) after CLP (Fig. 5B). These indicated that SP-A and SP-D proteins could influence cell apoptosis in the kidney of sepsis-induced AKI.
Fig. 5. Expression of Bcl-2 and caspase-3 in the kidney of septic WT and SP-A/SP-D KO mice.
Protein levels of Bcl-2 (A) and activated caspase-3 (B) in the kidney were determined by Western blotting analysis and quantified by densitometry. Protein levels were normalized to β-actin. Decreased level of Bcl-2 and increased level of activated caspase-3 were found in septic SP-A/SP-D KO mice compared to septic WT mice 6 h and 24 h post-CLP. Graphs represent the mean ± SEM, *p<0.05, **p<0.01. (n = 6 mice/group)
Kidney NF-κB activation
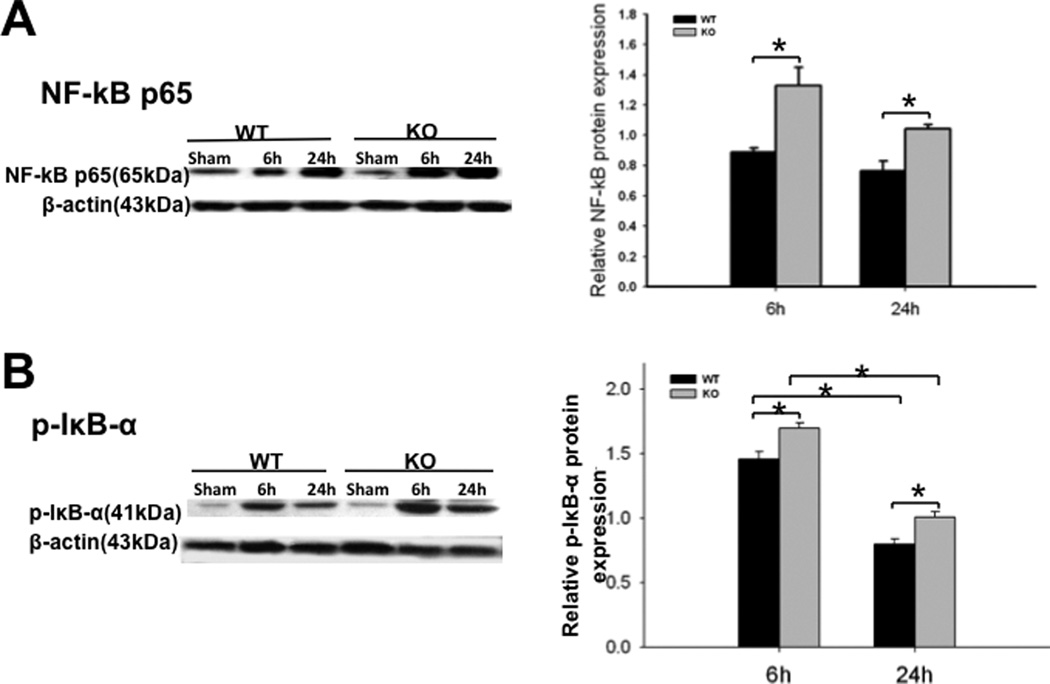
SP-A and SP-D are involved in the activation of the NF-κB signaling pathway (9–10, 22). Initiation of NF-κB activation requires phosphorylation of IκB-α. We therefore examined the levels of NF-κB p65 and phosphorylated IκB-α (p-IκB-α) in the kidney using Western blotting analysis with antibodies against NF-κBp65 and p-IκB-α. The results showed increased levels of NF-κB p65 and p-IκB-α in the kidney of septic mice compared to sham mice (Fig. 6, p<0.01). When compared with septic WT mice, the levels of NF-κB p65 (Fig. 6A, p<0.05) and p-IκB-α (Fig. 6B, p<0.01) were higher in the kidney of septic SP-A/SP-D KO mice 6 h and 24 h post-CLP, indicating that septic SP-A/SP-D KO mice had higher NF-κB activation in the kidney than septic WT mice.
Fig. 6. Expression of NF-κB p65 and phosphorylated IκB-α (p-IκB-α) in the kidney of septic WT and SP-A/SP-D KO mice.
Protein levels of NF-κB p65 (A) and p-IκB-α (B) in the kidneys were determined by Western blotting analysis and then quantified by densitometry. The levels were normalized to β-actin. Increased levels of NF-κB p65 and p-IκB-α were detected in the kidneys of septic SP-A/SP-D KO mice compared to septic WT mice 6 h and 24 h post-CLP. Graphs represent the mean ± SEM, *p<0.05, **p<0.01. (n = 6 mice/group)
Kidney proinflammatory cytokines
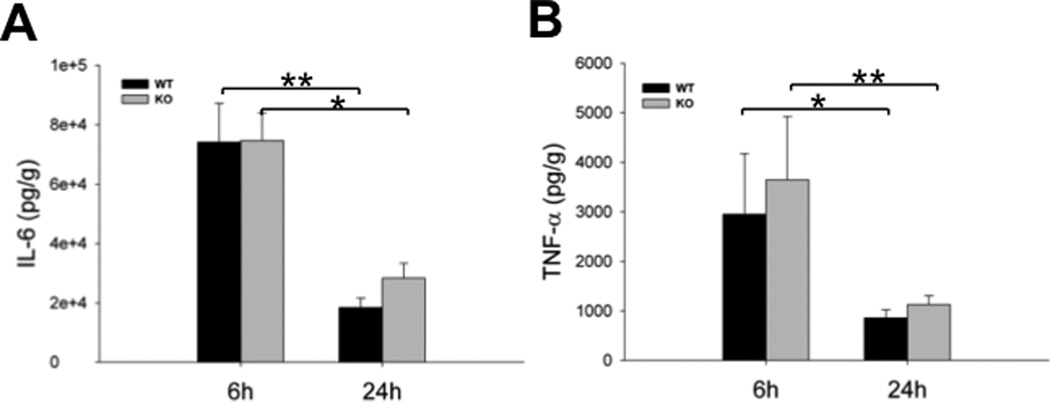
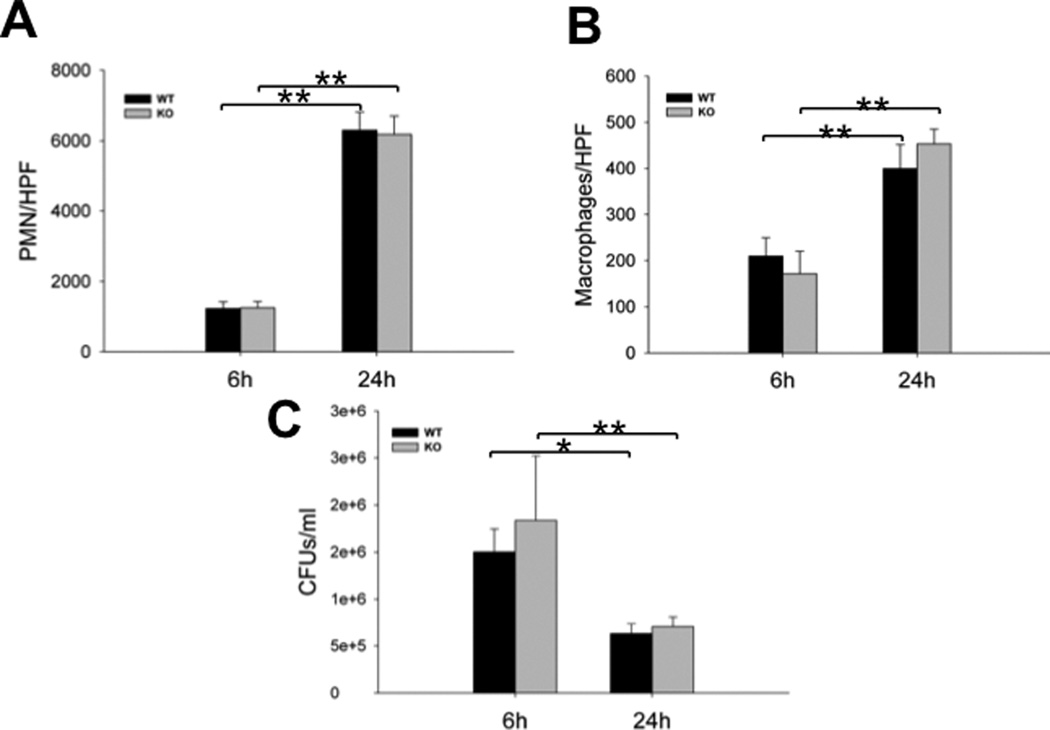
Cytokines and chemokines are related to the development of sepsis-induced AKI. The levels of two proinflammatory cytokines (IL-6 and TNF-α) were measured in kidney tissues by ELISA. Increased levels of IL-6 and TNF-α in septic mice were observed when compared with sham mice. The levels of IL-6 and TNF-α were 18459 ± 3129 pg/g & 28372 ± 4966 pg/g for IL-6 and 860 ± 165 pg/g & 1128 ± 174 pg/g for TNF-α 24 h post-CLP in the septic WT and SP-A/SP-D KO mice, respectively (Fig. 7) but only minimal levels (< 1 pg/g) of IL-6 and TNF-α were detected in the kidney of sham mice. Although IL-6 and TNF-α expression in the kidney of septic SP-A/SP-D KO mice 24 h post-CLP was increased, statistical analysis revealed no significant difference of either IL-6 or TNF-α between septic WT and SP-A/SP-D KO mice (p>0.05). We also examined proinflammatory cell counts and bacterial loads in the peritoneal lavage fluid (PLF) prepared from septic mice. No significant differences of cell counts and bacterial loads were observed between septic WT and SP-A/SP-D KO mice. However, the numbers of PMNs and macrophages increased, and CFUs reduced significantly in the PLF of both septic WT and SP-A/SP-D KO mice from 6 h to 24 h after CLP (Fig. 8).
Fig. 7. Cytokine production in the kidney of septic WT and SP-A/SP-D KO mice.
Kidneys were collected from septic WT and SP-A/SP-D KO mice 6 h and 24 h post-CLP. The levels of cytokine IL-6 (A) and TNF-α (B) were measured by ELISA. Compared with basal levels of IL-6 and TNF-α (sham mice), significantly increased levels of IL-6 and TNF-α were detected in the septic WT and SP-A/SP-D KO mice. However, no significant difference of either IL-6 or TNF-α was detected between septic WT and SP-A/SP-D KO mice 6 h and 24 h post-CLP. Graphs represent the mean ± SEM. *p<0.05, **p<0.01. (n = 6 mice/group)
Fig. 8. Cell counts and CFUs in the peritoneal lavage fluid of septic WT and SP-A/SP-D KO mice.
Peritoneal lavage fluid (PLF) was collected from septic WT and SP-A/SP-D KO mice 6 h and 24 h post-CLP. Slides containing cells from PLF were prepared by cytospin centrifugation and then stained by HEMA staining. PMNs (A) and macrophages (B) were counted at ×400 magnification. No significant difference of either PMNs or macrophages was found between septic WT and SP-A/SP-D KO mice. The CFUs (C) were determined by culturing the lavage fluid on LB plates. No significant difference of CFUs was found between septic WT and SP-A/SP-D KO mice. Graphs represent the mean ± SEM. *p<0.05, **p<0.01. (n = 6 mice/group)
Discussion
Sepsis is a major cause of AKI. SP-A and SP-D play important roles in innate immunity and in the modulation of inflammation in pulmonary infections (6, 11–12). We recently demonstrated SP-A and SP-D are expressed in renal epithelial cells (7) and some human SP-A and SP-D genetic variants are susceptible to urinary tract infection (23). It is unclear whether expression of SP-A and SP-D has an impact on the development of sepsis-induced AKI. In the present study, we demonstrate that mice lacking SP-A and SP-D expression exhibit decreased renal function and more severe kidney injury compared to WT mice in response to polymicrobial sepsis. Cellular and molecular analyses reveals SP-A and SP-D inhibit renal cell apoptosis and modulate vascular permeability in part by regulating the inflammatory NF-κB pathway in sepsis-induced AKI.
The histopathological changes of AKI vary depending on the cause (3). In the present study, mild vacuolization of tubular cells in the kidney was observed in the early phase of sepsis. i.e. 6 h post-CLP, severe vacuolization of tubular cells and the loss of brush border were found only in the late phase of sepsis i.e. 24 h post-CLP. When compared with septic WT mice, kidney injury was worse in septic SP-A KO and SP-A/SP-D KO mice, suggesting that SP-A KO and SP-A/SP-D KO mice are more susceptible to sepsis-induced AKI. Furthermore, the kidney injury (histopathological score) showed positive correlations with the increase of the creatinine and BUN levels in the CLP-induced sepsis.
Sepsis-induced AKI is associated with increased renal vascular permeability (19, 24). Our data showed septic SP-A/SP-D KO mice exhibit increased renal wet weight and more severe vascular permeability compared with septic WT mice. Collectively, these results suggested SP-A and SP-D have a protective role in maintaining the integrity of the kidney capillary membranes in AKI. These findings were consistent with previous reports which demonstrated a protective effect of SP-A and SP-D in bleomycin-induced lung injury (13) and LPS-induced pulmonary disease (14). Neutrophil and monocyte/macrophage infiltrations contribute to renal interstitial inflammation and represent one mechanism in the pathogenesis of sepsis-induced AKI (25). The immunohistochemical analysis revealed intensive monocytes/macrophages in the interstitial capillaries in the kidneys of septic WT and SP-A/SP-D KO mice but only few neutrophils existed (data not shown).
Tubular cell apoptosis of the kidney is another important mechanism in the pathogenesis of AKI (20). In this study, renal tubular cell apoptosis was increased at 6 h and 24 h after CLP, evidenced by TUNEL analysis and molecular analyses of two apoptosis-related biomarkers. Caspases proteins, a family of intracellular cysteine proteases, play a pivotal role in the initiation and execution of apoptosis; the member caspase-3 is an executor in the apoptosis while Bcl-2 acts as an inhibitor of apoptosis. The significantly increased level of activated caspase-3 (17 kDa) and the reduced level of anti-apoptosis protein Bcl-2 support the results from TUNEL analysis in the kidneys of septic mice after CLP treatment. There are increasing evidence that renal tubular cells die by apoptosis at the early phase of experimental model of acute toxic renal injury (26). Obvious cell apoptosis was observed at 6 h after CLP in this study. As shown in figures 4 and 5, increased TUNEL-positive cells and enhanced caspase-3 expression in the kidneys of septic SP-A/SP-D KO mice compared to septic WT mice demonstrated the protective effect of SP-A and SP-D to renal cells in sepsis-induced AKI. These findings were also consistent with previous observation that found the protective effect of SP-A against alveolar type II cell apoptosis in bleomycin-induced lung injury (13).
Nuclear factor-kappa B (NF-κB) is a well-known transcription factor that regulates many inflammation-related gene expression in response to various infections (27). When cells are stimulated, IκB of NF-kB/IκB complex in the cytoplasm is phosphorylated and dissociated from the complex, then NF-κB (p50 and p65) are translocated from the cytoplasm into the nucleus, rendering NF-κB activation. Endotoxemia stimulates the activation of renal NF-κB in vivo (28) and the inhibition of NF-κB activity ameliorates sepsis-induced AKI (29). In the present study, we demonstrated increased levels of NF-κBp65 and phosphorylated-IκB-α (p-IκB-α) in the kidneys of septic WT and SP-A/SP-D KO mice 6 h and 24 h post-CLP, with more pronounced expression in the septic SP-A/SP-D KO mice. Although the role of NF-κB signaling pathway in apoptosis is elusive, increasing evidence suggests positive correlation between apoptosis and NF-κB activation (30). Furthermore, increased caspase-3 expression is positively correlated with the NF-κBp65 level in kidney tissues from septic patients (20). In the present study, we observed increased NF-κB activation is associated with renal cell apoptosis in CLP-induced AKI. Collectively, our data provides two possible mechanisms for the amelioration of sepsis-induced AKI by SP-A and SP-D: 1) SP-A and SP-D may inhibit renal cell apoptosis by interacting with renal tubular cell receptors, such as TLR4 (31), which leads to down-regulate the activation of the NF-κB signaling pathway (32); 2) SP-A and SP-D proteins may influence the integrity of the kidney capillary membranes and thus reduce renal vascular permeability in sepsis-induced AKI.
Proinflammatory cytokines like TNF-α and IL-6 are important mediators to trigger the later systematic inflammation response and subsequently multi-organ failure in sepsis (33). Increased levels of TNF-α and IL-6 in the kidney tissues of septic WT and SP-A/SP-D KO mice were observed 6 h and 24 h post-CLP. Of interesting, no different levels of these proinflammatory cytokines were detected between septic WT and SP-A/SP-D KO mice although there were significant differences of NF-κB and p-IκB-α levels in the kidneys of septic WT and SP-A/SP-D mice. It is possible that the mice lacking SP-A and SP-D have changed renal immune homeostasis because it has been shown that SP-A/SP-D KO mice express significantly lower levels of IL-1β and IL-6 in myometrium during parturition (34). Furthermore, the imbalance of pro-inflammatory and anti-inflammatory cytokines may contribute to deterioration of sepsis and sepsis-induced AKI. Only two pro-inflammatory cytokines were assessed in this study, therefore, analysis of more kinds of cytokines and evaluation of the balance between pro-inflammatory and anti-inflammatory cytokines may explain the mechanisms of SP-A and SP-D function in the modulation of immune homeostasis of sepsis-induced AKI in the future study.
Massive leukocyte infiltration into the peritoneal cavity is commonly observed in CLP-induced sepsis. Innate immune responses to restrict bacterial spread are one of the most important defense mechanisms to prevent systemic infection. The enhanced migration of inflammatory cells, i.e. monocytes/macrophages and neutrophils, into the peritoneal cavity lead to increased expression of cytokines and chemokines in CLP-induced sepsis model (35). Although monocyte/macrophage infiltration and neutrophil influx were observed in the peritoneal cavity of septic WT and SP-A/SP-D KO mice 6 h and 24 h post-CLP, no significant difference was found between septic WT and SP-A/SP-D KO mice, suggesting that SP-A and SP-D may function in the local tissues in the sepsis-induced AKI. Therefore, future study will be needed whether exogenous SP-A and SP-D would restore the ability of immune homeostasis and reduce kidney injury in sepsis-induced AKI.
In summary, we conclude that SP-A and SP-D can attenuate kidney injury in sepsis-induced AKI model by inhibiting renal cell apoptosis, decreasing renal vascular permeability, and regulating renal inflammatory response through NF-κB pathway. These findings were obtained from SP-A/SP-D KO mouse model and further data are needed before these data might apply to humans.
Acknowledgements
We would like to thank Dr. S. Hawgood of The University of California San Francisco, CA for kindly providing SP-A KO and SP-A/SP-D KO mice. We also thank Dr. Q. Meng and all members of Prof. Nieman’s laboratory for their kind supports to this project.
This study is supported by NIH (HL096007) and NSFC (81201457).
Footnotes
This study was selected as one of the New Investigator Nominees at the 37th Annual Conference on Shock, held in Charlotte, North Carolina, June 7–10, 2014.
The authors have no relevant conflicts of interest.
References
- 1.Schrier RW, Wang W. Acute renal failure and sepsis. N Engl J Med. 2004;351(2):159–169. doi: 10.1056/NEJMra032401. [DOI] [PubMed] [Google Scholar]
- 2.Silvester W, Bellomo R, Cole L. Epidemiology, management, and outcome of severe acute renal failure of critical illness in Australia. Crit Care Med. 2001;29(10):1910–1915. doi: 10.1097/00003246-200110000-00010. [DOI] [PubMed] [Google Scholar]
- 3.Wan L, Bagshaw SM, Langenberg C, Saotome T, May C, Bellomo R. Pathophysiology of septic acute kidney injury: what do we really know? Crit Care Med. 2008;36(4 Suppl):S198–S203. doi: 10.1097/CCM.0b013e318168ccd5. [DOI] [PubMed] [Google Scholar]
- 4.Hubbard WJ, Choudhry M, Schwacha MG, Kerby JD, Rue LW, 3rd, Bland KI, Chaudry IH. Cecal ligation and puncture. Shock. 2005;24(Suppl 1):52–57. doi: 10.1097/01.shk.0000191414.94461.7e. [DOI] [PubMed] [Google Scholar]
- 5.Kaushal GP. Autophagy protects proximal tubular cells from injury and apoptosis. Kidney Int. 2012;82(12):1250–1253. doi: 10.1038/ki.2012.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giannoni E, Sawa T, Allen L, Wiener-Kronish J, Hawgood S. Surfactant proteins A and D enhance pulmonary clearance of Pseudomonas aeruginosa. Am J Respir Cell Mol Biol. 2006;34(6):704–710. doi: 10.1165/rcmb.2005-0461OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Madsen J, Kliem A, Tornoe I, Skjodt K, Koch C, Holmskov U. Localization of lung surfactant protein D on mucosal surfaces in human tissues. J Immunol. 2000;164(11):5866–5870. doi: 10.4049/jimmunol.164.11.5866. [DOI] [PubMed] [Google Scholar]
- 8.Gardai SJ, Xiao YQ, Dickinson M, Nick JA, Voelker DR, Greene KE, Henson PM. By binding SIRPalpha or calreticulin/CD91, lung collectins act as dual function surveillance molecules to suppress or enhance inflammation. Cell. 2003;115(1):13–23. doi: 10.1016/s0092-8674(03)00758-x. [DOI] [PubMed] [Google Scholar]
- 9.Sato M, Sano H, Iwaki D, Kudo K, Konishi M, Takahashi H, Takahashi T, et al. Direct binding of Toll-like receptor 2 to zymosan, and zymosan-induced NF-kappa B activation and TNF-alpha secretion are down-regulated by lung collectin surfactant protein A. J Immunol. 2003;171(1):417–425. doi: 10.4049/jimmunol.171.1.417. [DOI] [PubMed] [Google Scholar]
- 10.Yoshida M, Korfhagen TR, Whitsett JA. Surfactant protein D regulates NF-kappa B and matrix metalloproteinase production in alveolar macrophages via oxidant-sensitive pathways. J Immunol. 2001;166(12):7514–7519. doi: 10.4049/jimmunol.166.12.7514. [DOI] [PubMed] [Google Scholar]
- 11.Hawgood S, Ochs M, Jung A, Akiyama J, Allen L, Brown C, Edmondson J, et al. Sequential targeted deficiency of SP-A and -D leads to progressive alveolar lipoproteinosis and emphysema. Am J Physiol Lung Cell Mol Physiol. 2002;283(5):L1002–L1010. doi: 10.1152/ajplung.00118.2002. [DOI] [PubMed] [Google Scholar]
- 12.Hawgood S, Brown C, Edmondson J, Stumbaugh A, Allen L, Goerke J, Clark H, Poulain F. Pulmonary collectins modulate strain-specific influenza a virus infection and host responses. J Virol. 2004;78(16):8565–8572. doi: 10.1128/JVI.78.16.8565-8572.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goto H, Ledford JG, Mukherjee S, Noble PW, Williams KL, Wright JR. The role of surfactant protein A in bleomycin-induced acute lung injury. Am J Respir Crit Care Med. 2010;181(12):1336–1344. doi: 10.1164/rccm.200907-1002OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.King BA, Kingma PS. Surfactant protein D deficiency increases lung injury during endotoxemia. Am J Respir Cell Mol Biol. 2011;44(5):709–715. doi: 10.1165/rcmb.2009-0436OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang Z, Ni L, Javidiparsijani S, Hu F, Gatto LA, Cooney R, Wang G. Enhanced liver autophagic activity improves survival of septic mice lacking surfactant proteins A and D. Tohoku J Exp Med. 2013;231(2):127–138. doi: 10.1620/tjem.231.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yasuda H, Yuen PS, Hu X, Zhou H, Star RA. Simvastatin improves sepsis-induced mortality and acute kidney injury via renal vascular effects. Kidney Int. 2006;69(9):1535–1542. doi: 10.1038/sj.ki.5000300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thurman JM, Lucia MS, Ljubanovic D, Holers VM. Acute tubular necrosis is characterized by activation of the alternative pathway of complement. Kidney Int. 2005;67(2):524–530. doi: 10.1111/j.1523-1755.2005.67109.x. [DOI] [PubMed] [Google Scholar]
- 18.Wang G, Guo X, Diangelo S, Thomas NJ, Floros J. Humanized SFTPA1 and SFTPA2 transgenic mice reveal functional divergence of SP-A1 and SP-A2: formation of tubular myelin in vivo requires both gene products. J Biol Chem. 2010;285(16):11998–12010. doi: 10.1074/jbc.M109.046243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.King EG, Bauza GJ, Mella JR, Remick DG. Pathophysiologic mechanisms in septic shock. Lab Invest. 2014;94(1):4–12. doi: 10.1038/labinvest.2013.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Havasi A, Borkan SC. Apoptosis and acute kidney injury. Kidney Int. 2011;80(1):29–40. doi: 10.1038/ki.2011.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hotchkiss RS, Swanson PE, Freeman BD, Tinsley KW, Cobb JP, Matuschak GM, Buchman TG, Karl IE. Apoptotic cell death in patients with sepsis, shock, and multiple organ dysfunction. Crit Care Med. 1999;27(7):1230–1251. doi: 10.1097/00003246-199907000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Wu TT, Chen TL, Loon WS, Tai YT, Cherng YG, Chen RM. Lipopolysaccharide stimulates syntheses of toll-like receptor 2 and surfactant protein-A in human alveolar epithelial A549 cells through upregulating phosphorylation of MEK1 and ERK1/2 and sequential activation of NF-kappaB. Cytokine. 2011;55(1):40–47. doi: 10.1016/j.cyto.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 23.Liu J, Hu F, Liang W, Wang G, Singhal PC, Ding G. Polymorphisms in the surfactant protein a gene are associated with the susceptibility to recurrent urinary tract infection in chinese women. Tohoku J Exp Med. 2010;221(1):35–42. doi: 10.1620/tjem.221.35. [DOI] [PubMed] [Google Scholar]
- 24.Temmesfeld-Wollbruck B, Brell B, David I, Dorenberg M, Adolphs J, Schmeck B, Suttorp N, Hippenstiel S. Adrenomedullin reduces vascular hyperpermeability and improves survival in rat septic shock. Intensive Care Med. 2007;33(4):703–710. doi: 10.1007/s00134-007-0561-y. [DOI] [PubMed] [Google Scholar]
- 25.Langenberg C, Gobe G, Hood S, May CN, Bellomo R. Renal histopathology during experimental septic acute kidney injury and recovery. Crit Care Med. 2014;42(1):e58–e67. doi: 10.1097/CCM.0b013e3182a639da. [DOI] [PubMed] [Google Scholar]
- 26.Lerolle N, Nochy D, Guerot E, Bruneval P, Fagon JY, Diehl JL, Hill G. Histopathology of septic shock induced acute kidney injury: apoptosis and leukocytic infiltration. Intensive Care Med. 2010;36(3):471–478. doi: 10.1007/s00134-009-1723-x. [DOI] [PubMed] [Google Scholar]
- 27.May MJ, Ghosh S. Signal transduction through NF-kappa B. Immunol Today. 1998;19(2):80–88. doi: 10.1016/s0167-5699(97)01197-3. [DOI] [PubMed] [Google Scholar]
- 28.Ejima K, Layne MD, Carvajal IM, Kritek PA, Baron RM, Chen YH, Vom Saal J, et al. Cyclooxygenase-2-deficient mice are resistant to endotoxin-induced inflammation and death. FASEB J. 2003;17(10):1325–1327. doi: 10.1096/fj.02-1078fje. [DOI] [PubMed] [Google Scholar]
- 29.Hocherl K, Schmidt C, Kurt B, Bucher M. Inhibition of NF-kappaB ameliorates sepsis-induced downregulation of aquaporin-2/V2 receptor expression and acute renal failure in vivo. Am J Physiol Renal Physiol. 2010;298(1):F196–F204. doi: 10.1152/ajprenal.90607.2008. [DOI] [PubMed] [Google Scholar]
- 30.Zingarelli B. Nuclear factor-kappaB. Crit Care Med. 2005;33(12 Suppl):S414–S416. doi: 10.1097/01.ccm.0000186079.88909.94. [DOI] [PubMed] [Google Scholar]
- 31.Ramani V, Madhusoodhanan R, Kosanke S, Awasthi S. A TLR4-interacting SPA4 peptide inhibits LPS-induced lung inflammation. Innate Immunity. 2013;19(6):596–610. doi: 10.1177/1753425912474851. [DOI] [PubMed] [Google Scholar]
- 32.Wu YZ, Medjane S, Chabot S, Kubrusly FS, Raw I, Chignard M, Touqui L. Surfactant protein-A and phosphatidylglycerol suppress type IIA phospholipase A2 synthesis via nuclear factor-kappaB. Am J Respir Crit Care Med. 2003;168(6):692–699. doi: 10.1164/rccm.200304-467OC. [DOI] [PubMed] [Google Scholar]
- 33.Osuchowski MF, Welch K, Siddiqui J, Remick DG. Circulating cytokine/inhibitor profiles reshape the understanding of the SIRS/CARS continuum in sepsis and predict mortality. J Immunol. 2006;177(3):1967–1974. doi: 10.4049/jimmunol.177.3.1967. [DOI] [PubMed] [Google Scholar]
- 34.Montalbano AP, Hawgood S, Mendelson CR. Mice deficient in surfactant protein A (SP-A) and SP-D or in TLR2 manifest delayed parturition and decreased expression of inflammatory and contractile genes. Endocrinology. 2013;154(1):483–498. doi: 10.1210/en.2012-1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Craciun FL, Schuller ER, Remick DG. Early enhanced local neutrophil recruitment in peritonitis-induced sepsis improves bacterial clearance and survival. J Immunol. 2010;185(11):6930–6938. doi: 10.4049/jimmunol.1002300. [DOI] [PMC free article] [PubMed] [Google Scholar]