Abstract
Caffeine, standard treatment for apnea of prematurity, improves brainstem auditory processing. We hypothesized that caffeine also improves cortical differentiation of complex speech sounds. We used event-related potential methodology to measure responses to speech-sound contrasts in 45 intensive care neonates, stratified by cumulative exposure as no-, low-, and high-caffeine groups. Sound differentiation in the low-caffeine group and near-term no-caffeine infants was similar with repeated measures ANOVA controlling for gestational and postnatal age. However, a generalized estimating equation approach demonstrated that, at equivalent postnatal age, differentiation was reduced in the high-caffeine (gestational age 25 weeks) compared to the low-caffeine group (gestational age 28 weeks), reflecting the importance of maturity at birth (Z=2.77, p<0.006).
We conclude that caffeine improves measures of auditory processing associated with improved neurodevelopmental outcomes in preterm infants. However, current usage of caffeine for apnea of prematurity cannot fully compensate for the effects of brain immaturity on speech sound processing.
Keywords: event-related potential, caffeine, auditory, preterm, neonate
Introduction
Apnea of prematurity affects a majority of infants born at low gestational ages.1,2 The resultant hypoxemia can lead to decreased cerebral blood oxygenation3,4 and potential neural injury.5–7 Caffeine administration is the standard treatment to prevent apnea in preterm infants, as it increases respiratory drive by stimulating respiratory centers in the ventral medulla.8,9 Although studies have examined the short- and long-term effects of caffeine administration on chronic lung disease and neurodevelopment,6,10–12 little is known about how caffeine affects electrophysiologic function in the developing cortex of human infants. A recent neuroimaging study suggests that caffeine exposure may be associated with improved cortical white matter microstructure,1,2,13 but little research exists on the functional effects of caffeine in neonatal brains.
In infants with apnea of prematurity, brainstem neural processing (assessed using auditory brainstem responses) is faster in response to individual tones or clicks in patients receiving caffeine compared to controls.3,4,14 However, whether caffeine also affects cortical processing of complex auditory stimuli, such as speech sounds, is unknown. Event-related potential methodology records cortical brain activity time-locked to stimulus onset and has been used to measure preterm infants’ ability to differentiate speech sounds.5–7,15–18 In newborns requiring intensive care, event-related potential responses to speech sounds are highly predictive of cognitive and communication outcomes at 1 and 2 years of age.8,9,19,20 Furthermore, these responses are influenced by gestational age at birth and postnatal age, reflecting the effects of immaturity and experience on brain development and function.6,10–12,21
We hypothesized that caffeine exposure would be associated with improved cortical processing of auditory stimuli, or greater sound differentiation, after accounting for neural immaturity at birth. To test this hypothesis, we prospectively studied event-related potential responses in newborn infants throughout a range of gestational ages and analyzed the effects of caffeine exposure on neural processing of speech-sound differentiation.
Methods
Subjects
Subjects who met study criteria were selected from 2 prospective observational cohorts of infants cared for in the neonatal intensive care unit at Monroe Carell Jr Children’s Hospital at Vanderbilt between 01/07 and 01/10. The 2 studies in which the infants participated included medical data and event-related potential measurements prior to hospital discharge. The Vanderbilt Institutional Review Board approved both protocols. Inclusion criteria for the subset of infants in the present study were respiratory stability in room air without need for nasal continuous positive airway pressure, or high-flow nasal cannula at the time of testing. We excluded infants with congenital brain abnormalities or genetic syndromes, presence of severe white matter injury on any cranial ultrasound examination, neuropathic hearing loss, lack of available data on caffeine exposure, or exposure to any sedative agent within 24 hours of event-related potential testing. Prior to testing, all infants had serial cranial ultrasound examinations as part of the neonatal intensive care unit standard of care; a pediatric radiologist evaluated them for the presence of severe white matter injury defined as Grade III or IV intraventricular hemorrhage according to the Papile classification22 or periventricular leukomalacia. Infants also had routine hearing screening at approximately 34 weeks postmenstrual age, using auditory brainstem responses, performed by a pediatric audiologist to evaluate neuropathic hearing loss. All event-related potential testing was performed after 32 weeks postmenstrual age, when infants were considered clinically stable by the medical team and when they had reached the minimal testing head circumference requirements of 31 cm.
Caffeine Exposure
According to routine practice at our institution, infants born before 34 weeks gestational age were candidates to receive caffeine to prevent or treat apnea of prematurity. Caffeine is usually started within the first 72 hours after birth in very preterm infants, and typically discontinued by 34 weeks postmenstrual age or earlier if apnea has resolved (5 days without apnea). Caffeine is not used for therapeutic purposes other than apnea in our unit. We extracted data on daily doses of caffeine from pharmacy records and calculated cumulative exposure at the time of event-related potential testing. Cumulative caffeine exposure was also calculated as the algebraic sum of daily dose/kg and expressed in mg/kg.
Event-related potential response methodology
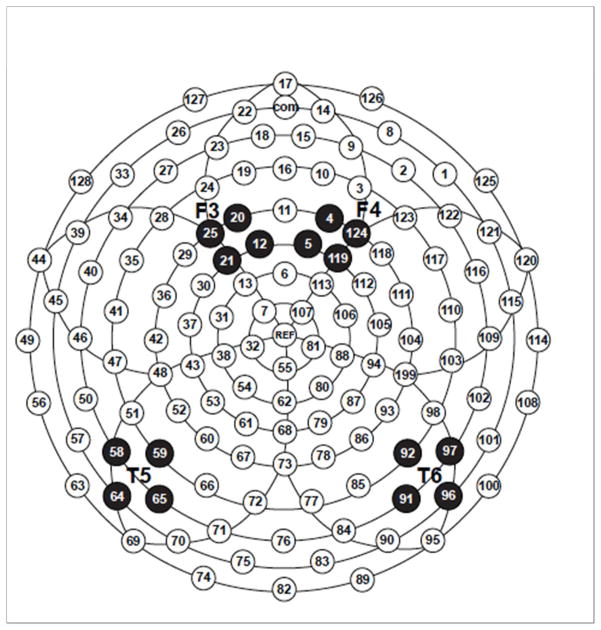
Our methodology has been previously described in detail.19–21 Briefly, six computer-synthesized consonant-vowel syllables (/ba/, /da/, /ga/, /bu/, /du/, /gu/), from the series employed by Stevens and Blumstein23 were used as stimuli. To record infant event-related potential responses, we used a high-density array of 124 Ag/AgCl electrodes embedded in soft sponges saturated with warm saline solution (Geodesic Sensor Net without the lower eye channels, EGI, Inc., Eugene, OR). Electrode impedance levels were below 40 kK before and after testing, while the low-pass filter was set to 30 Hz and the high-pass filter to 0.1 Hz. During acquisition, data were sampled at 250 Hz. All electrodes were referred to vertex and then re-referenced offline to an average reference. A subset of electrodes corresponding to frontal and temporal locations in the left and right hemisphere was analyzed (Figure 1). These locations are thought to reflect activity in the primary auditory cortices and higher processing areas.24
Figure 1. Event-related potential scalp location map.
F3: Frontal left F4: Frontal Right T5: Temporal Left T4 Temporal Right
Infants were tested in quiet-alert state while lying without restraint in the bassinet or in the caregiver’s arms with both ears unobstructed. The syllables were presented by a computer at 80 dB SPL(A) as measured at the infant’s ear through a speaker positioned approximately one meter above the midline of the infant’s head. The six sounds were each presented 25 times in random order, for a total of 150 trials. Inter-stimulus intervals varied randomly from 1600 to 2600 ms to prevent habituation to sound onset. Recording of the brainwaves was controlled by Net Station software (v. 4.2, EGI, Inc., Eugene, OR). Stimulus presentation was controlled by E-Prime (v. 1.2, PST, Inc., Pittsburgh, PA). The infant’s electroencephalogram (EEG) and behavior were continuously monitored so that the stimulus presentation occurred only when the infant was aligned with the speaker and the EEG was free of motor artifacts. Testing time took less than 10 minutes. Event-related potential data were analyzed and prepared as previously described.21 Inclusion of a participant’s data set in the overall analyses required that averages for each stimulus be based on a minimum of 10 trials. Mean amplitudes were calculated for each selected electrode location by averaging readings within 150–250 ms and 250–400 intervals after stimulus onset, based on previous studies in healthy newborn infants17 and neonatal intensive care unit patients.19–21
Statistical analysis
Infants were stratified into 3 groups based on caffeine exposure: no-caffeine, low-caffeine and high-caffeine groups divided along the median exposure. To examine the effects of caffeine on brain function, we began with a repeated-measures analysis of variance (RM-ANOVA). While this statistical model has been questioned,25 it most closely replicates past work in the field. In the present analysis, speech-sound differentiation was quantified as the absolute difference in mean amplitude between two contrasting auditory stimuli (e.g., /ba/-/ga/). These discrimination scores served as the dependent variables in an RM-ANOVA with caffeine exposure group (none, low, high) as the between-subject factor and sound contrast (/ba-ga/, /da-ga/, /bu-gu/, /du-gu/) X time window (150–250 ms, 250–400 ms) X electrode cluster (frontal, temporal) X hemisphere (left, right) within-subject factors. Huynh-Feldt correction was used for correlated errors that occur with violations of sphericity. Gestational age at birth and postnatal age at the time of event-related potential testing were entered as covariates. Significant main effects and interactions involving caffeine exposure group and sound contrast factors were followed by planned comparisons using one-way ANOVAs.
To examine the differences in speech-sound processing between low- and high-caffeine groups, we also performed a follow-up analysis using generalized estimating equations. We simultaneously examined /da-ga/ discrimination (the contrast most highly correlated with neurodevelopmental outcomes at 2 years of age) over 2 time-windows and 4 scalp locations and accounted for postnatal and gestational ages.
Results
Population characteristics
Of the 57 infants in the original cohort, 45 infants met criteria for inclusion in the study. These infants included 14 with no caffeine exposure and 31 treated with caffeine, including two who were still receiving a daily caffeine dose at the time of testing but had not received it within 12 hours of testing. These two infants were receiving 1 mg/kg/d and 3 mg/kg/d and were off caffeine the next day and 3 days later, respectively. Analyses were performed with and without these two infants without altering the results, so they were included in the final analysis. The mean length of time between last caffeine dose and event-related potential testing in infants no longer on caffeine was 27 days, with a minimum of 4 days and with a mean postmenstrual age at last caffeine dose of 33.5 weeks. Among caffeine-exposed infants, average daily doses over the course of their treatment ranged from 2.1 to 23.2 mg/kg/d and the total median exposure for all infants was 145.8 mg/kg. The median daily dose of 5.2 mg/kg reflects the recommended daily doses of 5–10 mg/kg/d. The wide range is due to additional doses when infants continued to have apnea and insufficient dosing was suspected and, on the lower end, to recommended doses that had not been adjusted for weight gain as infants approached 34 weeks post-menstrual age. Based on the median cumulative dose, exposed infants were divided into low- (n=10) and high- (n=21) caffeine groups. Table 1 summarizes characteristics of the three caffeine exposure groups. Groups did not differ statistically in postnatal age at testing or sex. As expected, gestational age and birth weight were lower in the caffeine-exposed groups than the unexposed group (p=0.001 for both), and lower in the high- than low- caffeine groups (p=0.007).
Table 1.
Study population characteristics
| No caffeine (N=14) | Low caffeine (N=10) | High caffeine (N=21) | |
|---|---|---|---|
| Sex (M/F) | 8/6 | 6/4 | 13/8 |
| Gestational age in weeks (mean, SD) | * 34.9 ± 3.5 | 28.7 ± 3.7 | ** 25.9 ± 1.8 |
| Postnatal age at ERP in days (mean, SD) | * 48.4 ± 31.7 | 70.8 ± 33.8 | N75.8 ± 25.5 |
| Postmenstrual age at ERP in weeks (mean, SD) | *41.1 ± 3.6 | 38.8 ± 4.4 | N 36.8 ± 3.7 |
| Cumulative caffeine at ERP (median, mg/kg) | 0 | 145.75 | 322.3 |
p for No caffeine vs. any caffeine (Low or High) < 0.01
p for Low vs High caffeine exposure groups < 0.01
p > 0.7 for Low vs High caffeine exposure groups
Caffeine Exposure Effects on Speech Sound Differentiation
Event-related potential measurements were successfully obtained in all infants. Artifact rejection rates were comparable across stimulus conditions with the final averages based on 14.8+/−3.7 trials per condition (/ba/: 14.8+/−3.8; /da/: 14.8+/−3.9; /ga/: 15.0+/−3.5; /bu/: 14.7+/−3.5; /du/: 14.8+/−3.8; /gu/: 15.1+/−4.0).
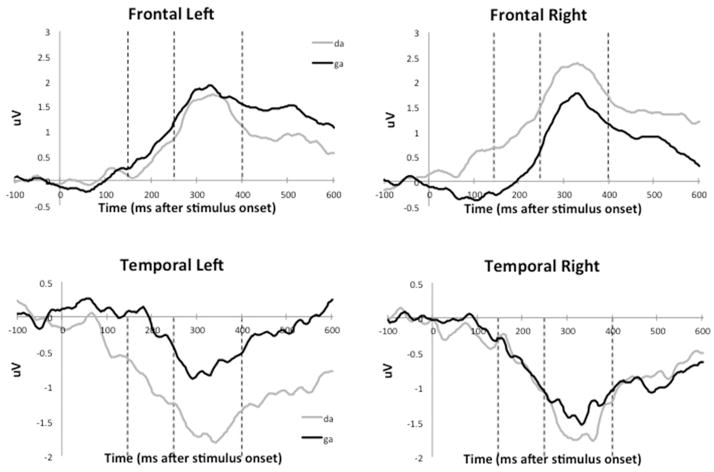
Figure 2 shows the grand average event-related potential waveform tracings for all 45 infants. Preterm infants do not demonstrate well-demarcated peaks in response to the speech-sound stimuli. Differences between responses to speech sounds are apparent in the early prespecified time-windows as amplitude differences between each tracing. The RM-ANOVA identified one significant interaction involving the variables of interest: caffeine exposure group X sound contrast X time window X electrode X hemisphere, with F(6,117)=2.18, p=.04 and partial η2=.10. Neither gestational age nor postnatal age made significant contributions to this interaction.
Figure 2. Event-related potential responses to speech sounds in intensive care neonates (Grand average waveforms of N = 45 patients).
Amplitude of the response to each of the sound stimuli in VV is represented by deflections from zero in the vertical axis. The frontal waveforms show a positive deflection after the stimulus onset while the temporal waveform shows a negative deflection reflecting the polarity reversal around the reference electrodes. The difference between the two tracings represents differences in cortical processes in response to the two sounds at various locations specified in Figure 1.
Follow-up one-way ANOVAs were used to narrow down the effect of caffeine exposure on speech-sound differentiation. Exposure-group differences were present for /da-ga/ contrast in the 150–250 ms window at the left frontal location, F(2,41)=3.35, p= .04, and right frontal location, F(2,41)=3.53, p=.03. Group differences were also observed at the left temporal location for the /da-ga/ F(2,41)=3.22, p=.05, and /ba-ga/, F(2,41)=3.33, p=.04, contrasts. The /da-ga/ difference at the left temporal location remained significant in the 250–400 ms interval, F(2,41)=3.77, p=.03.
No group differences in speech-sound differentiation were detected between the no-caffeine and low-exposure groups for any of the five event-related potential variables identified as sensitive to caffeine exposure (p values equal 0.11 to 0.73), even though mean values for sound discrimination appeared higher in the low-exposure group. Mean amplitudes in the high-exposure group were lower than the no-exposure group for the /da-ga/ contrast in the 150–250 ms interval at the left and right frontal electrodes, F(1,32)=4.36, p=.04 and F(1,32)=9.59, p=.004, respectively.
Comparison of low- and high-caffeine exposure effects on speech-sound discrimination
Speech-sound differentiation within 150–250 ms at the left frontal (/da-ga/: F(1,29)=7.09, p=.01) and left temporal locations (/da-ga/: F(1,29)=5.95, p=.02, /ba-ga/: F(1,29)=5.926, p=.02) was greater in the group with low-caffeine exposure than the high-exposure group. The left temporal /da-ga/ differences remained significant in the 250–400 ms window (F(1,29)=6.38, p=.01) locations. In all of these cases, infants in the low-exposure group evidenced greater sound differentiation than infants with high exposure to caffeine.
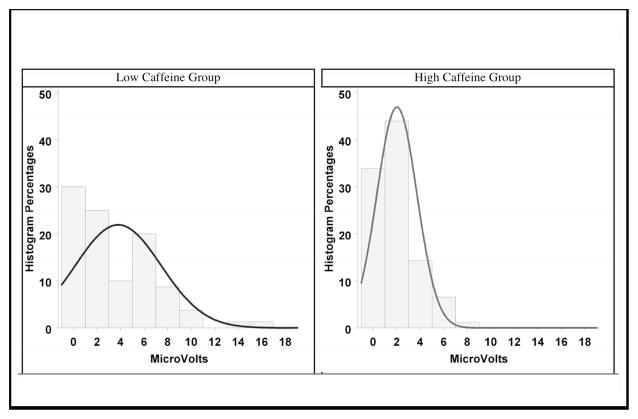
Histograms in Figure 3 show an obvious skew of the population and a Poisson-like increased variance in the group with higher mean caffeine exposure. The distributions are one-tailed, not two-tailed like the normal distribution, further justifying a secondary analysis with this model beyond the RM-ANOVA.26
Figure 3. Statistical distribution of speech sound differentiation scores in infants exposed to caffeine (Low and High).
Values on the Y-axis represent mean amplitudes in μV for the |ba − ga| differentiation.
The generalized estimating equations approach found better (/da-ga/) differentiation in the low-caffeine group (Z=2.77, p=.006) compared to the high-caffeine group (Table 2), confirming that the observed effect is related to caffeine differences in both groups and not to gestational or postnatal age differences alone.
Table 2.
Comparison of low and high caffeine exposure groups using a generalized estimating equation model
| Parameter | Estimate | Error | 95% CI | Z | Pr > |Z| |
|---|---|---|---|---|---|
| Intercept | 1.16 | 1.56 | −1.89, 4.21 | 0.74 | 0.46 |
| Caffeine | |||||
| Group | |||||
| Low | 0.72 | 0.26 | 0.21, 1.23 | 2.77 | 0.006 |
| High | 0 | 0 | 0, 0 | . | . |
| GA | −0.04 | 0.05 | −0.14, 0.07 | −0.70 | 0.48 |
| PNA | 0.01 | 0.00 | 0.00, 0.02 | 1.36 | 0.17 |
| Location | |||||
| Left Frontal | 0.21 | 0.19 | −0.16, 0.58 | 1.13 | 0.26 |
| Right Frontal | 0.04 | 0.17 | −0.29, 0.38 | 0.26 | 0.80 |
| Left Temporal | 0.28 | 0.18 | −0.07, 0.64 | 1.56 | 0.12 |
| Right Temporal | 0 | 0 | 0, 0 | . | . |
Pr >Z: probability
Discussion
Using event-related potentials, we found that caffeine exposure affected differentiation of speech sounds in preterm neonatal intensive care unit patients. Although preterm infants typically show reduced speech-sound discrimination compared to term infants, sound differentiation in the low-exposure group was similar to that of more mature infants not exposed to caffeine at equivalent postnatal age at testing. These results suggest that caffeine may improve cortical processing of auditory stimuli in preterm infants. Thus, caffeine may have contributed to accelerated brain maturation in the less immature infants, perhaps allowing auditory cortex function to be positively influenced by postnatal sound experience. Our work extends the findings of prior ABR studies in preterm infants showing that caffeine exposure can result in more optimal brainstem functioning as reflected in decreased interpeak latencies.14 A possible mechanism for caffeine’s effect on neonatal electrophysiologic function was postulated in a study of short-term effects of caffeine using amplitude-integrated EEG immediately after intravenous dosing.27 The authors speculated that the increased cortical activity demonstrated by enhanced continuity and amplitudes may be related to caffeine’s boosting of synaptic activity in preterm infants. To our knowledge, our study is the first to report the positive effects on cortical auditory function of caffeine treatment for apnea of prematurity.
Another possibility for caffeine’s effects of improved cortical function has been suggested by human and animal studies demonstrating that caffeine can improve intermittent hypoxia in preterm infants and through this mechanism, decrease perinatal white matter injury in mice.28,29 We can speculate that caffeine may have direct neuroprotective effects and may also decrease additional oxidative and inflammatory insults to the developing brain, thereby preserving cortical function.
We found that speech-sound differentiation was reduced in the high- compared to the low-caffeine exposure groups when tested at equivalent postnatal age. This is likely related to greater electrophysiologic immaturity of the brain in the high-exposure group,21 whose gestational age at birth was approximately 3 weeks earlier compared to the low-exposure group. We speculate that a minimal level of developmental maturity is needed to benefit from caffeine exposure and that caffeine alone is not sufficient to compensate for extreme prematurity. This interpretation is consistent with the differential effects of caffeine related to gestational age that have been reported in animal models for locomotor activity30 and seizure susceptibility.30,31 Postulated mechanisms for these differences include age- and brain-region-dependent distribution of A1/A2 receptors.32–34
Strengths of our study include the broad range of gestational ages in infants without severe white matter injury, and the caffeine exposures studied. Additionally, robust statistical methods accounted for both traditional analyses of event-related potentials and more clinically relevant analyses in a neonatal population. In particular, the RM-ANOVA approach had 3 important limitations: (1) imperfect Huynh-Feldt correction for the random effects of infant differences; (2) skewed non-normal distribution of sound discrimination scores, which appear closer to Poisson counts than to the normal distribution that RM-ANOVA presumes; (3) the unrealistic assumption of “compound symmetry,” or constant covariances across repeated measurements. The generalized estimating equations allowed us to avoid these limitations and provided an estimate of the simple main effect of caffeine without reporting 8 separate effects.35
We also acknowledge several limitations unavoidable due to the observational nature of the study. Caffeine administration was not controlled and blood levels of drug were not available. Thus, we can show only association rather than causation. Because caffeine dose was not directed by study protocol, we made a practical decision to separate exposure groups at the median cumulative caffeine dose. The relatively small sample size also limits in-depth analyses. Finally, our study was cross-sectional rather than longitudinal. A longitudinal study may answer the important question of whether caffeine facilitates maturation of cortical brain processing. In particular, repeated assessments of neural processing could help identify the timing of maximal caffeine effect on brain maturation.
In summary, our study shows that exposure to caffeine in preterm infants appears to improve cortical functioning as reflected by speech-sound discrimination, although it does not compensate for brain immaturity in extremely preterm infants. Because speech-sound discrimination is associated with cognitive and communication outcomes in early childhood,19,20 future studies should examine whether continued administration of caffeine age in the most immature infants would improve cortical processing as it does in the more mature infants.
Acknowledgments
Funding
This study was funded by a Turner-Hazinski Award from the Vanderbilt Department of Pediatrics, an 1R21 ES013730, the Gerber Foundation, Vanderbilt GCRC M01 RR 000095, NICHD P30HD15052 to the Vanderbilt Kennedy Center and Vanderbilt CTSA grant 1 UL1 RR024975.
Footnotes
Declaration of Conflicting Interests
None of the authors have any conflicts of interest.
Author Contributions
NM obtained grant funding for one of the studies, formulated the hypothesis, acquired the medical data, designed the initial analysis and wrote all drafts of the manuscript. JC collected the pharmacology data and contributed to the initial draft of the manuscript. AS refined the hypothesis and design and edited all drafts of the manuscript. WL performed and revised the statistical analysis and wrote the corresponding methods and discussion sections. JA obtained grant funding for one of the studies, contributed to the study design and edited the final draft of the manuscript. AK designed and implemented the testing paradigm, processed and analyzed the event-related potential data, directed the analysis and all drafts of the manuscript.
References
- 1.Eichenwald EC, Aina A, Stark AR. Apnea frequently persists beyond term gestation in infants delivered at 24 to 28 weeks. Pediatrics. 1997;100(3 Pt 1):354–359. doi: 10.1542/peds.100.3.354. [DOI] [PubMed] [Google Scholar]
- 2.Lorch SA, Srinivasan L, Escobar GJ. Epidemiology of apnea and bradycardia resolution in premature infants. Pediatrics. 2011;128(2):e366–e373. doi: 10.1542/peds.2010-1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yamamoto A, Yokoyama N, Yonetani M. Evaluation of change of cerebral circulation by SpO2 in preterm infants with apneic episodes using near infrared spectroscopy. Pediatrics International. 2003 doi: 10.1111/j.1442-200x.2003.01803.x. [DOI] [PubMed] [Google Scholar]
- 4.Urlesberger B, Kaspirek A, Pichler G. Apnoea of prematurity and changes in cerebral oxygenation and cerebral blood volume. Neuropediatrics. 1999 doi: 10.1055/s-2007-973453. [DOI] [PubMed] [Google Scholar]
- 5.Sinha SK, D’Souza SW, Rivlin E, Chiswick ML. Ischaemic brain lesions diagnosed at birth in preterm infants: clinical events and developmental outcome. Arch Dis Child. 1990;65(10 Spec):1017–1020. doi: 10.1136/adc.65.10_spec_no.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khairy M, Kokkotis A, Cormier C, Messmer D. Apnea is associated with neurodevelopmental impairment in very low birth weight infants. Journal of Perinatololgy. 2004 doi: 10.1038/sj.jp.7211182. [DOI] [PubMed] [Google Scholar]
- 7.Perlman JM, Volpe JJ. Episodes of apnea and bradycardia in the preterm newborn: impact on cerebral circulation. Pediatrics. 1985 [PubMed] [Google Scholar]
- 8.Martin RJ, Abu-Shaweesh JM. Control of breathing and neonatal apnea. Biol Neonate. 2005;87(4):288–295. doi: 10.1159/000084876. [DOI] [PubMed] [Google Scholar]
- 9.Ruggins NR. Pathophysiology of apnoea in preterm infants. Arch Dis Child. 1991;66(1 Spec):70–73. doi: 10.1136/adc.66.1_spec_no.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmidt B, Roberts RS, Davis P. Caffeine Therapy for Apnea of Prematurity — NEJM. N Engl J Med. 2006 doi: 10.1056/NEJMoa054065. [DOI] [PubMed] [Google Scholar]
- 11.Schmidt B, Roberts RS, Davis P. Long-term effects of caffeine therapy for apnea of prematurity. N Engl J Med. 2007 doi: 10.1056/NEJMoa073679. [DOI] [PubMed] [Google Scholar]
- 12.Howarth TM, Bork D, Dinu IA. Permanent bilateral sensory and neural hearing loss of children after neonatal intensive care because of extreme prematurity: a thirty-year study. Pediatrics. 2009 doi: 10.1542/peds.2008-2531. [DOI] [PubMed] [Google Scholar]
- 13.Doyle LWL, Cheong JJ, Hunt RWR, et al. Caffeine and brain development in very preterm infants. Ann Neurol. 2010;68(5):734–742. doi: 10.1002/ana.22098. [DOI] [PubMed] [Google Scholar]
- 14.Chen YJ, Liou CS, Tsai CH, Yeh TF. Effect of aminophylline on brain stem auditory evoked potentials in preterm infants. Arch Dis Child Fetal Neonatal Ed. 1994;71(1):F20–F23. doi: 10.1136/fn.71.1.f20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bisiacchi PS, Mento G, Suppiej A. Cortical auditory processing in preterm newborns: An ERP study. Biol Psychol. 2009;82(2):176–185. doi: 10.1016/j.biopsycho.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 16.Ceponiene R, Kushnerenko E, Fellman V, Renlund M, Suominen K, Näätänen R. Event-related potential features indexing central auditory discrimination by newborns. Brain Res Cogn Brain Res. 2002;13(1):101–113. doi: 10.1016/s0926-6410(01)00093-3. [DOI] [PubMed] [Google Scholar]
- 17.Kushnerenko E, Ceponiene R, Balan P, Fellman V, Huotilaine M, Näätäne R. Maturation of the auditory event-related potentials during the first year of life. Neuroreport. 2002;13(1):47–51. doi: 10.1097/00001756-200201210-00014. [DOI] [PubMed] [Google Scholar]
- 18.deRegnier R-A. Neurophysiologic evaluation of brain function in extremely premature newborn infants. Semin Perinatol. 2008;32(1):2–10. doi: 10.1053/j.semperi.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 19.Maitre NL, Slaughter JC, Aschner JL, Key AP. Hemisphere Differences in Speech-Sound Event-Related Potentials in Intensive Care Neonates: Associations and Predictive Value for Development in Infancy. J Child Neurol. 2013 doi: 10.1177/0883073813493502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maitre NL, Lambert WE, Aschner JL, Key AP. Cortical speech sound differentiation in the neonatal intensive care unit predicts cognitive and language development in the first 2 years of life. Dev Med Child Neurol. 2013;55(9):834–839. doi: 10.1111/dmcn.12191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Key APF, Lambert EW, Aschner JL, Maitre NL. Influence of gestational age and postnatal age on speech sound processing in NICU infants. Lit Discuss. 2012;49(5):720–731. doi: 10.1111/j.1469-8986.2011.01353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Papile L-A, Munsick-Bruno G, Schaefer A. Relationship of cerebral intraventricular hemorrhage and early childhood neurologic handicaps. J Pediatr. 1983;103(2):273–277. doi: 10.1016/s0022-3476(83)80366-7. [DOI] [PubMed] [Google Scholar]
- 23.Stevens KN, Blumstein SE. Invariant cues for place of articulation in stop consonants. J Acoust Soc Am. 1978;64(5):1358–1368. doi: 10.1121/1.382102. [DOI] [PubMed] [Google Scholar]
- 24.Key APF, Dove GO, Maguire MJ. Linking brainwaves to the brain: an ERP primer. Dev Neuropsychol. 2005;27(2):183–215. doi: 10.1207/s15326942dn2702_1. [DOI] [PubMed] [Google Scholar]
- 25.Nich C, Carroll K. Now you see it, now you don’t: a comparison of traditional versus random-effects regression models in the analysis of longitudinal follow-up data from a clinical trial. J Consult Clin Psychol. 1997;65(2):252–261. doi: 10.1037//0022-006x.65.2.252. [DOI] [PubMed] [Google Scholar]
- 26.Gardner W, Mulvey EP, Shaw EC. Regression analyses of counts and rates: Poisson, overdispersed Poisson, and negative binomial models. Psychol Bull. 1995;118(3):392–404. doi: 10.1037/0033-2909.118.3.392. [DOI] [PubMed] [Google Scholar]
- 27.Supcun S, Kutz P, Pielemeier W, Roll C. Caffeine Increases Cerebral Cortical Activity in Preterm Infants. The Journal of Pediatrics. 2010;156(3):490–491. doi: 10.1016/j.jpeds.2009.10.033. [DOI] [PubMed] [Google Scholar]
- 28.Rhein LM, Dobson NR, Darnall RA, et al. Effects of caffeine on intermittent hypoxia in infants born prematurely: a randomized clinical trial. JAMA Pediatr. 2014;168(3):250–257. doi: 10.1001/jamapediatrics.2013.4371. [DOI] [PubMed] [Google Scholar]
- 29.Back SAS, Craig AA, Luo NLN, et al. Protective effects of caffeine on chronic hypoxia-induced perinatal white matter injury. Ann Neurol. 2006;60(6):696–705. doi: 10.1002/ana.21008. [DOI] [PubMed] [Google Scholar]
- 30.Tchekalarova J, Kubova H, Mareš P. Postnatal caffeine exposure: effects on motor skills and locomotor activity during ontogenesis. Behav Brain Res. 2005;160(1):99–106. doi: 10.1016/j.bbr.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 31.Tchekalarova J, Kubová H, Mareš P. Effects of early postnatal caffeine exposure on seizure susceptibility of rats are age-and model-dependent. Epilepsy research. 2010 doi: 10.1016/j.eplepsyres.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 32.Londos C, Cooper DM, Wolff J. Subclasses of external adenosine receptors. Proc Natl Acad Sci U S A. 1980;77(5):2551–2554. doi: 10.1073/pnas.77.5.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guillet R, Kellogg CK. Neonatal caffeine exposure alters developmental sensitivity to adenosine receptor ligands. Pharmacol Biochem Behav. 1991;40(4):811–817. doi: 10.1016/0091-3057(91)90091-f. [DOI] [PubMed] [Google Scholar]
- 34.Montandon G, Bairam A, Kinkead R. Neonatal caffeine induces sex-specific developmental plasticity of the hypoxic respiratory chemoreflex in adult rats. AJP: Regulatory, Integrative and Comparative Physiology. 2008;295(3):R922–R934. doi: 10.1152/ajpregu.00059.2008. [DOI] [PubMed] [Google Scholar]
- 35.Laird NM, Ware JH. Random-Effects Models for Longitudinal Data. Biometrics. 2002;38(4):963–974. [PubMed] [Google Scholar]