Abstract
Sepsis is a complex medical condition characterized by a systemic inflammatory response in the setting of infection. We hypothesized that combining antibiotics plus an immunosuppressant would protect against the morbidity and mortality of polymicrobial sepsis in mice better than antibiotics alone. We used a murine cecal ligation and puncture model in which mice were treated either with imipenem plus cyclophosphamide or imipenem alone. Titration to a low cyclophosphamide dose revealed that combination therapy increased survival by 20% compared to imipenem alone (56% v. 36%, p < 0.001). To investigate the mechanism by which combination therapy did this, we reviewed quantitative and qualitative markers of the systemic immune response, end-organ damage, and the local immune response at the site of injury. Cyclophosphamide treatment was not associated with depletion of peripheral leukocytes or differences in pulmonary damage. However, mice that received combination therapy had higher plasma G-CSF levels than those treated with antibiotics alone. Additionally, mice treated with cyclophosphamide had higher levels of bacterial colonization in intestinal Peyer’s patch lymph nodes at 72 hours after the septic insult. Intraperitoneal macrophage phenotypes and phagocytosis activity did not differ between groups. We conclude that the inflammatory response plays a significant role in the mortality of polymicrobial sepsis and that the regulation of this element is both feasible and beneficial in this disease model.
Keywords: Severe sepsis, immunosuppression, immunomodulation, cecal ligation and puncture
Introduction
Sepsis remains a major problem in medicine, annually affecting approximately 750,000 patients and causing over 225,000 deaths per year in the United States (1). While its pathogenesis is incompletely understood, sepsis is thought to be an excessive, dysregulated host immune response to severe microbial infections. Under these extreme circumstances, elements of the body’s immune system that normally serve to protect against infection by destroying invading pathogens instead become “hyperactivated” and also destroy host tissues.
Despite decades of basic and clinical research, the only effective therapies for sepsis are anti-infective drugs and supportive care. Because current therapeutic paradigms have not substantially improved the clinical management of sepsis since the advent of antibiotics, effective therapies will likely require innovative approaches that radically depart from traditional strategies. The overarching hypothesis of our research is that sepsis consists of two pathogenic components, an invasive micro-organism and a dysregulated immune system. Consequently, an effective therapeutic strategy would have to target both the microbial pathogen and the pathological host immune response. Under this conceptual framework, a successful treatment regimen for sepsis would combine an anti-infective to control the microbial pathogen, with an immunosuppressant to control the pathological immune response.
Therefore, in this study, we specifically hypothesized that combination therapy consisting of an antibiotic plus an immunosuppressant would protect against the morbidity and mortality of polymicrobial sepsis in mice better than an antibiotic alone. Furthermore, we postulated that an immunosuppressant would protect against sepsis-induced morbidity by decreasing pro-inflammatory cytokine expression while promoting neutrophil activity.
To test our hypothesis, we utilized the murine cecal ligation and puncture (CLP) model, which features both a polymicrobial infection caused by the leakage of stool from the cecal puncture, and ischemic tissue damage from ligating the cecal segment of the large intestine. This method has long been considered the gold standard of modeling sepsis in mice (2). An outbred mouse strain was used to avoid producing genotype-specific results. To further strengthen the clinical relevance of the study, we used a treatment model in which mice did not receive any therapeutic intervention until after the initial septic insult. We began with titration of the cyclophosphamide (CyP) dose; once a positive survival benefit was observed, we investigated the mechanism of that survival advantage by examining the quantitative and qualitative immune response, end organ damage, and the local immune response at the site of injury.
Materials and Methods
Animals
All experimental procedures were done in full accordance with the policies of the Institutional Animal Care and Use Committee at UCSF. Male ICR outbred mice (Harlan Laboratories, Livermore, CA) greater than 5 weeks of age, weighing 25–30 grams and maintained under standard conditions were used in all experiments. All animals were housed in a specific pathogen free facility with no more than 5 mice per cage. Cages were cleaned, food and hydration were restocked, and animals were monitored for signs of fighting or other physical ailment on a daily basis.
Cecal Ligation and Puncture Model of Sepsis
Male ICR mice underwent cecal ligation and puncture (CLP) using a previously published CLP model of polymicrobial sepsis (3–4). Briefly, a midline incision was created through the skin and linea alba in isoflurane-anesthetized mice and the cecum identified. Approximately two-thirds of the cecum was ligated with 4-0 silk suture and a single puncture into the ligated portion made with an 18-gauge needle. A small amount of stool was extruded, 1mL of normal saline pre-warmed to 37.0°C was injected intraperitoneally for fluid resuscitation, and the abdomen was closed in two running layers using 4-0 silk suture. Another group of male ICR mice underwent a sham CLP operation in which the abdomen was opened, and the cecum identified and manipulated before being returned to the abdomen. At the end of the operation, all mice received a local anesthetic of subcutaneously injected 0.2 mL of bupivacaine hydrochloride (0.25%). At 12 and 18 hours post-operation, 0.05 mg/kg buprenorphine was injected subcutaneously for analgesia. From 12–24 hours postoperatively, mice were monitored every 15 minutes for activity and response to stimuli. If the animals demonstrated 4 consecutive evaluations of persistent and unimproved lethargy or moribund behavior, they were euthanized prior to the end of the study.
Cyclophosphamide Titration Experiments
Initial cyclophosphamide titrate experiments were performed in mice undergoing sham operations only to avoid confounding mortality added by CLP. Twenty-four hours after sham operation, selected doses (0–360 mg/kg) via two dosing regimens (single-dose and multiple-dose) of cyclophosphamide were injected intraperitoneally for 4–8 consecutive days beginning 24 hours after surgery. Per dosing regimen, 5 mice per treatment group were used. Serial complete blood counts (CBC) and absolute neutrophil counts (ANC) were obtained 12 hours after sham operation, and then every 24 hours for 28 days. Serial blood draws of 40 μl were obtained by retro-orbital capillary plexus puncture under isoflurane-induced anesthesia in alternating mice every other day to minimize the cumulative anemic effect. The neutrophil count was assessed to determine the lowest dose of cyclophosphamide required to achieve an ANC<100 cells/μl blood. The total dose of cyclophosphamide administered by single versus multiple dosing regimens was based on human studies. For severe multiple sclerosis, a total of 200 mg/kg CyP daily in four equal doses has been described (5). Published studies in mice show that a single 200 mg/kg dose of CyP readily induced neutropenia without significant mortality (6).
Imipenem and Cyclophosphamide Administration in CLP Model
After mice underwent CLP operation, they were given a broad-spectrum antibiotic (imipenem, 0.5 mg/mouse) 2 hours after surgery and then every 12 hours for 5 days. Due to the high mortality of the CLP model as well as planned serial euthanasia, a cohort of 8 to 24 mice were used per treatment group. From the previously obtained titration results, doses ranging from 10 mg/kg for 4 days (total dose 40 mg/kg) up to 20 mg/kg for 8 days (tota1 dose 60 mg/kg) were administered intraperitoneally beginning 24 hours after surgery. Serial CBC and ANC data were obtained 12 hours after CLP operation, and then every 24 hours for 28 days as described above without having to sacrifice animals. To obtain final survival effects of imipenem or imipenem with CyP, the final dosing regimen of 10 mg/kg for 6 days was used in a larger subset of animals (imipenem alone n= 83, imipenem + CyP n=69).
Specifically for cytokine, histology, MPO, and bacterial culture and colonization data, separate cohorts of animals in each treatment group (CLP operation followed by imipenem alone or imipenem with CyP were euthanized at 24 hour intervals up to 4 days post-operation. Five to ten animals were used per treatment group (details listed in Results and Figure Legends), and at the time of euthanasia, blood was collected via cardiac puncture and tissue samples from liver, spleen, and lymph nodes were obtained via open dissection of the abdominal cavity. All organs were flushed with 25°C normal saline before storage.
Cell Counts
Blood samples (40 μl) obtained as described above were anticoagulated with heparin (15 IU) and evaluated with a Hemavet 590 cell counter (Drew Scientific, Oxford, CT) according to the manufacturer’s instructions.
Blood Cultures
Two hours after mice underwent sham or CLP operation, mice were given imipenem (0.5 mg/mouse), cyclophosphamide (10 mg/kg/mouse), or saline vehicle. Animals were then euthanized 9 hours later and blood collected via cardiac puncture was used in serial dilutions and plated on blood agar and incubated at 37°C. Bacterial colony-forming units were counted every 24 hours for 4 days to assess bacterial burden.
Neutrophil Isolation
To isolate neutrophils, 300 μl of whole blood were layered onto 3 ml of Ficoll-Hypaque in a 15 ml Falcon tube (BD Falcon) and spun for 30 mins at 1,500 rpm using a Legend RT+ centrifuge (Sorvall). The pellet containing the polymorphonuclear cells (PMNs) and red blood cells was recovered. PMNs were separated from red blood cells via hypotonic lysis. These PMNs were then resuspended in HBSS solution. Half of these cells were incubated for 25 minutes at 25°C in a water bath with phorbol myristate acetate (PMA) added to a final concentration of 100ng/ml to activate the PMNs. The other half was kept at 4°C (unactivated batch). Both sets of cells were then used in the myeloperoxidase assay described below.
Lung Tissue Homogenization
Immediately following cardiac puncture, the left lung was isolated and weighed. The lung was then homogenized in 1 mL PBS/Complete Solution (made from 1 complete protease inhibitor tablet (Roche) in 50 mL PBS), using a PowerGen 700 (Fischer Scientific) for 5 seconds. The homogenate was centrifuged at 2000 rpm in a Legend RT+ centrifuge (Sorvall) for 10 minutes and the pellet discarded.
Myeloperoxidase Assay
190 μl of a standardized substrate was added to wells of a 96-well plate with 10 μl of lung homogenate (described above), 10 μl purified serum neutrophils (unactivated), or 10 μl purified serum neutrophils (activated with PMA as described above). End-point absorbance readings were taken at 460nm after 30 minutes incubation with a SpectraMAX Plus (Molecular Devices) absorbance reader. One unit of activity is defined as the ability to degrade 1 mole of hydrogen peroxide per minute at 25°C. Data were normalized to grams of lung tissue or μl of serum.
Lung Histology
The right lung was perfused with 10% formalin by injection into the right ventricle. The lungs were then inflated by installation of 10% formalin through the trachea using an 18-gauge angiocather. The lungs were then placed in 10% formalin for 24 hours. The lung tissue was subsequently transferred to 70% ethanol. After fixation, the right lung was stained with hematoxylin and eosin for histopathological analysis. The slides were then evaluated by a pathologist blinded to the study.
Cytokine Levels
Systemic cytokine profiles were analyzed to determine if differences existed when mice were treated with cyclophosphamide. Levels of IL-4, IL-6, IL-10, IL-13, IL-17, TNF-α, INF-γ, G-CSF, GM-CSF, and MIP-1β were checked at 48 hours post-CLP. Serum cytokine levels were measured using a flow-cytometry-based bead assay (BD, Franklin Lakes, New Jersey) per the manufacturer’s protocol.
Bacterial colonization
Samples of dissected intestinal Peyer’s patch lymph nodes, liver (0.5 mg), and spleen (0.1 mg) were collected at 48 and 72 hours after CLP from both treatment groups. Tissues were homogenized, plated on blood agar, and incubated under aerobic conditions for 24 hours at 37° C. A sham animal (abdomen opened and closed using the previously described technique) was used as a control for each experiment. After the incubation period, colonies were counted and colony-forming units per gram of tissue were calculated.
Intraperitoneal Macrophage Isolation
Cells were harvested via peritoneal lavage with Phosphate Buffered Saline (PBS) from mice peritoneal cavities at the time of euthanasia and washed with PBS. Cells were then incubated for 2 hours at 37°C in a humidified atmosphere containing 5% CO2. Non-adherent cells were then washed away and macrophages were quantified with a hemocytometer.
Arginase Assay
Intraperitoneal lavage (10 mL ice cold PBS) was performed on euthanized mice from both treatment groups at 48 and 72 hours post-CLP. Cells were counted, concentrations standardized, and then cells were lysed using Triton X-100 containing a protease inhibitor mixture. Arginase was activated with manganese cofactor, substrate (arginine) added, and reaction to the combination developed at 37°C for 1.5 hours. The reaction was then stopped with a 1:3:7 acid mixture (sulfuric acid: phosphoric acid: distilled H20). α-isonitrosopropiophenone was added to each of the samples, and absorbance was measured at 540 nm on a SpectraMAX Plus absorbance reader.
Candida albicans
A clinical isolate of Candida albicans was obtained from the laboratory of Dr. Suzanne Noble at UCSF and maintained at −80°C. The yeast cells were cultured in Yeast Extract Peptone Dextrose agar plates at 30°C for 3 days, washed in PBS, and quantified with a hemocytometer. The yeast were then heat killed at 65°C for 1 hour, diluted to a final concentration of 107 yeast particles/ml and stored at 4°C.
Phagocytic activity
Isolated macrophages were plated on four-well chamber slides (3x106 cells/ 400uL/ well; DMEM solution with 10% FBS and 1% Streptomycin; Lab-Tek®II Chamber Slide™) and incubated at 37°C within a humidified atmosphere of 5% CO2 for 24 hours. Following the incubation, 400uL (4x106) of heat killed yeast particles were added and incubated for 2 hours. The slides were then washed with PBS, fixed with methanol (Sigma), and stained with Giemsa solution (Sigma). Phagocytic activity index (percentage of cells that ingested yeast) and phagocytic index (number of yeast particles ingested per cell) were calculated by examining randomly selected sections on each slide under a 40X light microscope and counted by a laboratory assistant who was blinded to the study.
Statistical analysis
Survival was determined daily for 28 days after CLP or sham surgery. Differences in survival between the treatment groups were analyzed using a log-rank test. Data from peripheral cytokine assays were analyzed by unpaired t-tests. Bacterial colonization data were analyzed using Mann-Whitney tests. Phagocytosis and arginase activity were compared using unpaired t-tests.
Results
Cyclophosphamide Dose that Induces Neutropenia with an Acceptable Morbidity Profile in Sham-operated Mice
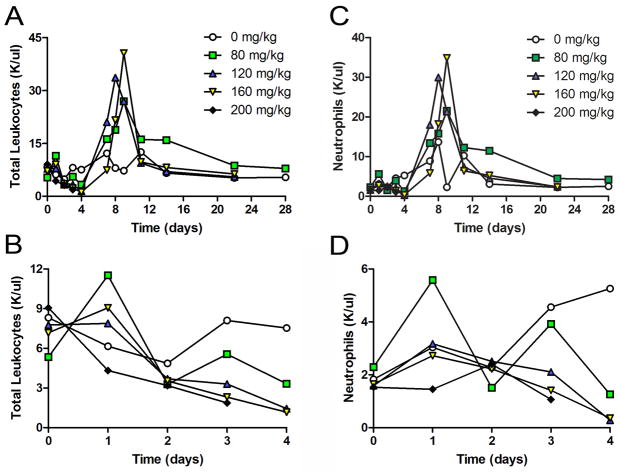
As expected, in sham-operated mice, cyclophosphamide induced relative leukopenia and neutropenia that were both dose- and time-dependent (Figure 1). The cell count nadirs occurred approximately 2–3 days after cyclophosphamide administration (days 4–5 after surgery), with the lowest mean neutrophil counts of 285–360 cells/μl observed in the 120 and 160 mg/kg groups. No animal developed an ANC of <100 cells/μl blood, the definition of absolute neutropenia in mice. Interestingly, and unlike in human studies, cyclophosphamide-induced neutropenia was rapidly followed by a spontaneous and robust neutrophilia, which peaked 7–8 days after cyclophosphamide administration. Total and absolute neutrophil counts returned to baseline after approximately 2 weeks.
Figure 1. Cyclophosphamide Dosage Effects on Cell Populations in Sham Operations.
Varying doses (0–200 mg/kg) of CyP or saline vehicle were injected intraperitoneally as single dose bolus 24 hours after sham surgery. There was no major difference in total leukocyte count amongst the doses (A). The expected neutropenia reached a nadir at days 4–6, which was followed by a spontaneous neutrophilia (B). The pattern of neutrophilia closely mirrored that observed for all white blood cell subsets (data not shown) (n=5 per group).
The injection of a single dose of cyclophosphamide produced considerable toxicity (Figure 2A). Whereas all of the treated mice lost 10–15% of their body weight over the first week, only the mice receiving >160 mg/kg of cyclophosphamide failed to regain the lost weight and demonstrated abnormal total weight gain for the 28-day experimental time period (Figure 2B). Importantly, the single injection of high-dose cyclophosphamide was fatal for the majority of animals, with an estimated LD50 ~140 mg/kg.
Figure 2. Effects of Single Dose Cyclophosphamide on Body Weight and Survival after Sham Operations.
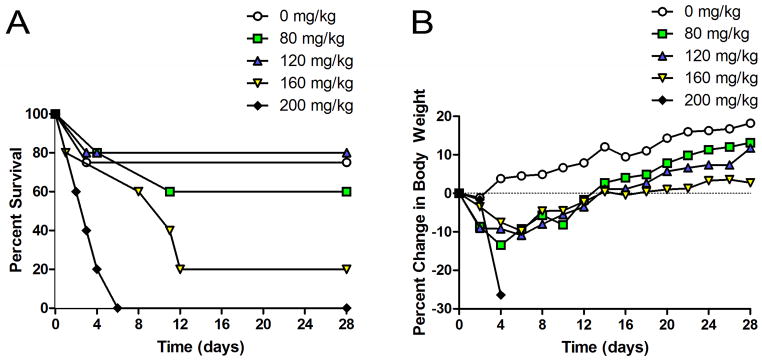
A single intraperitoneal bolus injection of CyP (0–200 mg/kg) or saline vehicle was given 24 hours after sham surgery. Single dose CyP yielded dose-dependent lethality (A) and weight loss (B) in mice that underwent sham surgery (n=5 mice per group).
Despite the lethality of these doses, they also failed to induce an absolute neutropenia. With the hope of reducing the treatment’s lethality while still inducing neutropenia, we next administered total doses of cyclophosphamide identical to those that proved lethal as bolus injections, only divided into four equal doses injected over consecutive days. Dividing the total dosage virtually eliminated the lethality of high-dose cyclophosphamide. These mice had a 80–100% overall survival rate that was not dose-dependent, despite our having administered total cyclophosphamide doses of 80–360 mg/kg for 4 days (Supplemental Figure 1A). Whereas all mice initially lost weight, their total weight gain over the 28-day study period was normal (Supplemental Figure 1B). All but the lowest dose regimen of cyclophosphamide (20 mg/kg/day for 4 days) induced a relative neutropenia in the mice (Supplemental Figure 1C).
Cyclophosphamide Dose that Induces Neutropenia with an Acceptable Morbidity Profile as Measured by 28-day Change in Body Weight and Survival in CLP-treated Mice
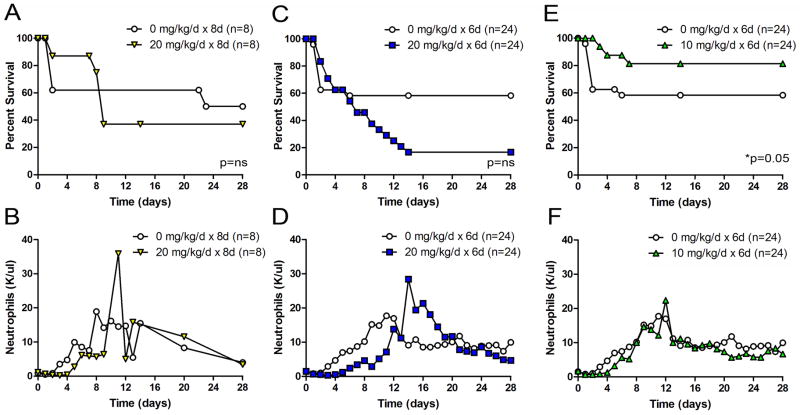
Mice that underwent CLP and imipenem treatment with cyclophosphamide showed a dose-dependent increase in survival. At the higher ends of the dosage spectrum (20 mg/kg for eight days – Figure 3A and 20 mg/kg for six days – Figure 3C), there was a trend toward increased mortality, although it did not achieve statistical significance. We discovered a treatment protocol (10 mg/kg for six days) that provided long-term protection against CLP-induced death (Figure 3E, *p=0.05). This treatment protocol was used for all further experiments. Importantly, this dose as well as the higher doses was too low to cause neutropenia (Figure 3B, 3D, and 3F) or the spontaneous neutrophilia that was usually observed after high dose single bolus cyclophosphamide treatment (Figure 1). In fact, neutrophil, eosinophil, monocyte, and lymphocyte counts were all equivalent, indicating that for peripheral blood cells, there was no evidence of leukocyte depletion by cyclophosphamide (data not shown). These findings suggested a very narrow therapeutic index by which cyclophosphamide confers its protective benefit. The increased morbidity associated with higher cyclophosphamide doses became irrelevant in this low-dose treatment model. These findings also suggested that quantitative depletion of neutrophils was not the mechanism by which cyclophosphamide protected these mice in the setting of severe sepsis.
Figure 3. Effect of multi-dose cyclophosphamide on survival and neutrophil counts after CLP.
Survival and neutrophil counts were observed in male ICR mice after CLP-induced sepsis for 28 days after treatment with imipenem and either saline vehicle (white circles) or Cyclophosphamide (non-circle, colored points). The cyclophosphamide-treated animals received 4–8 daily intraperitoneal injections of cyclophosphamide (10–20 mg/kg) after CLP via an 18g needle and 1 perforation. Low dose cyclophosphamide is associated with a survival advantage but no change in circulating neutrophils in mice that underwent CLP. There was a trend toward decreased survival in higher CyP dosing regiments (A and C), but there was significant increased survival with lower dose CyP (Part E, *p=0.05). Serial measurements of peripheral neutrophil counts were obtained. The data are cumulative of separate experiments (n=8–24 per group).
Combination Antibiotic and Immunosuppressant Therapy Results in Better Survival
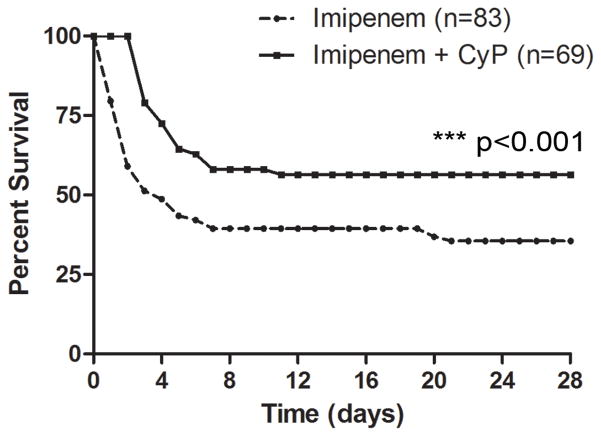
After titration of cyclophosphamide to a beneficial dose (10 mg/kg for six days), subsequent experiments examined the exact difference in survival rate for mice that received combination antibiotic and CyP therapy and those that received antibiotic therapy alone. Even with antibiotic treatment, mortality was approximately 60%, demonstrating the severe nature of this sepsis model, but remarkably, mice treated with cyclophosphamide and imipenem survived at a 20% greater rate than those that received only imipenem (28-day survival 56% vs 36%; Figure 4, ***P < 0.001, n= 83 control, n=69 treatment). This difference demonstrated that combination therapy was substantially protective.
Figure 4. Combination Therapy with Antibiotic and Cyclophosphamide Improves Survival in CLP Model.
Survival increases by 20% when cyclophosphamide is added to antibiotic treatment (56%) compared to imipenem alone (36%). n=83 control, n=69 treatment, ***p < 0.001.
Exclusion of a Potential Confounder: Cyclophosphamide lacks Antimicrobial Activity
Next, we investigated if the cyclophosphamide was working simply by acting as an antibiotic with direct bactericidal activity rather than through an immunosuppressive mechanism. At 2 hours after CLP, mice were given saline vehicle, imipenem alone (0.5 mg/mouse), cyclophosphamide alone (8 mg/kg/mouse), and cultures were obtained from blood, liver, spleen, and lymph nodes 9 hours later. The mice that received imipenem had few positive blood cultures, and those that received cyclophosphamide had positive blood culture counts that did not differ significantly from those in mice that underwent CLP but no other treatment (Supplemental Figure 2, n=10 per group). These findings suggest that cyclophosphamide’s protective effect is not mediated through an antimicrobial activity.
Qualitative Systemic Immune Response
As quantitative differences in peripheral leukocytes were not the mechanism behind the survival advantage of combination therapy, we began to investigate functional differences. Since mortality in our model was highest within the first 96 hours after the septic insult (Figure 4), we focused subsequent experiments on evaluating effector function of neutrophils. Peripheral blood leukocytes were obtained 48 hours after the initial septic insult, a time point at which mice had received antibiotics with or without cyclophosphamide. Myleoperoxidase activity of these neutrophils was not different between groups (Supplemental Figure 3, n=10 per group).
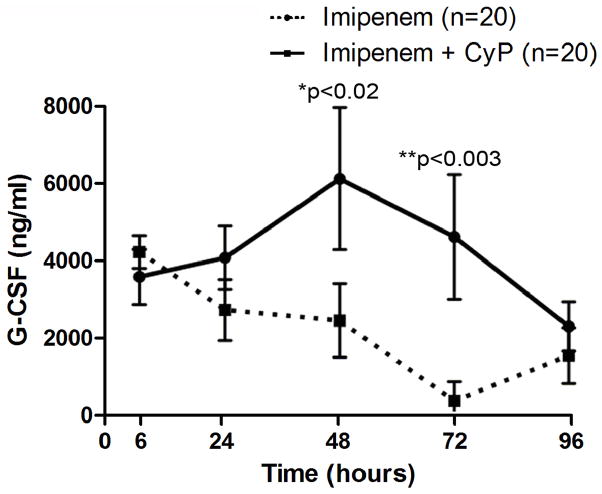
Analysis of systemic cytokine levels (IL-4, IL-6, IL-10, IL-13, IL-17, TNF-α, INF-γ, G-CSF, GM-CSF, and MIP-1β) at 48 hours post-CLP showed much variability between individuals, but no difference across groups. However, in time-course experiments, mice treated with cyclophosphamide and imipenem had a relatively longer duration of elevated G-CSF levels than mice treated with antibiotic alone. As shown in Figure 5, this elevation of G-CSF is statistically significant at 48 (*p < 0.02) and at 72 hours (**p < 0.003). All other cytokine levels were not significantly different between groups (n = 20, data not shown).
Figure 5. G-CSF is elevated in CLP Model with Antibiotic and CyP Treatment.
After mice underwent CLP operation, and given imipenem alone or with cyclophosphamide (10 mg/kg for 4 days), blood was collected at various timepoints and analysis of G-CSF levels were determined. Differences in G-CSF between mice that received combination treatment with cyclophosphamide and imipenem and those that received imipenem alone are statistically significant at 48 (*p < 0.02, n=20) and at 72 hours (**p < 0.003, n=20). Error bars indicate standard deviation.
End Organ Damage
Efforts to understand end-organ damage in our model included a thorough investigation of lung tissue. Histological slides (evaluated by a pathologist blinded to the study) of lung tissue at 48 hours post-CLP showed no quantifiable differences between combination therapy compared to imipenem alone (n=30, representative slides in Supplemental Figure 4). There were no significant differences between groups when lung wet-to-dry ratios were calculated (n=10, data not shown). Myeloperoxidase activity of neutrophils obtained from lung tissue homogenate and bronchial alveolar lavage were not different between groups (Supplemental Figure 3, n=10 per group).
Local Immune Response
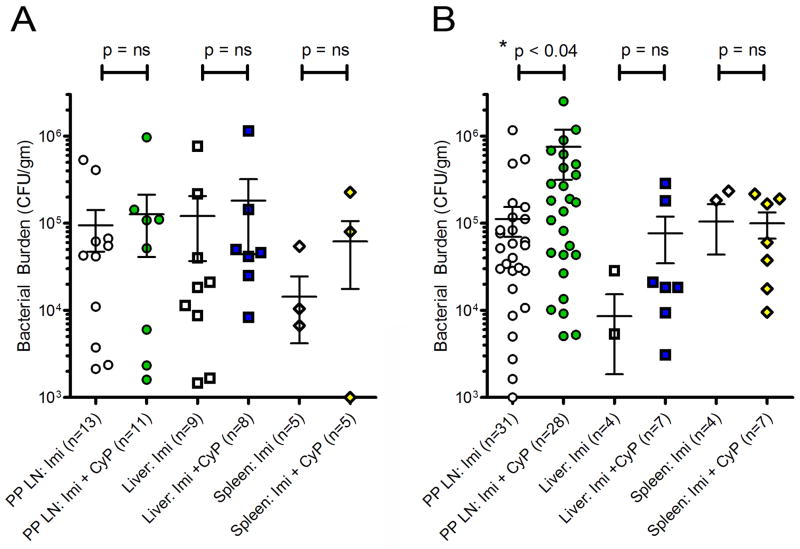
Characterization of the local immune response began with culture of intraperitoneal lavage fluid. No difference was observed between mice that received imipenem alone and those that received impenem and CyP (n=10, data not shown). Subsequently, the bacterial colonization of Peyer’s patch lymph nodes, liver, and spleen at 48 and 72 hours post-CLP was quantified. No differences were seen between treatment groups at 48 hours (Figure 6A) in the Peyer’s patch lymph nodes, liver, or spleen. At 72 hours (Figure 6B), liver and spleen colonization was also similar between groups. However, bacterial burden of the Peyer’s patch lymph nodes was significantly higher 72 hours after CLP in mice that received combination treatment (Figure 6B, *p <0.04).
Figure 6. Antibiotic and Cyclophosphamide Treatment in CLP leads to Increased Bacterial Burden in Peyer’s Patch Lymph nodes in Later Sepsis.
After mice underwent CLP operation, mice were given imipenem alone or imipenem with cyclophosphamide (10 mg/kg for 4 days). Intestinal Peyer’s patch (PP) lymph nodes, liver and spleen were harvested 48 hours later and 48 hours(n=13 control, n=12 treatment) and 72 hours (n=31 control, n=28 treatment) and processed for bacterial colonization. Box plot demonstrate no change in bacterial burden in any tissues at 48 hours (A). At 72 hours (Part B), the bacterial burden of the PP lymph nodes was significantly higher (*p<0.04) in animals receiving imipenem with CyP versus imipenem alone; there was no difference between treatment groups in liver or spleen samples. (Within figure, “n” represent number of individual specimens of LN, liver or spleen from mice; not all mice yielded bacterial culture results that could be represented.)
As macrophages are believed to be common scavengers and first responders to microorganisms within lymph nodes, we subsequently examined macrophage phenotype using two separate assays. M1 activated macrophages are known to be pro-inflammatory and have higher levels of arginase activity than their anti-inflammatory phenotype, the M2 activated macrophage. The number of intraperitoneal macrophages isolated at 48 or 72 hours post-CLP in control groups receiving imipenem alone versus treatment imipenem with CyP was not significantly different (data not shown, n=5). The expression of arginase at 48 or 72 hours post-CLP also did not differ between control and treatment groups (Supplemental Figure 5, n=5). Phagocytosis by these same macrophages was also measured. Neither the percentage of cells that ingested yeast nor the number of yeast particles ingested per cell varied across groups (n=23 at 48 hours, n=34 at 72 hours, data not shown).
Discussion
The concept of treating sepsis with immunosupressants is not novel, but it has never been successfully applied in humans. Previously promising targets and their therapies shown to be beneficial in animal models of sepsis also failed to translate into clinical advances (7). It is also inherently controversial that dampening the immune response in the face of an active, systemic infection might be beneficial. However, we hypothesized that in severe sepsis, the inflammatory response is the larger insult, especially when antibiotics are used to control the infection. It was with this logic that numerous attempts at targeting the inflammatory component of sepsis were previously attempted. Initially, high-dose corticosteroids were tried. Patients were observed to have decreased levels of IL-6 and TNF-α, but there was no clinical benefit (8). Broad corticosteroids (9) and specific therapies against key inflammatory mediators of sepsis were also attempted, including IL-1 receptor antagonism, anti-TNF antibodies, and anti-LPS antibodies, but without clinical success (10–12). Though in prior mouse models of sepsis, treatment with antibiotic with G-CSF and novel antimicrobial peptide LL-37 did increase survival (13). Also, studies in septic patients who received G-CSF supplementation to bolster neutrophil function revealed no demonstrable benefit (14–17). Accordingly, sepsis has proven to be an extremely complex process wherein attempts to block specific signal pathways have proven unsuccessful, likely due to the redundancy inherent to the host immune system.
Given this complexity, we initially hypothesized that immunosuppression directly targeting effector cell populations of the innate immune system might be able to overcome the challenge of redundant signaling pathways that may have hampered previous efforts. Therefore, we used the immunosuppressant cyclophosphamide. The active metabolite of this nitrogen mustard precursor is as an alkylating agent that damages DNA, triggers the apoptosis of immune effector cells, and thus inhibits the host immune response without targeting a specific inflammatory signaling pathway. While the mechanism of action of cyclophosphamide in our model remains unclear, we suspect that it acts broadly to dampen the immediate immune response. Our results demonstrate that cyclophosphamide acts to protect against early mortality in the setting of severe sepsis. Thus, we infer that early immune effector cells are modulated to decrease the immune-based insult.
Our study also shows that in a model of severe sepsis, cyclophosphamide used together with the antibiotic imipenem provided a significant survival advantage over imipenem alone. Mice that received combination treatment survived nearly twice as long as those that received only imipenem. Since previous attempts at immunomodulating specific parts of the immune system have had little benefit, it is important that we identify key differences of our model that might explain why our study was successful. Two such aspects are disease severity and specificity of the immunomodulating agent. In a less severe model of sepsis, the contribution of the inflammatory component may be smaller, and in such a scenario, use of an immunomodulator could potentially be deleterious. Various studies that used corticosteroids as immunomodulators in patients with sepsis have had mixed results. A meta-analysis of corticosteroid use in the setting of septic shock showed an increased rate of shock reversal over the first 28 days of illness and decreased length of intensive care unit stay, but no overall survival advantage (8). Thus, corticosteroid use may have more benefit in more severe settings.
Additionally, our findings differed from several trials in which G-CSF administered to patients with severe sepsis showed no clear survival benefit (14–17). A meta-analysis of these trials was recently published verifying a lack of a survival benefit for individuals receiving G-CSF (15). These trials were premised on the theory that the overwhelming immune response seen early in the course of sepsis is either ineffective at a functional level or that the large immune response leads to later paralysis. Although adjunct G-CSF therapy is now considered unable to rescue the immune system either early or late in the course of sepsis, we found that mice with elevated G-CSF levels were more likely to survive. When trying to reconcile our data—that higher endogenous G-CSF levels yield a survival benefit—with prior data that exogenous G-CSF supplementation does not improve survival, we are forced to consider G-CSF as a marker of disease severity, rather than a tool to be clinically manipulated. If G-CSF is an indication of immune function, with higher levels being produced by an immunocompromised host in an attempt to self-rescue, then our observations support these prior studies. Thus, we propose that cyclophosphamide-mediated immune damage creates higher G-CSF levels that indicate a more immunosuppressed, and thus better fitted to survive, host.
The local immune response appears to be limited in immunosuppressed mice. Higher bacterial colonization in these mice suggests an anti-inflammatory state. Surprisingly, the mice with a dramatically higher bacterial burden in their intestinal Peyer’s patch lymph nodes were those same mice that went on to survive at a higher rate. This is in contrast to a previously published model of bacteremia-induced sepsis (18), in which mice treated with cyclophosphamide, though they had increased bacterial burden (in blood and solid organs: liver, spleen lung), they all succumbed to sepsis and death. What may explain this difference is these authors administered a single, high-dose 200 mg/kg regimen, which we found increased mortality in sham operated mice. The mechanism by which the local immune system is altered by the administration of cyclophosphamide remains a mystery, as both functional and phenotypical studies of macrophages showed similar characteristics between groups.
The theory of “immune paralysis” that occurs late in the course of sepsis is novel and relevant to our study. Currently, many believe that immune paralysis is detrimental to the host by incapacitating the immune system (19–20). We hypothesize that the paralysis relevant to our model occurs well before the later pathologic paralysis seen in non-immunomodulated hosts. Furthermore, cyclophosphamide-generated paralysis may rescue the immune system from later immune exhaustion.
There are several important limitations of our study. Foremost, we recognize the difficulty in translating murine studies to humans, the lack of clinical data to support immunosuppression thus far, our lack of a mechanism, and a narrow therapeutic window. We examined end-organ damage only in the lung, however examining other organ systems such as the kidney (affected early in septic shock) or the GI tract (possibly impacted through cyclophosphamide’s effect on rapidly dividing cells) may be revealing. Additionally, other studies have shown that very early events after CLP may be responsible for the final outcome. It will be interesting to compare early changes with the late changes presented in our study. Our cytokine analysis in blood may have been affected due to animal variability, which has been observed by others after CLP. Future studies should complement our results with quantitative RT-PCR of inflammatory cytokines within relevant organs. Blood cultures as well as bacterial burden in end organs could be collected at several time points or with increased cohorts of mice to verify our negative data. Infiltration of neutrophils into the lung and liver is a hallmark of CLP; although no difference was seen histologically or in MPO studies of the lung; hepatic infiltration remains to be examined. Isolation of peritoneal macrophages after CLP can be complicated due to the infiltration of other cell types, however we did not perform a flow cytometric analysis of the isolated population. Additionally, it is possible that the peritoneal macrophages have become saturated in their phagocytic capacity. Future studies should compare the phagocytic activity of naïve macrophages from non-operated mice to that of macrophages from manipulated mice. Also, we did not characterize macrophage polarization beyond M1 and M2. Future studies should examine additional markers of activation.
In conclusion, we have created a model of severe septic shock that carries high levels of early mortality. We targeted the innate immune system by using an agent (cyclophosphamide) that acts primarily against rapidly dividing cells. Although we found no benefit in depleting the absolute number of early immune responders, we did find a great survival benefit in altering the function of those early responders. These data may suggest a new functional effect of the well-known immunosuppressant cyclophosphamide. Our model overcomes the overwhelming immune insult early in the course of sepsis by decreasing the function of early immune responders. Importantly, our therapeutic regimen may then protect against late septic mortality, specifically because our immunosuppressant does not completely deplete circulating leukocytes. We hypothesize that these cells are thus able to self-rescue at a point during the critical phase of the illness when the possession of a robust immune response transitions from deleterious to beneficial.
Supplementary Material
Varying doses (0–90 mg/kg/day) of CyP or saline vehicle were injected intraperitoneally for 4 consecutive days 24 hours after sham surgery, thus total doses ranged from 80mg/kg to 360 mg/kg. Unlike higher dose bolus injections, dividing doses eliminated lethality, with overall survival rates ranging from 80–100 % (A), There was no major difference in weight loss or regain (B) or in the neutrophil count (C), which reached a nadir at days 4–6, which was followed by a spontaneous neutrophilia (n=5 per group).
Two hours after mice underwent sham or CLP operation, mice were given saline vehicle, imipenem (0.5 mg/mouse), or cyclophosphamide (8 mg/kg/mouse). Blood cultures from samples collected 9 hours post-operation showed a reduction in CLP-induce bacteremia with imipenem (*p<0.05) but not with cyclophosphamide. No mice undergoing sham operation had bactremia (n=10 per group).
After mice underwent CLP operation, mice were given imipenem alone or imipenem with cyclophosphamide (10 mg/kg for 4 days). Lung tissue and peripheral neutrophils were then obtained 48 hours later and tested for MPO activity. MPO activity was unchanged with the addition of cyclophosphamide in lung, unactivated peripheral neutrophils or activated neutrophils (n=10 per group).
After mice underwent CLP operation, mice were given imipenem alone or imipenem with cyclophosphamide (10 mg/kg for 4 days). Lung was then obtained 48 hours later and histologic markers of inflammation severity were evaluated by a pathologist blinded to treatment group. There was no different in the degree of pulmonary inflammation in the two groups (n=10 per group).
After mice underwent CLP operation, mice were given imipenem alone or imipenem with cyclophosphamide (10 mg/kg for 4 days). Lung tissue and peripheral neutrophils were then obtained 48 hours later and tested for MPO activity. MPO activity was unchanged with the addition of cyclophosphamide in lung, unactivated peripheral neutrophils or activated neutrophils (n=10 per group).
Acknowledgments
This study was partially funded by the NIH grant 1 R01 GM095967.
Footnotes
No author has conflicts of interest to disclose.
References
- 1.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29(7):1303–10. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Dejager L, Pinheiro I, Dejonckheere E, Libert C. Cecal ligation and puncture: the gold standard model for polymicrobial sepsis? Trends Microbiol. 2011;19(4):198–208. doi: 10.1016/j.tim.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 3.Baker CC, Chaudry IH, Gaines H, Baue AE. Evaluation of factors affecting mortality following sepsis in a murine cecal ligation and puncture model. Surgery. 1983;94:331–335. [PubMed] [Google Scholar]
- 4.Hubbard WJ, Choudhry MA, Schwacha MG, Kerby JD, Rue LW, Bland KI, Chaudry IH. Cecal ligation and puncture. Shock. 2005;24 (Suppl 1):52–57. doi: 10.1097/01.shk.0000191414.94461.7e. [DOI] [PubMed] [Google Scholar]
- 5.Riedemann NC, Guo RF, Ward PA. Novel strategies for the treatment of sepsis. Nat Med. 2003;9(5):517–24. doi: 10.1038/nm0503-517. [DOI] [PubMed] [Google Scholar]
- 6.Hirsh M, Carmel J, Kaplan V, Livne E, Krausz MM. Activity of lung neutrophils and matrix metalloproteinases in cyclophosphamide-treated mice with experimental sepsis. Int J Exp Pathol. 2004;85(3):147–57. doi: 10.1111/j.0959-9673.2004.00385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Osuchowski MF, Remick DG, Lederer JA, Lang CH, Aasen AO, Aibiki M, Azevedo LC, Bahrami S, Boros M, Cooney R, et al. Abandon the mouse research ship? not just yet! Shock. 2014;41(6):463–75. doi: 10.1097/SHK.0000000000000153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, Peterson E, Tomlanovich M. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;8;345(19):1368–77. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 9.Annane D, Bellissant E, Bollaert PE, Briegel J, Confalonieri M, De Gaudio R, Keh D, Kupfer Y, Oppert M, Meduri GU. Corticosteroids in the treatment of severe sepsis and septic shock in adults: a systematic review. JAMA. 2009;301(22):2362–75. doi: 10.1001/jama.2009.815. [DOI] [PubMed] [Google Scholar]
- 10.Lefering R, Neugebauer EA. Steroid controversy in sepsis and septic shock: a meta-analysis. Crit Care Med. 1995;23(7):1294–303. doi: 10.1097/00003246-199507000-00021. [DOI] [PubMed] [Google Scholar]
- 11.Opal SM, Fisher CJ, Jr, Dhainaut JF, Vincent JL, Brase R, Lowry SF, Sadoff JC, Slotman GJ, Levy H, Balk RA, Shelly MP, Pribble JP, LaBrecque JF, Lookabaugh J, Donovan H, Dubin H, Baughman R, Norman J, DeMaria E, Matzel K, Abraham E, Seneff M. Confirmatory interleukin-1 receptor antagonist trial in severe sepsis: a phase III, randomized, double-blind, placebo-controlled, multicenter trial. The Interleukin-1 Receptor Antagonist Sepsis Investigator Group. Crit Care Med. 1997;25(7):1115–24. doi: 10.1097/00003246-199707000-00010. [DOI] [PubMed] [Google Scholar]
- 12.Reinhart K, Menges T, Gardlund B, Harm Zwaveling J, Smithes M, Vincent JL, Tellado JM, Salgado-Remigio A, Zimlichman R, Withington S, Tschaikowsky K, Brase R, Damas P, Kupper H, Kempeni J, Eiselstein J, Kaul M. Randomized, placebo-controlled trial of the anti-tumor necrosis factor antibody fragment afelimomab in hyperinflammatory response during severe sepsis: The RAMSES Study. Crit Care Med. 2001;29(4):765–9. doi: 10.1097/00003246-200104000-00015. [DOI] [PubMed] [Google Scholar]
- 13.Cirioni O, Ghiselli R, Tomasinsig L, Orlando F, Silvestri C, Skerlavaj B, Riva A, Rocchi M, Saba V, Zanetti M, Scalise G, Giacometti A. Efficacy of LL-37 and granulocyte colony-stimulating factor in a neutropenic murine sepsis due to pseudomonas aeruginosa. Shock. 2008;30:443–448. doi: 10.1097/SHK.0b013e31816d2269. [DOI] [PubMed] [Google Scholar]
- 14.Mohammad RA. Use of granulocyte colony-stimulating factor in patients with severe sepsis or septic shock. Am J Health Syst Pharm. 2010;67(15):1238–45. doi: 10.2146/ajhp090325. [DOI] [PubMed] [Google Scholar]
- 15.Bo L, Wang F, Zhu J, Li J, Deng X. Granulocyte-colony stimulating factor (G-CSF) and granulocyte-macrophage colony stimulating factor (GM-CSF) for sepsis: a meta-analysis. Crit Care. 2011;15(1):R58. doi: 10.1186/cc10031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meisel C, Schefold JC, Pschowski R, Baumann T, Hetzger K, Gregor J, Weber-Carstens S, Hasper D, Keh D, Zuckermann H, Reinke P, Volk HD. Granulocyte-macrophage colony-stimulating factor to reverse sepsis-associated immunosuppression: a double-blind, randomized, placebo-controlled multicenter trial. Am J Respir Crit Care Med. 2009;180(7):640–8. doi: 10.1164/rccm.200903-0363OC. [DOI] [PubMed] [Google Scholar]
- 17.Root RK, Lodato RF, Patrick W, Cade JF, Fotheringham N, Milwee S, Vincent JL, Torres A, Rello J, Nelson S. Multicenter, double-blind, placebo-controlled study of the use of filgrastim in patients hospitalized with pneumonia and severe sepsis. Pneumonia Sepsis Study Group. Crit Care Med. 2003;31(2):367–73. doi: 10.1097/01.CCM.0000048629.32625.5D. [DOI] [PubMed] [Google Scholar]
- 18.Chung HM, Cartwright MM, Bortz DM, Jackson TL, Younger JG. Dynamical system analysis of staphylococcus epidermidis bloodstream infection. Shock. 2008;30:518–526. doi: 10.1097/SHK.0b013e31816a0b77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hotchkiss RS, Coopersmith CM, McDunn JE, Ferguson TA. The sepsis seesaw: tilting toward immunosuppression. Nat Med. 2009;15(5):496–7. doi: 10.1038/nm0509-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Eng J Med. 2003;348(2):138–50. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Varying doses (0–90 mg/kg/day) of CyP or saline vehicle were injected intraperitoneally for 4 consecutive days 24 hours after sham surgery, thus total doses ranged from 80mg/kg to 360 mg/kg. Unlike higher dose bolus injections, dividing doses eliminated lethality, with overall survival rates ranging from 80–100 % (A), There was no major difference in weight loss or regain (B) or in the neutrophil count (C), which reached a nadir at days 4–6, which was followed by a spontaneous neutrophilia (n=5 per group).
Two hours after mice underwent sham or CLP operation, mice were given saline vehicle, imipenem (0.5 mg/mouse), or cyclophosphamide (8 mg/kg/mouse). Blood cultures from samples collected 9 hours post-operation showed a reduction in CLP-induce bacteremia with imipenem (*p<0.05) but not with cyclophosphamide. No mice undergoing sham operation had bactremia (n=10 per group).
After mice underwent CLP operation, mice were given imipenem alone or imipenem with cyclophosphamide (10 mg/kg for 4 days). Lung tissue and peripheral neutrophils were then obtained 48 hours later and tested for MPO activity. MPO activity was unchanged with the addition of cyclophosphamide in lung, unactivated peripheral neutrophils or activated neutrophils (n=10 per group).
After mice underwent CLP operation, mice were given imipenem alone or imipenem with cyclophosphamide (10 mg/kg for 4 days). Lung was then obtained 48 hours later and histologic markers of inflammation severity were evaluated by a pathologist blinded to treatment group. There was no different in the degree of pulmonary inflammation in the two groups (n=10 per group).
After mice underwent CLP operation, mice were given imipenem alone or imipenem with cyclophosphamide (10 mg/kg for 4 days). Lung tissue and peripheral neutrophils were then obtained 48 hours later and tested for MPO activity. MPO activity was unchanged with the addition of cyclophosphamide in lung, unactivated peripheral neutrophils or activated neutrophils (n=10 per group).