Abstract
In the present study, the outbreak patterns of bovine brucellosis in Korea from 2000 to 2011 were analyzed to understand the epidemiological evolution of this disease in the country. A total of 85,521 brucella reactor animals were identified during 14,215 outbreaks over the 12-year study period. The number of bovine brucellosis cases increased after 2003 and peaked in 2006 before decreasing thereafter. The majority of the bovine brucellosis cases were Korean native cattle, Han Woo. The numbers of human brucellosis cases and cattle outbreaks increased and decreased in the same pattern. The correlation coefficient for human and bovine cases per year was 0.96 (95% confidence interval = 0.86~0.99; p < 10-3). The epidemiological characteristics of bovine brucellosis appeared to be affected by the intensity of eradication programs that mainly involved a test-and-slaughter policy. Findings from the present study were based on freely available statistics from web pages maintained by government agencies. This unlimited access to information demonstrates the usefulness of government statistics for continually monitoring the health of animal populations.
Keywords: brucellosis, cattle, epidemiology, eradication, Korea
Introduction
Bovine brucellosis caused by Brucella (B.) abortus has been one of the most important endemic animal diseases in several countries for several decades including Korea. This condition exerts a significant socioeconomic impact due to decreased productivity of animals such as the loss of breeding cattle, abortion, delayed growth, and reduced milk yield [4] as well as zoonotic effects [8]. Outbreaks of bovine brucellosis not only affect farmers and veterinarians but also employees of related industries, the public health, and society in general [19].
A monitoring and surveillance system for bovine brucellosis based on a test-and-slaughter policy was established in the 1960s and has been in operation for more than 50 years following the first official case in Korea in 1956 [27]. According to the Act on the Prevention of Contagious Animal Diseases, brucellosis is a Class 2 notifiable animal disease and is currently regulated by the standard operation procedure (SOP) for tuberculosis and brucellosis implemented by the Ministry of Agriculture, Food, and Rural Affairs (MAFRA). The SOP was last amended in March 2014 [20]. Once a reactor is confirmed on a cattle farm, all individual animals reared in the same farm and those that may be epidemiologically associated are tested within 10 days after detecting the first reactor. The outbreak farm can obtain official certification of the end of the brucellosis outbreak when no additional reactors are confirmed by at least two consecutive tests conducted within the next 30~60 days [20].
Prior to 2000, implementation of the bovine brucellosis control program in Korea concentrated on dairy cattle. Thus, the majority of reactors in Korea were Holstein dairy cows. Outbreaks involving Han Woo, Korean native beef cattle (scientific name: Bos taurus coreanae), have been sporadically reported since the early 1990s with the number of outbreaks increasing in the early 2000s [26]. An intensive eradication program that imposed strict control measures on HanWoo and dairy cattle was implemented in 2004. Since then, various supplementary measures were subsequently added. For example, any cow that was not certified as "brucella-free" could not be sold after June 2004 and could not be slaughtered as of March 2005. In July 2006, complementary periodical tests of high-risk populations (e.g., animals inhabiting high-prevalence region, large herds, and middleman holding lots) were added. A compulsory annual test was instituted in January 2008 for all cows aged 1 year or older. In addition, brucella-free certification was extended to include bulls. In 2004, the Korean government compensated 100% of the market price for culled animals. The payment was reduced to 60% between November 2006 and June 2008. After July 2008, owners of the reactors were compensated 80% of the market price [31]. Vaccination of cattle with the RB51 strain was initiated in 1998. However, this program was immediately eliminated due to unexpected side effects including abortion, premature birth, and decreased milk yield. In the present study, epidemiological patterns of bovine brucellosis outbreaks during the first 11 years of the 21st century were analyzed to understand the epidemiological evolution of this disease in Korea.
Materials and Methods
Definitions
The term reactor (or reactor animal) refers to animals that produce positive serological reactions to the defined tests for brucellosis in cattle. Brucellosis tests analyze raw milk and/or serum. Bulk raw milk from dairy herds is tested using the milk ring test (MRT). Individual serum tests are performed for cattle aged 12 months or older in dairy herds that showed a positive reaction or for beef cattle. A primary evaluation is performed using the Rose Bengal plate test (RBT). Serum samples that produce positive reactions in the primary test are subject to confirmatory evaluation using an ELISA, complement fixation test, or tube agglutination test. A farm with at least one confirmed reactor is designated as an outbreak farm.
The geographical locations of farms were classified based on the administrative districts of Korea. First, the farms were classified according to the 16 provinces and metropolitan cities before being grouped into five regions. The northeastern region corresponded to Gangwon province. Seoul, Incheon, and Gyeonggi province were included in the northwestern region. Daejeon, Chungnam, and Chungbuk provinces comprised the central region. Busan, Daegu, Ulsan, Gyeongbuk, and Gyeongnam provinces formed the southeastern region. Gwangju, Jeonbuk, Jeonnam, and Jeju provinces represented the southwestern region. If a farm was located in an administrative community with a name that ended with 'dong', it was classified as an urban area. Otherwise the location was classified as a rural area. March to May were designated as spring, June to August as summer, September to November as autumn, and December to February as winter.
Data sources
Information for individual cattle was identified by unique numbers that were traced from the date of birth to sale of the animal as beef. Newborn calves were reported within 5 days of birth to the designated registration agency. An ear tag was attached within 7 days of birth in the case of beef cattle. This period was extended to 30 days for dairy cattle. All transfers, change of ownership, or deaths were declared within 5 days.
The numbers of animals tested and those with positive reactions for bovine brucellosis were obtained from the Agriculture, Food and Rural Affairs Statistics Yearbook 2013 of the MAFRA. In the yearbook, cases of infectious livestock diseases (as heads) from 2001 to 2012 are recorded [21]. Statistics related to infectious animal diseases such as the number of outbreak farms and reactor animals per unit of time (month and year) or region (province and metropolitan city) were extracted from the webpages maintained by the Animal and Plant Quarantine Agency (QIA) that provides information about notifiable animal diseases [11].
Data for individual cattle and farms, including the address, date of final diagnosis, breed, and number of reactor animals as well as outbreak duration, were obtained from the webpages of the Korean Animal Health Integrated System (KAHIS) operated by the QIA [12]. The unit of record in the KAHIS is a farm, and the detection type for each record is marked as 'new (the first diagnosis of reactor(s) on a farm)' or 'continued (detection of reactors during the test subsequent to the first detection)' based on the SOP [20]. The number of outbreaks considered only 'new' detections of farms, while number of 'reactor animals' concerned both of 'new' and 'continued' detections. To obtain the records of control measures, the annually published Plan of Operation and Implementation for Animal Disease Control was consulted [22]. Statistics for human cases of brucellosis were obtained from the Infectious Disease Surveillance Yearbook 2011 produced by the Korean Center for Disease Control [14].
Statistical analysis
The animal-associated annual incidence rate (hereafter referred to as the incidence rate) was estimated as the proportion of reactors animals among those tested. The incidence rate was determined for 1,000 animals. The 95% confidence interval (C.I.) was calculated according to an exact binomial method using NCSS2007 (NCSS, USA).
An episode was defined as a period from the detection of one or more reactors to the end of a brucellosis outbreak on a farm. The number of reactors per episode was calculated by determining the sum of reactor animals recorded from the first (new) detection to the last 'continued' detection on the same farm. After officially verifying the end of an outbreak, any other 'new' detection incident was regarded as a recurrence and dealt with as a separate episode. A choropleth map representing the number of outbreaks in each province was produced using the statistical geographical system website of the Statistics Korea, Ministry of Strategy and Finance [15]. Spearman's rank correlation coefficients were estimated for the number of outbreaks involving farms and animals per month or region as well as human incidences (newly confirmed patients) and outbreak farms per year using NCSS2007 (NCSS).
Results
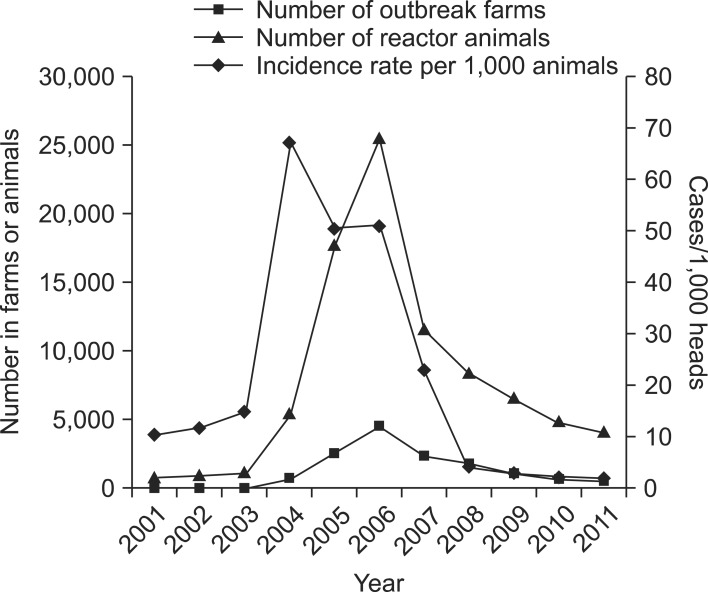
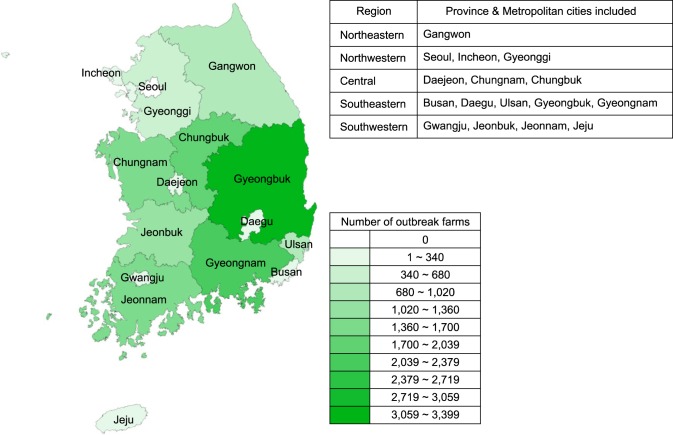
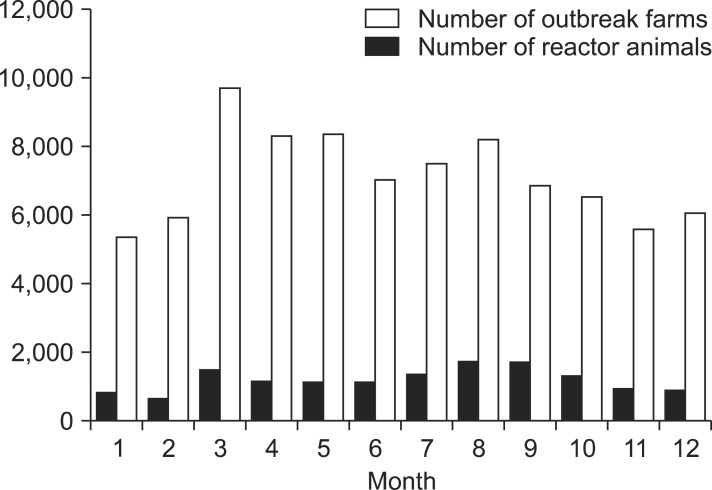
A total of 85,521 brucella reactor animals were identified for 14,215 outbreaks between 2001 and 2011. The numbers of brucellosis cases both at the individual animal and farm levels started to increase after 2003, peaked in 2006, and decreased thereafter. The incidence rates showed the same trend although a 2-year lag was observed. A lag of 2 years was observed in the trends between incidence rate and number of outbreaks (and reactor animals). The incidence rate started to increase and then decreased 2 years before the increase and decrease of number of outbreaks (Fig. 1). The majority of farms with at least one reactor raised Han Woo (13,251 farms; 93.22%) and was located in rural areas (13,278 farms; 93.41%). The highest number of outbreaks was found in Gyeongbuk province (3,398, 23.93%); Gyeongnam province had the second greatest number with 2,114 (14.87%) farms. Both provinces are in the southeastern region of Korea. Chungbuk (1,734; 12.20%), Jeonnam (1,696; 11.93%), Chungnam (1,673; 11.77%), and Jeonbuk (1,062; 7.47%) provinces each had over 1,000 outbreaks (Fig. 2). The first and second highest numbers of outbreaks were detected in August (1,734 farms; 12.20%) and September (1,693; 11.91%), respectively. However, reactor animals were identified (Fig. 3) mainly in March (9,473 animals; 11.39%), May (8,365; 9.78%), and April (8,308; 9.71%). Correlation coefficients for the number of outbreaks and reactor animals were 0.64 (95% C.I. = 0.10 to 0.89; p < 0.05) for the months and 0.95 (95% C.I. = 0.87 to 0.98; p < 10-3) for the regions. The median number of reactor animals per episode was two, and 43.07% (6,122) of the episodes involved only one reactor. Out of the 13,320 cattle farms with confirmed brucellosis outbreaks, 6.1% experienced a recurrence of brucellosis (Table 1).
Fig. 1.
Annual number of bovine brucellosis outbreaks and reactor animals, and the incidence rate for individual animals in Korea from 2001 to 2011.
Fig. 2.
Geographical distribution of bovine brucellosis cases confirmed between 2001 and 2011 according to province and metropolitan city regions.
Fig. 3.
Monthly distribution of the number of reactor animals and bovine brucellosis outbreaks observed between 2001 and 2011.
Table 1.
Epidemiological characteristics of bovine brucellosis cases in Korea from 2001 to 2011
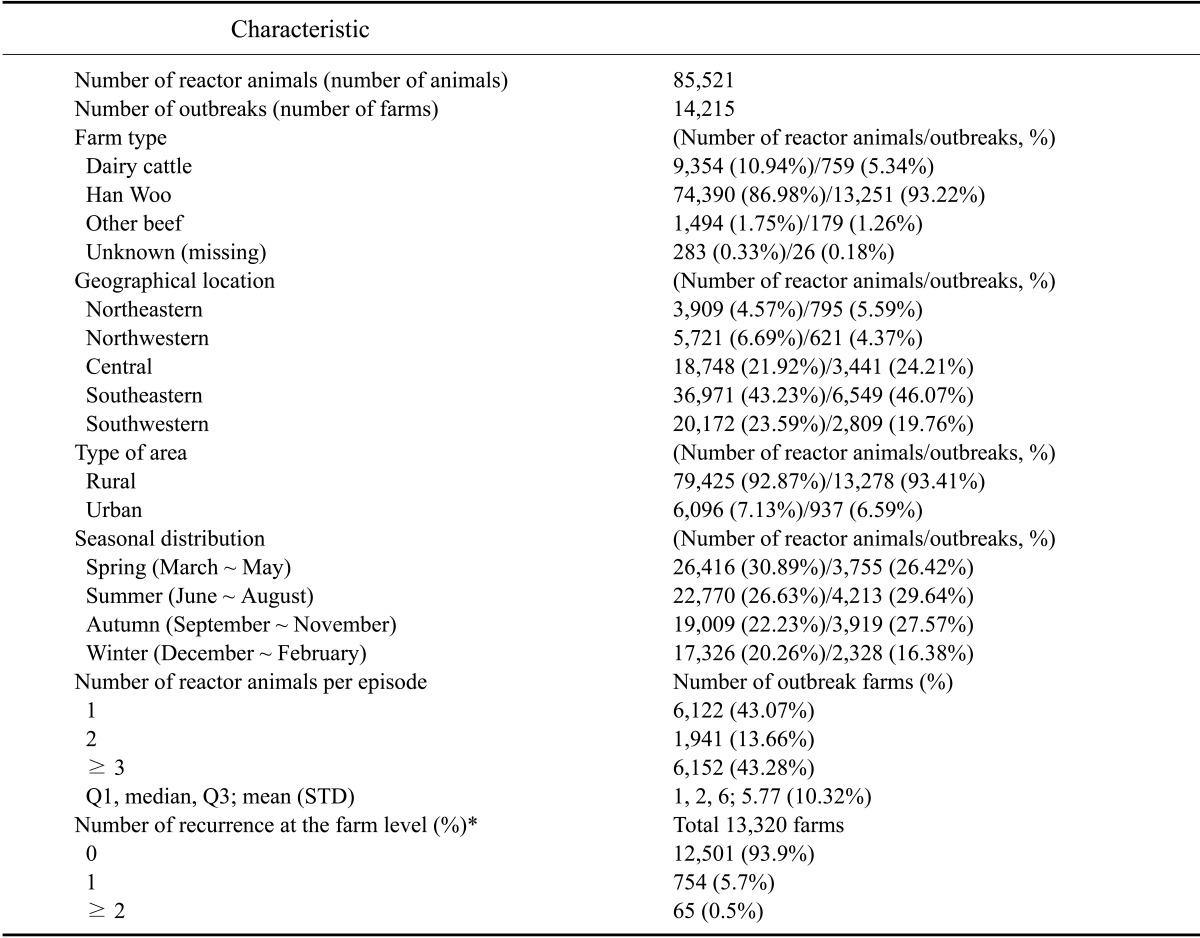
*Number of outbreak farms that previously experienced at least one outbreak.
Association of bovine brucellosis and humans
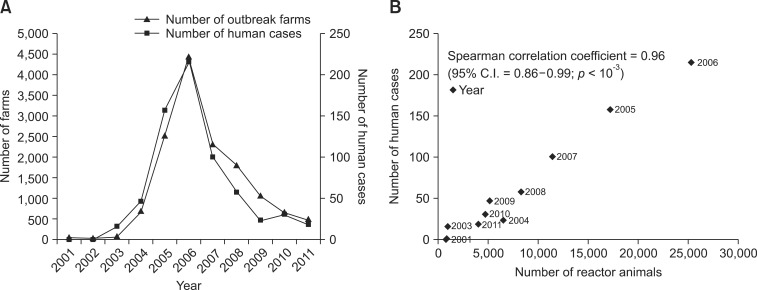
The two line plots showing the number of outbreaks among cattle and human cases showed consistent patterns of increasing and decreasing incidences (panel A in Fig. 4). No human case was confirmed in 2001 and only one case was officially registered in 2002. However, the number of human brucellosis cases increased sharply to 215 in 2006 when the number of outbreaks also reached a peak among animals. The number of human cases and bovine outbreaks declined starting in 2007. The correlation coefficient between human cases and number of outbreaks in cattle per year was 0.96 (95% C.I. = 0.86 to 0.99; p < 10-3, panel B in Fig. 4).
Fig. 4.
Human and bovine brucellosis cases. (A) The number of human cases and bovine outbreaks. (B) Correlation between the number of human cases and reactor animals.
Discussion
Activities related to addressing bovine brucellosis have been amended in Korea over the last decade through continuous efforts of all parties concerned including farmers, livestock related industries, and animal health authorities. A reinforced surveillance system implemented in 2004, known as the eradication program of bovine brucellosis, resulted in a remarkable decrease of brucellosis cases among both cattle and humans [23]. It seems that the transmissibility of bovine brucellosis decreased due to the removal of reservoirs by culling reactor animals within the recommended timeframe of the intensive test program [31].
The incidence rate is a relative indicator that takes the at-risk population and time into consideration. This rate increased and decreased with the number of outbreak farms and reactor animals during our study period. Current government statistics data on bovine brucellosis test and outbreak of government statistics does not allow calculating cattle breed-specific incidence rates. The incidence rates presented in this study is an overall estimate combined with Han Woo and dairy cattle. The interpretation of the bovine brucellosis incidence rate in Korea need to be considered in conjunction with that 87% of the cattle population in Korea is Han Woo [21]. The present study also showed that the majority (86.98%) of reactors were also Han Woo. From the fact that the animal populations and reactors mainly consist of Han Woo, we can infer that the incidence rate for Han Woo is similar to that of the whole cattle population in Korea.
Prior to 2000 when beef herds were not included in the official surveillance program [26], the number of animals tested for bovine brucellosis was higher in the northwest near the capital area than other regions. Consequently, the northwest region was found to have the highest number of outbreaks and infected animals. Concentrated surveillance of high-risk populations initiated in 2004 as an intensive test program resulted in a decreased number of bovine brucellosis cases in this region [26,31]. Meanwhile, Gyeongbuk province had the greatest number of bovine brucellosis outbreaks and the central region contained the second highest number of bovine brucellosis cases. Areas with high numbers of bovine brucellosis outbreaks were distributed along a diagonal axis (northwest to southeast) across Korea. This epidemiological characteristic appears to be affected by the intensity of the surveillance program [23].
The fiscal year in Korea is a calendar year and the budget for testing brucellosis also covers the same period. According to the budgeting system, surveillance begins in March. In addition, the severe cold weather of January and February often delays the initiation of intensive testing. The highest number of reactor animals confirmed in March could be explained by these specific fieldwork conditions in Korea. In the current study, each month was assigned to a specific season as was usual. However, the seasonal characteristics of brucellosis outbreak occurrence in Korea are actually transforming due to climate change. The definitions of seasons in Korea might also vary in the coming years.
The patterns of human and bovine brucellosis cases were similar, and a very high positive correlation coefficient of 0.96 was observed. Using a regression model, we found that the reduction in bovine brucellosis incidences was directly related to the number of human cases [17]. In addition, brucellosis in humans and cattle was caused by B. abortus infections and the human isolates were clustered together with the animal isolates; a significant genetic correlation with regional distribution was observed [9]. The human brucellosis cases in Korea were related to contact with infected cattle during animal-associated activities such as calving or artificial insemination [18,26]. Domestic cattle are the main reservoirs for the transmission of bovine brucellosis to other animals, including wildlife and humans [13,23]. Therefore, education about sanitary techniques, especially for animal-related professionals, should be regularly provided to prevent the emergence of zoonotic diseases [24,29]. The eradication of brucellosis from livestock populations is the main approach for preventing brucellosis in humans [32].
Countries that have successfully eradicated brucellosis often report the combined, efficient, and effective efforts conducted by the veterinary, medical, public health, and agricultural sectors as well as other government services and non-government organizations [1,6,7,25]. On the other hand, a lack of collaboration has been found in areas where eradication has not been achieved [5,10]. These findings reflect the importance of long-term monitoring and surveillance programs for which multi-disciplinary cooperation is essential. The prevalence of a disease is the most important determinant of the effectiveness of an eradication program. For example, a significant association was observed between the past and current status of brucellosis occurrence in Sicily, Italy [3]. In Korea, the incidences of brucellosis have decreased since the mid-2000s. For both 2012 and 2013 (data not published), the incidence rates were < 0.2% at the herd level, which fulfills the guidelines of the Terrestrial Animal Health Code of the World Organization for Animal Health [28].
Previous studies conducted in Korea have shown that large herds used to bring animals from the outside and raise animals with substandard hygienic conditions. This in turn increased the risk for brucellosis outbreaks on cattle farms [16,26,30]. To eradicate bovine brucellosis in Korea, an improved surveillance program tailored to address actual situations should be implemented. The findings of the present study were based on freely available statistics retrieved from the webpages of governmental agencies. Unlimited access to information facilitates continuous monitoring of the health of animal populations. The eradication program specific for bovine brucellosis has been proven to be economically efficient [2] although its economic impact on the country has not been estimated. A cost-benefit analysis of the eradication program should therefore be performed in the near future.
Acknowledgments
This study was funded by the Research and Development Program of the Animal and Plant Quarantine Agency (project no. I-1543068-2014-15-01), Korea.
Footnotes
There is no conflict of interest.
References
- 1.Arambulo P., 3rd International programs and veterinary public health in the Americas-success, challenges, and possibilities. Prev Vet Med. 2008;86:208–215. doi: 10.1016/j.prevetmed.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 2.Bernués A, Manrique E, Maza MT. Economic evaluation of bovine brucellosis and tuberculosis eradication programmes in a mountain area of Spain. Prev Vet Med. 1997;30:137–149. doi: 10.1016/s0167-5877(96)01103-8. [DOI] [PubMed] [Google Scholar]
- 3.Calistri P, Iannetti S, Atzeni M, Di Bella C, Schembri P, Giovannini A. Risk factors for the persistence of bovine brucellosis in Sicily from 2008 to 2010. Prev Vet Med. 2013;110:329–334. doi: 10.1016/j.prevetmed.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 4.Corbel MJ. Brucellosis: an overview. Emerg Infect Dis. 1997;3:213–221. doi: 10.3201/eid0302.970219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coulibaly ND, Yameogo KR. Prevalence and control of zoonotic diseases: collaboration between public health workers and veterinarians in Burkina Faso. Acta Trop. 2000;76:53–57. doi: 10.1016/s0001-706x(00)00090-5. [DOI] [PubMed] [Google Scholar]
- 6.Economides P. Control of zoonoses in Cyprus. Rev Sci Tech. 2000;19:725–734. doi: 10.20506/rst.19.3.1246. [DOI] [PubMed] [Google Scholar]
- 7.Grossklaus D. Zoonoses control-new challenges in health protection of consumers. Berl Munch Tierarztl Wochenschr. 2001;114:420–427. [PubMed] [Google Scholar]
- 8.Hässig M, Paganini C, Simmen-Fritschy B, Knab C, Herren M. Perception of zoonosis in veterinary medicine and society. Schweiz Arch Tierheilkd. 2010;152:355–362. doi: 10.1024/0036-7281/a000083. [DOI] [PubMed] [Google Scholar]
- 9.Her M, Kang SI, Kim JW, Kim JY, Hwang IY, Jung SC, Park SH, Park MY, Yoo H. A genetic comparison of Brucella abortus isolates from animals and humans by using the MLVA assay. J Microbiol Biotechnol. 2010;20:1750–1755. [PubMed] [Google Scholar]
- 10.John K, Kazwala R, Mfinanga GS. Knowledge of causes, clinical features and diagnosis of common zoonoses among medical practitioners in Tanzania. BMC Infect Dis. 2008;8:162. doi: 10.1186/1471-2334-8-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.KAHIS. Korea Animal Health Integrated System. Anyang: Animal and Plant Quarantine Agency; 2013. Statistics on notifiable animal diseases occurrence. [Google Scholar]
- 12.KAHIS. Korea Animal Health Integrated System. Anyang: Animal and Plant Quarantine Agency; 2013. Information on animal disease occurrence. [Google Scholar]
- 13.Kim JY, Her M, Kang SI, Lee K, Lee HK, Jung SC. Epidemiologic relatedness between Brucella abortus isolates from livestock and wildlife in South Korea. J Wildl Dis. 2013;49:451–454. doi: 10.7589/2012-07-188. [DOI] [PubMed] [Google Scholar]
- 14.Korea Centers for Disease Control and Prevention. Public Health Weekly Report. Vol. 5. Osong: Korea Centers for Disease Control and Prevention; 2012. Infectious Diseases Surveillance Yearbook, 2011; p. 6.p. 7.p. 25.p. 32.p. 34.p. 40.p. 100.p. 342.p. 406. [Google Scholar]
- 15.KOSTAT. Statistical Geographic Information Service 2001-2011. Daejeon: Stastics Korea; 2013. [Google Scholar]
- 16.Lee BY, Higgins IM, Moon OK, Clegg TA, McGrath G, Collins DM, Park JY, Yoon HC, Lee SJ, More SJ. Surveillance and control of bovine brucellosis in the Republic of Korea during 2000-2006. Prev Vet Med. 2009;90:66–79. doi: 10.1016/j.prevetmed.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 17.Lee HS, Her M, Levine M, Moore GE. Time series analysis of human and bovine brucellosis in South Korea from 2005 to 2010. Prev Vet Med. 2013;110:190–197. doi: 10.1016/j.prevetmed.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 18.Lim HS, Min YS, Lee HS. Investigation of a series of brucellosis cases in Gyeongsangbuk-do during 2003-2004. J Prev Med Public Health. 2005;38:482–488. [PubMed] [Google Scholar]
- 19.Marcotty T, Thys E, Conrad P, Godfroid J, Craig P, Zinsstag J, Meheus F, Boukary AR, Badé MA, Sahibi H, Filali H, Hendrickx S, Pissang C, Van Herp M, van der Roost D, Thys S, Hendrickx D, Claes M, Demeulenaere T, van Mierlo J, Dehoux JP, Boelaert M. Intersectoral collaboration between the medical and veterinary professions in low-resource societies: The role of research and training institutions. Comp Immunol Microbiol Infect Dis. 2013;36:233–239. doi: 10.1016/j.cimid.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 20.Ministry of Agriculture, Food and Rural Affairs (KR) Standard operation procedure on tuberculosis and brucellosis. 2014. Mar 17, Notice 2014-13. [Google Scholar]
- 21.Ministry of Agriculture, Food and Rural Affairs. Agriculture, Food and Rural Affairs Statistics Yearbook 2013. Sejong: Ministry of Agriculture, Food and Rural Affairs; 2013. [Google Scholar]
- 22.Ministry of Agriculture, Food and Rural Affairs. Plan of Operation and Implementation for Animal Disease Control 2014. Sejong: Ministry of Agriculture, Food and Rural Affairs; 2014. [Google Scholar]
- 23.Park SY, Kim TJ, Yoon H, Kim JY, Lee MJ, Lee WC. A retrospective study of the extensive eradication program for brucellosis outbreaks and control in Korea, 2002-2009. Jpn J Infect Dis. 2012;65:427–429. [PubMed] [Google Scholar]
- 24.Snedeker KG, Anderson MEC, Sargeant JM, Weese JS. A survey of Canadian public health personnel regarding knowledge, practice and education of zoonotic diseases. Zoonoses Public Health. 2012;60:519–525. doi: 10.1111/zph.12029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Turner AJ. Endemic disease control and regulation in Australia 1901-2010. Aust Vet J. 2011;89:413–421. doi: 10.1111/j.1751-0813.2011.00811.x. [DOI] [PubMed] [Google Scholar]
- 26.Wee SH, Nam HM, Kim CH. Emergence of brucellosis in cattle in the Republic of Korea. Vet Rec. 2008;162:556–557. doi: 10.1136/vr.162.17.556. [DOI] [PubMed] [Google Scholar]
- 27.Wee SH, Kim CH, More SJ, Nam HM. Mycobacterium bovis in Korea: an update. Vet J. 2010;185:347–350. doi: 10.1016/j.tvjl.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 28.World Organisation for Animal Health. Terrestrial Animal Health Code. 23rd eds. Paris: OIE; 2014. Chap. 8.4. Infection with Brucella abortus, B. melitensis and B. suis. [Google Scholar]
- 29.Yoo SJ, Choi YS, Lim HS, Lee K, Park MY, Chu C, Kang YA. Seroprevalence and risk factors of brucellosis among slaughterhouse workers in Korea. J Prev Med Public Health. 2009;42:237–242. doi: 10.3961/jpmph.2009.42.4.237. [DOI] [PubMed] [Google Scholar]
- 30.Yoon H, Nam HM, Kim CH, Park CK, Kang MI, Wee SH. Risk factors for bovine brucellosis in Korea: a case control study. Korean J Vet Public Health. 2006;30:77–87. [Google Scholar]
- 31.Yoon H, Moon OK, Her M, Carpenter TE, Kim YJ, Jung S, Lee SJ. Impact of bovine brucellosis eradication programs in the Republic of Korea. Prev Vet Med. 2010;95:288–291. doi: 10.1016/j.prevetmed.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 32.Zinsstag J, Schelling E, Roth F, Bonfoh B, de Savigny D, Tanner M. Human benefits of animal interventions for zoonosis control. Emerg Infect Dis. 2007;13:527–531. doi: 10.3201/eid1304.060381. [DOI] [PMC free article] [PubMed] [Google Scholar]