Abstract
Cadmium (Cd) is a well-known hepatotoxic environmental pollutant. We used rat hepatocytes as a model to study oxidative damage induced by Cd, effects on the antioxidant systems, and the role of N-acetylcysteine (NAC) in protecting cells against Cd toxicity. Hepatocytes were incubated for 12 and 24 h with Cd (2.5, 5, 10 µM). Results showed that Cd can induce cytotoxicity: 10 µM resulted in 36.2% mortality after 12 h and 47.8% after 24 h. Lactate dehydrogenase, aspartate aminotransferase, and alanine aminotransferase activities increased. Additionally, reactive oxygen species (ROS) generation increased in Cd-treated hepatocytes along with malondialdehyde levels. Glutathione concentrations significantly decreased after treatment with Cd for 12 h but increased after 24 h of Cd exposure. In contrast, glutathione peroxidase activity significantly increased after treatment with Cd for 12 h but decreased after 24 h. superoxide dismutase and catalase activities increased at 12 h and 24 h. glutathione S-transferase and glutathione reductase activities decreased, but not significantly. Rat hepatocytes incubated with NAC and Cd simultaneously had significantly increased viability and decreased Cd-induced ROS generation. Our results suggested that Cd induces ROS generation that leads to oxidative stress. Moreover, NAC protects rat hepatocytes from cytotoxicity associated with Cd.
Keywords: cadmium, hepatocytes, oxidative stress, rat
Introduction
Cadmium (Cd) is a hazardous environmental and industrial toxicant that has been classified as a type I carcinogen by the International Agency for Research on Cancer and the US National Toxicology Program [32]. Cd accumulates in soil and water before entering the food chain and accumulating in the final links. Human exposure occurs mainly through cigarette smoke, occupation, food, water, and dermal absorption [25]. The toxic effects of Cd depend on the dose, concentration, route, and exposure time [4,6]. In the human body, Cd has a biological half-life of more than 20 years.
Cd is toxic to several tissues such as kidney, liver, lung, testis, and bone. Most notably, Cd causes hepatotoxicity and nephrotoxicity following acute and chronic exposure, respectively. The mechanisms responsible for Cd toxicity are dependent on cell type. However, the mechanism by which Cd induces cytotoxicity remains unclear. Several studies have shown that Cd modulates toxic effects at least through oxidative stress-associated mechanisms. Oxidative stress caused by excessive reactive oxygen species (ROS) production can damage tissues and cells by altering lipid peroxidation as well as protein or nucleic acid structure and function [30], interfering with antioxidant enzymes [16], inhibiting energy metabolism [27], altering thiol proteins [1], and affecting enzyme (superoxide dismutase, catalase) activities [21].
Cd has only one oxidation state and thus cannot generate free radicals directly. However, Cd can indirectly promote the generation of ROS such as superoxide anions, hydroxyl radicals, and hydrogen peroxide [29]. Lopez et al. [24] reported that Cd induces ROS generation and lipid peroxidation in cultured cortical neurons. Odewumi et al. [32] found that Cd damages cultured rat liver cells. Nemmiche [29] suggested that Cd can induce oxidative stress in Wistar rat blood, liver, and brain. These findings suggest that oxidative stress is important for Cd-associated toxicity. Reagents such as hesperetin [20], quercetin [34], selenium [30], N-acetylcysteine (NAC) [32], and diallyl tetrasulfide [28] act as chelators and/or antioxidants, and have been used to prevent Cd-induced cytotoxicity in animal models or cell lines. In the present investigation, we examined the ability of Cd to affect ROS generation and oxidative stress in rat hepatocytes. We also assessed the beneficial role of NAC in protecting cells against Cd-induced toxicity and ROS production.
Materials and Methods
Materials
Cadmium acetate (CdAC2); 2,7-dichlorodihydrofluorescein diacetate (H2DCF-DA), penicillin, and streptomycin were purchased from Sigma-Aldrich (USA). Insulin, fetal bovine serum (FBS), penicillin-streptomycin solution, and Leibovitz-15 (L-15) medium were obtained from Gibco Laboratories (USA). 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) and trypsin were from Amresco (USA). Lactate dehydrogenase (LDH), aspartate aminotransferase (AST), alanine aminotransferase (ALT), malondialdehyde (MDA), glutathione (GSH), glutathione peroxidase (GPx), glutathione S-transferase (GST), glutathione reductase (GR), superoxide dismutase (SOD), and catalase (CAT) kits were purchased from Jiancheng Bioengineering Institute (China). Cell culture plates were obtained from Corning (USA). Other reagents (for example: NaCl, KCl) used were available locally and of analytical grade.
Hepatocyte isolation, culture, and treatment
Rat hepatocytes were prepared by trypsin perfusion as previously described by Lin [22] with some modifications. Sprague-Dawley rats were anesthetized with sodium pentobarbital (40 mg/kg body weight; intraperitoneal injection). The liver was perfused through the portal vein at a flow rate of 25 mL/min with 150 mL of 25 mM sodium phosphate buffer (pH 7.6) to remove the blood. The buffer was replaced with 200 mL of the same buffer supplemented with 0.18% trypsin, 40 mM CaCl2, and 5 mg of trypsin inhibitor, and the liver was perfused for another 10 min at a rate of 18 mL/min.
To produce a single-cell suspension of hepatic parenchymal cells, the liver was removed, passed through a sieve (stainless stell mesh), washed (phosphate buffer solution), suspended in Percoll [19], and centrifuged (130 × g for 15 min at 4℃). The hepatocytes were then resuspended and washed twice with washing medium (L-15 medium). Cell viability was determined by trypan blue exclusion and was higher than 95%. After a final wash, the isolated hepatocytes were resuspended at a density of 5 × 105 cells/mL in L-15 medium (pH 7.6) supplemented with 10% FBS, 5 mg/L insulin, 4 mg/L dexamethasone, 100,000 IU/L penicillin, and 100 mg/L streptomycin. The cells (2.5 × 106 cells) were plated in collagen-precoated 6-well culture plates. The cell cultures were maintained at 37℃ in a humidified 5% CO2-air atmosphere. After 2 h of incubation, the cultures were washed with serum-free L-15 medium and then incubated in L-15 medium (with serum) for 20 h before being used for subsequent experiments. Next, the cell cultures were washed with L-15 medium and incubated with serum-free L-15 medium containing 0, 2.5, 5, or 10 µM Cd for a specific period of time (0.75, 1.5, 6, 12 or 24 h). NAC (1, 2 mM) was also added simultaneously with the Cd for 1.5 or 24 h.
Cell viability assay
After treatment with various concentrations of Cd for 12 or 24 h, hepatocyte viability was measured with an MTT assay as previously described [26]. The cells were washed with phosphate buffer saline (PBS) and incubated at 37℃ for 2 h in fresh culture medium containing MTT (100 µL/mL of medium). The hepatocytes were then washed with PBS and lysed with dimethylsulfoxide (DMSO). Colored formazan crystals that formed after the conversion of tetrazolium salt by mitochondrial dehydrogenases were solubilized in DMSO and the optical density was measured by spectrophotometry with microplate reader (Tecan, Australia) at 570 nm.
Assessment of LDH, AST, and ALT
After hepatocytes were treated with Cd, the medium from each well was collected to measure the activities of LDH, AST, and ALT that had been released using commercial kits.
Measurement of ROS production
H2DCF-DA was used to measure ROS formation. H2DCF-DA enters cells where it is transformed into 2,7-dichlorodihydrofluorescein (H2DCF) by intracellular stearases. H2DCF is then oxidized into fluorescent DCF by hydrogen peroxide. Thus, the fluorescent intensity is proportional to the amount of peroxides produced by the cells. Following exposure to Cd, the hepatocytes were trypsinized and washed with ice-cold PBS. Afterward, 1 mL of PBS containing 50 µM H2DCF-DA was added and the cells were incubated for 30 min at 37℃. Fluorescence emission from DCF was analyzed by FACSscan flow cytometry by the fluorescence intensity (FL-1, 530 nm).
Cell homogenate preparation
After different treatments, the hepatocytes were trypsinized, harvested, and pelleted by centrifugation (3,000 × g for 5 min at 4℃). The cells were resuspended in isotonic Tris HCl buffer (20 mM, pH 7.4), centrifuged at 3,000 × g for 5 min at 4℃, and rinsed twice with the Tris HCl buffer. Next, the cells were lysed in hypotonic Tris HCl buffer by five freeze thaw cycles. The resulting cell homogenate was centrifuged at 3,000 × g for 10 min at 4℃. The supernatant was stored at -80℃ for the subsequent biochemical assays as described by Nzengue et al. [31].
Quantitative protein determination
Protein content in the supernatant of cell homogenate preparation was measured by the Bradford method using crystalline BSA as a standard as previously described [38].
Estimation of lipid peroxidation
Lipid peroxidation was estimated by measuring thiobarbituric acid reactive substances (TBARS) and expressed in terms of MDA content according to the method of Esterbauer and Cheeseman [9]. Briefly, a 200-µL aliquot of hepatocytes supernatant (10%, w/v) was mixed thoroughly with an aqueous solution of thiobarbituric acid (0.5%, 1 mL) and heated at 100℃ for 30 min in a water bath. The suspension was cooled to room temperature, and then centrifuged at 3,500 × g for 10 min at room temperature. The resulting pink supernatant was analyzed by spectrophotometry at 532 nm. TBARS concentration of the samples was calculated using the extinction coefficient of MDA (1.56 × 105 M-1cm-1) given that 99% of TBARS exists as MDA.
Determination of intracellular reduced GSH levels
GSH levels in all the experimental groups of hepatocytes were measured as previously described by Ellman [8]. A 500 µL of hepatocyte supernatant and 2 mL 5% TCA were mixed to precipitate the protein contents of the suspension. After centrifugation (10,000 × g for 5 min, room temperature), the supernatant was collected and combined with 5,5'-Dithiobis-(2-nitrobenzoic acid) (DTNB) solution (Ellman's reagent). Absorbance was measured at 412 nm. A standard curve was generated using different known concentrations of GSH solution (0, 20, 40, 60, 80, 100 µmol/L) and used to calculate GSH contents of the hepatocytes.
Assessment of antioxidant enzyme activity
GPx activity was assayed as previously described by Flohe and Ginzler [10]. The reaction between GSH remaining after GPx action and 5,5'-dithiobis-2-nitrobenzoic acid forms a complex with a maximum absorbance at 412 nm. GST activity was determined spectrophotometrically using dichloro-2,4-dinitrobenzene as a substrate [13].
GR activity was measured according to the method of Smith et al. [38]. GR can utilize NADPH to convert metabolized glutathione (GSSG) into reduced GSH. SOD activity was assessed according to the method of Kakkar et al. [17] in which the inhibition of NADPH-phenazine methosulphate nitroblue tetrazolium formazon formation of was measured spectrophotometrically at 560 nm. CAT activity was assayed colorimetrically using dichromate acetic acid reagent as previously described by Bonaventura et al. [3].
Statistical analysis
All results are presented as the mean ± standard deviation (SD). Differences were assessed using an analysis of variance (ANOVA) and group mean values were compared by Duncan's multiple range test (DMRT). P values of 0.05 or less were considered significant.
Results
Effect of Cd on cell viability
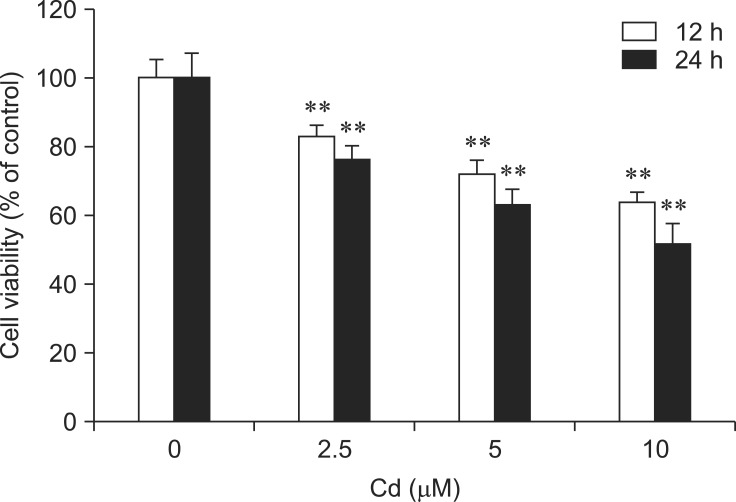
The effect of Cd on rat hepatocyte viability is presented in Fig. 1. Cell viability was reduced in Cd-treated hepatocytes compared to normal hepatocytes incubated in L-15 medium alone. The results showed that different concentrations of Cd (2.5~10 µM) significantly decreased viability of the rat hepatocytes (p < 0.01).
Fig. 1.
Effect of cadmium (Cd) on the viability of rat hepatocytes. The cells were incubated with 0, 2.5, 5, and 10 µM Cd for 12 and 24 h. Viability was assessed by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) colorimetric assay. Each experiment was repeated six times and data are expressed as the mean ± SD (**p < 0.01).
Effect on marker enzyme activity
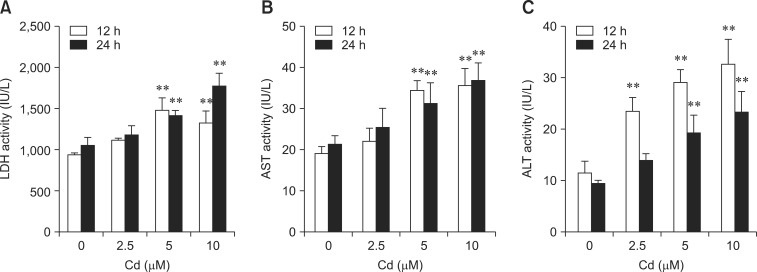
LDH, AST, and ALT activities are shown in Fig. 2. After 12 h, the level of LDH and AST activities significantly increased after treatment with 5 and 10 µM Cd. ALT activity significantly increased after treatment with 2.5, 5, and 10 µM Cd (p < 0.01). The levels of LDH, AST, and ALT activity significantly increased after treatment with 5 and 10 µM Cd for 24 h (p < 0.01).
Fig. 2.
Effects of Cd on (A) lactate dehydrogenase (LDH) activity, (B) aspartate aminotransferase (AST) activity, and (C) alanine aminotransferase (ALT) activity in rat hepatocytes. Cells were incubated with 0, 2.5, 5, and 10 µM Cd for 12 and 24 h. Each experiment was repeated six times and data are expressed as the mean ± SD (**p < 0.01).
Effect of Cd on ROS generation
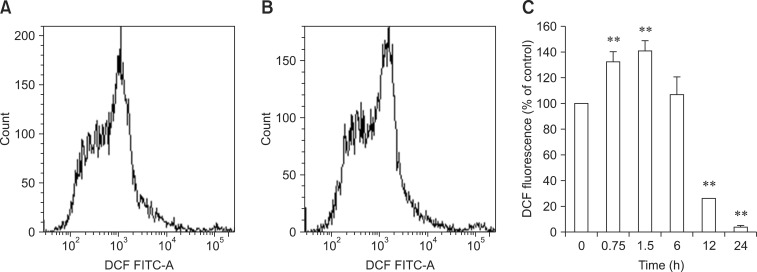
To evaluate the involvement of ROS in the development of Cd-induced oxidative stress in the hepatocytes, we monitored ROS generation over time. The hepatocytes were exposed to 5 µM Cd, and the level of DCF fluorescence after 0.75, 1.5, 6, 12, and 24 h was monitored by flow cytometry. As shown in panel C in Fig. 3, ROS generation increased significantly as early as 0.75 h after Cd exposure, peaked at 1.5 h, and began to decrease thereafter. ROS production eventually dropped below that found in the untreated control at 12 and 24 h.
Fig. 3.
Effect of Cd on reactive oxygen species (ROS) production. Hepatocytes were treated with 5 µM of Cd for 45 min or 1.5, 6, 12, and 24 h. The cells were then stained with DCFH2-DA. Intracellular ROS levels were measured by flow cytometry as described in the Materials and Methods section. Results are expressed as a representative histogram (A, control; B, 5 µM Cd; respectively 1.5 h) and mean fluorescence obtained from the histogram statistics (C). Each bar represents the mean ± SD (n = 3). **p < 0.01 and *p < 0.05 compared to the control. DCF: dichlorofluorescein, FITC: fluorescein isothiocyanate.
Oxidative stress markers
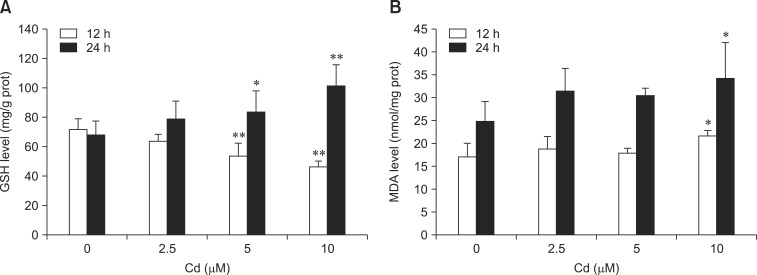
Two oxidative stress markers were evaluated to monitor oxidative changes in the hepatocytes after 12 or 24 h of incubation with various concentrations of Cd. The panel A in Fig. 4 shows GSH levels in the hepatocytes. This protein is thought to offer cytoprotection against Cd toxicity. Our results showed that GSH levels significantly decreased after treatment with 5 and 10 µM Cd for 12 h, but increased after treatment with 5 and 10 µM for 24 h (p < 0.05 or p < 0.01). MDA levels increased significantly only after 24 h of treatment with 10 µM Cd (panel B in Fig. 4), indicating that Cd induced lipid peroxidation in the hepatocytes (p < 0.05).
Fig. 4.
Effects of Cd on (A) glutathione (GSH) and (B) malondialdehyde (MDA) levels in rat hepatocytes. Cells were treated with 0, 2.5, 5, and 10 µM Cd for 12 and 24 h. Each experiment was repeated six times and data are expressed as the mean ± SD (*p < 0.05 and **p < 0.01).
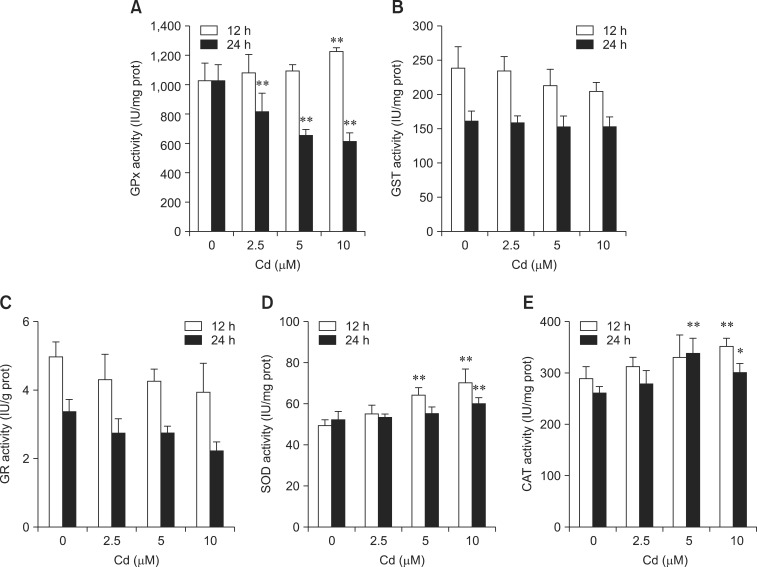
Antioxidant enzyme activity
GPx activity significantly increased after treatment with 10 µM only after 12 h, but decreased after exposure to 2.5, 5, and 10 µM for 24 h (p < 0.01) (panel A in Fig. 5). A negative correlation was observed between GPx activity and GSH content. GST and GR activities decreased after 12 or 24 h of incubation (panels B and C in Fig. 5) but not significantly. As presented in panel D in Fig. 5, SOD activity increased after the hepatocytes were treated with 5 and 10 µM Cd (p < 0.01). CAT activity significantly increased after exposure to 10 µM Cd for 12 h. After 24 h of incubation with 5 and 10 µM Cd, CAT activity was also significantly elevated (p < 0.05 or p < 0.01; panel E in Fig. 5). Taken together, these findings demonstrate that Cd affected intracellular GPx, CAT, and SOD activities but not GST or GR activities.
Fig. 5.
Effects of Cd on (A) glutathione peroxidase (GPx), (B) glutathione S-transferase (GST), (C) glutathione reductase (GR), (D) superoxide dismutase (SOD), and (E) catalase (CAT) activities. Hepatocytes were treated with 2.5, 5, and 10 µM Cd. GPx, GST, GR, SOD, and CAT activities were measured after 12 and 24 h. Each experiment was repeated six times and data are expressed as the mean ± SD (*p < 0.05 and **p < 0.01).
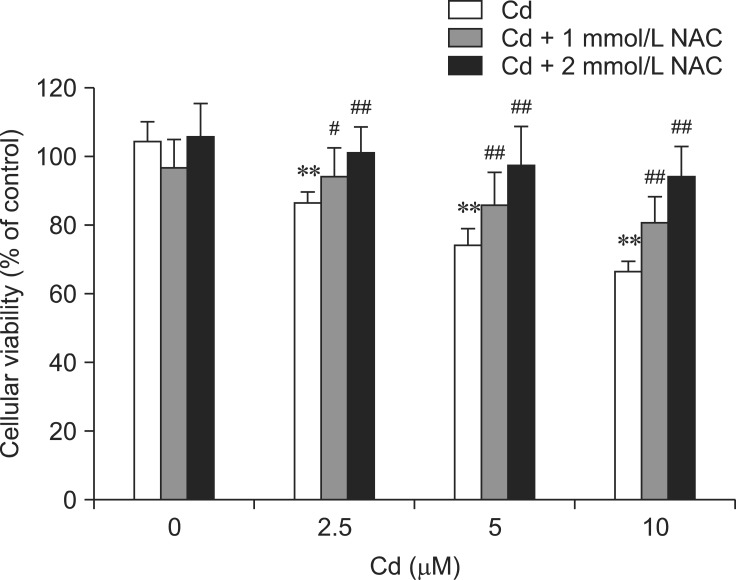
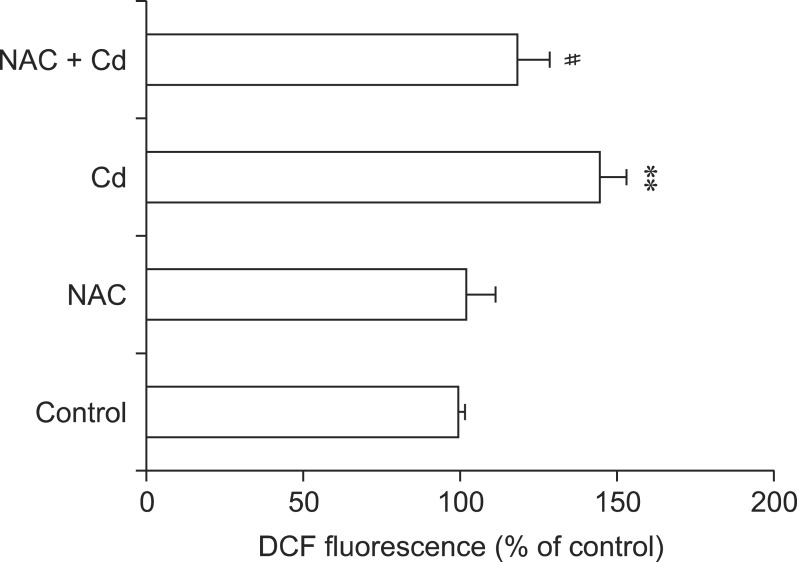
Influence of NAC on cytotoxicity and ROS generation induced by Cd
Rat hepatocytes were incubated with 1 or 2 mM NAC along with 2.5, 5, or 10 µM Cd simultaneously for 12 h. NAC significantly increased hepatocyte viability reduced by Cd in a dose-dependent manner (p < 0.05 or p < 0.01; Fig. 6). To determine whether or not oxidative stress was linked to Cd treatment, the hepatocytes were incubated with 2 mM NAC and 5 µM Cd for 1.5 h when Cd-induced ROS generation peaked. The effect of NAC on ROS production was monitored by DCFH2-DA staining. As shown in Fig. 7, incubation with NAC resulted in almost complete inhibition of Cd-associated ROS generation at 1.5 h.
Fig. 6.
Effect of N-acetylcysteine (NAC) on Cd-induced cytotoxicity in hepatocytes. Cells were incubated with NAC (1 or 2 mM) and Cd (2.5, 5, or 10 µM) simultaneously for 24 h. An MTT assay was then performed to evaluate cytotoxicity. Each experiment was repeated six times and data are expressed as the mean ± SD (**p < 0.01, #p < 0.05, and ##p < 0.01 compared to cells treated with Cd alone).
Fig. 7.
Inhibitory effect of NAC on Cd-induced ROS generation (as monitored by DCF fluorescence). The cells were incubated with NAC (2 mM) and Cd (5 µM) at the same time for 1.5 h. Bars represent the mean ± SD (n = 3). **p < 0.01 and #p < 0.05 compared to cells treated with Cd alone.
Discussion
Cd can damage rat liver cells by inducing oxidative stress and mitochondrial dysfunction in vivo [23]. We decided to use rat primary hepatocytes in our research. The biggest advantage of primary cultured cells is that these cells better represent live tissues. Biological traits of the cells do not change much when cultured. Thus, primary cultures are ideal for in vitro toxicity studies.
Cell viability is directly associated with membrane integrity and cell membrane structure. In the present study, cellular damage was detected by measuring the percentage of cell viability and the activities of membrane enzymes LDH, AST, and ALT. The levels of them are related to the status and function of hepatic cells. High levels of LDH, AST and ALT are hallmarks of liver damage. Changes in the activities of these enzymes have been used to study cell viability and cell membrane permeability [37]. In the present study, incubation of hepatocytes with Cd reduced the percentage of cell viability compared to untreated hepatocytes. The activities of LDH, AST, and ALT also increased in the hepatocytes incubated with Cd.
The intracellular antioxidant system includes different free radical scavenging antioxidant enzymes along with non-enzyme antioxidants such as GSH. GPx, GST, GR, SOD, and CAT mutually form a defense against ROS. GPx can convert H2O2 and GSH into water and GSSG. GR and GPx also maintain the intracellular redox status [18]. SOD and CAT are the most important enzymes that act against the toxic effects of oxygen metabolism. SOD transforms O2- into H2O2 and H2O [37]. CAT is a hemeprotein that catalyses the reduction of H2O2 into water and oxygen, and thus protects the cell from oxidative damage by H2O2 and OH [5]. Thus, these cellular antioxidants have important roles in the elimination of free radicals, and equilibrium exists between these factors under normal physiological conditions. When excess free radicals are produced due to toxin exposure, this equilibrium is lost and oxidative insult consequently occurs [37].
Controversies exist in the literature as to whether Cd promotes the increase or decrease of the intracellular GSH levels [12,24]. We observed that Cd decreased the intracellular levels of reduced GSH at 12 h but increased at 24 h after exposure. At the earlier time point (12 h), we believe that hepatocytes try to protect themselves against the Cd toxicity. And reduced GSH was thought one of the most important systems present in the hepatocytes. After a longer period of time (24 h), the reduced GSH levels increased. Cd may induce the transcription of genes involved in GSH biosynthesis in some cell types as indicated by results obtained by Nzengue [32] and Hatcher et al. [14] in HaCaT and A459 cells.
Our results demonstrated that Cd exposure increased SOD and CAT activities after 12 and 24 h. GPx activity increased at 12 h and decreased at 24 h. Increases in the activities of SOD, CAT, and GPx suggest that there are groups of hepatocytes that are resistant to Cd because the cells are able to produce enzymes that confer protection against oxidative stress. Increases in these enzymatic activities could be due to elevated enzyme expression. Oxidative damage provokes cellular responses in an effort to compensate for the overload of ROS formation [7]. Data showing the effect of Cd on antioxidant enzymes are contradictory [31,35]. This diversity of results may be due to differences among cell types, the Cd concentration used, and exposure time. The reason why GPx activity decreased at 24 h in the current study may be due to the fact that Cd interacts directly with GPx which decreases the efficiency of the enzyme to remove peroxides and H2O2.
Funakoshi et al. [24] reported that lipid peroxidation in hepatocytes increases after 12 h of Cd exposure. Elevated ROS levels, however, are observed prior to increases in lipid peroxidation. If present in excess, ROS initiate peroxidative cell damage [11]. Bolduc along with Shih et al. [2,36] reported that 100 or 50 µM Cd elicited a burst of ROS production at 3 h in normal human lung fibroblast MRC-5 cells, and 30 min in human enterocytic-like Caco-2 cells. These findings suggest that ROS helps increase lipid peroxidation promoted by Cd exposure. Based on data from our present study, there appeared to be an ROS burst occurring within 2 h. The level of ROS was significantly decreased at 12 and 24 h in our investigation. However, the GSH level along with the activities of SOD and CAT were increased at the same time. These results indicate that increased antioxidant and enzyme activities following Cd treatment may be due to decreased ROS generation. Liu et al. [23] observed that GSH content negatively correlates with ROS production in vivo after rats were exposed to Cd for 16 h, and speculated that ROS has a vital role in the inhibition of antioxidant enzyme activity. Several mechanisms underlying Cd cytotoxicity have been proposed including lipid peroxidation, interference with mitochondrial function, and interaction with cellular thiol ligands [15]. Our data support the hypothesis that oxidative stress serves as a common mediator of Cd cytotoxicity.
NAC is known as a precursor for GSH synthesis and as an antioxidant agent able to scavenge ROS [39]. In our study, a significant increase in cellular viability and decreased ROS generation were observed when the rat hepatocytes were incubated with Cd and NAC at the same time. This observation was in agreement with those from a previous study in anterior pituitary cells [33], indicating that either NAC accumulation during the pre-incubation period was insufficient or that formation of an impermeable NAC-Cd complex is required for protection. Hepatocytes pretreated with NAC and exposed to Cd in the absence of NAC were not protected against Cd cytotoxicity (date not shown). Nzengue et al. [15,31] suggested that cellular viability is significantly decreased when HaCaT and A549 cells are incubated with Cd and NAC at the same time. In they experiments, NAC restored cellular viability when incubated with the cells prior to Cd exposition. A cumulative toxic effect of NAC and Cd under experimental conditions could result from an interaction between NAC and Cd in HaCaT cells or the culture medium. NAC can chelate Cd and facilitate its uptake by cells, leading to increased cellular Cd levels.
In conclusion, our results suggested that ROS is involved in the development of oxidative stress generated by Cd. Cd can induce lipid peroxidation and change the activities of antioxidant enzymes. NAC can help protect Cd-exposed cell populations against the toxic effects of this metal.
Acknowledgments
This study was supported by the Research Fund for Doctor of Henan University of Science and Technology (09001489), China. We thank Dr. Mao-zhi Hu (Yangzhou University) for technical assistance with the flow cytometry analysis.
Footnotes
There is no conflict of interest.
References
- 1.Belyaeva EA, Korotkov SM. Mechanism of primary Cd2+-induced rat liver mitochondria dysfunction: discrete modes of Cd2+ action on calcium and thiol-dependent domains. Toxicol Appl Pharmacol. 2003;192:56–68. doi: 10.1016/s0041-008x(03)00255-2. [DOI] [PubMed] [Google Scholar]
- 2.Bolduc JS, Denizeau F, Jumarie C. Cadmium-induced mitochondrial membrane-potential dissipation does not necessarily require cytosolic oxidative stress: studies using rhodamine-123 fluorescence unquenching. Toxicol Sci. 2004;77:299–306. doi: 10.1093/toxsci/kfh015. [DOI] [PubMed] [Google Scholar]
- 3.Bonaventura J, Schroeder WA, Fang S. Human erythrocyte catalase: an improved method of isolation and a reevaluation of reported properties. Arch Biochem Biophys. 1972;150:606–617. doi: 10.1016/0003-9861(72)90080-x. [DOI] [PubMed] [Google Scholar]
- 4.Bridges CC, Zalups RK. Molecular and ionic mimicry and the transport of toxic metals. Toxicol Appl Pharmacol. 2005;204:274–308. doi: 10.1016/j.taap.2004.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chance B, Greenstein DS, Roughton FJW. The mechanism of catalase action. I. Steady state analysis. Arch Biochem Biophys. 1952;37:301–321. doi: 10.1016/0003-9861(52)90194-x. [DOI] [PubMed] [Google Scholar]
- 6.Chin TA, Templeton DM. Protective elevations of glutathione and metallothionein in cadmium-exposed mesangial cells. Toxicology. 1993;77:145–156. doi: 10.1016/0300-483x(93)90145-i. [DOI] [PubMed] [Google Scholar]
- 7.Dalton TP, Shertzer HG, Puga A. Regulation of gene expression by reactive oxygen. Annu Rev Pharmacol Toxicol. 1999;39:67–101. doi: 10.1146/annurev.pharmtox.39.1.67. [DOI] [PubMed] [Google Scholar]
- 8.Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 9.Esterbauer H, Cheeseman KH. Determination of aldehydic lipid peroxidation products: malonaldehyde and 4-hydroxynonenal. Methods Enzymol. 1990;186:407–421. doi: 10.1016/0076-6879(90)86134-h. [DOI] [PubMed] [Google Scholar]
- 10.Flohé L, Günzler WA. Assays of glutathione peroxidase. Methods Enzymol. 1984;105:114–121. doi: 10.1016/s0076-6879(84)05015-1. [DOI] [PubMed] [Google Scholar]
- 11.Funakoshi T, Ueda K, Shimada H, Kojima S. Effects of dithiocarbamates on toxicity of cadmium in rat primary hepatocyte cultures. Toxicology. 1997;116:99–107. doi: 10.1016/s0300-483x(96)03533-0. [DOI] [PubMed] [Google Scholar]
- 12.Gaubin Y, Vaissade F, Croute F, Beau B, Soleilhavoup JP, Murat JC. Implication of free radicals and glutathione in the mechanism of cadmium-induced expression of stress proteins in the A549 human lung cell-line. Biochim Biophys Acta. 2000;1495:4–13. doi: 10.1016/s0167-4889(99)00149-4. [DOI] [PubMed] [Google Scholar]
- 13.Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249:7130–7139. [PubMed] [Google Scholar]
- 14.Hatcher EL, Chen Y, Kang YJ. Cadmium resistance in A549 cells correlates with elevated glutathione content but not antioxidant enzymatic activities. Free Radic Biol Med. 1995;19:805–812. doi: 10.1016/0891-5849(95)00099-j. [DOI] [PubMed] [Google Scholar]
- 15.Hinkle PM, Kinsella PA, Osterhoudt KC. Cadmium uptake and toxicity via voltage-sensitive calcium channels. J Biol Chem. 1987;262:16333–16337. [PubMed] [Google Scholar]
- 16.Hussain T, Shukla GS, Chandra SV. Effects of cadmium on superoxide dismutase and lipid peroxidation in liver and kidney of growing rats: in vivo and in vitro studies. Pharmacol Toxicol. 1987;60:355–358. doi: 10.1111/j.1600-0773.1987.tb01526.x. [DOI] [PubMed] [Google Scholar]
- 17.Kakkar P, Das B, Viswanathan PN. A modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Biophys. 1984;21:130–132. [PubMed] [Google Scholar]
- 18.Ketterer B. Detoxication reactions of glutathione and glutathione transferases. Xenobiotica. 1986;16:957–973. doi: 10.3109/00498258609038976. [DOI] [PubMed] [Google Scholar]
- 19.Kreamer BL, Staecker JL, Sawada N, Sattler GL, Hsia MT, Pitot HC. Use of a low-speed, iso-density percoll centrifugation method to increase the viability of isolated rat hepatocyte preparations. In Vitro Cell Dev Biol. 1986;22:201–211. doi: 10.1007/BF02623304. [DOI] [PubMed] [Google Scholar]
- 20.Leelavinothan P, Kalist S. Beneficial effect of hesperetin on cadmium induced oxidative stress in rats: an in vivo and in vitro study. Eur Rev Med Pharmacol Sci. 2011;15:992–1002. [PubMed] [Google Scholar]
- 21.Lemarié A, Lagadic-Gossmann D, Morzadec C, Allain N, Fardel O, Vernhet L. Cadmium induces caspase-independent apoptosis in liver Hep3B cells: role for calcium in signaling oxidative stress-related impairment of mitochondria and relocation of endonuclease G and apoptosis-inducing factor. Free Radic Biol Med. 2004;36:1517–1531. doi: 10.1016/j.freeradbiomed.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 22.Lin WC, Liao YC, Liau MC, Lii CK, Sheen LY. Inhibitory effect of CDA-II, a urinary preparation, on aflatoxin B1-induced oxidative stress and DNA damage in primary cultured rat hepatocytes. Food Chem Toxicol. 2006;44:546–551. doi: 10.1016/j.fct.2005.08.029. [DOI] [PubMed] [Google Scholar]
- 23.Liu T, He W, Yan C, Qi Y, Zhang Y. Roles of reactive oxygen species and mitochondria in cadmium-induced injury of liver cells. Toxicol Ind Health. 2011;27:249–256. doi: 10.1177/0748233710386408. [DOI] [PubMed] [Google Scholar]
- 24.López E, Arce C, Oset-Gasque MJ, Cañadas S, González MP. Cadmium induces reactive oxygen species generation and lipid peroxidation in cortical neurons in culture. Free Radic Biol Med. 2006;40:940–951. doi: 10.1016/j.freeradbiomed.2005.10.062. [DOI] [PubMed] [Google Scholar]
- 25.Méplan C, Mann K, Hainaut P. Cadmium induces conformational modifications of wild-type p53 and suppresses p53 response to DNA damage in cultured cells. J Biol Chem. 1999;274:31663–31670. doi: 10.1074/jbc.274.44.31663. [DOI] [PubMed] [Google Scholar]
- 26.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 27.Müller L. Consequences of cadmium toxicity in rat hepatocytes: mitochondrial dysfunction and lipid peroxidation. Toxicology. 1986;40:285–295. doi: 10.1016/0300-483x(86)90061-2. [DOI] [PubMed] [Google Scholar]
- 28.Murugavel P, Pari L. Effects of diallyl tetrasulfide on cadmium-induced oxidative damage in the liver of rats. Hum Exp Toxicol. 2007;26:527–534. doi: 10.1177/0960327107073810. [DOI] [PubMed] [Google Scholar]
- 29.Nemmiche S, Chabane-Sari D, Guiraud P. Role of α-tocopherol in cadmium-induced oxidative stress in Wistar rat's blood, liver and brain. Chem Biol Interact. 2007;170:221–230. doi: 10.1016/j.cbi.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 30.Newairy AA, El-Sharaky AS, Badreldeen MM, Eweda SM, Sheweita SA. The hepatoprotective effects of selenium against cadmium toxicity in rats. Toxicology. 2007;242:23–30. doi: 10.1016/j.tox.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 31.Nzengue Y, Steiman R, Garrel C, Lefèbvre E, Guiraud P. Oxidative stress and DNA damage induced by cadmium in the human keratinocyte HaCaT cell line: role of glutathione in the resistance to cadmium. Toxicology. 2008;243:193–206. doi: 10.1016/j.tox.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 32.Odewumi CO, Badisa VLD, Le UT, Latinwo LM, Ikediobi CO, Badisa RB, Darling-Reed SF. Protective effects of N-acetylcysteine against cadmium-induced damage in cultured rat normal liver cells. Int J Mol Med. 2011;27:243–248. doi: 10.3892/ijmm.2010.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Poliandri AHB, Cabilla JP, Velardez MO, Bodo CCA, Duvilanski BH. Cadmium induces apoptosis in anterior pituitary cells that can be reversed by treatment with antioxidants. Toxicol Appl Pharmacol. 2003;190:17–24. doi: 10.1016/s0041-008x(03)00191-1. [DOI] [PubMed] [Google Scholar]
- 34.Prabu SM, Muthumani M, Shagirtha K. Quercetin potentially attenuates cadmium induced oxidative stress mediated cardiotoxicity and dyslipidemia in rats. Eur Rev Med Pharmacol Sci. 2013;17:582–595. [PubMed] [Google Scholar]
- 35.Renugadevi J, Prabu SM. Quercetin protects against oxidative stress-related renal dysfunction by cadmium in rats. Exp Toxicol Pathol. 2010;62:471–481. doi: 10.1016/j.etp.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 36.Shih CM, Ko WC, Wu JS, Wei YH, Wang LF, Chang EE, Lo TY, Cheng HH, Chen CT. Mediating of caspase-independent apoptosis by cadmium through the mitochondria-ROS pathway in MRC-5 fibroblasts. J Cell Biochem. 2004;91:384–397. doi: 10.1002/jcb.10761. [DOI] [PubMed] [Google Scholar]
- 37.Sinha M, Manna P, Sil PC. Taurine, a conditionally essential amino acid, ameliorates arsenic-induced cytotoxicity in murine hepatocytes. Toxicol In Vitro. 2007;21:1419–1428. doi: 10.1016/j.tiv.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 38.Smith IK, Vierheller TL, Thorne CA. Assay of glutathione reductase in crude tissue homogenates using 5,5'-dithiobis(2-nitrobenzoic acid) Anal Biochem. 1988;175:408–413. doi: 10.1016/0003-2697(88)90564-7. [DOI] [PubMed] [Google Scholar]
- 39.Wispriyono B, Matsuoka M, Igisu H, Matsuno K. Protection from cadmium cytotoxicity by N-acetylcysteine in LLC-PK1 cells. J Pharmacol Exp Ther. 1998;287:344–351. [PubMed] [Google Scholar]