Abstract
Health-related quality of life (HRQL) measures provide information about disease assessment; however, healthcare providers may be reluctant to use HRQL assessments as scores can be difficult to interpret. We sought to identify levels for impaired pain-related HRQL in children with sickle cell disease (SCD). Children (n=251) completed the PedsQL™ Generic Core Scales and PedsQL™ SCD Module in a multisite study. Using children’s item scores on the Pain and Hurt and Pain Impact scales of the PedsQL™ SCD Module, High, Intermediate, and Low Functioning groups were created. We compared functioning groups to the Pain and Hurt and Pain Impact scale scores to determine levels representing high and low HRQL. These scores were compared to disease severity and the PedsQL™ Generic Core Scales. Scores of 60 or below on the PedsQL™ SCD Pain and Hurt and Pain Impact scales were associated with severe disease and met requirements for impaired functioning on the PedsQL™ Generic Core Scales. Scores of 81 or higher on the Pain and Hurt and the Pain Impact scales can be considered consistent with good HRQL in those domains in SCD. Alternately, scores of 60 or lower are cause for concern and suggest areas of HRQL impairment in SCD.
Keywords: children, health-related quality of life, patient-reported outcomes, pediatrics, sickle cell disease, PedsQL
INTRODUCTION
Consider the following scenario: A child with sickle cell disease (SCD) completes the PedsQL™ SCD Module, a disease-specific assessment of pediatric health-related quality of life (HRQL), while attending clinic for a routine check-up. Upon scoring the assessment, the healthcare provider notes the child’s mean scores on the Pain and Hurt and Pain Impact scales are 66.67 and 52.50. How should the healthcare provider interpret the child’s report of functioning with regard to pain? One possibility the healthcare provider might consider is the child’s scores in relation to the normative mean provided in a previous study with the PedsQL™ SCD Module scales (66.7 and 54.0).1 Using this method, the healthcare provider may conclude child’s scores are similar to the average scores from published data and, therefore, not a concern. This conclusion would not be entirely correct.
Many physicians and medical researchers have begun to focus on more patient-centered methods of disease assessment by incorporating measurement of HRQL. HRQL has been defined as “the child’s physical, emotional and social well-being, as reported by the caregiver or by the child directly.”2 HRQL scores are often reported using means/medians with statistical tests highlighting differences between patient groups.3–5 However, minimal research has examined approaches to facilitate greater clinical utility of HRQL instruments. Healthcare providers may be unfamiliar with the scoring of different HRQL measures and may not understand or have an intuitive sense of a “good” HRQL score.3–5 Therefore, research is needed to provide information that will increase the clinical utility of HRQL data in patients with chronic illness and provide guidelines for interpretation of scores.
Given current limitations healthcare providers may face in understanding HRQL scores, we sought to characterize HRQL data from a sample of children with SCD using methodology designed to increase interpretability of HRQL data for clinicians.5 Children with SCD are an ideal example population given the common complications they experience including frequent painful episodes and psychological and social difficulties.6 As such, children with SCD have comparable or worse HRQL in baseline health relative to other chronic illness populations.7 Specifically, a recent study showed that the median Total score on the PedsQL™ Generic Core Scales for children with sickle cell disease was 68.3.8 Comparatively, studies of obese children and children with cancer who were undergoing treatment reported mean Total scores on the PedsQL™ Generic Core Scales of 67.0 and 72.2, respectively.9, 10 The goal of the present study was to provide additional information about interpretations of pain-related HRQL scores in a SCD population to facilitate use of HRQL data in clinical practice.
METHODS
Participants
Participants were a convenience sample of children ages 5–18 years with a diagnosis of SCD (any genotype), confirmed by a treating physician, at one of five SCD clinics across the United States including the Medical College of Wisconsin/Children’s Hospital, Milwaukee; University of Texas Southwestern/Children’s Medical Center, Dallas; Baylor College of Medicine/Texas Children’s Hospital, Houston; Jonathan Jaques Children’s Cancer Center/Miller Children’s Hospital, Long Beach, CA; and University of Alabama at Birmingham/Children’s of Alabama. Each clinic’s institutional review board approved the study, and informed consent/assent was obtained.1
Measures
The PedsQL™ Sickle Cell Disease Module
Patients completed the Pediatric Quality of Life Inventory™ Sickle Cell Disease Module (PedsQL™ SCD).1 The PedsQL™ SCD includes 43 items covering nine scales. The current study focuses on two scales: 1) Pain and Hurt and 2) Pain Impact. Pain is a fundamental symptom of SCD, and these scales demonstrated the strongest measurement properties for patient self-report.1 Thus, pain scale scores should have great clinical relevance in the assessment of HRQL of children with SCD. The 9-item Pain and Hurt scale includes four items assessing overall pain (e.g., “I hurt a lot”) and five items assessing pain in specific areas of the body (e.g., “I hurt in my arms”). The 10-item Pain Impact scale includes items assessing difficulties resulting from pain (e.g., “I miss school when I have pain”).
Pediatric Quality of Life Inventory™ 4.0 Generic Core Scales (PedsQL™ Generic Core Scales)
Patients completed the PedsQL™ Generic Core Scales.11 This measure includes 23 items encompassing four scales: Physical Functioning, Emotional Functioning, Social Functioning, and School Functioning. This study focuses on the Physical and School Functioning scores, as well as Total Score. Social and Emotional Functioning were not examined independently because our prior research suggests children with SCD do not differ from their African American, non-SCD peers on these scales.8 Children are considered at-risk for HRQL impairment on these scales if their scores fall one standard deviation below the population sample mean.12
Scoring the PedsQL™ Generic Core Scales and the SCD Module
Both the PedsQL™ Generic Core Scales and PedsQL™ SCD have child self-report forms designed for ages 5–7 (young child), 8–12 (child), and 13–18 (adolescent) years. For both instruments, children ages 8 years and older report how much of a problem each item has been for them during the past one month. PedsQL™ assessments include a Likert response scale (0 = never a problem; 1 = almost never a problem; 2 = sometimes a problem; 3 = often a problem; 4 = almost always a problem) for each item in each scale. Children ages 5 – 7 years use a simplified, 3-point rating scale in which emotive faces (i.e., happy, sad, and neutral) accompany responses.11 Items are reverse-scored and transformed to a 0–100 scale where higher scores indicate better HRQL. A detailed description of scoring can be found via the PedsQL™ website.13
Disease Severity
Disease severity was determined using criteria defined in our previous research and was established a priori as mild or severe.1 Specifically, children who experienced an overt stroke, acute chest syndrome, or three or more hospitalizations for painful events in the prior three years were classified as having severe disease. Children who did not experience one or more of the aforementioned criteria were classified as having mild disease.
Procedure
Data were collected between 2010 and 2012 using a cross-sectional design. The present study was part of a larger project exploring the psychometric properties of the PedsQL™ SCD.1 Because pain is a primary symptom of SCD, we focused on the Pain and Hurt and Pain Impact scales from the PedsQL™ SCD which are likely to have high clinical utility. Previous research suggests dichotomized scores are more informative for clinicians.4, 5 Thus, similar to previous research, we concentrated on scores at one end of the Likert scale.14 Specifically, we dichotomized children’s scores by counting the number of times they responded to items with “never” or “almost never” a problem (indicating better HRQL). Because we wanted to capture the two extremes of HRQL (high and low), we created three levels of functioning based on the dichotomized item counts: High Functioning, Intermediate Functioning, and Low Functioning.
The cut-offs for item counts were chosen to create a balanced trichotomy that was applicable to both pain scales. The Low Functioning level was created first and included children who answered “never” or “almost never” a problem on 3 items or less for each scale, indicating that pain or the impact of pain during the past month was a problem. The cut-off of 3 was chosen to represent the lower 30–33% of the sample. The High Functioning level included children who answered “never” or “almost never” on the Likert scale for 7 or more items for each scale indicating they rarely experienced pain or were rarely impacted by pain during the past month. The cut-off of 7 was chosen to represent the upper 30–33% of the sample. Finally, the Intermediate Functioning level included children who answered “never” or “almost never” on 4 – 6 items for each scale, indicating they sometimes experienced pain or were sometimes impacted by pain during the past month. HRQL scale scores were calculated for the Pain and Hurt and Pain Impact Scales per developer’s instructions. Scores of both scales were categorized into five levels representing units of twenty (0–20; 21–40; 41–60; 61–80; 81–100). This distribution was chosen as it provided more accurate data than quartiles and larger distributions (i.e., 10 levels representing units of 10).
Using multivariate frequency distributions (i.e., crosstabs) via SPSS, we compared Pain and Hurt and Pain Impact scale scores to the Low/Intermediate/High Functioning counts to determine the proportion of answers of the functioning counts within each grouped scale score. Total percentages were graphed to view trends in Pain and Hurt and Pain Impact scales. This procedure allowed us to determine scale scores that represent distinct levels of HRQL related to pain that can aid the clinician in understanding the interpretation of the numerical score.
Once functioning levels related to pain were established, a chi-square test for independence was conducted to determine whether the HRQL scores at the different HRQL levels distinguished between mild and severe disease. Further, we explored whether children who had a mean score on the scales at or below the lowest level for HRQL on the PedsQL™ SCD Pain scales would also be considered at-risk for HRQL impairment as measured by the PedsQL™ Generic Core Scales. We computed additional multivariate frequency distributions and chi-square tests for independence to compare children’s scores on the PedsQL™ SCD Pain scales to scores on PedsQL™ Generic Core Scales for School, Physical, and Total Score. Previous work has identified children with chronic disease as having impaired HRQL using the PedsQL™ Generic Core Scales.12 Additionally, recent work demonstrated that children who reported higher levels of symptoms (i.e., a lot of pain) also had lower HRQL on the PedsQL™ Generic Core Scales.15 Therefore, we hypothesized the majority of children in our sample would have PedsQL™ Generic Core Scales scores in the impaired range, particularly if they also have mean HRQL scores in the lowest group on the Pain and Hurt and Pain Impact scales.11, 16
RESULTS
Study Population
Two-hundred fifty-one children (51.8% female) participated in this study. Children’s average age was 11.47 years (SD = 3.84). Complete demographics are available in the original study examining psychometric properties of the PedsQL™ SCD.1 Two-hundred forty-three children completed enough items on the PedsQL™ SCD to calculate scores for the Pain and Hurt scale, and 239 completed enough items to calculate scores for the Pain Impact scale.
Interpretation of PedsQL™ SCD Pain and Hurt Scores
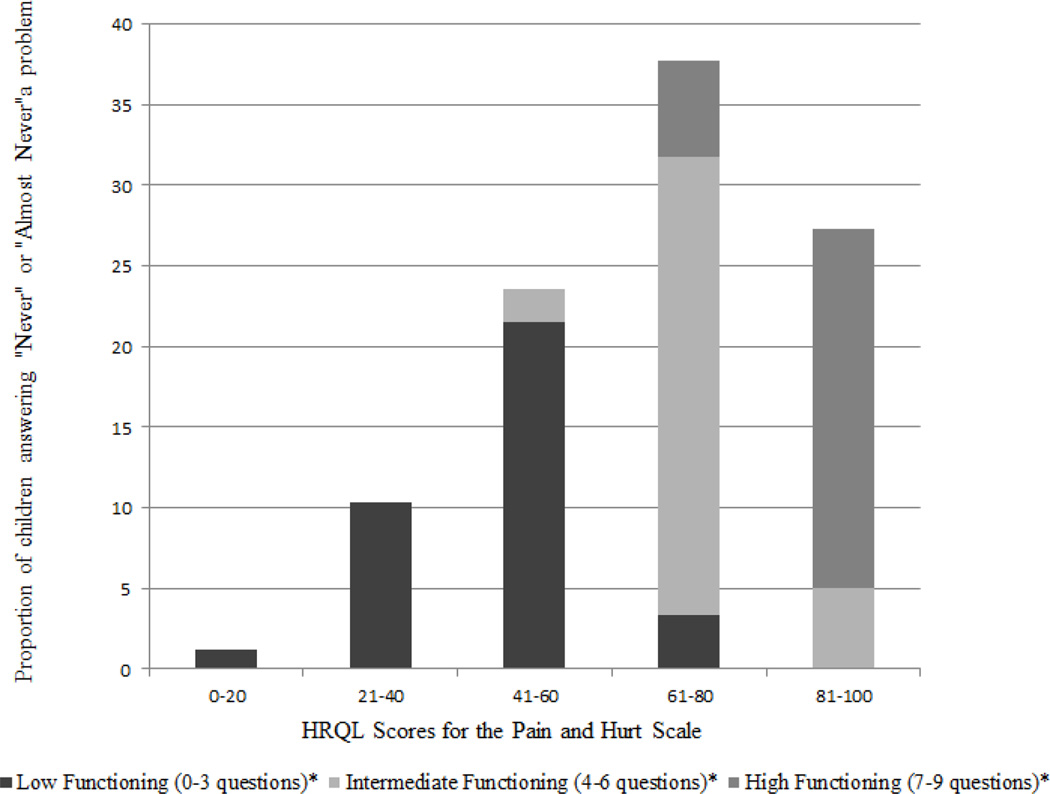
Results are presented by Functioning level below and in Figure 1, which depicts the proportion of children in each Functioning level relative to their overall Pain and Hurt scale scores.
Fig. 1.
Distribution of Pain and Hurt HRQL Scores
* Refers to the number of questions on a 9-item scale that were answered as "never" or "almost never" a problem.
Low Functioning
Of 89 children at the Low Functioning level (M = 46.53, SD = 12.89), the majority had Pain and Hurt scores of 60 or lower (n = 80). Thus, over 90% of patients who were classified as Low Functioning reported they ‘always,’ ‘almost always,’ or ‘sometimes’ had pain in the past month for the majority of the items on the scale.
High Functioning
Children at the High Functioning level (n = 68) had the highest scores (M = 89.28, SD = 9.40) supporting better HRQL. All of these children had Pain and Hurt mean scores over 60, and nearly 80% had scores over 80. These children report infrequent experiences of pain over the past month (i.e., reporting “never” or “almost never” on at least 7 items of the scale).
Intermediate Functioning
Children at the Intermediate Functioning level (n = 86) had great variability in their Pain and Hurt scale scores. These children had Pain and Hurt scores ranging 52 – 87 (M = 71.76, SD = 7.79); however, 80% of scores fell in the 61 – 80 range.
Interpretation of PedsQL™ SCD Pain Impact Scores
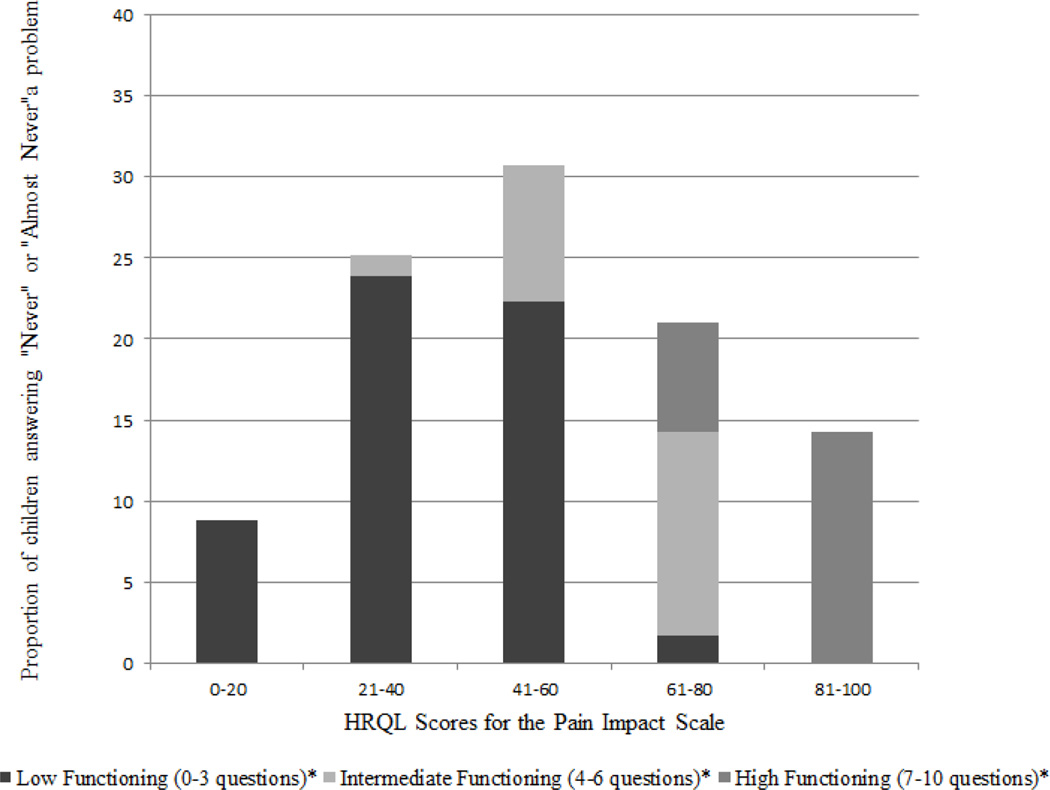
Results are presented in Figure 2 and below by Functioning level. Figure 2 depicts the proportion of children in each Functioning level relative to their overall Pain Impact scale scores.
Fig. 2.
Distribution of Pain Impact HRQL Scores.
* Refers to the number of questions on a 10-item scale that were answered as "never" or "almost never" a problem.
Low Functioning
Of 135 children at the Low Functioning level (M = 37.18, SD = 14.94), most (97%) had Pain Impact HRQL scores of 60 or lower. Thus, most children who scored at the Low Functioning level were likely to be troubled by pain moderately to frequently over the past month.
High Functioning
Children at the High Functioning level (n = 51) had the highest Pain Impact scale scores (M = 88.99, SD = 10.51) supporting better HRQL. All of these children had Pain Impact mean scores over 70, and over 75% had scores over 80. In fact, all children whose scores ranged 81 – 100 were at the High Functioning level. These children report less impact of pain over the past month.
Intermediate Functioning
Children at the Intermediate Functioning level (n = 53) had Pain Impact scores ranging 37.5 – 80 (M = 62.99, SD = 10.64). A little over half of children in this group (57%) had Pain Impact scores in the 61 – 80 range, and 43% had scores of 60 or lower.
Disease Severity
In the study sample, 41.1% of children were classified as having severe disease (n = 132).
PedsQL™ SCD Pain and Hurt Scores
Of those children with severe disease, nearly half (46.6%) had Pain and Hurt scale scores in the lowest level of 60 or below. Alternatively, only 20.4% of children with Pain and Hurt scores above 80 were classified as having severe disease. The majority of children with mild disease had Pain and Hurt scores in the intermediate range of 61 – 80 (41.9%) or had HRQL scores in the highest group above 80 (30.1%). A chi-square test for independence revealed HRQL scores were significantly different among mild and severe disease classifications for the Pain and Hurt scale, χ2 (2,n = 239) = 9.044, p = 0.011.
PedsQL™ SCD Pain Impact Scores
Unlike the Pain and Hurt scale, HRQL scores for the Pain Impact scale did not distinguish between mild and severe disease, χ2(2, n = 239) = 2.779, ns. Children with the lowest HRQL scores (60 or below) comprised the majority of both mild and severe disease classifications. Of the children with severe disease, 70.2% had HRQL scores below 60; similarly, 60% of children with mild disease had HRQL scores below 60.
Comparison to the PedsQL™ Generic Core Scales
As expected, multivariate frequency distributions revealed the majority of children scored at or below previously defined impaired levels for School Functioning (HRQL score ≤ 62.99), Physical Functioning (HRQL score ≤ 72.98), and Total Score (HRQL score ≤ 69.71) on the PedsQL™ Generic Core Scales.12 Of the 239 children in the study sample for the PedsQL™ SCD Pain and Hurt Scale, 59.4% had scores in the impaired range for School Functioning, 54.8% had scores in the impaired range for Physical Functioning, and 51.0% had Total scores in the impaired range. Further, for the PedsQL™ SCD Pain Impact Scale, 59.8% had scores in the impaired range for School Functioning, 55.6% had scores in the impaired range for Physical Functioning, and 50.6% had Total scores in the impaired range. Also as expected, chi-square tests for independence revealed children who had HRQL scores of 60 and below tended to have impaired HRQL as measured by the PedsQL™ Generic Core Scales. This relationship held true for both pain scales of the PedsQL™ SCD module (Table 1).
Table 1.
Comparison between the PedsQL™ SCD Module HRQL Scores for Pain and the PedsQL™ Generic Core Scales
| PedsQL™ SCD Module HRQL scores for Pain Scales |
||||
|---|---|---|---|---|
| Pain and Hurt (n = 239) | ||||
| PedsQL™ Generic Core Scales |
HRQL: 0 – 60 (n = 86) |
HRQL: 61 – 80 (n = 91) |
HRQL: 81 – 100 (n = 62) |
χ2 |
| School % Impaired (n = 142) | 46.5 | 38.0 | 15.5 | 25.43*** |
| Physical % Impaired (n = 131) | 49.6 | 32.1 | 18.3 | 24.22*** |
| Total % Impaired (n = 122) | 51.6 | 32.8 | 15.6 | 29.13*** |
| Pain Impact (n = 239) |
||||
| HRQL: 0 – 60 (n = 154) |
HRQL: 61 – 80 (n = 50) |
HRQL: 81 – 100 (n = 35) |
||
| School % Impaired (n = 143) | 76.2 | 17.5 | 6.3 | 26.64*** |
| Physical % Impaired (n = 133) | 79.7 | 14.3 | 6.0 | 32.40*** |
| Total % Impaired (n = 122) | 82.0 | 13.9 | 4.1 | 36.62*** |
Note.
p < 0.001
DISCUSSION
Health-related quality of life (HRQL) assessments provide information that helps healthcare providers administer the most effective care to patients. Particularly in the SCD population, where disease experiences vary greatly, incorporating patients’ perspectives is important when providing care. Our study demonstrated how HRQL scores on the PedsQL™ SCD can be interpreted in a clinically meaningful way. Results indicated children with mean scores on the PedsQL™ SCD pain scales ranging 81 – 100 had low levels of pain, and thus, better HRQL; children with scores ranging 61 – 80 had intermediate levels of pain; and children with scores ranging 0 – 60 should be considered to have poor HRQL related to pain. Additionally, children with scores 60 or less tended to have severe disease and impaired HRQL on the School Functioning, Physical Functioning, and total scores of the PedsQL™ Generic Core Scales. These results supported our hypotheses.
Scores of 60 or below and 81 or above offer healthcare providers an intuitive sense of good and poor HRQL on the PedsQL™ SCD. The importance of acknowledging scores of 60 and below is supported further because children in this group were found to have impaired scores in School and Physical Functioning as well as impaired Total scores on the PedsQL™ Generic Core Scales. Scores of 81 or above indicated a high likelihood of good HRQL. Patients who scored above this level reported fewer instances of pain and less impact of pain over the past month and would likely have fewer active disease symptoms and fewer hospital visits. The wide range of scores at the Intermediate level can make it difficult to interpret HRQL. Some of these children may be doing fairly well while others may not. Children with scores ranging 61 – 80 should be questioned further about their HRQL to determine how to tailor care if needed. Recognizing these threshold levels provides clinicians with a more intuitive sense of whether or not HRQL is impaired. Armed with this information, healthcare providers can more easily identify poor HRQL and more readily provide and tailor needed care.
Recall the scenario presented in the introduction in which a child scored 66.67 and 52.50 on the Pain and Hurt and Pain Impact scales, respectively. Although these scores likely represent average HRQL related to pain,1 this result can be misleading as many children with SCD have impaired HRQL at baseline. In fact, the current study’s findings would suggest this child’s scores are relatively low. With a Pain and Hurt score between 61 and 80 and a Pain Impact Score below 60, the healthcare provider now has information to recognize that this child may need increased care for pain and/or further inquiry into the child’s experience of pain.
For the PedsQL™ SCD Pain and Hurt scale, children with scores of 60 or below had more severe disease than children with higher scores. There was no distinction between severe and mild disease based on HRQL scores for the Pain Impact Scale. In this study and similar to our prior work, we classified disease severity based on phenotypic expression of the disease.1 Specifically, children must have had acute care utilization related to vaso-occlusive episodes or must have experienced an overt stroke to be classified as severe, and pain at home was not incorporated into the classification. Because of this classification, it is important to examine children’s scores on both the Pain and Hurt and Pain Impact scales. The Pain and Hurt scale describes the level of pain the child has experienced; thus, more pain is likely to lead to more acute care utilization and more severe disease. The Pain Impact scale describes children’s evaluations of how their pain influences their lives. It is understandable that any amount of pain may negatively impact the child, regardless of whether they seek acute care or treat their pain at home. It is not surprising, then, that the Pain Impact scale did not distinguish between mild and severe disease.
As others have suggested, HRQL scores need to be more accessible and understandable to healthcare providers.4, 5 Using our recommended levels as a complement to the traditional PedsQL™ scoring methodology, can provide meaningful data that has clinical utility. Although the specific levels presented here are not transferable to other HRQL assessments, the methodology used here could be applied to other measures of HRQL.
This study used cross-sectional data from children aged 5 – 18 years. Although the PedsQL™ provides age-appropriate formats, we were not able to follow our participants over time. Because disease progression over time is an important aspect of HRQL, future studies should collect longitudinal data to capture the changing nature of disease and allow results to be stratified by age.
Our results contribute to the validity of the recently developed PedsQL™ SCD as the levels we determined align with cases of pediatric SCD presented during clinic visits. However, because the PedsQL™ SCD is new, more studies are needed to test the levels presented here. As this instrument is utilized increasingly, especially within clinical settings, we expect greater understanding of HRQL scores by healthcare providers who care for children with SCD. By using PRO assessments during clinic, we hope healthcare professionals will be able to provide more patient-centered care to their patients and improve physician-patient communication.
CONCLUSION
Based on our sample, scores of 81 or higher on the PedsQL™ SCD pain scales should be considered good HRQL functioning. These children likely have had less pain that was less severe than children with lower scores and/or are managing their symptoms well. Children with scores of 60 or below (as illustrated in the case example in the introduction) should be monitored more closely as they are likely suffering from more pain and are more impacted by pain than other children with SCD.
Acknowledgements
Source of Funding: This work was supported by a grant from the National Institutes of Health (U54 HL090503, Project 3 and CTSI 1-UL1-RR031973).
We would like to acknowledge the assistance of Raymond G. Hoffman, Ph.D. and Ke Yan, Ph.D. from the Department of Pediatrics, Biostatistics at the Medical College of Wisconsin who we consulted regarding the statistical development of the levels.
Footnotes
Conflict of Interest: Dr. Varni holds the copyright and the trademark for the PedsQL™ and receives financial compensation from the Mapi Research Trust, which is a nonprofit research institute that charges distribution fees to for-profit companies that use the Pediatric Quality of Life Inventory™. For the remaining authors none were declared.
References
- 1.Panepinto JA, Torres S, Bendo CB, et al. PedsQL™ Sickle Cell Disease Module: Feasibility, reliability, and validity. Pediatr Blood Cancer. 2013;60:1338–1344. doi: 10.1002/pbc.24491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spilker B. Quality of life and pharmacoeconomics in clinical trials. Philadelphia, PA: Lippincott Williams and Wilkins; 1996. [Google Scholar]
- 3.Panepinto JA. Health-related quality of life in sickle cell disease. Pediatr Blood Cancer. 2008;51:5–9. doi: 10.1002/pbc.21557. [DOI] [PubMed] [Google Scholar]
- 4.Guyatt GH, Schunemann HJ. How can quality of life researchers make their work more useful to health workers and their patients? Qual Life Res. 2007;16:1097–1105. doi: 10.1007/s11136-007-9223-3. [DOI] [PubMed] [Google Scholar]
- 5.Schunemann HJ, Akl EA, Guyatt GH. Interpreting the results of patient reported outcome measures in clinical trials: The clinician's perspective. Health Qual Life Outcomes. 2006;4:62. doi: 10.1186/1477-7525-4-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Noll RB, Reiter-Purtill J, Vannatta K, et al. Peer relationships and emotional well-being of children with sickle cell disease: A controlled replication. Child Neuropsychol. 2007;13:173–187. doi: 10.1080/09297040500473706. [DOI] [PubMed] [Google Scholar]
- 7.Panepinto JA, Bonner M. Health-related quality of life in sickle cell disease: Past, present, and future. Pediatr Blood Cancer. 2012;59:377–385. doi: 10.1002/pbc.24176. [DOI] [PubMed] [Google Scholar]
- 8.Panepinto JA, Pajewski NM, Foerster LM, et al. The performance of the PedsQL™ Generic Core Scales in children with sickle cell disease. J Pediatr Hematol Oncol. 2008;30:666–673. doi: 10.1097/MPH.0b013e31817e4a44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schwimmer JB, Burwinkle TM, Varni JW. Health-related quality of life of severely obese children and adolescents. JAMA. 2003;289:1813–1819. doi: 10.1001/jama.289.14.1813. [DOI] [PubMed] [Google Scholar]
- 10.Varni JW, Burwinkle TM, Katz ER, et al. The PedsQL™ in pediatric cancer. Cancer. 2002;94:2090–2106. doi: 10.1002/cncr.10428. [DOI] [PubMed] [Google Scholar]
- 11.Varni JW, Seid M, Kurtin PS. PedsQL™ 4.0: Reliability and validity of the pediatric quality of life inventory version 4.0 generic core scales in healthy and patient populations. Med Care. 2001;39:800–812. doi: 10.1097/00005650-200108000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Varni JW, Burwinkle TM, Seid M, et al. The PedsQL™ 4.0 as a pediatric population health measure: Feasibility, reliability, and validity. Ambul Pediatr. 2003;3:329–341. doi: 10.1367/1539-4409(2003)003<0329:tpaapp>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 13.Varni JW. The PedsQL™ scoring algorithm. 2012 Oct 1; Available at: http://www.pedsql.org/score.html.
- 14.Uzark K, King E, Spicer R, et al. The clinical utility of health-related quality of life assessment in pediatric cardiology outpatient practice. Congenit Heart Dis. 2013;8:211–218. doi: 10.1111/chd.12002. [DOI] [PubMed] [Google Scholar]
- 15.Kim J, Chung H, Amtmann D, et al. Symptoms and quality of life indicators among children with chronic medical conditions. Disabil Health J. 2014;7:96–104. doi: 10.1016/j.dhjo.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fayers PM, Hand DJ. Factor analysis, causal indicators and quality of life. Qual Life Res. 1997;6:139–150. doi: 10.1023/a:1026490117121. [DOI] [PubMed] [Google Scholar]