Abstract
Natural toxic substances have a bitter taste and their ingestion sends signals to the brain leading to aversive oral sensations. In the present study, we investigated chronological changes in c-Fos immunoreactivity in the nucleus tractus solitarius (NTS) to study the bitter taste reaction time of neurons in the NTS. Equal volumes (0.5 mL) of denatonium benzoate (DB), a bitter tastant, or its vehicle (distilled water) were administered to rats intragastrically. The rats were sacrificed at 0, 0.5, 1, 2, 4, 8, or 16 h after treatment. In the vehicle-treated group, the number of c-Fos-positive nuclei started to increase 0.5 h after treatment and peaked 2 h after gavage. In contrast, the number of c-Fos-positive nuclei in the DB-treated group significantly increased 1 h after gavage. Thereafter, the number of c-Fos immunoreactive nuclei decreased over time. The number of c-Fos immunoreactive nuclei in the NTS was also increased in a dose-dependent manner 1 h after gavage. Subdiaphragmatic vagotomy significantly decreased DB-induced neuronal activation in the NTS. These results suggest that intragastric DB increases neuronal c-Fos expression in the NTS 1 h after gavage and this effect is mediated by vagal afferent fibers.
Keywords: bitter tastant, c-Fos, nucleus tractus solitarius, vagal afferent fibers, vagotomy
Introduction
Specific sensors lining the oral cavity and digestive tract play important roles in the regulation of ingestive and digestive functions [3,16]. There are two types of taste sensor receptors, type 1 (T1R) and type 2 (T2R), in the oral cavity and digestive tract. Heterodimers of T1R family members can detect sweet and L-amino acid tastants. The T2R family consists of more than 30 diverse members that can detect bitter tastants. T2Rs are present in the human and rodent gastrointestinal tract, and are activated by bitter tastants such as denatonium benzoate (DB) and phenylthiocarbamide (PTC) [19,21]. DB is a very bitter compound and an exogenous ligand of T2R4 [19]. Ingestion of exogenous bitter tastants serves as a central warning signal against potentially harmful substances [1].
c-Fos is widely used as an indirect marker of neuronal activity because this factor is expressed when neurons signal [2]. In addition, c-Fos is also used for the activation of neuronal taste perception [8,10,12,22]. It has been reported that gustatory stimulation induces c-Fos-like immunoreactivity in various regions of the brain including the nucleus tractus solitarius (NTS) [8,12,18]. Increased c-Fos expression in the NTS 2 h after gavage was also reported. Intragastric administration of T2R ligands increased food intake during the first 30 min and elevated octanoyl along with total ghrelin levels in plasma within 40 min of gavage [11], but inhibited gastric emptying in mice [11]. Few reports on the chronological and dose-dependent changes of neuronal activation by DB have been published. Therefore, we conducted the present study to investigate the chronological changes of neuronal activation by intragastric administration of DB, a ligand for T2R, in the rat NTS.
Materials and Methods
Experimental animals
Male Sprague-Dawley rats (n = 128) were purchased from the Japan SLC (Japan). The animals were used at 6 weeks of age and housed under standard laboratory conditions with adequate temperature (22℃) and humidity (60%) as well as a 12-h light/dark cycle. All rats had free access to solid diet (D12450Bi; Research Diets, USA) and tap water. Handling and care of the rats conformed to guidelines that comply with current international laws and policies (National Institutes of Health [NIH] Guide for the Care and Use of Laboratory Animals, NIH Publication No. 85-23, 1985, revised 1996). The study protocols were also approved by the Institutional Animal Care and Use Committee (IACUC) of Seoul National University, Korea (approval no. SNU-120103-10). All experiments were conducted to minimize both the number of animals used and their suffering due to the procedures performed.
Subdiaphragmatic vagotomy
The animals (n = 5 in each group) were anesthetized with a mixture of 2.5% isoflurane (Baxtor, USA) in 33% oxygen and 67% nitrous oxide. Subdiaphragmatic vagotomy was performed according to the technique described by Gschossmann et al. [5]. Briefly, a midline laparotomy incision was made to expose the peritoneal cavity. The external muscle coat of the esophagus 1 cm from the cardia distally was stripped. Since vagal branches are connected to the muscle coat, this results in a subdiaphragmatic vagotomy. To ensure completeness of the vagotomy, the left gastric artery was isolated and stripped of all connective tissues and nerves, including an accessory branch of the vagus nerve. Another group of rats underwent a sham operation. The animals were housed individually after the surgery and received a liquid diet (Ensure; Abbott Laboratories, USA) with water ad libitum. The rats were allowed to recover for 7~10 days before the subsequent experiment.
Intragastric gavage with DB
The rats (n = 7 per group) were treated with either 0.5 mL of distilled water or 0.5 mL of DB (30 mg/kg; Sigma, USA) after a 12-h fast. To monitor the dose-dependent effects of DB on c-Fos expression, the animals received 0.5 mL DB (30 mg/kg, 100 mg/kg, or 300 mg/kg, n = 5 for each dosage) intragastrically after a 12-h fast.
Tissue processing
For histology, vehicle- or DB-treated rats were anesthetized with 30 mg/kg Zoletil 50 (Virbac, France) at 0 (immediately after gavage), 0.5 (30 min), 1, 2, 4, 8, or 16 h after gavage (n = 7 for each time point). The rats were then perfused transcardially with 0.1 M phosphate-buffered saline (PBS, pH 7.4) followed by 4% paraformaldehyde in 0.1 M phosphate buffer (PB, pH 7.4). Brains were then removed and postfixed in 4% paraformaldehyde in 0.1 M phosphate buffer for 12 h at room temperature. The brains were cryoprotected by infusion with 30% sucrose overnight at 4℃ and cut coronally into 30-µm-thick sections using a cryostat (Leica Biosystems, Germany). The sections were transferred to six-well plates (SPL life science, Korea) containing PBS for further processing.
Immunohistochemistry for c-Fos
To assess c-Fos expression, the sections were carefully processed simultaneously under identical conditions. Brain tissue sections from -12.80 mm and -13.30 mm posterior to the bregma according to the rat atlas [14] were selected for each animal. The sections were sequentially treated with 0.3% hydrogen peroxide (H2O2) in PBS for 15 min at room temperature and 5% normal goat serum (Vector Laboratories, USA) in 0.1 M PBS. They were next incubated with rabbit anti-c-Fos antibody (1 : 10,000; EMD Millipore, USA) overnight at room temperature, and subsequently exposed to biotinylated goat anti-rabbit IgG (1 : 200; Vector Laboratories) with streptavidin peroxidase complex for 2 h at room temperature (1 : 200, Vector Laboratories). Next, antibody binding was visualized by staining with 3,3'-diaminobenzidine tetrahydrochloride (Sigma). c-Fos-positive nuclei in all groups were examined using an image analysis system equipped with a computer-based Charge-Coupled Device camera (Optimas 6.5 software; CyberMetrics, USA). The number of c-Fos-positive cells was counted per section and the average was calculated.
Statistical analysis
All statistical analyses were carried out using GraphPad Prism 4.0 (GraphPad Software, USA). The data are presented as the mean ± standard error of the mean (SEM) for each group. Differences between the two groups were analyzed by Student's t-test. Multiple parameters were assessed by a one-way or two-way analysis of variance (ANOVA). P-values < 0.05 were considered statistically significant.
Results
Effects of vehicle on c-Fos expression
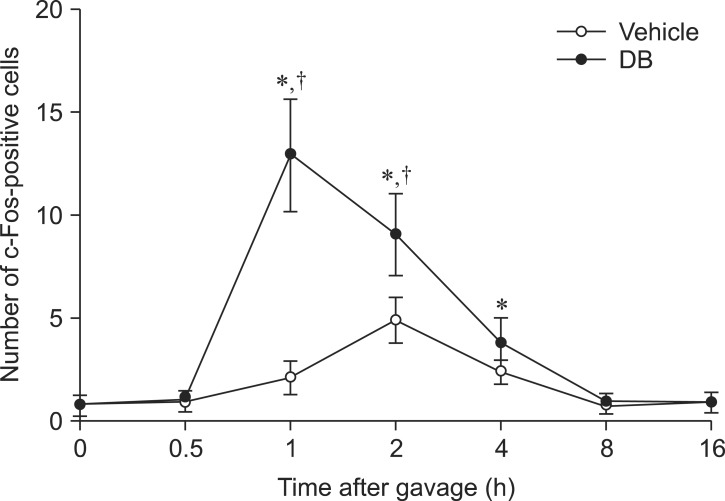
In the vehicle-treated group, few c-Fos-positive nuclei were detected in the NTS 0 h after gavage (panel A in Fig. 1). The average number of c-Fos-positive nuclei was 0.89 ± 0.38 per section. The number of c-Fos-positive nuclei increased in the NTS 30 min after gavage and peaked 2 h after gavage (panels B-D in Fig. 1) although differences between groups were not significant. In the 2-h group, the number of c-Fos-positive nuclei was 4.91 ± 1.11 per section (Fig. 2). Thereafter, the number of c-Fos-positive nuclei decreased in a time-dependent manner (panels E-G in Fig. 1, and Fig. 2). At 8 and 16 h after gavage, the number of c-Fos-positive nuclei was similar to that found 0 h after gavage (panels F and G in Fig. 1, and Fig. 2).
Fig. 1.
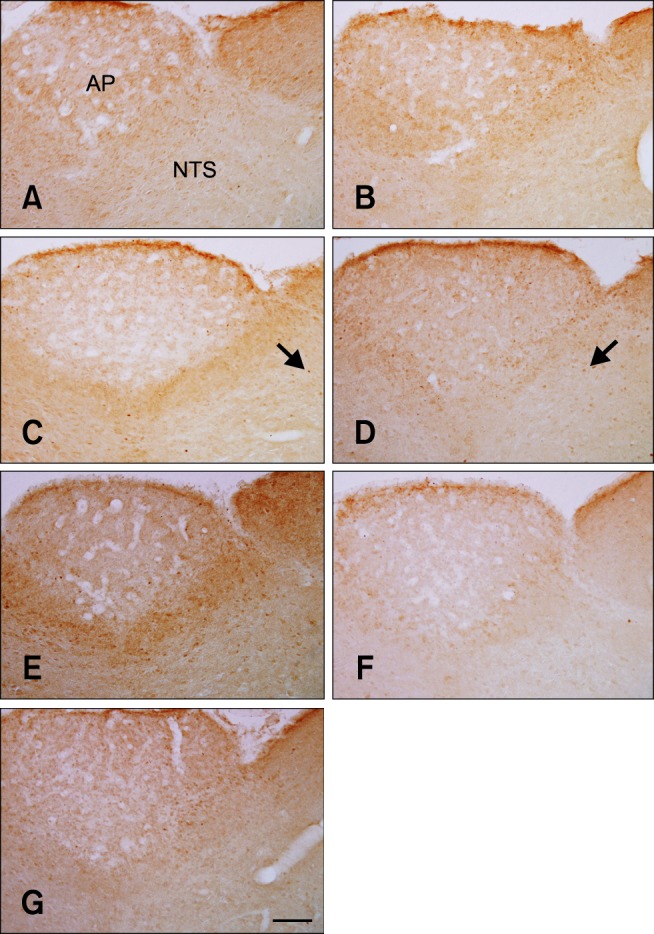
c-Fos-specific immunohistochemistry in the area postrema (AP) and nucleus tractus solitarius (NTS) of rats at 0 (A), 0.5 (B), 1 (C), 2 (D), 4 (E), 8 (F), and 16 h (G) after gavage with distilled water. Few c-Fos-positive nuclei were observed in the NTS 0 h after gavage. The number of c-Fos-positive nuclei (arrows) was increased 1~2 h after gavage and decreased thereafter over time. Scale bar = 50 µm.
Fig. 2.
The number of c-Fos-positive nuclei per section for each group at 0, 0.5, 1, 2, 4, 8, and 16 h after gavage with distilled water or denatonium benzoate (DB) (n = 7 per group; *p < 0.05 vs. the 0 h post-gavage group; †p < 0.05 vs. the vehicle-treated group). Data are presented as the mean ± SEM.
Effects of DB on c-Fos expression
In the DB-treated group, few c-Fos-positive nuclei were observed in the NTS 0 h after gavage (panel A in Fig. 3). The number of c-Fos-positive nuclei slightly increased in the NTS 30 min after gavage compared to that observed in the vehicle-treated group (Fig. 2, and panel B in Fig. 3). The number of c-Fos-positive nuclei peaked 1 h after gavage and was significantly higher than that found in the vehicle-treated group (Fig. 2, and panel C in Fig. 3). The number of c-Fos-positive nuclei was 12.9 ± 2.75 per section (Fig. 2). Thereafter, the number of c-Fos-positive nuclei decreased in a time-dependent manner although the number of these nuclei remained high in the DB-treated group compared to that in the vehicle-treated group at the 4-h time point (Fig. 2). At 8 and 16 h after gavage, the number of c-Fos-positive nuclei was similar than that found in the vehicle-treated group (Fig. 2, and panels F and G in Fig. 3).
Fig. 3.
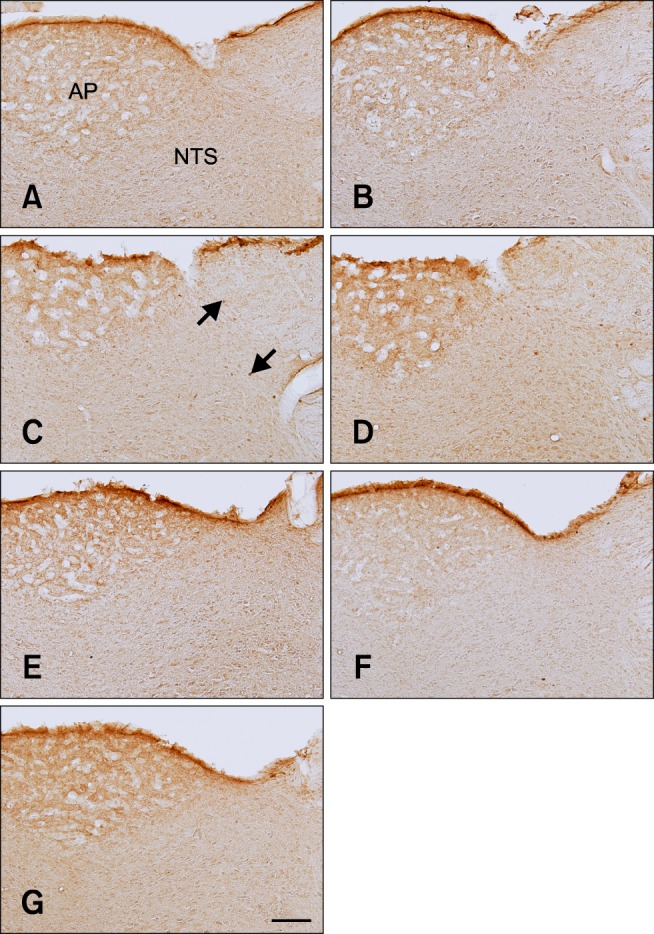
c-Fos-specific immunohistochemistry in the NTS of rats at 0 (A), 0.5 (B), 1 (C), 2 (D), 4 (E), 8 (F), and 16 h (G) after gavage with DB. Few c-Fos-positive nuclei were observed in the NTS 0 h after gavage. c-Fos-positive nuclei (arrows) were abundantly detected 1 h after gavage and the number decreased thereafter over time. The distribution pattern of c-Fos-positive nuclei 8 or 16 h after gavage was similar to that found 0 h after gavage. Scale bar = 50 µm.
Dose-dependent effects of DB on c-Fos expression
In the vehicle-treated group, few c-Fos-positive nuclei were observed in the NTS 1 h after gavage (panel A in Fig. 4). In the DB-treated animals, c-Fos-positive nuclei were abundantly detected in the NTS 1 h after gavage (panels B-E in Fig. 4). Interestingly, the number of c-Fos-positive nuclei increased in a dose-dependent manner. The number of nuclei in the 300 mg/kg DB-treated group was about 3.36-fold of that found in the 30 mg/kg DB-treated group.
Fig. 4.
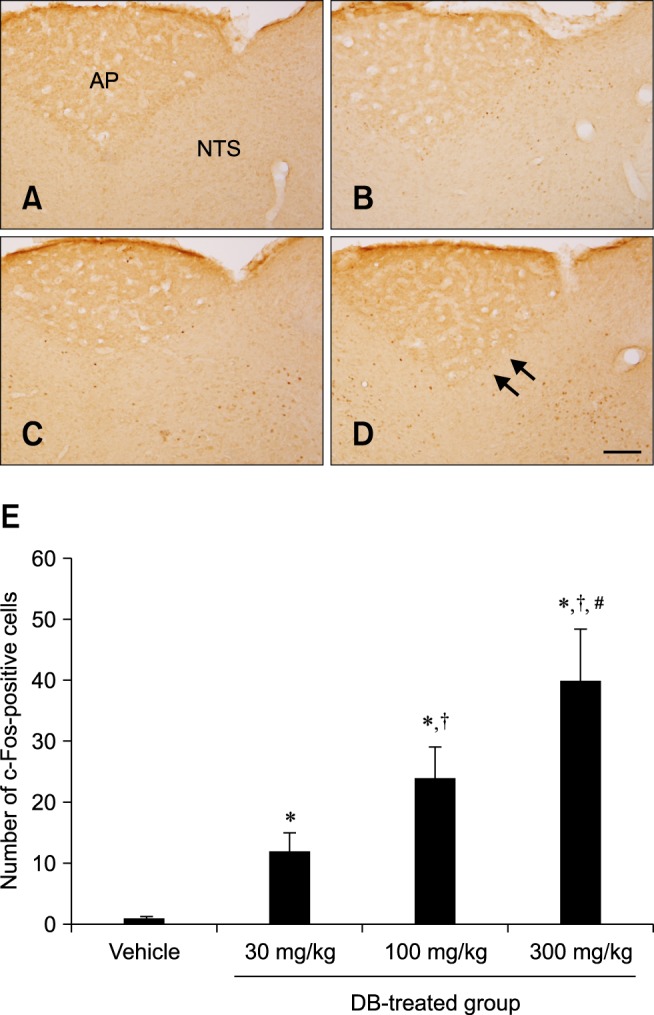
c-Fos-specific immunohistochemistry in the NTS of rats 1 h after gavage with vehicle (A) or 30 mg/kg (B), 100 mg/kg (C), and 300 mg/kg (D) DB. In the vehicle-treated group, few c-Fos-positive nuclei were observed in the NTS. In the DB-treated group, the number of c-Fos-positive nuclei (arrows) was dose-dependently increased in the NTS. (E) The number of c-Fos-positive nuclei per section for each group (n = 5 per group; *p < 0.05 vs. the vehicle-treated group, †p < 0.05 vs. the 30 mg/kg DB-treated group, #p < 0.05 vs. the 100 mg/kg DB-treated group). Data are presented as the mean ± SEM. Scale bar = 50 µm.
Effects of vagotomy on DB-induced c-Fos expression
In the sham group, some c-Fos-positive nuclei were detected in the NTS 1 h after DB gavage (panel A in Fig. 5) whereas only a few c-Fos-positive nuclei were observed in the NTS of the vagotomized group 1 h after DB gavage (panel B in Fig. 5). In the vagotomized group, the number of c-Fos-positive nuclei (0.78 ± 0.42 per section) was significantly lower than that in the sham group (panel C in Fig. 5).
Fig. 5.
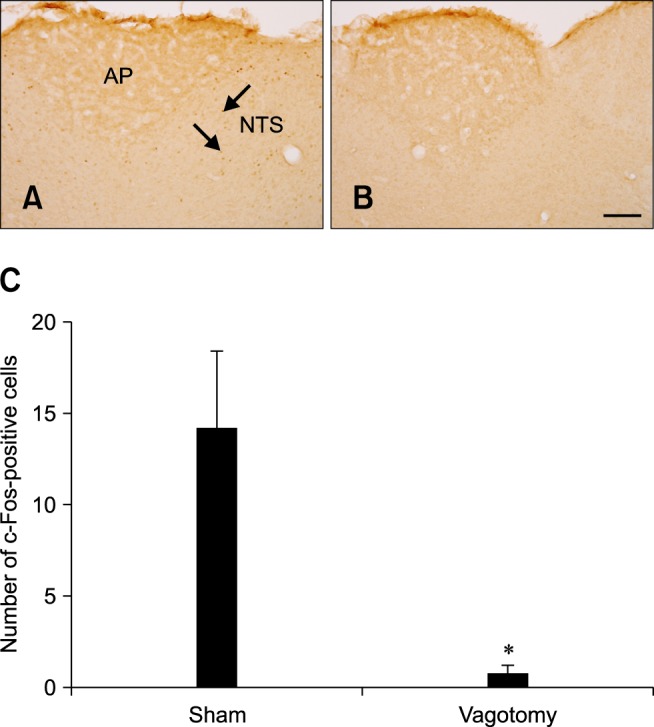
c-Fos expression in the NTS of rats 1 h after gavage with DB and a sham operation (A) or vagotomy (B). In the sham-operated group, c-Fos-positive nuclei (arrows) were observed in the NTS. In the vagotomy group, few c-Fos-positive nuclei were detected. (C) The number of c-Fos-positive nuclei per section for each group (n = 5 per group; *p < 0.05 vs. the sham-operated group). Data are presented as the mean ± SEM. Scale bar = 50 µm.
Discussion
Nociception and perception of bitter tastants are facilitated by T2R-expressing cells including brush cells in the rat gastrointestinal tract [9,13,15,17]. Detection and perception induce signal transduction by vagal afferents, and play an important role in gut-brain communication. Activation of vagal afferents causes neuron excitation in the NTS followed by reflex activation of the vagal efferent neurons that alters gastrointestinal function [4]. In the current study, we monitored chronological changes of c-Fos-positive cells in the NTS following T2R ligand gavage at various time points that mimics neuronal taste perception [8,12,22]. The number of c-Fos-positive nuclei in the NTS increased 1 h after gavage with distilled water although this increase was not statistically significant. In contrast, c-Fos-positive nuclei numbers in the NTS significantly increased 2 h after gavage before decreasing in a time-dependent manner. These results suggest that volume distension of the stomach may enhance neuronal c-Fos expression in the NTS. In the DB-treatment group, the number of c-Fos-positive nuclei was significantly increased 1 h after DB gavage. This finding indicates that gavage with a T2R ligand further activates the neuronal expression of c-Fos in the NTS along with volume distension.
It was reported that c-Fos expression does not significantly increased 2 h after gavage with DB alone although this procedure tends to increase the number of c-Fos-positive neurons in the NTS compared to control conditions [6]. In the present study, we observed a further increase of c-Fos expression in the NTS 2 h after DB gavage. We also observed a dose-dependent increase of c-Fos-positive cells in the NTS at the same time points. The discrepancy between our data and findings published in the literature may be associated with the dosages used. It has been reported that antinoceptive effects in the brain are mediated by the gastric vagal nerve [5]. Additionally, DB serotonin delivered by intragastric administration has been found to discharge in the brain via the vagus nerve and is blocked by p-chlorophenylalanine that selectively depletes brain serotonin [19].
We also performed subdiaphragmatic vagotomy to investigate the correlation between vagal afferents and increased c-Fos expression in the NTS after DB gavage. The vagotomized group showed a significant loss of c-Fos expression in the NTS after DB gavage. This result was similar to ones from a previous study indicating that increases in the number of Fos-positive NTS neurons by a T2R ligand mixture are prevented by subdiaphragmatic vagotomy [7]. Thus, DB potently activates the NTS soon after gavage via the vagal afferent pathway. In conclusion, our results demonstrated that DB, a T2R ligand, enhances neuronal c-Fos expression in the NTS in a dose-dependent manner after gavage through the vagal afferents.
Acknowledgments
This research was supported by the Korea Food Research Institute (grant no. E0131203).
Footnotes
There is no conflict of interest.
References
- 1.Akabas MH, Dodd J, Al-Awqati Q. A bitter substance induces a rise in intracellular calcium in a subpopulation of rat taste cells. Science. 1988;242:1047–1050. doi: 10.1126/science.3194756. [DOI] [PubMed] [Google Scholar]
- 2.Dragunow M, Faull R. The use of c-fos as a metabolic marker in neuronal pathway tracing. J Neurosci Methods. 1989;29:261–265. doi: 10.1016/0165-0270(89)90150-7. [DOI] [PubMed] [Google Scholar]
- 3.Furudono Y, Ando C, Kobashi M, Yamamoto C, Yamamoto T. The role of orexigenic neuropeptides in the ingestion of sweet-tasting substances in rats. Chem Senses. 2005;30(Suppl 1):i186–il87. doi: 10.1093/chemse/bjh176. [DOI] [PubMed] [Google Scholar]
- 4.Grundy D. Sensory signals from the gastrointestinal tract. J Pediatr Gastroenterol Nutr. 2005;41(Suppl 1):S7–S9. doi: 10.1097/01.scs.0000180286.58988.cf. [DOI] [PubMed] [Google Scholar]
- 5.Gschossmann JM, Mayer EA, Miller JC, Raybould HE. Subdiaphragmatic vagal afferent innervation in activation of an opioidergic antinociceptive system in response to colorectal distension in rats. Neurogastroenterol Motil. 2002;14:403–408. doi: 10.1046/j.1365-2982.2002.00345.x. [DOI] [PubMed] [Google Scholar]
- 6.Hao S, Dulake M, Espero E, Sternini C, Raybould HE, Rinaman L. Central Fos expression and conditioned flavor avoidance in rats following intragastric administration of bitter taste receptor ligands. Am J Physiol Regul Integr Comp Physiol. 2009;296:R528–R536. doi: 10.1152/ajpregu.90423.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hao S, Sternini C, Raybould HE. Role of CCK1 and Y2 receptors in activation of hindbrain neurons induced by intragastric administration of bitter taste receptor ligands. Am J Physiol Regul Integr Comp Physiol. 2008;294:R33–R38. doi: 10.1152/ajpregu.00675.2007. [DOI] [PubMed] [Google Scholar]
- 8.Harrer MI, Travers SP. Topographic organization of Fos-like immunoreactivity in the rostral nucleus of the solitary tract evoked by gustatory stimulation with sucrose and quinine. Brain Res. 1996;711:125–137. doi: 10.1016/0006-8993(95)01410-1. [DOI] [PubMed] [Google Scholar]
- 9.Höfer D, Püschel B, Drenckhahn D. Taste receptor-like cells in the rat gut identified by expression of α-gustducin. Proc Natl Acad Sci U S A. 1996;93:6631–6634. doi: 10.1073/pnas.93.13.6631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Houpt TA, Berlin R, Smith GP. Subdiaphragmatic vagotomy does not attenuate c-Fos induction in the nucleus of the solitary tract after conditioned taste aversion expression. Brain Res. 1997;747:85–91. doi: 10.1016/s0006-8993(96)01221-8. [DOI] [PubMed] [Google Scholar]
- 11.Janssen S, Laermans J, Verhulst PJ, Thijs T, Tack J, Depoortere I. Bitter taste receptors and α-gustducin regulate the secretin of ghrelin with functional effects on food intake and gastric emptying. Proc Natl Acad Sci USA. 2011;108:2094–2099. doi: 10.1073/pnas.1011508108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.King CT, Travers SP, Rowland NE, Garcea M, Spector AC. Glossopharyngeal nerve transection eliminates quinine-stimulated fos-like immunoreactivity in the nucleus of the solitary tract: implications for a functional topography of gustatory nerve input in rats. J Neurosci. 1999;19:3107–3121. doi: 10.1523/JNEUROSCI.19-08-03107.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mueller KL, Hoon MA, Erlenbach I, Chandrashekar J, Zuker CS, Ryba NJ. The receptors and coding logic for bitter taste. Nature. 2005;434:225–229. doi: 10.1038/nature03352. [DOI] [PubMed] [Google Scholar]
- 14.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 6th ed. Amsterdam: Academic Press; 2007. pp. 139–144. [Google Scholar]
- 15.Rozengurt N, Wu SV, Chen MC, Huang C, Sternini C, Rozengurt E. Colocalization of the α-subunit of gustducin with PYY and GLP-1 in L cells of human colon. Am J Physiol Gastrointest Liver Physiol. 2006;291:G792–G802. doi: 10.1152/ajpgi.00074.2006. [DOI] [PubMed] [Google Scholar]
- 16.Sternini C. Taste receptors in the gastrointestinal tract. IV. Functional implications of bitter taste receptors in gastrointestinal chemosensing. Am J Physiol Gastrointest Liver Physiol. 2007;292:G457–G461. doi: 10.1152/ajpgi.00411.2006. [DOI] [PubMed] [Google Scholar]
- 17.Sutherland K, Young RL, Cooper NJ, Horowitz M, Blackshaw LA. Phenotypic characterization of taste cells of the mouse small intestine. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1420–G1428. doi: 10.1152/ajpgi.00504.2006. [DOI] [PubMed] [Google Scholar]
- 18.Travers JB, Urbanek K, Grill HJ. Fos-like immunoreactivity in the brain stem following oral quinine stimulation in decerebrate rats. Am J Physiol. 1999;277:R384–R394. doi: 10.1152/ajpregu.1999.277.2.R384. [DOI] [PubMed] [Google Scholar]
- 19.Uneyama H, Tanaka T, Torii K. Gut nutrient sensing by the abdominal vagus. Nihon Yakurigaku Zasshi. 2004;124:210–218. doi: 10.1254/fpj.124.210. [DOI] [PubMed] [Google Scholar]
- 20.Wu SV, Chen MC, Rozengurt E. Genomic organization, expression, and function of bitter taste receptors (T2R) in mouse and rat. Physiol Genomics. 2005;22:139–149. doi: 10.1152/physiolgenomics.00030.2005. [DOI] [PubMed] [Google Scholar]
- 21.Wu SV, Rozengurt N, Yang M, Young SH, Sinnett-Smith J, Rozengurt E. Expression of bitter taste receptors of the T2R family in the gastrointestinal tract and enteroendocrine STC-1 cells. Proc Natl Acad Sci U S A. 2002;99:2392–2397. doi: 10.1073/pnas.042617699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamamoto T, Sawa K. Comparison of c-Fos-like immunoreactivity in the brainstem following intraoral and intragastric infusions of chemical solutions in rats. Brain Res. 2000;866:144–151. doi: 10.1016/s0006-8993(00)02242-3. [DOI] [PubMed] [Google Scholar]