Abstract
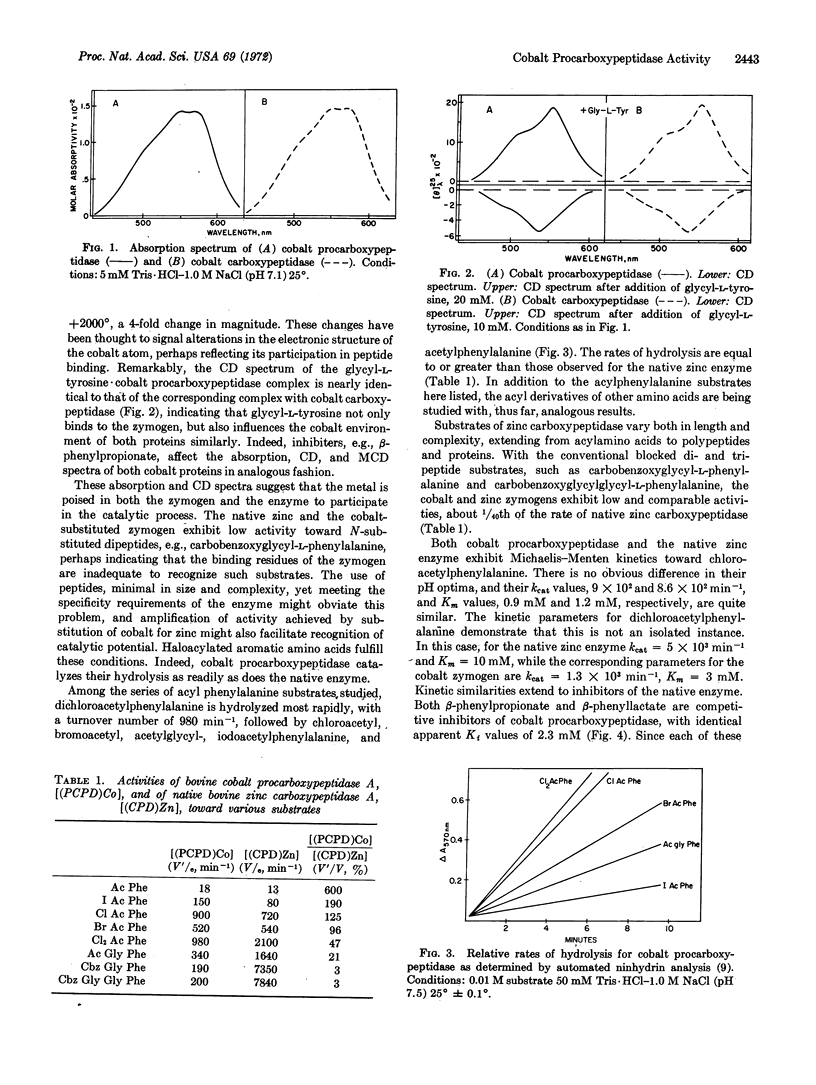
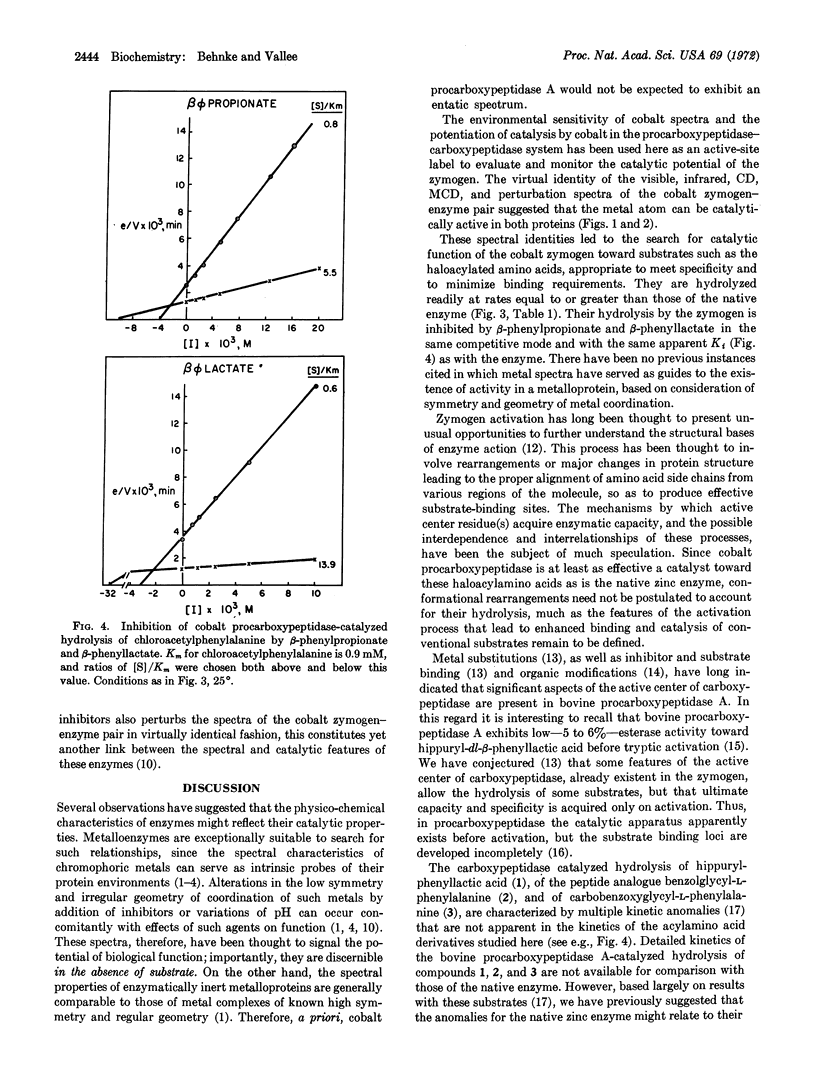
The spectra of functionally essential, chromophoric metal atoms of metalloenzymes are thought to reflect catalytic potential [Vallee and Williams (1968) Proc. Nat. Acad. Sci. USA 59, 498]. The spectra of cobalt procarboxypeptidase and their perturbations by substrates and inhibitors are virtually the same as those of cobalt carboxypeptidase; both are consistent with a distorted tetrahedral geometry about the cobalt atom, suggesting irregular coordination geometries and low symmetries of metal-binding sites. We have, therefore, examined the enzymatic properties of cobalt procarboxypeptidase and found that it catalyzes the hydrolysis of a series of haloacylated amino acids at rates equal to or greater than those of native zinc carboxypeptidase. These observations demonstrate the existence both of the catalytic and of the binding sites for haloacylated amino acids of the zymogen, even before activation. Hence, the magnitude of conformational changes at the catalytic site thought to accompany its activation must be very small, no matter what their magnitude might be elsewhere in the molecule. The data support the entatic state hypothesis and suggest that it may profitably guide the exploration of catalytic potential of metalloproteins and metal-protein complexes.
Keywords: circular dichroism, catalytic site, binding site, zinc, magnetic circular dichroism
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auld D. S., Vallee B. L. Kinetics of carboxypeptidase A. II. Inhibitors of the hydrolysis of oligopeptides. Biochemistry. 1970 Feb 3;9(3):602–609. doi: 10.1021/bi00805a022. [DOI] [PubMed] [Google Scholar]
- Behnke W. D., Wade R. D., Neurath H. Interactions of the endopeptidase subunit of bovine procarboxypeptidase A-S6. Biochemistry. 1970 Oct 13;9(21):4179–4188. doi: 10.1021/bi00823a021. [DOI] [PubMed] [Google Scholar]
- Davies R. C., Riordan J. F., Auld D. S., Vallee B. L. Kinetics of carboxypeptidase A. I. Hydrolysis of carbobenzoxyglycyl-l-phenylalanine, benzoylglycyl-l-phenylalanine, and hippuryl-dl-beta-phenyllactic acid by metal-substituted and acetylated carboxypeptidases. Biochemistry. 1968 Mar;7(3):1090–1099. doi: 10.1021/bi00843a029. [DOI] [PubMed] [Google Scholar]
- Freisheim J. H., Walsh K. A., Neurath H. The activation of bovine procarboxypeptidase A. II. Mechanism of activation of the succinylated enzyme precursor. Biochemistry. 1967 Oct;6(10):3020–3028. doi: 10.1021/bi00862a008. [DOI] [PubMed] [Google Scholar]
- Kay J., Kassell B. The autoactivation of trypsinogen. J Biol Chem. 1971 Nov;246(21):6661–6665. [PubMed] [Google Scholar]
- Latt S. A., Vallee B. L. Spectral properties of cobalt carboxypeptidase. The effects of substrates and inhibitors. Biochemistry. 1971 Nov;10(23):4263–4270. doi: 10.1021/bi00799a017. [DOI] [PubMed] [Google Scholar]
- NEURATH H. MECHANISM OF ZYMOGEN ACTIVATION. Fed Proc. 1964 Jan-Feb;23:1–7. [PubMed] [Google Scholar]
- Piras R., Vallee B. L. Procarboxypeptidase A-carboxypeptidase A interrelationships. Metal and substrate binding. Biochemistry. 1967 Jan;6(1):348–357. doi: 10.1021/bi00853a052. [DOI] [PubMed] [Google Scholar]
- THIERS R. E. Contamination in trace element analysis and its control. Methods Biochem Anal. 1957;5:273–335. doi: 10.1002/9780470110218.ch6. [DOI] [PubMed] [Google Scholar]
- Vallee B. L., Riordan J. F., Bethune J. L., Coombs T. L., Auld D. S., Sokolovsky M. A model for substrate binding and kinetics of carboxypeptidase A. Biochemistry. 1968 Oct;7(10):3547–3556. doi: 10.1021/bi00850a032. [DOI] [PubMed] [Google Scholar]
- Vallee B. L., Riordan J. F., Johansen J. T., Livingston D. M. Spectro-chemical probes for protein conformation and function. Cold Spring Harb Symp Quant Biol. 1972;36:517–531. doi: 10.1101/sqb.1972.036.01.066. [DOI] [PubMed] [Google Scholar]
- Vallee B. L., Williams R. J. Metalloenzymes: the entatic nature of their active sites. Proc Natl Acad Sci U S A. 1968 Feb;59(2):498–505. doi: 10.1073/pnas.59.2.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R. J. The entatic state. Cold Spring Harb Symp Quant Biol. 1972;36:53–62. doi: 10.1101/sqb.1972.036.01.010. [DOI] [PubMed] [Google Scholar]
- YAMASAKI M., BROWN J. R., COX D. J., GREENSHIELDS R. N., WADE R. D., NEURATH H. PROCARBOXYPEPTIDASE A-S6. FURTHER STUDIES OF ITS ISOLATION AND PROPERTIES. Biochemistry. 1963 Jul-Aug;2:859–866. doi: 10.1021/bi00904a039. [DOI] [PubMed] [Google Scholar]


