Abstract
Sustained attention and reinforcement are posited as causal mechanisms in Attention-Deficit/Hyperactivity Disorder (ADHD), but their interaction has received little empirical study. In two studies, we examined the impact of performance-based reinforcement on sustained attention over time, or vigilance, among 9- to 12-year-old children. Study 1 demonstrated the expected vigilance deficit among children with ADHD (n=25; 12% female) compared to typically developing (TD) controls (n=33; 22% female) on a standard continuous performance task (CPT). During a subsequent visit, reinforcement improved attention more among children with ADHD than controls. Study 2 examined the separate and combined effects of reinforcement and acute methylphenidate (MPH) on CPT performance in children with ADHD (n=19; 21% female). Both reinforcement and MPH enhanced overall target detection and attenuated the vigilance decrement that occurred in no-reinforcement, placebo condition. Cross-study comparisons suggested that the combination of MPH and reinforcement eliminated the vigilance deficit in children with ADHD, normalizing sustained attention. This work highlights the clinically and theoretically interesting intersection of reinforcement and sustained attention.
Keywords: Attention-Deficit/Hyperactivity Disorder, ADHD, sustained attention, vigilance, Continuous performance task, reinforcement, methylphenidate
Attention Deficit/Hyperactivity Disorder (ADHD) is a common childhood-onset disorder that is characterized by developmentally inappropriate and impairing symptoms of inattention, hyperactivity and impulsivity (American Psychiatric Association, 2013). As the name of the disorder suggests, there is considerable heterogeneity in the symptom presentation among children with ADHD, as well as heterogeneity in the cognitive and motivational processes theorized to cause those symptoms (e.g., Castellanos, Sonuga-Barke, Milham, & Tannock, 2006; Nigg, Willcutt, Doyle, & Sonuga-Barke, 2005).
Among the cognitive processes implicated in ADHD, attention has long been of interest (e.g., Douglas, 1972; Douglas, 1983). The majority of children with ADHD exhibit attention problems in multiple settings (Fabiano et al., 2006). In the laboratory, attention is commonly examined with continuous performance tasks (CPTs; see reviews by Huang-Pollock, Karalunas, Tam, & Moore, 2012; Losier, McGrath, & Klein, 1996). The A-X CPT (Rosvold, Mirsky, Sarason, Bransome, & Beck, 1956) is prototypic, requiring participants to detect the presentation of infrequent target stimuli (a “X” preceded by an “A”) among a string of non-target letters over an extended period of time (10+ minutes). CPTs consistently reveal impaired target detection (i.e., reduced hit rate) among children with ADHD compared to typically developing children, with an average effect size of .62 across 39 studies (Huang-Pollock et al., 2012).
Although these data are consistent with an attentional deficit in ADHD, they fall short of evaluating whether the deficit is evident from the beginning of the task or whether the problem is with sustained attention over time, or vigilance. In the real world, attention must be maintained over long periods of time to successfully complete school work, sports activities, and even conversations with friends and family. Despite the theoretical and clinical significance of sustained attention, surprisingly few CPT studies present attention data as a function of time. Among those that do, there is evidence of a small-to-moderate vigilance deficit in ADHD (i.e., a steeper decline in hit rate over time), but there is also substantial variability across studies (mean d=.38; SD=.37; n=7; see Huang-Pollock et al., 2012).
In everyday life, the degree to which we attend to any given stimulus or activity depends in part on motivation (e.g., Lang, Bradley, & Cuthbert, 1997). Indeed, several theories of ADHD emphasize dysregulation of reinforcement in the form of an elevated reinforcement threshold (Haenlein & Caul, 1987) or reduced ability of delayed reinforcement to maintain control over behavior (Sagvolden, Johansen, Aase, & Russell, 2005; Tripp & Wickens, 2008). However, it is not necessary to pit cognitive and reinforcement models against one another; performance is increasingly viewed as an interaction of cognition and motivation (see Castellanos et al., 2006; Douglas, 1999). This perspective is implicit in behavioral treatments for ADHD, one of two frontline interventions for the disorder (American Academy of Pediatrics, 2001; Pelham & Fabiano, 2008): reinforcement is used to enhance desired behavior, effects, which may be mediated by improvements in cognitive processes such as sustained attention.
The impact of reinforcement on cognitive processes in ADHD is the focus of a growing but varied literature (see reviews by Luman, Oosterlaan, & Sergeant, 2005; Luman, Tripp, & Scheres, 2010). The most straightforward test of reinforcement effects on sustained attention in ADHD would be compare hit rates over time from a traditional no-reinforcement CPT to a CPT with continuous trial-by-trial reinforcement. Surprisingly, Study 1 of the present manuscript is the first such investigation of which we are aware. Prior CPT studies (Rubia, Halari, et al., 2009; Rubia, Smith, et al., 2009; Rubia, Smith, & Taylor, 2007; Solanto, Wender, & Bartell, 1997) have not employed a no-reinforcement baseline condition that would be comparable to a standard CPT. Instead, a performance feedback condition was compared to performance feedback + additional reinforcement. Because delivering reinforcement provides feedback about performance and because feedback alone can improve performance (e.g., Annett, 1969; Craighead, Kazdin, & Mahoney, 1976; Kluger & DeNisi, 1996), these studies likely underestimate the degree to which reinforcement enhances attention relative to standard testing conditions. (The parallel medication study would compare two active doses or medications; the absence of a no-medication/placebo condition limits conclusions about efficacy.) Thus, the absence of reinforcement condition differences for overall hit rate in two of the four CPT studies and the similar absence of an effect on vigilance in the one study that examined attention over time (Solanto et al., 1997) should be interpreted with caution. Although it is certainly possible that reinforcement does not improve sustained attention in ADHD, accepting the null seems premature in the absence of a study that provides a stronger test of the hypothesis.
Study 1 examined the effect of reinforcement on sustained attention during the commonly used A-X CPT (Halperin, Greenblatt, Wolf, & Young, 1991; Halperin et al., 1988; Huang-Pollock, Nigg, & Halperin, 2006) among children with ADHD and typically developing (TD) children. Children completed two visits approximately 1 week apart. Given the small literature on true vigilance deficits in ADHD (Huang-Pollock et al., 2012), the first visit consisted of a standard A-X CPT. We hypothesized that children with ADHD would show a steeper decline in hit rate as a function of time. The second visit employed a modified task that explicitly contrasted periods of reinforcement and no-reinforcement. We hypothesized that children with ADHD would obtain fewer hits than controls, particularly during later portions of the task, and that reinforcement would improve hit rate, particularly for children with ADHD. To test whether reinforcement specifically ameliorates the vigilance deficit in ADHD, we evaluated the Group × Reinforcement × Time interaction.
Study 1
Methods
Participants
Children aged 9 to 12 years old with ADHD-Combined Type (n=25) and a typically developing control group (TD, n=33) participated in a study of the effect of reinforcement on neurocognitive processes. Typically developing participants were recruited through community advertisement and from local schools. Participants in the ADHD group were recruited from a university-based clinic in addition to flyers posted in medical offices.
Screening included an initial telephone call followed by collection of parent and teacher ratings on a DSM-IV symptom checklist (Disruptive Behavior Disorder Rating Scale [DBD-RS]; Pelham, Fabiano, & Massetti, 2005; Pelham, Gnagy, Greenslade, & Milich, 1992) and Impairment Rating Scale (IRS; Fabiano et al. 2006). All children included in the ADHD group were required to have 6 or more symptoms of inattention and 6 or more symptoms of hyperactivity/impulsivity on the DBD-RS (with parent/teacher overlap in each domain), and clinically significant impairment on the IRS, consistent with a diagnosis of ADHD Combined type. Prospective members of the typically developing control group were required to exhibit fewer than four symptoms on the combined parent and teacher DBD-RS within each symptom domain (inattention and hyperactivity/impulsivity).
Parents of children meeting the ratings scale criteria were invited to complete a structured computerized clinical interview (Diagnostic Interview Schedule for Children Version IV [DISC-IV], Shaffer, Fisher, Lucas, Dulcan, & Schwab-Stone, 2000). A DISC-IV diagnosis of ADHD Combined type was required to be eligible for the ADHD group; typical levels of comorbidity with Oppositional-Defiant Disorder (ODD; n=11) and Conduct Disorder (n=2) were observed. To be eligible for the control group, the child had to be free of an externalizing disorder on the DISC-IV. Children were excluded from the study if they had an IQ below 80, history of a pervasive developmental disorder or psychosis or were taking psychiatric medication other than stimulant treatment for ADHD.
Table 1 provides demographic and diagnostic data for the participants in Study 1. Children with ADHD and TD controls were comparable on all demographic variables and did not significantly differ on IQ. As intended, children with ADHD scored higher on all externalizing disorder symptom measures than did TD children.
Table 1. Sample Characteristics: Study 1a,b,c.
| Measure | ADHD (n=25) | TD (n=33) | p-value |
|---|---|---|---|
| Age | 10.8 (1.1) | 10.9 (1.0) | 0.84 |
| Gender (male:female) | 22:3 | 27:6 | 0.52 |
| Race/Ethnicity(n) | |||
| Hispanic | 2 | 2 | 0.77 |
| African American | 2 | 3 | 0.93 |
| Caucasian | 22 | 28 | |
| WISC Full Scale IQ | 108 (12) | 113 (12) | 0.14 |
|
| |||
| ADHD Symptoms | |||
| Inattention DBD-RS | |||
| Parent report | 22 (4) | 2 (2) | <0.001 |
| Teacher report | 18 (7) | 2 (2) | <0.001 |
| Hyperactivity/Impulsivity DBD-RS | |||
| Parent report | 20 (4) | 1 (2) | <0.001 |
| Teacher report | 16 (6) | 1 (2) | <0.001 |
| CBCL Attention Problems t-score | 72 (8) | 51 (2) | <0.001 |
| TRF Attention Problems t-score | 66 (7) | 51 (3) | <0.001 |
|
| |||
| ODD Symptoms | |||
| DBD-RS | |||
| Parent report | 13 (5) | 1 (1) | <0.001 |
| Teacher report | 8 (7) | 1 (1) | <0.001 |
| CBCL ODD Problems t-score | 66 (9) | 51 (2) | <0.001 |
| TRF ODD Problems t-score | 61 (8) | 51 (3) | <0.001 |
|
| |||
| CD Symptoms | |||
| DBD-RS | |||
| Parent report | 4 (3) | .2 (.5) | <0.001 |
| Teacher report | 3 (3) | 1 (3) | <0.001 |
| CBCL CD Problems t-score | 65 (9) | 52 (3) | <0.001 |
| TRF CD Problems t-score | 61 (8) | 51 (3) | <0.001 |
All measures are reported as means (SD) unless otherwise indicated.
p-values reflect tests for unequal variances when applicable.
ADHD=Attention-Deficit/Hyperactivity Disorder; TD=Typically-Developing; WISC=Weschler Intelligence Scale for Children; DBD-RS=Disruptive Behavior Disorders Ratings Scale; CBCL=Child Behavior Checklist; TRF=Teacher Report Form; ODD=Oppositional Defiant Disorder; CD=Conduct Disorder.
Setting and Procedure
Procedures were approved by an Institutional Review Board at the university where the research was conducted. Children visited the university for two full days, approximately 1 week apart, in groups of 2 to 5. The structure of each visit included several testing sessions of various neurocognitive processes (i.e., attention, intrasubject variability, working memory (see Strand et al., 2012), inhibition, and delay discounting). The focus of this manuscript is on the sustained attention results as measured by a CPT. Testing was interspersed with group activities (e.g., sports and board games) and meals. Children completed the CPT during different timeslots counterbalanced across children. Participants actively taking stimulant medication (n=15) discontinued use at least 24 h prior to each testing day.
The first day served as the baseline visit, during which participants completed a battery of neurocognitive tasks. The tasks did not have a reinforcement manipulation and children only earned a modest number of points throughout the day for following the rules for appropriate behavior and completing the tasks. Points received during the baseline visit were not contingent on task performance. Children exchanged their points at the conclusion of the baseline visit for toys and gift cards in a “store” where the value of the items was converted for approximately one U.S. cent for each point. Children completed the tasks in individual rooms that contained a computer and chair. A research assistant remained in the room during testing.
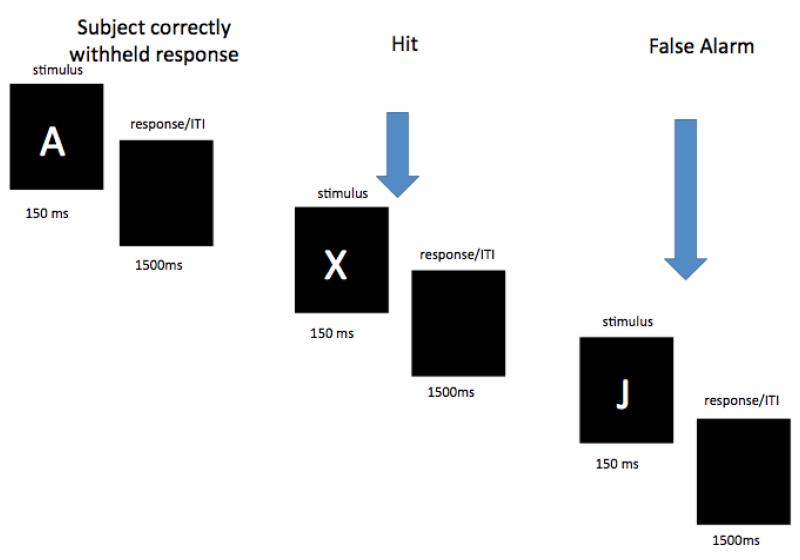
An A-X CPT (Rosvold et al., 1956) was selected to examine sustained attention. The CPT completed at the baseline visit was modestly adapted from that of Halperin and colleagues (Halperin et al., 1991; Halperin et al., 1988; Huang-Pollock et al., 2006). Children were instructed to press the spacebar of a standard keyboard when they saw the letter “X” but only when it was immediately preceded by an “A” (see Figure 1). Prior to the test blocks, children completed a practice of 20 trials (600ms stimulus duration; 2000ms response window; three targets). This was followed by a continuous stream of 4 100-trial epochs, each of which included 10 targets (X preceded by A) and 90 non-targets (including 5 “X’s” not preceded by an “A” and 17 “A’s” without “X’s” immediately after them). Trials consisted of a 150ms letter presentation followed by a 1500ms response window. Task duration was 11 minutes. Letters (1 cm × 1cm) were presented in the center of a 38 cm Dell CRT monitor. The task was programmed in E-prime (Psychology Software Tools, Pittsburgh, PA).
Figure 1.
A schematic of the baseline visit CPT. Three trials are depicted. Arrows indicate the trials on which the participant responded.
The second day served as the reinforcement visit, during which participants completed the same battery of neurocognitive tasks that were modified to contrast performance under reinforcement and no-reinforcement conditions. During the reinforcement blocks, children are provided with immediate feedback on each trial and earn points based on their task performance that could be exchanged for prizes and gift cards at the end of the day.
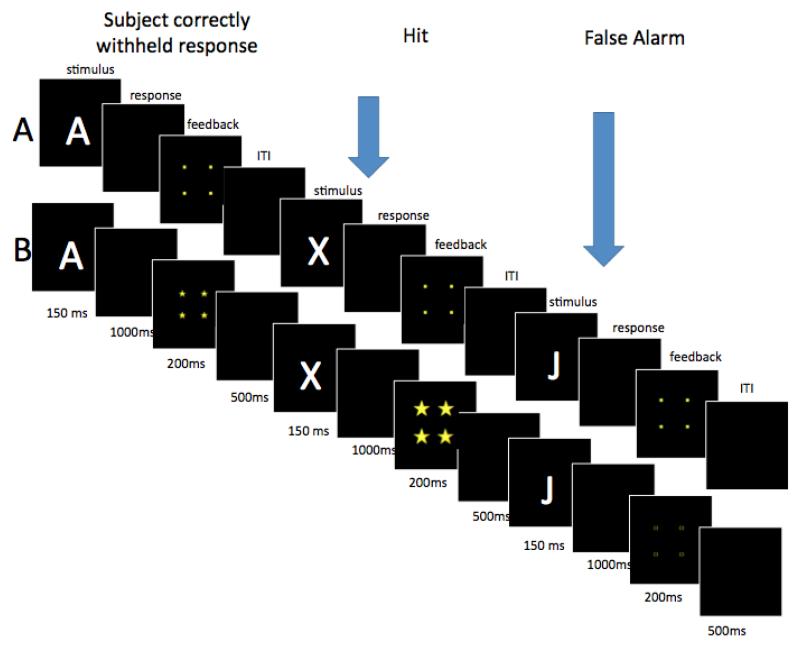
The CPT completed during the reinforcement visit was modified from the standard CPT completed at the baseline visit in several ways. The trial structure of the CPT with the reinforcement manipulation was modified to accommodate the presentation of immediate feedback (see Figure 2), such that they consisted of a 150ms stimulus presentation, 1000ms response period, 200ms feedback period, and 500ms interstimulus interval (the 1500ms ISI was extended to accommodate the feedback)1. In addition, concern about ceiling effects based on a previous investigation (unpublished) led the investigators to extend the length of the reinforcement CPT. Therefore, the test portion of the task consisted of 800 trials that were presented in four blocks of 200 trials, which each again contained 10 percent targets.
Figure 2.
A schematic of the reinforcement visit CPT. Three trials are depicted from the (A) no-reinforcement and (B) reinforcement conditions. Arrows indicate the trials on which the participant responded.
Previous research with children with ADHD suggests the order in which participants experience reinforcement and no-reinforcement conditions may impact performance (Huang-Pollock, Mikami, Pfiffner, & McBurnett, 2007; Shiels et al., 2008). In particular, if the no reinforcement condition occurs during the latter part of the task, there is a large decline in performance likely due to both the removal of reinforcement and a vigilance decrement. Pilot data (unpublished) suggested quickly alternating reinforcement conditions best guard against these influences (Strand et al., 2012). Therefore, the reinforcement conditions alternated across the four task blocks (whether children began with reinforcement or not was counterbalanced across children) and each block contained 2 100-trial epochs, resulting in 4 100-trial epochs per reinforcement condition. Each block was ~6 minutes long, and the test blocks were administered in approximately 25 minutes. Prior to each block, children were informed whether the reinforcement or no-reinforcement condition was next. The time to administer the directions took approximately 20 seconds to 1 minute.
During the feedback period of both conditions, a square (5 cm × 5 cm) was presented in the center of the screen (see Figure 2). During the reinforcement blocks, this square was composed of one of three different possible symbols depending on the participant’s response; large stars (1 cm × 1 cm) were presented for correctly identified targets (i.e., press for “A” followed by “X”) indicating 10 points earned, small stars (.5cm × .5 cm) were presented for correct rejections (i.e., do not press for letters other than “A” followed by “X”) indicating one point earned, and hollow squares (.5cm × .5 cm) were presented for incorrect responses (i.e., press for letter other than “A” followed by “X” or failure to press for “A” followed by “X”) indicating no points were earned. Children were told how many points they earned at the end of each reinforcement block. During no reinforcement blocks, small solid squares (.5cm ×.5cm) were presented on every trial regardless of response, and were therefore uninformative but served to maintain a similar trial timing and structure to the reinforcement condition.
Data Reduction and Analysis
Similar to many previous investigations (Huang-Pollock et al., 2012; Willcutt, Doyle, Nigg, Faraone, & Pennington, 2005), the primary dependent variable from the CPT was target detection, or percent “hits” ([(number of targets identified / number of targets presented] * 100%). Percent hits was calculated within each 100-trial epoch. Percent false alarms (responses to non-target letters; e.g., a “J” or an “X” not immediately preceded by an “A”) was examined in parallel to ensure that any increase in hits was indicative of improved target detection and not simply an increase in overall responding.
For the standard CPT, hits and false alarms were analyzed in separate 2 Group × 4 Time (100-trial epochs) ANOVAs. For the effect of time, the linear and quadratic orthogonal polynomials were examined in lieu of the omnibus test, as they more precisely model decrements in attention over time (i.e., vigilance decrement).
For the reinforcement-manipulation CPT, hits and false alarms were analyzed in separate 2 Group × 2 Reinforcement × 4 Time (100-trial epochs) ANOVAs. Reinforcement Order (reinforcement first v. reinforcement second) was included in the model as an additional between-subjects factor, but the results for this counterbalancing factor are presented in detail only when they interact with group. To examine if reinforcement normalized vigilance in children with ADHD, we contrasted hits obtained by children with ADHD during the reinforcement condition with hits obtained by TD children during the no reinforcement condition
Results
Visit 1 – Baseline/Standard CPT
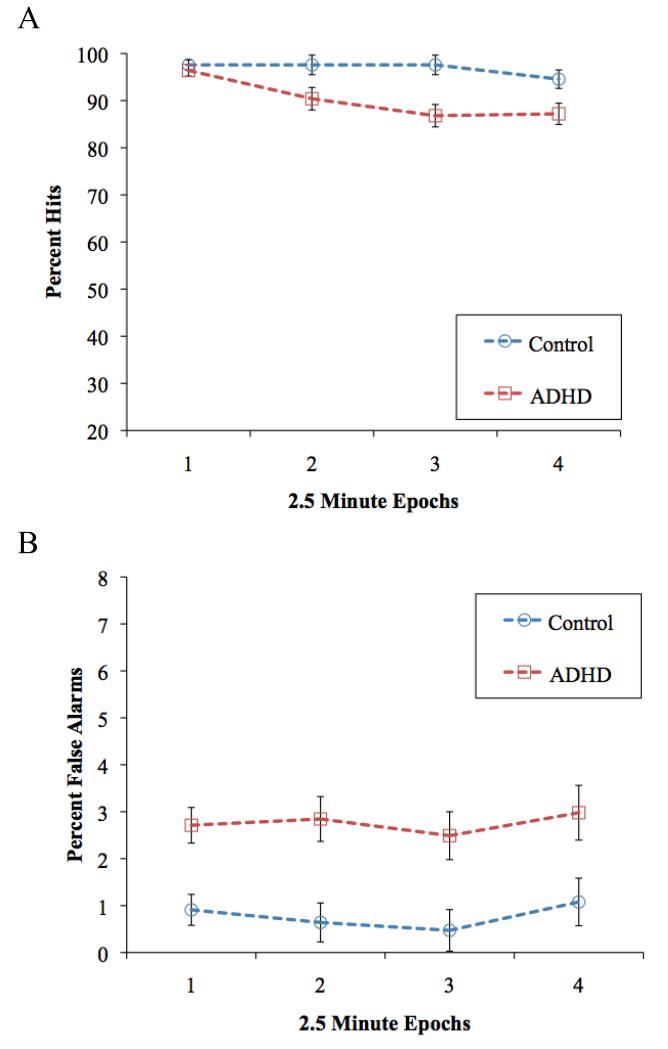
Figure 3A presents mean percent hits for all Group × Time conditions in the standard CPT completed at the baseline visit. As expected, the TD group had more hits overall than children with ADHD, group: F(1,56) = 13.3, p = .001, d = .88, and there was decrease in hits over time, time linear: F(1, 56) = 13.5, p = .001, d=.252; time quadratic F<1. These main effects were qualified by a Group × Time linear interaction, F(1, 56) = 4.1, p = .049, d = .43; also Group × Time quadratic F(1,56) =3.0, p = .09, such that hits for the two groups were comparable in the first epoch (p = .46) whereas the hit rate was significantly worse in the ADHD group in epochs 2 through 4 (ps < .03; see Figure 3A).
Figure 3.
Mean percent (A) hits and (B) false alarms for all Group × Epoch conditions during the baseline visit. Error bars reflect standard error.
False alarms for all Group × Time conditions are presented in Figure 3B. Children with ADHD committed more false alarms overall than TD controls, F(1, 56)= 16.1, p < .001, d = .95. In contrast to the vigilance decrement seen for hits, false alarms did not change over the course of the task, time linear and quadratic and Group × Time linear and quadratic: Fs < 1.
Visit 2 - Reinforcement-Manipulation CPT
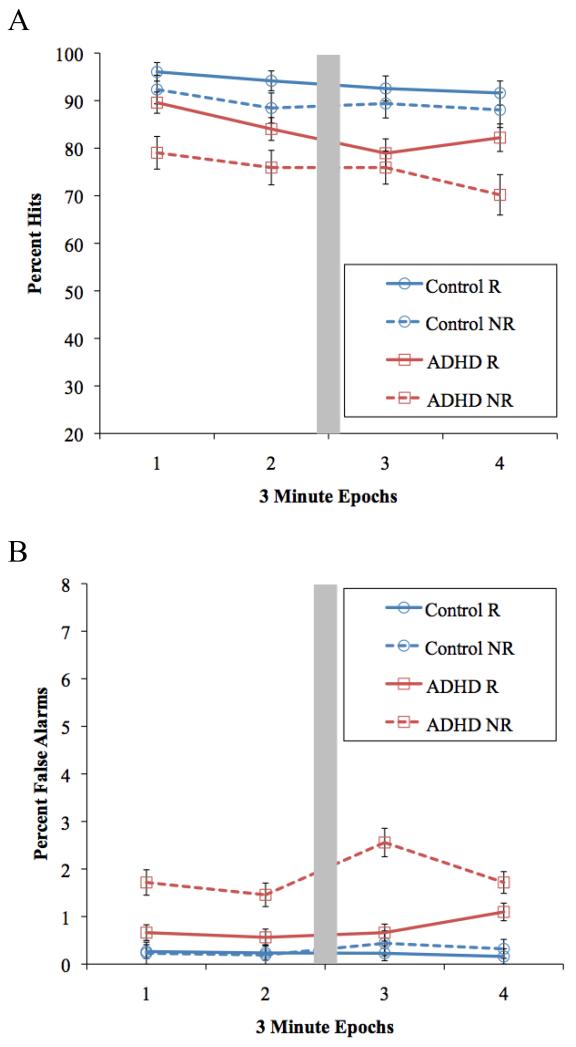
Figure 4A presents the mean percent hits for all Group × Reinforcement × Time conditions. Overall, typically developing children had more hits than children with ADHD, group: F(1, 54) = 13.9, p < .001, d = .86, and reinforcement led to greater hits on average, compared to no reinforcement, F(1, 54) = 24.9, p < .001, d = .83. Consistent with our predictions, reinforcement tended to improve overall hit rate more for children with ADHD (p = .002, d = .99) than for typically developing controls (p = .005, d = .74), Group Reinforcement F(1,54) = 3.1, p = .08, d=.47.
Figure 4.
Mean percent (A) hits and (B) false alarms for all Group × Reinforcement × Epoch conditions in Study 1, Reinforcement visit. The grey box represents the brief time allowed for instructions following every two epochs. Error bars reflect standard error. R= Reinforcement, NR= No Reinforcement.
With respect to vigilance, there was a marginal Group × Reinforcement × Time quadratic interaction: F(1, 54) = 3.6, p = .06, d=.48; Group × Reinforcement × Time linear, F<1. As expected under no-reinforcement conditions, target detection decreased over time, linear and quadratic Fs (1, 54) = 3.9 and .03, ps = .05 and .99, ds = .96 and .03. However, this vigilance decrement during no-reinforcement was not greater among the ADHD group, Group × Time linear and quadratic Fs < 1. Because there was no deficit to ameliorate, we did not complete the tests of whether reinforcement normalized sustained attention for children with ADHD.
False alarms for all Group × Reinforcement × Time conditions are presented in Figure 4 B. Overall, children with ADHD had more false alarms than TD controls, group: F(1, 54) = 31.5, p < .001, d = 1.18, and false alarms tended to increase over the course of the task, time linear: F(1, 54) = 3.9, p = .05. However, this effect was driven by children with ADHD who tended to commit more false alarms over time, p = .07, whereas TD children did not, p = .70, Group × Time linear interaction: F(1, 54) = 2.8, p = .10, d=.44; Group × Time quadratic F<1.
Reinforcement reduced the rate of false alarms among children with ADHD (p <.001, d = 1.6), whereas typically developing controls committed few false alarms during the reinforcement and no reinforcement conditions (p = .23, d = .28), Group × Reinforcement F(1,54) = 30.5, p < .001, d = 1.21. The differential impact of reinforcement on ADHD versus control children did not significantly vary over time, Group × Reinforcement × Time linear and quadratic Fs < 2.01.3
Study 1 Discussion
Results from the standard CPT completed on the baseline day of Study 1 replicated previous investigations reporting a lower target detection (“hit”) rate and higher false alarm rate among children with ADHD compared to typically developing controls (Epstein et al., 2003; Nigg et al., 2005; Willcutt et al., 2005). More importantly, the difference in hit rate developed over time. Children with ADHD performed just as well as controls during the initial minutes of the task. However, as time on task increased, children with ADHD exhibited a steeper decline in their detection of targets. These data are consistent with the small literature suggesting a deficit in sustained attention, or vigilance, in children with ADHD (Huang-Pollock et al., 2006, 2012).
Although the group difference in sustained attention was apparent by the second epoch of 100 trials, less than five minutes into testing, it is important to note that performance overall was quite high. Ceiling effects are a common problem in sustained attention tasks commonly used in ADHD research (Halperin et al., 1988, 1991; Huang-Pollock, 2006, 2012; see also Halperin, Trampush, Miller, Marks, & Newcorn, 2008); they use easily discriminable stimuli (as opposed to subtle variations or changes; e.g., Huang-Pollock et al., 2012) and are much shorter in duration than classic vigilance tasks (see Mackworth, 1969) and common real-world situations (e.g., school) that place demands on attention over extended periods of time.
Despite the high levels of performance, reinforcement tended to improve hit rate to a greater extent in children with ADHD than controls, as predicted. These data are novel in demonstrating such an effect (c.f., Rubia, Halari, et al., 2009; Rubia, Smith et al., 2009; Rubia, Smith, & Taylor, 2007), perhaps because of our strong contrast between the reinforcement and no-reinforcement conditions (see introduction). However, the Study 1 data are quite limited in addressing the ability of reinforcement to ameliorate or normalize a vigilance deficit – because we did not observe a vigilance deficit during the no-reinforcement condition. It is possible that vigilance deficit observed during the baseline visit was attenuated by the multiple breaks during the task – children had to sustain their attention for only six-minutes at a time, compared to twice that in baseline session. Therefore, Study 2 examined effects of reinforcement on vigilance during longer uninterrupted test periods.
In addition, Study 2 included tests of stimulant medication. Methylphenidate (MPH), a frontline treatment for ADHD (Greenhill et al., 2002), improves overall target detection in children with ADHD (Epstein et al., 2006; Riccio, Waldrop, Reynolds, & Lowe, 2001). Surprisingly, only one published ADHD study has examined the impact of MPH and reinforcement on sustained attention over time (Solanto et al. 1997). The results of that study suggested that a high dose of MPH (0.6 kg/mg) was more beneficial than modest monetary contingencies (earn/lose $.01US for correct/incorrect responses), but neither condition significantly attenuated the vigilance decrement in hit rate relative to a feedback-only condition.
Study 2 examined the separate and combined effects of reinforcement (as in Study 1) and a moderate dose of MPH (0.3 mg/kg) on CPT performance in a fully within-subjects design. Following our observations from Study 1, children completed a CPT that was four times as long as the typical A-X CPT (e.g., baseline of Study 1), roughly equal to the typical 45-minute classroom period children experience multiple times per day. Based on previous research, we predicted that: 1) both reinforcement and MPH would improve vigilance relative to condition without MPH or reinforcement, and 2) the combination of MPH and reinforcement would be more effective than either reinforcement or MPH alone. To provide tests of the degree to which reinforcement and/or MPH normalize vigilance, additional tests used the baseline data for control children in Study 1 as comparison for Study 2 ADHD data of comparable duration.
Study 2
Methods
Participants
Children with ADHD-Combined Type aged 9-12 years old (n=19) participated. Demographic and clinical characteristics of the children are presented in Table 2. All children had participated in a previous study of the effects of stimulant medication (Shiels et al., 2009; Spencer et al., 2009) or reinforcement (Study 1, n=124). Diagnostic and inclusion/exclusion criteria were comparable to those used in Study 1.
Table 2. Sample Characteristics: Study 2a,b.
| Measure | ADHD (n=19) |
|---|---|
| Age | 11.1 (0.8) |
| Gender (male:female) | 15:4 |
| Race/Ethnicity (n) | |
| Hispanic | 0 |
| African American | 2 |
| Caucasian | 17 |
| WISC Full Scale IQ | 108 (10) |
|
| |
| ADHD Symptoms | |
| Inattention DBD-RS | |
| Parent report | 22 (2) |
| Teacher report | 15 (7) |
| Hyperactivity/Impulsivity DBD-RS | |
| Parent report | 19 (4) |
| Teacher report | 15 (7) |
| CBCL Attention Problems t-score | 73 (7) |
| TRF Attention Problems t-score | 65 (10) |
|
| |
| ODD Symptoms | |
| DBD-RS | |
| Parent report | 12 (5) |
| Teacher report | 9 (7) |
| CBCL ODD Problems t-score | 67 (9) |
| TRF ODD Problems t-score | 62 (9) |
|
| |
| CD Symptoms | |
| DBD-RS | |
| Parent report | 4 (3) |
| Teacher report | 3 (3) |
| CBCL CD Problems t-score | 66 (8) |
| TRF CD Problems t-score | 61 (10) |
All measures are reported as means (SD) unless otherwise indicated.
ADHD=Attention-Deficit/Hyperactivity Disorder; WISC=Weschler Intelligence Scale for Children; DBD-RS=Disruptive Behavior Disorders Ratings Scale; CBCL=Child Behavior Checklist; TRF=Teacher Report Form; ODD=Oppositional Defiant Disorder; CD=Conduct Disorder.
Setting and Procedure
Children visited a university camp on two consecutive days to allow for a 2-day double-blind, placebo-controlled medication assessment. The daily activities were similar to Study 1. Participants completed a modified version of the reinforcement CPT from Study 1 on both days of the study. The value of points remained constant across studies.
Children currently taking stimulant medication (n=16) discontinued their medication at least 24 hours prior to the each visit. The employed medication was long-acting OROS methylphenidate (OROS-MPH). Doses were rounded to the nearest available equivalent of 0.3 mg/kg t.i.d. immediate release MPH (median=0.29mg/kg t.i.d.; SD=0.02) as this is typical stimulant dose for an elementary school aged child (Greenhill et al., 2002). The same number of opaque capsules was administered each morning to maintain blinding; placebo capsules were filled with micronized methylcellulose. Medication order was counterbalanced across participants. Cognitive testing was conducted between 1.5 and 8.5 hours after medication administration, well within the therapeutic window of OROS-MPH (e.g., Pelham et al., 2001); for each child, the CPT was performed at the same time of day for both visits.
Study 2 CPT
As in Study 1, the Study 2 CPT alternated between reinforcement and no-reinforcement conditions (order counterbalanced across participants and consistent across visits). However, we doubled the number of 100-trial epochs in each uninterrupted testing block to make each test block comparable in duration to the standard CPT completed at the baseline visit of Study 1 (~12 minutes; see also Halperin et al., 1991; Huang-Pollock et al., 2006). There was a brief (~20-second) transition between blocks. Thus, the task lasted approximately 49 minutes. All other CPT parameters were identical to Study 1.
Data Reduction and Analysis
The main dependent variables from the CPT were percent hits and false alarms calculated for each 100-trial epoch within each Reinforcement × Medication (Placebo vs. MPH) condition. Hits and false alarms were analyzed in separate 4 Treatment (no reinforcement + placebo, reinforcement alone, MPH alone, reinforcement + MPH) × 8 Time ANOVAs. As in Study 1, time effects were evaluated using orthogonal polynomials; we limited our evaluation to the linear, quadratic, and cubic trends. Although the treatments could be analyzed within a 2 × 2 factorial, we again used orthogonal contrasts to test the effects of interest: (1) no treatment (no reinforcement + placebo) vs. single treatments (average of reinforcement alone, MPH alone), (2) reinforcement alone vs. MPH alone, and (3) single treatment (average of reinforcement alone and MPH alone vs. reinforcement + MPH). The order in which reinforcement and MPH were delivered were included as between-subjects factors to account for variance attributable to these manipulations but effects involving medication or reinforcement order were not interpreted due to the small sample size.
When one or more treatment conditions improved vigilance (i.e., interacted with time) in Study 2, we conducted additional tests to determine whether treatment normalized sustained attention. We did this by comparing Study 2 ADHD data to Study 1 control data. Given the extensive literature on AX-CPTs with 400 uninterrupted trials (e.g., Halperin et al., 1991; Huang-Pollock et al., 2006), we focused on the 400-trial baseline data from Study 1 for the controls and the first 4 100-trial epochs in Study 2 for children with ADHD. If there was a vigilance deficit (Study 1 controls vs. Study 2 ADHD during the no-reinforcement+placebo condition), we tested the degree to which the single treatments and/or combination of reinforcement and methylphenidate normalized vigilance.
Results
Hits
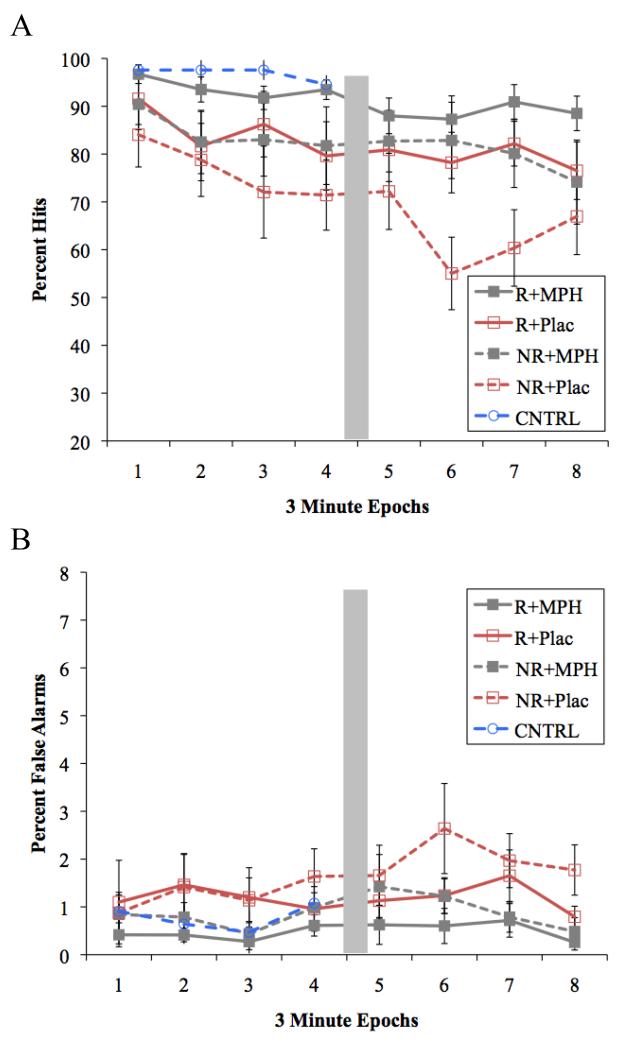
Averaged across epochs, the single treatments (the average of MPH alone and reinforcement alone) increased hit rate compared to no reinforcement + placebo, F(1,15) = 21.5, p <.001, d = 1.27 (see Figure 5A). Hit rate was very similar for reinforcement alone and MPH alone, F(1,15) =.001, p=.97, d=.20. Finally, the combination of reinforcement and MPH resulted in further improvement in hits compared to either treatment alone, F(1,15)=4.5, p=.05, d = .66.
Figure 5.
Mean percent (A) hits and (B) false alarms for all Medication × Reinforcement × Epoch conditions in Study 2. Performance for TD controls during the baseline visit is re-presented for normalization analyses. The grey box represents the brief time allowed for instructions following every four epochs. Error bars reflect standard error. R= Reinforcement, NR= No Reinforcement, MPH= Methylphenidate, Plac= Placebo.
Consistent with a vigilance decrement, hit rate decreased over the course of the task, Time linear and quadratic: Fs(1,15) = 23.2, 8.6, ps <.001 and = .010, ds =1.46, 1.1 respectively. The single treatments (reinforcement alone, MPH alone) attenuated the vigilance decrement compared to the no reinforcement + placebo condition, Treatment × Time linear and cubic, Fs(1, 15) = 3.8 and 9.3, ps = .07, .01, ds=.61, 1.27, respectively. As can be seen in Figure 5A, the difference in hit rate between no reinforcement + placebo and the single treatments generally grew larger over time (epochs 1 to 8 ps= .06, .07, .02, .05, .05, .002, .002, .08 respectively), although there was notable variability in the latter half of the time course of the no reinforcement + placebo condition. The vigilance decrement over time did not vary between reinforcement alone and MPH alone, Fs<1, nor did it significantly differ between the single treatments and their combination, Fs<1.8, ps > .20, ds<.54.
To determine whether treatment normalized the vigilance deficit for children with ADHD, we compared hit rates between children with ADHD in Study 2 and control children in Study 1 (see Figure 5A, Epochs 1-4). Consistent with a vigilance deficit, hit rate declined more across epochs among children with ADHD during the reinforcement+placebo condition than among the Study 1 controls during the baseline CPT (Control data in Figure 5 are copied from Figure 3), Group × Time linear and quadratic Fs=8.04 and 2.60, ps=.007 and .11, ds=.77 and .12; Group F=20.15, p<.001, d=1.1. This vigilance deficit remained when contrasting the single treatments (average of reinforcement alone and MPH alone) for the children with ADHD to baseline for controls (Group × Time linear and quadratic Fs=4.12 and 4.37, ps=.05 and .001, ds=.57 and .58; Group F=12.59, p=0.001, d=.92). However, the combination of reinforcement+ MPH among children with ADHD yielded sustained attention that was no longer significantly different from controls, Group Time linear and quadratic Fs .21 and 1.78, ps .65 and .19, ds=.13 and .03; main effect of group F=3.25, p=0.08, d=.51; main effects of time linear and quadratic Fs=6.26 and .01, ps=.02 and .93, d=.35 and .06. These results suggest that the reinforcement+MPH combination normalized sustained attention in children with ADHD.
False alarms
As can be seen in Figure 5B, false alarm rate increased over the course of the task in Study 2, time quadratic and cubic: Fs(1,15) = 3.4, 7.4, ps = .087, .016, ps = .33, .54 respectively. As predicted, receiving either MPH or reinforcement tended to reduce the number of false alarms overall compared to no treatment, F(1,15) = 3.2, p = .095, d = .48. There was no difference in the effectiveness of reinforcement alone compared to MPH alone on false alarm rate, F(1, 15) = 0.6, p = .445, d=0.21. The combination of reinforcement+MPH reduced false alarms compared to the single treatments, F(1, 54) = 8.2, p = .012, d = .79. Because interactions between treatment and time were all non-significant, Fs<2.6, ps > .13, no vigilance “normalization” tests were conducted for false alarms.
General Discussion
Deficient sustained attention and an atypical response to reinforcement are central constructs in ADHD psychopathology (e.g., Douglas, 1972, 1983; Haenlein and Caul, 1987; Nigg et al., 2005; Huang-Pollock et al., 2012; Luman et al., 2010). Study 1 examined whether sustained attention (i.e., performance over time, or vigilance) is weaker in children with ADHD compared to typically developing children and whether reinforcement differentially improved attention in children with ADHD. Study 2 examined the separate and combined effects of reinforcement and stimulant medication (MPH) on sustained attention in children with ADHD. Together, these studies demonstrated a vigilance deficit in children with ADHD. Reinforcement improved sustained attention in children with ADHD; a therapeutic dose of methylphenidate, a stimulant medication, had a comparable effect. The combination of reinforcement and stimulant medication normalized sustained attention in children with ADHD. Each of these findings is discussed in more detail below along with comparisons to the existing literature.
Vigilance Deficits
Although dozens of CPT studies report a lower overall hit rate in children with ADHD compared to control children (Epstein et al., 2003; Huang-Pollock et al., 2006; Nigg et al., 2005; Willcutt et al., 2005), surprisingly few studies have evaluated group differences in sustained attention over time, or vigilance (see Huang-Pollock et al., 2012). In Study 1, the data from the baseline session (see Figure 3) demonstrated the predicted vigilance deficit (e.g., Borger & van der Meere, 2000; Hooks, Milich, & Lorch, 1994; Huang-Pollock et al., 2006): as time on task increased, children with ADHD were less able to sustain their attention to detect targets.
Although control children did not exhibit a significant vigilance decrement during the baseline visit, this is likely the result of the short task duration (~12 minutes) of this commonly used CPT. CPTs of greater duration (30-min to 2 hours) reveal vigilance decrements even in healthy adults (see Mackworth, 1969). In retrospect, it is surprising that we have come to use such brief tasks of sustained attention in the ADHD literature. Both the sensitivity of CPTs to individual differences and the ecological validity of these tasks for relating to the duration of typical academic and recreational activities school children experience daily would be enhanced by increasing the duration of the typical CPT several-fold in future research.
In the present work, the changes in attention over time were not always linear, sometimes not even monotonic (see Figures 3-5). Although part of the variability across epochs may be due to error of measurement (there are only 10 targets per epoch) and to the occasional breaks in our reinforcement-manipulation CPT, similar patterns are evident in other studies (e.g., Huang-Pollock et al., 2006). The vigilance decrement not be a continuous gradual decrease in target detection but rather increases in the frequency or duration of intermittent periods of “tuning out”, after which attention rebounds for some time (see Mackworth, 1969). Stated differently, attention may become increasingly variable over time. Indeed, intra-subject variability on a smaller time scale has become the focus an important focus of recent work in ADHD. Moment-to-moment variability in reaction time is consistently greater among children with ADHD than among TD controls (Epstein, Langberg, et al., 2011; Nigg et al., 2005; Tamm et al., 2012). This variability is due to occasional very slow reaction times that are thought to reflect lapses in attention and impaired attention regulation (e.g, Leth-Steensen, Elbaz, & Douglas, 2000). Consistent with this perspective, a recent study with healthy adults suggests that intra-subject RT variability increases over the course of a 3-hour task (Wang, Ding, & Kluger, 2014). Future work on sustained attention in ADHD may benefit from integrating hit rate and RT data over the course of very long tasks, with developmentally appropriate durations chosen to reflect demands common in daily life (e.g., school class periords, sports and games). Doing so will better characterize the vigilance deficit in ADHD and allow for better evaluations of the impact of reinforcement, stimulants, and other theory- and/or therapy-based manipulations of sustained attention.
Reinforcement and Methylphenidate
Unfortunately, we did not have the benefit of this hindsight when designing the reinforcement manipulation CPT for Study 1, which required only 6 minutes of continuous performance at a time. The repeated interruptions of the task to switch reinforcement condition did eliminate major order effects (c.f. Huang-Pollock et al., 2007; Shiels et al., 2008), but they also limited our ability to detect changes in vigilance. Indeed, there was no group difference in vigilance during the no-reinforcement blocks of the task, precluding any test of whether reinforcement attenuated or normalized a vigilance deficit in ADHD. It was some consolation that reinforcement tended to improve overall hit rate more among children with ADHD than among controls. As discussed following Study 1 above, these novel data are consistent with theories of ADHD that emphasize reinforcement in ADHD (e.g., Haenlein and Caul, 1987; Sagvolden et al., 2005; Tripp and Wickens, 2008). However, determining the impact of reinforcement on vigilance required an additional experiment with a longer-duration CPT.
To provide such a context, Study 2 employed a 45-minute CPT. Among the Study 2 sample of children with ADHD, the vigilance decrement under standard or no-treatment (no reinforcement, placebo) conditions was significantly attenuated by reinforcement alone and MPH alone. Hit rates for reinforcement alone and MPH alone were nearly identical, (Figure 5). Compared to the single treatments, the combination of reinforcement+MPH resulted in a further improvement in overall hit rate, but not vigilance, across the 8-epoch time series. Importantly, the beneficial effects of reinforcement and MPH on target detection (hit rate) were not simply due an increase in overall responding, as these treatments decreased responding to non-targets (i.e., false alarms) in Studies 1 and 2.
The robust effects of reinforcement on vigilance observed in Study 2 may be reconciled with the absence of such effects in the one prior study in the area (Solanto et al., 1997) by considering the relative strength of the two manipulations. Compared to Solanto et al., we used a lower dose of MPH (.3 vs. .6 mg/kg) but a stronger reinforcer. Clinically, the impact of stimulants and behavior therapy depend on relative dose (Fabiano et al., 2007); the combined results of Solanto et al. and the present studies suggest that the same is true in the laboratory.
Dosing is also relevant to our cross-study evaluation of normalization. Compared to control children from Study 1, children with ADHD in Study 2 exhibited a vigilance deficit in the absence of treatment (i.e., no reinforcement+placebo; see Figure 5a Epochs 1-4). Although reinforcement alone and .3 mg/kg MPH alone did not normalize vigilance, the combination treatment (i.e., reinforcement + MPH) did. That is, the robust vigilance deficit in the absence of treatment (d=.77) was completely eliminated in the reinforcement+MPH condition (d=.13). To our knowledge, these data are the first demonstration that combined behavioral and stimulant treatment can normalize sustained attention over time in children with ADHD. Higher doses of either treatment alone may have a similar effect (e.g., Fabiano et al., 2007).
“Dose” of reinforcement is a function of the type and magnitude of the reinforcer and the schedule of reinforcement. The continuous schedule of reinforcement (FR1) used in the present studies is not feasible in most school settings. On the other hand, providing only summary feedback and a “prize” at the end of a task may not be sufficient to reinforce desired behavior (e.g., Epstein, Brinkman, et al., 2011), particularly given the theorized sensitivity of children with ADHD to immediate consequences (Sagvolden et al., 2005; Sonuga-Barke, 2002). However, there is a wide range of possibilities between these two extremes. For example, a teacher might provide reinforcement every five minutes or after each worksheet of a multi-sheet assignment. This highlights the need to directly compare multiple lean-to-rich schedules of reinforcement in children with ADHD (Douglas & Parry, 1983; Tripp & Wickens, 2008). The impact of reinforcement schedule may vary depending on the magnitude of each individual reinforcer, and it may be useful to consider the two concurrently (e.g., Luman, Van Meel, Oosterlaan, Sergeant, & Geurts, 2009).
Finally, it is unclear whether reinforcement (or medication) effects on vigilance and other cognitive functions in ADHD persist beyond one or two sessions. In fact, there is good evidence from preclinical work with rats and from research with food reinforcement with humans that reinforcers lose their effectiveness with repeated presentations. We hypothesize that this habituation of reinforcer effectiveness occurs more quickly among children with ADHD, though stimulants and the use of a variety of reinforcers may offset the proposed deficit (Lloyd, Medina, Hawk, Fosco, & Richards, 2014). We hope to test these hypotheses in upcoming experiments.
Overall, the present extends the surprisingly small ADHD literature on vigilance, or sustained attention over time. This work is novel in demonstrating that reinforcement improves vigilance in children with ADHD. The size of the reinforcement effect was comparable to that obtained with a therapeutic dose of stimulant medication. Further, the combination of reinforcement and MPH normalized vigilance in children with ADHD relative to typically developing controls. Rather than providing evidence for motivational theories over cognitive models of ADHD, the present work calls attention to the intersection of cognition and reinforcement (e.g., Douglas, 1999; Uebel et al., 2010) for understanding basic mechanisms of ADHD and its treatment with behavior therapy and stimulant medication.
Acknowledgments
We thank Dominica Vito for coordination of the project, Mark Kogutowski for computer programming, and Sarah Spencer and Mike Strand for their assistance with data collection. We appreciate the time and effort taken by the families who participated in these studies. This research was supported by grants from the National Institute of Mental Health (R01MH069434 and 3R01MH069434-04S1) to Larry Hawk.
Footnotes
Although a1500-ms response window is typical (e.g., Halperin et al., 1991; Huang Pollock et al., 2006), the vast majority of hits occur within the first 1000 ms (e.g., 98% in Study 1 Baseline CPT), suggesting that the change response window change did not have a major impact on the data.
For fully within-sujects comparisons, , where d3′=(mean-0)/standard deviation (Cohen, 1988).
Finally, false alarm rates were higher, on average, among children with ADHD that received reinforcement first compared to children with ADHD that received the reinforcement second (means [SDs] = 1.80 [1.28] and 1.09 [.90], respectively, p = .03), whereas reinforcement order did not affect the false alarm rate in controls (p = .55); Group × Reinforcement Order, F(1, 54) = 4.5, p = .04; all other reinforcement order effect ps > .18).
At the request of a reviewer, we compared the two subsamples in supplemental analyses. Hit rates were comparable during the baseline condition of Study 2 (no reinforcement + placebo condition, p=.20).
References
- American Academy of Pediatrics Clinical practice guidelines: Treatment of the school-age child with attention-deficit/hyperactivity disorder. Pediatrics. 2001;108:1–16. [Google Scholar]
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 5th ed. American Psychiatric Association; Arlington, VA: 2013. [Google Scholar]
- Annett John. Feedback and human behaviour: The effects of knowledge of results, incentives and reinforcement on learning and performance. Penguin; Harmondsworth: 1969. [Google Scholar]
- Borger N, van der Meere J. Visual behaviour of ADHD children during an attention test: an almost forgotten variable. Journal of Child Psychology Psychiatry. 2000;41:525–532. doi: 10.1017/s0021963000005655. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Sonuga-Barke EJ, Milham MP, Tannock R. Characterizing cognition in ADHD: beyond executive dysfunction. Trends in Cognitive Sciences. 2006;10:117–123. doi: 10.1016/j.tics.2006.01.011. doi: 10.1016/j.tics.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Cohen Jacob. Statistical power analysis for the behavioral sciences. 2nd ed. L. Erlbaum Associates; Hillsdale, N.J.: 1988. [Google Scholar]
- Craighead WE, Kazdin AE, Mahoney MJ. Behavior modification: Principles, issues, and applications. Houghton Mifflin; Boston: 1976. [Google Scholar]
- Douglas VI. Stop, look and listen: The problem of sustained attention and impulse control in hyperactive and normal children. Canadian Journal of Behavioural Science. 1972;4:259–282. [Google Scholar]
- Douglas VI. Attention and cognitive problems. In: Rutter M, editor. Developmental neuropsychiatry. Guilford Press; New York: 1983. pp. 280–329. [Google Scholar]
- Douglas VI. Cognitive control processes in attention-deficit/hyperactivity disorder. In: Quay HC, Hogan AE, editors. Handbook of disruptive behavior disorders. Kluwer Academic Publishers; Dordrecht, Netherlands: 1999. pp. 105–138. [Google Scholar]
- Douglas VI, Parry PA. Effects of reward on delayed reaction time task performance of hyperactive children. Journal of Abnormal Child Psychology. 1983;11:313–326. doi: 10.1007/BF00912094. [DOI] [PubMed] [Google Scholar]
- Epstein JN, Brinkman WB, Froehlich T, Langberg JM, Narad ME, Antonini TN, Altaye M. Effects of stimulant medication, incentives, and event rate on reaction time variability in children with ADHD. Neuropsychopharmacology. 2011;36:1060–1072. doi: 10.1038/npp.2010.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein JN, Conners CK, Hervey AS, Tonev ST, Arnold LE, Abikoff HB, Wigal T. Assessing medication effects in the MTA study using neuropsychological outcomes. Journal of Child Psychology and Psychiatry. 2006;47:446–456. doi: 10.1111/j.1469-7610.2005.01469.x. [DOI] [PubMed] [Google Scholar]
- Epstein JN, Erkanli A, Conners CK, Klaric J, Costello JE, Angold A. Relations between continuous performance test performance measures and ADHD behaviors. Journal of Abnormal Child Psychology. 2003;31:543–554. doi: 10.1023/a:1025405216339. [DOI] [PubMed] [Google Scholar]
- Epstein JN, Langberg JM, Rosen PJ, Graham A, Altaye M. Evidence for higher reaction time variability for children with ADHD on a range of cognitive tasks including reward and event rate manipulations. Neuropsychology. 2011;25:427–441. doi: 10.1037/a0022155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiano GA, Pelham WE, Jr., Gnagy EM, Burrows-Maclean L, Coles EK, Chacko A, Robb JA. The single and combined effects of multiple intensities of behavior modification and methylphenidate for children with attention deficit hyperactivity disorder in a classroom setting. School Psychology Review. 2007;36:195–216. [Google Scholar]
- Fabiano GA, Pelham WE, Jr., Waschbusch DA, Gnagy EM, Lahey BB, Chronis AM, Burrows-MacLean L. A practical measure of impairment: psychometric properties of the impairment rating scale in samples of children with Attention Deficit Hyperactivity Disorder and two school-based samples. Journal of Clinical Child and Adolescent Psychology. 2006;35:369–385. doi: 10.1207/s15374424jccp3503_3. [DOI] [PubMed] [Google Scholar]
- Greenhill LL, Pliszka S, Dulcan MK, Bernet W, Arnold V, Beitchman J, Stock S. Practice parameter for the use of stimulant medications in the treatment of children, adolescents, and adults. Journal of the American Academy of Child and Adolescent Psychiatry. 2002;41(2 Suppl):26S–49S. doi: 10.1097/00004583-200202001-00003. [DOI] [PubMed] [Google Scholar]
- Haenlein M, Caul WF. Attention deficit disorder with hyperactivity: a specific hypothesis of reward dysfunction. Journal of the American Academy of Child Adolescent Psychiatry. 1987;26:356–362. doi: 10.1097/00004583-198705000-00014. doi: 10.1097/00004583-198705000-00014. [DOI] [PubMed] [Google Scholar]
- Halperin JM, Greenblatt ER, Wolf LE, Young G. Subtype analysis of comission errors on the continuous perfromance test in children. Developmental Psychology. 1991;7:207–217. [Google Scholar]
- Halperin JM, Trampush JW, Miller CJ, Marks DJ, Newcorn JH. Neuropsychological outcome in adolescents/young adults with childhood ADHD: profiles of persisters, remitters and controls. Journal of Child Psychology and Psychiatry. 2008;49:958–966. doi: 10.1111/j.1469-7610.2008.01926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halperin JM, Wolf LE, Pascualvaca DM, Newcorn JH, Healey JM, O’Brien JD, Young JG. Differential assessment of attention and impulsivity in children. Journal of the American Academy of Child and Adolescent Psychiatry. 1988;27:326–329. doi: 10.1097/00004583-198805000-00010. [DOI] [PubMed] [Google Scholar]
- Hooks K, Milich R, Lorch EP. Sustained and selective attention in boys with Attention-Deficit Hyperactivity disorder. Journal of Clinical Child Psychology. 1994;23:69–77. [Google Scholar]
- Huang-Pollock CL, Karalunas SL, Tam H, Moore AN. Evaluating vigilance deficits in ADHD: A meta-analysis of CPT performance. Journal of Abnormal Psychology. 2012;121:360–371. doi: 10.1037/a0027205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang-Pollock CL, Mikami AY, Pfiffner L, McBurnett K. ADHD subtype differences in motivational responsivity but not inhibitory control: evidence from a reward-based variation of the stop signal paradigm. Journal of Clinical Child and Adolescent Psychology. 2007;36:127–136. doi: 10.1080/15374410701274124. [DOI] [PubMed] [Google Scholar]
- Huang-Pollock CL, Nigg JT, Halperin JM. Single dissociation findings of ADHD deficits in vigilance but not anterior or posterior attention systems. Neuropsychology. 2006;20:420–429. doi: 10.1037/0894-4105.20.4.420. [DOI] [PubMed] [Google Scholar]
- Kluger AN, DeNisi A. The effects of feedback interventions on performance: A historical review, a meta-analysis, and a preliminary feedback intervention theory. Psychological Bulletin. 1996;119:254–284. [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Motivated attention: Affect, activation, and action. Attention and orienting: Sensory and motivational processes. 1997:97–135. [Google Scholar]
- Leth-Steensen C, Elbaz ZK, Douglas VI. Mean response times, variability, and skew in the responding of ADHD children: a response time distributional approach. Acta Psychologica. 2000;104:167–190. doi: 10.1016/s0001-6918(00)00019-6. [DOI] [PubMed] [Google Scholar]
- Lloyd DR, Medina DJ, Hawk LW, Fosco WD, Richards JB. Habituation of reinforcer effectiveness. Frontiers in Integrative Neuroscience. 2014;7:107. doi: 10.3389/fnint.2013.00107. doi: 10.3389/fnint.2013.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losier BJ, McGrath PJ, Klein RM. Error patterns on the continuous performance test in non-medicated and medicated samples of children with and without ADHD: a meta-analytic review. Journal of Child Psychology and Psychiatry and Allied Disciplines. 1996;37:971–987. doi: 10.1111/j.1469-7610.1996.tb01494.x. [DOI] [PubMed] [Google Scholar]
- Luman M, Oosterlaan J, Sergeant JA. The impact of reinforcement contingencies on AD/HD: a review and theoretical appraisal. Clinical Psychology Review. 2005;25:183–213. doi: 10.1016/j.cpr.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Luman M, Tripp G, Scheres A. Identifying the neurobiology of altered reinforcement sensitivity in ADHD: a review and research agenda. Neuroscience and Biobehavioral Reviews. 2010;34:744–754. doi: 10.1016/j.neubiorev.2009.11.021. [DOI] [PubMed] [Google Scholar]
- Luman M, Van Meel CS, Oosterlaan J, Sergeant JA, Geurts HM. Does reward frequency or magnitude drive reinforcement-learning in attention-deficit/hyperactivity disorder? Psychiatry Research. 2009;168:222–229. doi: 10.1016/j.psychres.2008.08.012. [DOI] [PubMed] [Google Scholar]
- Mackworth Jane F. Vigilance and habituation: a neuropsychological approach. Penguin; Harmondsworth: 1969. [Google Scholar]
- Nigg JT, Willcutt EG, Doyle AE, Sonuga-Barke EJ. Causal heterogeneity in attention-deficit/hyperactivity disorder: do we need neuropsychologically impaired subtypes? Biological Psychiatry. 2005;57:1224–1230. doi: 10.1016/j.biopsych.2004.08.025. [DOI] [PubMed] [Google Scholar]
- Pelham WE, Jr., Fabiano GA. Evidence-based psychosocial treatments for attention-deficit/hyperactivity disorder. Journal of Clinical Child and Adolescent Psychology. 2008;37:184–214. doi: 10.1080/15374410701818681. doi: 10.1080/15374410701818681. [DOI] [PubMed] [Google Scholar]
- Pelham WE, Jr., Fabiano GA, Massetti GM. Evidence-Based Assessment of Attention Deficit Hyperactivity Disorder in Children and Adolescents. Journal of Clinical Child and Adolescent Psychology. 2005;34:449–476. doi: 10.1207/s15374424jccp3403_5. [DOI] [PubMed] [Google Scholar]
- Pelham WE, Jr., Gnagy EM, Burrows-Maclean L, Williams A, Fabiano GA, Morrisey SM, Morse GD. Once-a-day Concerta methylphenidate versus three-times-daily methylphenidate in laboratory and natural settings. Pediatrics. 2001;107:E105. doi: 10.1542/peds.107.6.e105. [DOI] [PubMed] [Google Scholar]
- Pelham WE, Jr., Gnagy EM, Greenslade KE, Milich R. Teacher ratings of DSM-III-R symptoms for the disruptive behavior disorders. Journal of the American Academy of Child and Adolescent Psychiatry. 1992;31:210–218. doi: 10.1097/00004583-199203000-00006. [DOI] [PubMed] [Google Scholar]
- Riccio CA, Waldrop JJ, Reynolds CR, Lowe P. Effects of stimulants on the continuous performance test (CPT): Implications for CPT use and interpretation. The Journal of Neuropsychiatry and Clinical Neurosciences. 2001;13:326–335. doi: 10.1176/jnp.13.3.326. [DOI] [PubMed] [Google Scholar]
- Rosvold HE, Mirsky AF, Sarason I, Bransome ED, Jr., Beck L. A continuous performance test of brain damage. Journal of Consulting Psychology. 1956;20:343–350. doi: 10.1037/h0043220. [DOI] [PubMed] [Google Scholar]
- Rubia K, Halari R, Cubillo A, Mohammad AM, Brammer M, Taylor E. Methylphenidate normalises activation and functional connectivity deficits in attention and motivation networks in medication-naive children with ADHD during a rewarded continuous performance task. Neuropharmacology. 2009;57:640–652. doi: 10.1016/j.neuropharm.2009.08.013. [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Halari R, Matsukura F, Mohammad M, Taylor E, Brammer MJ. Disorder-specific dissociation of orbitofrontal dysfunction in boys with pure conduct disorder during reward and ventrolateral prefrontal dysfunction in boys with pure ADHD during sustained attention. The American Journal of Psychiatry. 2009;166:83–94. doi: 10.1176/appi.ajp.2008.08020212. [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith A, Taylor E. Performance of children with attention deficit hyperactivity disorder (ADHD) on a test battery of impulsiveness. Child Neuropsychology. 2007;13:276–304. doi: 10.1080/09297040600770761. [DOI] [PubMed] [Google Scholar]
- Sagvolden T, Johansen EB, Aase H, Russell VA. A dynamic developmental theory of attention-deficit/hyperactivity disorder (ADHD) predominantly hyperactive/impulsive and combined subtypes. The Behavioral and Brain Sciences. 2005;28:397–419. doi: 10.1017/S0140525X05000075. [DOI] [PubMed] [Google Scholar]
- Shaffer D, Fisher P, Lucas CP, Dulcan MK, Schwab-Stone ME. NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): Description, differences from previous versions, and reliability of some common diagnoses. Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39:28–38. doi: 10.1097/00004583-200001000-00014. [DOI] [PubMed] [Google Scholar]
- Shiels K, Hawk LW, Jr., Lysczek CL, Tannock R, Pelham WE, Jr., Spencer SV, Waschbusch DA. The effects of incentives on visual-spatial working memory in children with attention-deficit/hyperactivity disorder. Journal of Abnormal Child Psychology. 2008;36:903–913. doi: 10.1007/s10802-008-9221-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiels K, Hawk LW, Reynolds B, Mazzullo RJ, Rhodes JD, Pelham WE, Gangloff BP. Effects of methylphenidate on discounting of delayed rewards in attention deficit/hyperactivity disorder. Experimental and Clinical Psychopharmacology. 2009;17:291–301. doi: 10.1037/a0017259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solanto MV, Wender EH, Bartell SS. Effects of methylphenidate and behavioral contingencies on sustained attention in attention-deficit hyperactivity disorder: a test of the reward dysfunction hypothesis. Journal of Child and Adolescent Psychopharmacology. 1997;7:123–136. doi: 10.1089/cap.1997.7.123. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJ. Psychological heterogeneity in AD/HD--a dual pathway model of behaviour and cognition. Behavioural Brain Research. 2002;130:29–36. doi: 10.1016/s0166-4328(01)00432-6. [DOI] [PubMed] [Google Scholar]
- Spencer SV, Hawk LW, Jr., Richards JB, Shiels K, Pelham WE, Jr., Waxmonsky JG. Stimulant treatment reduces lapses in attention among children with ADHD: The effects of methylphenidate on intra-individual response time distributions. Journal of Abnormal Child Psychology. 2009;37:805–816. doi: 10.1007/s10802-009-9316-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand MT, Hawk LW, Jr., Bubnik M, Shiels K, Pelham WE, Jr., Waxmonsky JG. Improving working memory in children with attention-deficit/hyperactivity disorder: The separate and combined effects of incentives and stimulant medication. Journal of Abnormal Child Psychology. 2012;40:1193–1207. doi: 10.1007/s10802-012-9627-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamm L, Narad ME, Antonini TN, O’Brien KM, Hawk LW, Jr., Epstein JN. Reaction time variability in ADHD: a review. Neurotherapeutics. 2012;9:500–508. doi: 10.1007/s13311-012-0138-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripp G, Wickens JR. Research review: dopamine transfer deficit: A neurobiological theory of altered reinforcement mechanisms in ADHD. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2008;49:691–704. doi: 10.1111/j.1469-7610.2007.01851.x. [DOI] [PubMed] [Google Scholar]
- Uebel H, Albrecht B, Asherson P, Börger NA, Butler L, Chen W, Banaschewski T. Performance variability, impulsivity errors and the impact of incentives as gender-independent endophenotypes for ADHD. Journal of Child Psychology and Psychiatry. 2010;51:210–218. doi: 10.1111/j.1469-7610.2009.02139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Ding M, Kluger BM. Change in intraindividual variability over time as a key metric for defining performance-based cognitive fatigability. Brain and Cognition. 2014;85C:251–258. doi: 10.1016/j.bandc.2014.01.004. doi: 10.1016/j.bandc.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willcutt Erik G., Doyle Alysa E., Nigg Joel T., Faraone Stephen V., Pennington Bruce F. Validity of the executive function theory of Attention-Deficit/Hyperactivity Disorder: A meta-analytic review. Biological Psychiatry. 2005;57:1336–1346. doi: 10.1016/j.biopsych.2005.02.006. [DOI] [PubMed] [Google Scholar]