Abstract
Objective
Although regular physical activity is associated with lower all-cause and disease-specific mortality among breast cancer survivors (BCS), most BCS do not meet its recommended guidelines. Attention function, a domain of cognition, is essential for daily tasks such as exercising, a form of planned physical activity. We tested the hypotheses that lower self-reported attention function in BCS would be associated with less exercise and higher body-mass index (BMI) by comparing a group of 505 young BCS (45 years or younger at diagnosis and 3–8 years post-treatment) to 466 acquaintance controls (AC).
Methods
The groups were compared on self-reported physical and psychological outcomes. Mplus software was used to perform confirmatory structural equation modeling (SEM) with a robust maximum likelihood estimator to evaluate hypothesized relationships among variables. The criteria for good model fit were: having RMSEA<.06, CFI>.95, and SRMR<.08. Modification indices were used to better fit the model.
Results
The final model demonstrated good fit, with RMSEA=.05, CFI=.98, and SRMR=.03. After controlling for demographics, parameter estimates revealed that, compared to AC, young BCS reported worse attention function (p<.001), more depressive symptoms (p<.001), and more fatigue (p<.001). Controlling for fatigue, depression, and anxiety, better attention function was associated with a greater likelihood of exercise in the past 3 months (p=.039), which in turn was associated with a lower BMI (p<.001).
Conclusions
The significant association between attention function and physical activity, if confirmed in a longitudinal study, will provide new targets for interventions aimed at improving physical activity and decreasing BMI among BCS.
Background
In 2012 there were 2.8 million breast cancer survivors (BCS) living in the United States [1]. Although most are cancer-free, many suffer from treatment-related physical and psychosocial sequelae such as being overweight or obese that adversely affect overall physical health [2]. Additionally, post-menopausal BCS who are overweight or obese with a high body mass index (BMI) have a greater risk of cancer recurrence [3–4] and are more likely to have co-morbid conditions such as cardiovascular disease, leading to increased morbidity and mortality [5]. Higher BMI is inversely related to physical activity [6–8], and more physical activity is related to higher health-related quality of life (HRQOL) [9–10]. Inadequate physical activity has emerged as a significant contributor to all-cause and breast cancer-related deaths, as seen in several studies in which BCS with lower physical activity had a higher mortality rate than those engaged in an ideal amount of physical activity [11–12]. In addition, the American Heart Association recommends lifestyle interventions aimed at promoting physical activity for all women, including BCS, for prevention of cardiovascular disease [13]. Despite the significant benefits of physical activity for breast cancer survivorship, after diagnosis and treatment only about a quarter of BCS adhere to the national exercise recommendations of ≥ 150 minutes/week of moderate physical activity [14]. Therefore, examining the determinants of physical activity among BCS is essential to inform intervention development that will lead to increased adherence to physical activity recommendations.
Correlates of physical activity were examined in the Women’s Healthy Eating and Living study, a large randomized trial that showed BCS in ethnic minorities or with lower education levels had lower physical activity [15]. After adjusting for demographic, psychosocial, and cancer-related variables, dietary intake was associated with physical activity; BCS with lower physical activity also had significantly lower adherence to the National Cancer Institute’s dietary recommendations. Studies have shown that patient-related problems such as fatigue, pain, illness, and lack of motivation along with environmental barriers such as lack of time and facilities to exercise are associated with less physical activity [16–17]. Studies looking at the early survivorship period, 3 weeks to 6 months post-treatment, showed that factors that predict physical activity participation varied between working and non-working women. General determinants of inadequate physical activity such as lack of social support and time were more significant in working women, whereas cancer-related determinants such as fatigue and physical illness significantly predicted reduced physical activity in non-working women [18]. To date, most studies have been completed in the year following treatment instead of addressing health behaviors many years after assimilating back into routine life, such as in the late survivorship period. Research is needed to understand the determinants of physical activity in survivors after they have been integrated back into routine life.
We hypothesize that in the late survivorship period, an important yet overlooked determinant of physical activity could be cognitive deficits. Such deficits, particularly in the domains of working memory, attention, and executive function, have been described in BCS [19–20]. Structural and functional neuroimaging studies have shown decreased frontal gray-matter density in chemotherapy-treated BCS in the late survivorship period with accompanying difficulties in self-reported executive functioning [21–22]. Initiation of and adherence to physical activity require cognitive skills such as goal setting, self-monitoring, and self-motivational strategies, as well as enhancement of self-efficacy and problem-solving skills [23]. In BCS, the impact of subtle yet potentially consequential cognitive deficits on initiating and maintaining a program of exercise, a form of repetitive, structured, and intentional physical activity, has never been examined. Therefore, we hypothesized that adherence to exercise would be more difficult in BCS compared to acquaintance controls (AC) and, consequently, BCS would exercise less and have higher BMI.
The purpose of this study was to assess attention function, exercise, and BMI, as well as their inter-relationships, in young breast cancer survivors (YBCS), 45 years or younger at diagnosis who were 3–8 years post-treatment, as compared to a healthy AC group. We chose to study YBCS instead of older survivors due to the unique challenges faced by this group compared to older survivors. YBCS not only experience worse psycho-social sequelae such as worse stress, depression, and HRQOL than older breast cancer survivors, but they also experience worse physical sequelae [24]. For example, YBCS tend to gain more weight after diagnosis than older survivors as they experience chemotherapy-related menopausal changes [24]. Our recent findings have shown YBCS to have worse attention function than older survivors (in press). Therefore, targeting modifiable behavioral health outcomes such as weight gain among YBCS by promoting exercise should not only improve weight but also HRQOL, stress, and depression. Hence, exploring predictors of exercise behavior is pivotal in this younger cohort.
We tested the fit of a confirmatory structural equation model (SEM) path model (i.e., a set of path regression equations, estimated simultaneously, among observed variables) that included the following hypothesized relationships: 1) YBCS have worse attention function than AC (as well as more trait anxiety, depression, and fatigue) after adjusting for demographic variables; 2) decreased attention function is associated with less exercise after adjusting for the co-variates of trait anxiety, fatigue, and depression; and 3) more exercise is associated with lower BMI in both YBCS and AC, after adjusting for demographic variables.
Methods
Sample
Young Breast Cancer Survivor
YBCS considered for study entry were selected from the breast cancer clinical trials conducted through the Eastern Cooperative Oncology Group (ECOG) at 97 institutions. Patients in these ECOG trials had received adjuvant chemotherapy for Stage I-III breast cancer, using a variety of chemotherapy regimens, or combined chemotherapy and hormonal therapy following surgical treatment with mastectomy or lumpectomy. In addition, some women received involved-field radiotherapy. Additional eligibility included being 45 or younger and being 3 to 8 years from initial treatment without a breast cancer recurrence. The women had been diagnosed between 1996 and 2001. Survivors were eligible to participate if they resided in the continental United States, were 18 years of age or older, spoke English, and were able to complete the background questionnaire. All survivors were asked to give informed consent and complete mailed questionnaires. Out of a total of 744 eligible survivors, 86% consented to the study and 67% (n=505) completed all data collection.
Acquaintance controls
AC were identified by survivor participants. Each participant was asked to submit the names of 3 women who were within ± 5 years of the survivor and were of similar education and race. Matched controls were ineligible if they had a history of cancer or were personal friends of the survivor. All control subjects were required to give informed consent and complete mailed questionnaires. Out of a total of 1013 AC, 46% agreed to participate and 40% (n=404) completed the questionnaires.
Procedures
This study was approved by the Institutional Review Board (IRB) of Indiana University, the coordinating site for the study, and the local IRBs from each recruiting site. Measurement of demographic variables: (age, education, income, occupation, and marital status), as well as medical history variables (breast cancer stage and treatment, other health conditions, gynecological history, and co-morbidities [the sum of reported co-morbid conditions] were collected through self-report and medical records. BMI was calculated using self-reported height and weight obtained at the time of survey. Patient-reported outcomes were elicited to assess physical, psychological, social, and spiritual functioning as well as overall HRQOL. A self-administered survey that included the measured outcomes was mailed with an informed consent form and postage-paid return envelope.
Measurement of Physical Health
Fatigue was measured by the Functional Assessment of Cancer Therapy Fatigue Subscale (FACT-F), a 13-item instrument [25]. Attention function was measured using the Attention Function Index (AFI) questionnaire to measure participants’ self-reported effectiveness in cognitive functioning [26]. The AFI is a 13-item questionnaire that assesses perceived changes in attention, working memory, and higher-level executive functions needed to set, plan, and execute goals and tasks of daily living. It has been shown to have consistent validity and reliability, with an internal consistency co-efficient of 0.92 (Cronbach’s α) in a sample of women with breast cancer [26].
Measurement of Psychological Indices (depression and trait anxiety)
Depression was measured by the CES-D, which is a summated 20-item scale that measures the presence and severity of depressive symptoms in both clinical and general populations [27]. Trait anxiety was measured using the State-Trait Anxiety Inventory (STAI) that includes 40 items [28].
Measurement of Exercise
Exercise as a form of physical activity was measured with a binary indicator of having regularly performed any of the following activities over the past 3 months: playing strenuous racquet sports (singles tennis, paddleball, etc.), playing other strenuous sports (basketball, soccer, or other sports involving running), riding a bicycle, swimming or walking, and or running/jogging in a physical activity program.
Statistical Analysis
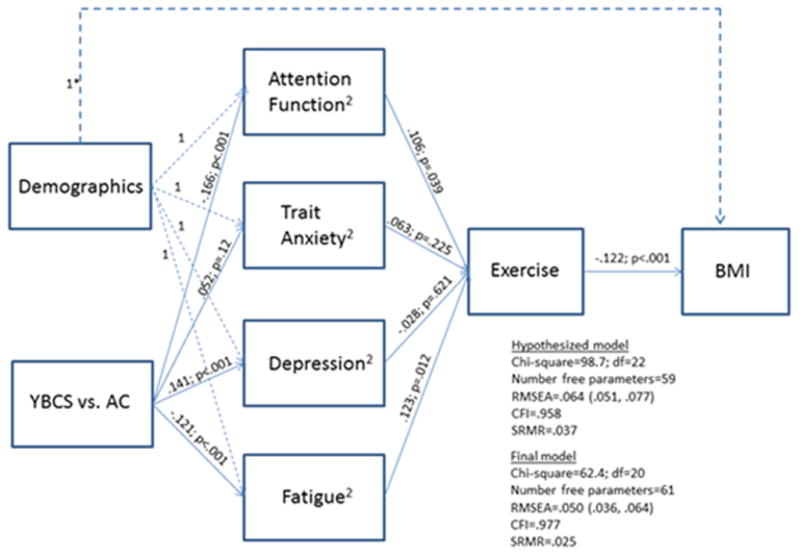
SEM was used to test a hypothesized path model of associations among a set of self-reported outcomes on a sample of YBCS and AC. Endogenous measures (i.e., dependent variables) included attention function, trait anxiety, depression, fatigue, exercise, and BMI. Exogenous variables (i.e., independent variables) included various demographic characteristics and a group variable indicating YBCS versus AC. Mplus software [29] was used to evaluate model fit, estimate and test path coefficients arising from structural equations, and estimate and test indirect and total effects. A goodness-of-fit chi-square p-value < 0.05 indicated statistically significant deviation from good model fit. However, large sample sizes (as in this study) have high power to detect minor deviations from good fit; therefore, greater emphasis was placed on fit indices that are less dependent on sample size. Adequate model fit was defined as comparative fit index (CFI) > .95, root mean square error of approximation (RMSEA) < .06, and standardized root mean square residual (SRMR) <.08, according to Hu and Bentler [30]. The covariance matrix used for analysis was based upon all available data from all subjects. The final model for our data is shown in Figure 1. The only (minor) differences between the initial hypothesized model and the final model were that modification indices suggested “freeing” the married and education paths to BMI, that is, not specifying any expected values for these variables.
Figure 1.
Results
Table 1 contains differences among measured variables for all women in the sample (YBCS and AC). Average age was significantly lower among the YBCS compared to AC (p=0.0027). In addition, significantly fewer YBCS had a college education compared to AC (p=.0020). Approximately 1/2 reported having incomes greater than $75,000 in both groups, with no difference between the groups at any income level. About 4/5 reported being married in both groups. The unadjusted p value for exercise was 0.019, with approximately 3/4 of the YBCS compared to 2/3 AC reporting exercising regularly over the past 3 months at the time of the survey. Approximately 2/3 also reported having an occupation categorized as manager/proprietor/clerical or professional according to the Barona scale [31]. These occupation distributions varied significantly according to YBCS and AC, with larger and smaller proportions of YBCS having incomes >$75,000 and between $30,000 and $75,000, respectively (p=.0321). Average BMI for both groups fell in the overweight category with no significant difference. The unadjusted p values for state anxiety, fatigue, and attention function indicated more anxiety symptoms, more fatigue, and worse attention function among YBCS compared to AC.
Table 1.
Descriptive statistics by group N=895
| Variable | Young BCS (N=498) | Acquaintance control (N=397) | p-value* |
|---|---|---|---|
| Age, mean (SD) | 45.3 (4.7) | 46.6 (7.1) | .0027 |
| Years of education >=16, N (%) | 217 (43.7) | 209 (54.2) | .0020 |
| Income, N (%) | .1141 | ||
| <$30,000 | 48 (9.8) | 37 (9.5) | |
| $30,000–$75,000 | 172 (35.3) | 163 (42.0) | |
| >$75,000 | 268 (54.9) | 188 (48.5) | |
| Occupation, N (%) | |||
| Semi-skilled/skilled | 82 (16.5) | 44 (4.9) | .0321 |
| Not in labor force | 73 (14.7) | 81 (20.5) | |
| Manager/proprietor/clerical | 185 (37.2) | 146 (37.0) | |
| Professional | 157 (31.6) | 124 (31.4) | |
| Currently married, N (%) | 412 (83.2) | 318 (80.5) | .2927 |
| Attention function, mean (SD) | 6.6 (1.8) | 7.1 (1.6) | <.0001 |
| Exercise, N (%) | 356 (71.5) | 254 (64.1) | .0191 |
| Trait anxiety, mean (SD) | 36.4 (10.2) | 35.4 (10.2) | .0191 |
| Depression, mean (SD) | 10.9 (9.5) | 8.4 (8.6) | .1427 |
| Fatigue, mean (SD) | 39.0 (10.4) | 41.3 (9.3) | .0007 |
| BMI, mean (SD) | 27.9 (6.2) | 27.8 (7.1) | .8600 |
Table 1 provides descriptive statistics for the sample. The relationships in the hypothesized model, including the group comparisons on attention function and psychological scale scores adjusted for demographics, are tested and shown in Figure 1.
Comparisons for continuous variables were done using t-test; comparisons on categorical variables were done using chi-square test.
Figure-1 shows the results of the tests for the hypothesized relationships, including the group comparisons (YBCS vs. AC) for attention function and psychological scale scores adjusted for demographics. Standardized beta regression coefficients (stb) are shown with associated p-values.
The hypothesized model showed reasonable goodness of fit, with RMSEA=.06, CFI=.96 and SRMR=.04, and it had a chi-square of 98.7 (22 df) and 59 free parameters. The final modified model contained only two additional paths that were allowed to be estimated based on modification indices and clinical relevance. The final modified model had a chi-square of 62.4 (20 df) and 61 free parameters, p<.0001. Thus, as expected, the large sample size resulted in a statistically significant detection of model misfit. More importantly, the goodness of fit indices indicated a good fitting model: RMSEA=.05, CFI=.98, and SRMR=.03. In the modified model, parameter estimates revealed that, compared to AC, YBCS reported significantly worse attention function (stb=−0.17, p<.001), more depressive symptoms (stb=0.14, p<.001), and more fatigue (stb=−0.12, p<.001). Better attention function (stb=0.11, p=.039) and less fatigue (stb=0.12, p=.012) were significantly associated with a higher likelihood of regularly exercising in the past 3 months, and having regularly exercised in the past 3 months (stb=−0.12, p<.001), being currently married (stb=−0.14, p=.001), and having education>=16 years (stb=−0.16, p<.001) were all significantly associated with a lower BMI.
Among the demographic variables, there were significant relationships between income, education, and occupation, respectively, and attention function, trait anxiety, depression, and fatigue. Compared to subjects (YBCS and AC) with an income greater than $75,000, those below $30,000 experienced poorer attention (standardized beta regression coefficient [stb]= −0.13, p=.002) and more symptoms of anxiety (stb=0.18, p=<.001), depression (stb=0.21, p<.001), and fatigue (stb=−0.16, p=<.001). Similarly, those with incomes between $30,000 and $75,000 experienced poorer attention (stb=−0.11, p=.002) and more symptoms of anxiety (stb=0.11, p=.003), depression (stb=0.11, p=.002), and fatigue (p=−0.11, p=.002). In addition, those who were not in the labor force, compared to professionals, experienced poorer attention (stb=−0.09, p=.027) and more symptoms of anxiety (stb=0.08, p=.048), depression (stb=0.12, p=.003), and fatigue (stb=−0.14, p=.002). Finally, those with a college degree, compared to those without, had fewer symptoms of anxiety (stb=−0.08, p=.027), depression (stb=−0.10, p=.011), and fatigue (stb=0.08, p=.043).
Indirect effects of group (YBCS vs. AC) on exercise and group on BMI were also investigated (Table 2). The total indirect effects were significant for each of the two sets of paths (p<.012). Individual pieces of the indirect effects showed that group had a significant indirect effect on exercise via attention function (p=.047) and fatigue (p=.022). Group had a marginal indirect effect on BMI via attention function and exercise (p=.08) and fatigue and exercise (p=.064).
Table 2.
Indirect effects from group to exercise and BMI.
| Effect | ||
|---|---|---|
| Z statistic | P-value | |
| Group1 to Exercise | ||
| Total indirect | −3.77 | <.001 |
| Group --> Attention Function --> Exercise | −1.98 | .047 |
| Group --> Trait Anxiety --> Exercise | 0.91 | .365 |
| Group --> Depression --> Exercise | −0.49 | .622 |
| Group --> Fatigue --> Exercise | −2.29 | .022 |
| Group1 to BMI | ||
| Total indirect | 2.52 | .012 |
| Group --> Attention Function --> Exercise--> | 1.75 | .080 |
| Group --> Trait Anxiety --> Exercise--> BMI | −0.87 | .387 |
| Group --> Depression --> Exercise--> BMI | 0.49 | .627 |
| Group --> Fatigue --> Exercise--> BMI | 1.85 | .064 |
Group indicates group membership: Young breast cancer survivor group vs. acquaintance controls.
Discussion
To our knowledge this is the first study conducted in the late survivorship period to examine the specific relationships of breast cancer-associated attention-function deficits to exercise and BMI, and to simultaneously test a set of hypothesized relationships in an SEM path model by comparing YBCS to AC. We were able to confirm our first hypothesis that YBCS had worse attention function than AC as well as more trait anxiety, depression, and fatigue after adjusting for demographic variables. We were also able to confirm our second and novel hypothesis that attention function was positively associated with exercise after adjusting for trait anxiety, depression, and fatigue. And finally, our third hypothesis was also confirmed, namely, that more exercise was associated with lower BMI. Therefore, our final model demonstrated that, after adjusting for co-variates, significant relationships remained among attention function, exercise, and BMI. These hypotheses were tested in the context of an SEM model that fit the data very well.
The cognitive deficits experienced by YBCS have been previously shown [32]. In addition, we have recently shown that YBCS report poorer HRQOL, including worse attention function, compared to older breast cancer survivors who were diagnosed between 55–70 years of age (in press). This may be because YBCS are more likely than older survivors to be employed and have family responsibilities such as caring for minor children at home, all of which can lead to competing time demands that can negatively impact HRQOL [33]. This time constraint may further strain the cognitive reserve of YBCS, leading to poorer attention function, which can also further negatively impact self-regulatory capacity and can lead to lower exercise behavior [34–35]. Cognition involves numerous functional domains such as intelligence, language, memory, attention, and executive function. Attention and executive functions are critical for organization and task efficiency. Daily activities at work and home need intact cognitive organizational circuitry for completion of tasks in a timely fashion. Problems with attention and executive functions can negatively impact incorporation of both simple and complex tasks throughout everyday living. Daily physical activity including exercise, which is a planned, repetitive, and structured event, needs careful planning and attention to “fit it in” to one’s life. The ability to plan for incorporating exercise into a daily routine is probably more important for YBCS than AC or older survivors because of higher strain on cognitive reserve. This study suggests that healthcare providers need to pay particularly close attention to YBCS who have lower attention function since they may need help with planning for exercise in their lives.
Our study showed greater psychological distress among YBCS than AC. A recent review indicated YBCS have poorer HRQOL, higher psychosocial distress associated with premature menopause and infertility issues, along with greater weight gain compared to older survivors [24]. Increased caloric intake was not observed among these survivors and not thought to be a contributor towards this energy imbalance. Increasing physical activity can not only mitigate the weight gain but can also help in decreasing psychological distress. Based on our study, innovative strategies such as cognitive retraining in addition to behavioral interventions may lead to increased physical activity initiation and adherence, especially for YBCS.
Our results are similar to those of the Childhood Cancer Survivor Study (CCSS), which showed that long-term adult survivors of childhood cancers who had neurocognitive deficits in task efficiency were less likely to meet the Centers for Disease Control’s recommendations for weekly physical activity [36]. In the CCSS study, the instrument used to measure cognition was the CCSS-NCQ, a self-report and validated measure of neurocognition. This instrument measured 4 main domains, namely task efficiency, memory, organization, and emotional regulation. The task-efficiency construct is a measure of attention, similar to the AFI scale used in our study. It is worth noting that cancer-associated cognitive deficits in attention or executive functioning can also impact other health behaviors that may influence overall BMI and HRQOL. For example, the CCSS study also showed that adult survivors of childhood cancers who had attention-function deficits were more likely to smoke than those without attention deficits [37]. Therefore, attention-function deficits may impact multiple health behaviors such as diet, exercise, and smoking that can all impact BMI and HRQOL.
In this study, YBCS had a significantly higher likelihood of engaging in regular exercise (odds ratio=1.40, p value=0.019) than AC; however, it should be noted that we purposely did not hypothesize a direct and unadjusted effect of group on exercise in the SEM path model because we hypothesized that the scientifically sensible path of influence was that group had an effect on attention function and other psychological variables that in turn had effects on exercise. Our hypothesis received support as indicated by significant indirect effects from group to exercise through attention function and psychological variables of fatigue, depression, and trait anxiety.
Our third hypothesis involved the relationship between exercise and BMI. Exercise explained a significant amount of the variance on BMI. As demonstrated in previous studies, [38] we found that exercise was inversely related to BMI for both YBCS and AC. For the interested reader, there was no significant bivariate (unadjusted) mean difference on BMI between YBCS and AC. However, we purposely did not include that direct path in our model because we hypothesized a scientifically sensible path of influence from group to BMI. Specifically, our hypothesis of an indirect relationship between group and BMI through attention function and psychological mediators, as well as exercise, was confirmed. However, it should be noted that the indirect effect from group to BMI was significant only when considering the total indirect effect from group to BMI through the combined set of attention-function and psychological mediators (anxiety, depression, and fatigue), as well as through exercise. The specific indirect effects from group to BMI through mediators (attention function, anxiety, depression, or fatigue), were marginally significant but only through attention function and exercise and through fatigue and exercise. Thus, it may be important to consider the complex combined effect of attention function and fatigue (adjusted for depression and anxiety) on exercise and, in turn, the effect of exercise on BMI when attempting to understand the relationship between survivorship and BMI.
Our study had several limitations relative to generalizing these findings. The cross-sectional data collection and non-experimental design of the study make it impossible to verify the causal directionality of these relationships. It is unclear if exercise improves neurocognitive functioning such as attention in YBCS or if the survivors with poorer attention function have difficulty managing their daily activities to the extent that they cannot incorporate exercise in their busy lives. Certainly having good attention function can help individuals with time management skills so they can accommodate exercise habits. It seems reasonable from the final theoretical model that attention function would predict exercise, but only a longitudinal study could clearly address this issue.
The accuracy of self-report is frequently discussed. However, reporting of attention function is not likely to be biased in a negative direction. That is, women would not be likely to report decreased attention function if it wasn’t a problem. There were no objective assessments of physical activity, which was measured as self-reported exercise since this was a cross-sectional, observational study. Although self-report may not give a totally accurate assessment of physical activity, it is feasible and inexpensive. We recognize that no assessment for physical activity is perfect and, since it was not a primary outcome in our study, we kept its assessment brief and did not use established self-measure instruments. We acknowledge that we may have not captured light-to-moderate physical-activity, which may be more prevalent among YBCS than more strenuous exercise; therefore, exercise may be underreported in our study. However, strenuous exercise is a more memorable activity and easy to recall, thus, less likely to involve recall bias. Moreover, we used a binary variable (Yes/No) for any exercise in the previous 3 months. One would assume that this was a broad assessment that did not take into account duration, intensity, frequency, and physical domain of exercise, yet we saw greater variability in exercise among YCBS than AC. Assessment of exercise over 3 months instead of a whole year or longer also meant that the time interval was less prone to recall bias, but still long enough to account for any temporary changes in lifestyle due to external causes. BMI was also calculated by self-reported height and weight at time of data collection. Self-report may tend to under-report actual weight for those with higher BMI. This potential systematic bias would probably have little effect on our findings because this bias would likely be similar for YBCS and AC. Furthermore, the bias might slightly decrease the variance of BMI, but this would tend to conservatively dampen rather than strengthen the relationship between exercise and BMI in our study.
Another limitation of our study is that less than 50% of the AC approached consented to the study, but the scores of our AC group for psychological variables of trait anxiety, depression, and fatigue were similar to the population norms. Our AC participation rate is comparable to the general rate of enrollment in current epidemiologic studies involving healthy controls [39]. Our study involved a fairly homogeneous cohort of women enrolled on ECOG trials, which typically include mostly Caucasians and women of a higher socio-economic class. Although we adjusted for demographic variables, self-reported outcomes of exercise or physical activity and other HRQOL variables are worse for ethnically underrepresented women following breast-cancer treatment, which could potentially further strengthen our model [40]. Lastly, we did not include measurements of other health-behaviors such as diet, smoking, and alcohol intake, all of which can influence exercise and BMI.
In conclusion, we used SEM to test a unique and novel hypothesis about relationships among attention function, exercise, and BMI in a large study of young breast cancer survivors in the late survivorship period, comparing them to acquaintance controls. Interventions among BCS aimed at enhancing attention function could potentially increase adherence to physical activity, including exercise, and decrease BMI, leading to a decrease in breast cancer recurrence and cardiovascular morbidities and enhancement of HRQOL. A longitudinal, prospective study to look at the association of cognitive deficits measured both objectively and by self-report with physical activity and other health behaviors is planned to further confirm our findings.
Acknowledgments
This study was conducted by the Eastern Cooperative Oncology Group (Robert L. Comis, M.D.) and supported in part by Public Health Service Grants, CA37403, CA49883, CA17145, CA86726, and by the National Cancer Institute Division of Cancer Prevention, National Institutes of Health, and the Department of Health and Human Services. Its content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute. It was also funded by American Cancer Society Grant-RSGPB-04-089-01 PBP to Dr. Victoria Champion. Dr. Pradhan is a postdoctoral fellow in Training in Behavioral Oncology and Cancer Control program supported by the National Cancer Institute of the National Institutes of Health under Award Number R25CA117865.
References
- 1.Howlader NNA, Krapcho M, Garshell J, Neyman N, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Cho H, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA, editors. SEER Cancer Statistics Review, 1975–2010. 2013 Available from: http://seer.cancer.gov/csr/1975_2010/
- 2.Elme A, et al. Obesity and physical inactivity are related to impaired physical health of breast cancer survivors. Anticancer Res. 2013;33(4):1595–602. [PubMed] [Google Scholar]
- 3.Vance V, et al. Weight gain in breast cancer survivors: prevalence, pattern and health consequences. Obes Rev. 2011;12(4):282–94. doi: 10.1111/j.1467-789X.2010.00805.x. [DOI] [PubMed] [Google Scholar]
- 4.Zhao G, et al. Trends in modifiable lifestyle-related risk factors following diagnosis in breast cancer survivors. Journal of Cancer Survivorship. 2013:1–7. doi: 10.1007/s11764-013-0295-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nichols HB, et al. Body mass index before and after breast cancer diagnosis: associations with all-cause, breast cancer, and cardiovascular disease mortality. Cancer Epidemiol Biomarkers Prev. 2009;18(5):1403–9. doi: 10.1158/1055-9965.EPI-08-1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blanchard CM, Stein K, Courneya KS. Body mass index, physical activity, and health-related quality of life in cancer survivors. Med Sci Sports Exerc. 2010;42(4):665–71. doi: 10.1249/MSS.0b013e3181bdc685. [DOI] [PubMed] [Google Scholar]
- 7.Courneya KS, et al. Associations among exercise, body weight, and quality of life in a population-based sample of endometrial cancer survivors. Gynecol Oncol. 2005;97(2):422–30. doi: 10.1016/j.ygyno.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 8.Irwin ML, et al. Physical activity levels among breast cancer survivors. Med Sci Sports Exerc. 2004;36(9):1484–91. [PMC free article] [PubMed] [Google Scholar]
- 9.Inoue-Choi M, et al. Adherence to the World Cancer Research Fund/American Institute for Cancer Research recommendations for cancer prevention is associated with better health-related quality of life among elderly female cancer survivors. J Clin Oncol. 2013;31(14):1758–66. doi: 10.1200/JCO.2012.45.4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inoue-Choi M, Robien K, Lazovich D. Adherence to the WCRF/AICR guidelines for cancer prevention is associated with lower mortality among older female cancer survivors. Cancer Epidemiol Biomarkers Prev. 2013;22(5):792–802. doi: 10.1158/1055-9965.EPI-13-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holick CN, et al. Physical activity and survival after diagnosis of invasive breast cancer. Cancer Epidemiol Biomarkers Prev. 2008;17(2):379–86. doi: 10.1158/1055-9965.EPI-07-0771. [DOI] [PubMed] [Google Scholar]
- 12.Irwin ML, et al. Randomized controlled trial of aerobic exercise on insulin and insulin-like growth factors in breast cancer survivors: the Yale Exercise and Survivorship study. Cancer Epidemiol Biomarkers Prev. 2009;18(1):306–13. doi: 10.1158/1055-9965.EPI-08-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mosca L, et al. Effectiveness-based guidelines for the prevention of cardiovascular disease in women--2011 update: a guideline from the american heart association. Circulation. 2011;123(11):1243–62. doi: 10.1161/CIR.0b013e31820faaf8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bellizzi KM, et al. Health behaviors of cancer survivors: examining opportunities for cancer control intervention. J Clin Oncol. 2005;23(34):8884–93. doi: 10.1200/JCO.2005.02.2343. [DOI] [PubMed] [Google Scholar]
- 15.Hong S, et al. Correlates of physical activity level in breast cancer survivors participating in the Women’s Healthy Eating and Living (WHEL) Study. Breast Cancer Res Treat. 2007;101(2):225–32. doi: 10.1007/s10549-006-9284-y. [DOI] [PubMed] [Google Scholar]
- 16.Blaney JM, et al. Cancer survivors’ exercise barriers, facilitators and preferences in the context of fatigue, quality of life and physical activity participation: a questionnaire-survey. Psychooncology. 2013;22(1):186–94. doi: 10.1002/pon.2072. [DOI] [PubMed] [Google Scholar]
- 17.Ottenbacher AJ, et al. Exercise among breast and prostate cancer survivors--what are their barriers? J Cancer Surviv. 2011;5(4):413–9. doi: 10.1007/s11764-011-0184-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Charlier C, et al. The contribution of general and cancer-related variables in explaining physical activity in a breast cancer population 3 weeks to 6 months post-treatment. Psychooncology. 2013;22(1):203–11. doi: 10.1002/pon.2079. [DOI] [PubMed] [Google Scholar]
- 19.Anderson-Hanley C, et al. Neuropsychological effects of treatments for adults with cancer: a meta-analysis and review of the literature. J Int Neuropsychol Soc. 2003;9(7):967–82. doi: 10.1017/S1355617703970019. [DOI] [PubMed] [Google Scholar]
- 20.Correa DD, Ahles TA. Neurocognitive changes in cancer survivors. Cancer J. 2008;14(6):396–400. doi: 10.1097/PPO.0b013e31818d8769. [DOI] [PubMed] [Google Scholar]
- 21.McDonald BC, et al. Frontal gray matter reduction after breast cancer chemotherapy and association with executive symptoms: a replication and extension study. Brain Behav Immun. 2013;30(Suppl):S117–25. doi: 10.1016/j.bbi.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kesler SR, Kent JS, O’Hara R. Prefrontal cortex and executive function impairments in primary breast cancer. Arch Neurol. 2011;68(11):1447–53. doi: 10.1001/archneurol.2011.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Artinian NT, et al. Interventions to promote physical activity and dietary lifestyle changes for cardiovascular risk factor reduction in adults: a scientific statement from the American Heart Association. Circulation. 2010;122(4):406–41. doi: 10.1161/CIR.0b013e3181e8edf1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Howard-Anderson J, et al. Quality of life, fertility concerns, and behavioral health outcomes in younger breast cancer survivors: a systematic review. J Natl Cancer Inst. 2012;104(5):386–405. doi: 10.1093/jnci/djr541. [DOI] [PubMed] [Google Scholar]
- 25.Yellen SB, et al. Measuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. J Pain Symptom Manage. 1997;13(2):63–74. doi: 10.1016/s0885-3924(96)00274-6. [DOI] [PubMed] [Google Scholar]
- 26.Cimprich B, Visovatti M, Ronis DL. The Attentional Function Index--a self-report cognitive measure. Psychooncology. 2011;20(2):194–202. doi: 10.1002/pon.1729. [DOI] [PubMed] [Google Scholar]
- 27.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Measure. 1997;1(3):385–401. [Google Scholar]
- 28.Spielberger CDGR, Lushene R, Vagg P, Jacobs G, editors. Manual for the State-Trait Anxiety Inventory (Form Y) IV. Consulting Psychologists Press Inc; Palo Alto, CA: 1983. [Google Scholar]
- 29.Muthen LK, Muthen BO. Mplus usesr’s guide fifth edition. Muthen & Muthen; Los angeles: 1998–2007. [Google Scholar]
- 30.Hu LT, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: conventional criteria versus new alternatives. Seminars in Adolescent Medicine. 1999;6(1):1–55. [Google Scholar]
- 31.Spreen O. In: A Compendium of Neuropsychological tests: Adminstrations, Norms, and Commentary. 2. Spreen O, editor. New York, New York: Oxford University Press; 1998. [Google Scholar]
- 32.Brezden CB, et al. Cognitive function in breast cancer patients receiving adjuvant chemotherapy. J Clin Oncol. 2000;18(14):2695–701. doi: 10.1200/JCO.2000.18.14.2695. [DOI] [PubMed] [Google Scholar]
- 33.Bloom JR, et al. Then and now: quality of life of young breast cancer survivors. Psychooncology. 2004;13(3):147–60. doi: 10.1002/pon.794. [DOI] [PubMed] [Google Scholar]
- 34.Gaillard AW. Comparing the concepts of mental load and stress. Ergonomics. 1993;36(9):991–1005. doi: 10.1080/00140139308967972. [DOI] [PubMed] [Google Scholar]
- 35.Arndt J, et al. Broadening the cancer and cognition landscape: the role of self-regulatory challenges. Psychooncology. 2014;23(1):1–8. doi: 10.1002/pon.3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krull KR, et al. Neurocognitive functioning and health-related behaviours in adult survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. Eur J Cancer. 2011;47(9):1380–8. doi: 10.1016/j.ejca.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kahalley LS, et al. Attentional and executive dysfunction as predictors of smoking within the Childhood Cancer Survivor Study cohort. Nicotine Tob Res. 2010;12(4):344–54. doi: 10.1093/ntr/ntq004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shaw K, et al. Exercise for overweight or obesity. Cochrane Database Syst Rev. 2006;(4):CD003817. doi: 10.1002/14651858.CD003817.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Galea S, Tracy M. Participation rates in epidemiologic studies. Ann Epidemiol. 2007;17(9):643–53. doi: 10.1016/j.annepidem.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 40.Paxton RJ, et al. Associations among physical activity, body mass index, and health-related quality of life by race/ethnicity in a diverse sample of breast cancer survivors. Cancer. 2012;118(16):4024–31. doi: 10.1002/cncr.27389. [DOI] [PMC free article] [PubMed] [Google Scholar]


