Abstract
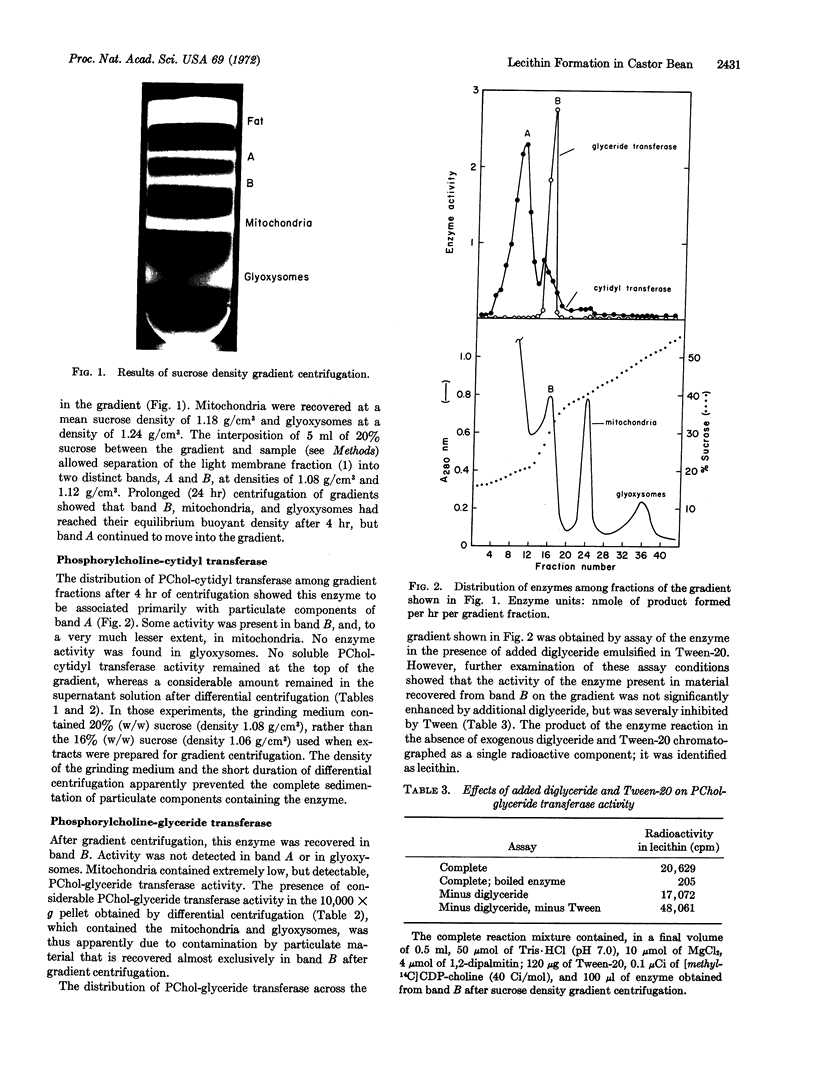
The occurrence and subcellular distribution of enzymes of the cytidine diphosphate choline pathway of lecithin synthesis have been examined. Choline kinase (EC 2.7.1.32) was completely soluble, while phosphorylcholine-cytidyl transferase (EC 2.7.7.15) and phosphorylcholine-glyceride transferase (EC 2.7.8.2) were associated with particulate fractions. Although components sedimenting at 10,000 to 100,000 × g contained both enzymes, phosphorylcholine-cytidyl transferase and particularly phosphorylcholine-glyceride transferase were present in the 10,000 × g pellet, which contained the major organelles, mitochondria, and glyoxysomes. When the crude homogenate was centrifuged on a sucrose density gradient, four major bands of particulate protein were recovered. A band at density 1.24 g/cm3 contained the glyoxysomes and was devoid of phosphorylcholine-cytidyl transferase and phosphorylcholine-glyceride transferase activity. Enzyme activity was barely detectable in the mitochondria, at density 1.18 g/cm2. Phosphorylcholine-glyceride transferase was found almost exclusively in a sharp band at density 1.12 g/cm3, and phosphorylcholinecytidyl transferase was found in the uppermost band at density 1.08 g/cm3. Thus, for the synthesis of lecithin in their membranes, the glyoxysomes and mitochondria depend on enzymes elsewhere in the cell; the final two steps in lecithin formation occur, apparently exclusively, in separate particulate cell components.
Keywords: lecithin, membranes, glyoxysomes, mitochondria, endoplasmic reticulum
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BORKENHAGEN L. F., KENNEDY E. P. The enzymatic synthesis of cytidine diphosphate choline. J Biol Chem. 1957 Aug;227(2):951–962. [PubMed] [Google Scholar]
- Bracker C. E., Grove S. N. Continuity between cytoplasmic endomembranes and outer mitochondrial membranes in fungi. Protoplasma. 1971;73(1):15–34. doi: 10.1007/BF01286408. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Kartenbeck J. Outer mitochondrial membrane continuous with endoplasmic reticulum. Protoplasma. 1971;73(1):35–41. doi: 10.1007/BF01286409. [DOI] [PubMed] [Google Scholar]
- Johnson K. D., Kende H. Hormonal Control of Lecithin Synthesis in Barley Aleurone Cells: Regulation of the CDP-Choline Pathway by Gibberellin. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2674–2677. doi: 10.1073/pnas.68.11.2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungalwala F. B., Dawson R. M. Phospholipid synthesis and exchange in isolated liver cells. Biochem J. 1970 Apr;117(3):481–490. doi: 10.1042/bj1170481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungalwala F. B., Dawson R. M. The origin of mitochondrial phosphatidylcholine within the liver cell. Eur J Biochem. 1970 Feb;12(2):399–402. doi: 10.1111/j.1432-1033.1970.tb00865.x. [DOI] [PubMed] [Google Scholar]
- KENNEDY E. P. Biosynthesis of phospholipides. Fed Proc. 1957 Sep;16(3):847–853. [PubMed] [Google Scholar]
- Kaiser W., Bygrave F. L. Incorporation of choline into the outer and inner membranes of isolated rat liver mitochondria. Eur J Biochem. 1968 May;4(4):582–585. doi: 10.1111/j.1432-1033.1968.tb00253.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- McCaman R. E., Cook K. Intermediary metabolism of phospholipids in brain tissue. 3. Phosphocholine-glyceride transferase. J Biol Chem. 1966 Jul 25;241(14):3390–3394. [PubMed] [Google Scholar]
- McMurray W. C., Dawson R. M. Phospholipid exchange reactions within the liver cell. Biochem J. 1969 Mar;112(1):91–108. doi: 10.1042/bj1120091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morré D. J., Merritt W. D., Lembi C. A. Connections between mitochondria and endoplasmic reticulum in rat liver and onion stem. Protoplasma. 1971;73(1):43–49. doi: 10.1007/BF01286410. [DOI] [PubMed] [Google Scholar]
- Morré D. J., Nyquist S., Rivera E. Lecithin Biosynthetic Enzymes of Onion Stem and the Distribution of Phosphorylcholine-Cytidyl Transferase among Cell Fractions. Plant Physiol. 1970 Jun;45(6):800–804. doi: 10.1104/pp.45.6.800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PHILLIPS A. H., LANGDON R. G. Hepatic triphosphopyridine nucleotide-cytochrome c reductase: isolation, characterization, and kinetic studies. J Biol Chem. 1962 Aug;237:2652–2660. [PubMed] [Google Scholar]
- Ruby J. R., Dyer R. F., Skalko R. G. Continuities between mitochondria and endoplasmic reticulum in the mammalian ovary. Z Zellforsch Mikrosk Anat. 1969;97(1):30–37. doi: 10.1007/BF00331868. [DOI] [PubMed] [Google Scholar]
- SCHNEIDER W. C. INTRACELLULAR DISTRIBUTION OF ENZYMES. XIII. ENZYMATIC SYNTHESIS OF DEOXYCYTIDINE DIPHOSPHATE CHOLINE AND LECITHIN IN RAT LIVER. J Biol Chem. 1963 Nov;238:3572–3578. [PubMed] [Google Scholar]
- Stoffel W., Schiefer H. G. Biosynthesis and composition of phosphatides in outer and inner mitochondrial membranes. Hoppe Seylers Z Physiol Chem. 1968 Aug;349(8):1017–1026. doi: 10.1515/bchm2.1968.349.2.1017. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Tolbert N. E., Gohlke A. F. Choline kinase and phosphorylcholine phosphatase in plants. Plant Physiol. 1966 Feb;41(2):307–312. doi: 10.1104/pp.41.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEISS S. B., SMITH S. W., KENNEDY E. P. The enzymatic formation of lecithin from cytidine diphosphate choline and D-1,2-diglyceride. J Biol Chem. 1958 Mar;231(1):53–64. [PubMed] [Google Scholar]
- WILGRAM G. F., KENNEDY E. P. INTRACELLULAR DISTRIBUTION OF SOME ENZYMES CATALYZING REACTIONS IN THE BIOSYNTHESIS OF COMPLEX LIPIDS. J Biol Chem. 1963 Aug;238:2615–2619. [PubMed] [Google Scholar]