Abstract
Cancer-induced inflammation results in accumulation of myeloid cells. It has become increasingly evident that tumor-dependent factors condition myeloid cells toward an immunosuppressive and pro-tumorigenic phenotype. These myeloid cells include progenitors and progeny of monocytes, granulocytes, macrophages, and dendritic cells. Myeloid cells are not simply bystanders in malignancy or barometers of disease burden. Reflecting their dynamic and plastic nature, myeloid cells manifesta continuum of cellular differentiation and are intimately involved at all stages of neoplastic progression. They can promote tumorigenesis through both immune-dependent and independent mechanisms and can dictate response to therapies. A greater understanding of the inherent plasticity and relationships among myeloid subsets is needed to inform therapeutic targeting. New clinical trials are being designed to modulate the activities of myeloid cells in cancer, which may be essential to maximize the efficacy of both conventional cytotoxic and immune-based therapies for solid tumors.
BACKGROUND
Cancer vaccines are designed to induce tumor-antigen-specific cytolytic T cells, but are rarely effective at eliminating established tumors. This inefficiency potentially reflects a tolerized response and/or a limited endogenous T cell repertoire specific forthenon-mutated, self-proteins that represent the majority of targetable tumor antigens. The adoptive transfer of T cells engineered to express high affinity receptors against tumor/self-antigens may, in principle, overcome some of the obstacles faced in engendering an endogenous T cell response (1, 2). However, even when transferred in high numbers, these tailored T cells will likely encounter multiple mechanisms of cancer-associated immunosuppression that interfere with tumor eradication.
The accumulation of hematopoietic-derived, immunosuppressive cells is now recognized as a primary mechanism employed by tumors to evade elimination by cytotoxic T lymphocytes (3). Cell subsets from both the lymphoid (e.g. regulatory T cells) and myeloid lineages can regulate T lymphocytes; this review focuses on pathways co-opted by tumors that instruct myeloid complicity in cancer progression. In this review, we discuss: 1) the ontogeny of myeloid cells involved in cancer; 2) the pathways initiated by tumors that instruct myeloid accumulation and trafficking; 3) the fate of myeloid cells in malignancy; and 4) the obstacles that must be overcome to successfully translate the targeting of myeloid cells to enhance cancer therapy. We also discuss specific aspects of pancreatic ductal adenocarcinoma (PDA) as a noteworthy example of the challenges presented by this class of cells to effective immune strategies.
The extraordinary plasticity, rapid turnover, and capacity to present antigen to T cells position the myeloid compartment as an attractive focal point for potentiating targeted therapies. However, the heterogeneity and dynamic nature of the myeloid lineage also render its rational targeting a daunting task. A better understanding of the relationships among myeloid progenitors and progeny should help elucidate treatment strategies for solid tumors.
Disrupted myeloid homeostasis: a continuum of cellular differentiation and plasticity
Hematopoiesis represents a dynamic and hierarchical process of cell-fate decisions governed by both intrinsic (e.g., transcription factors) and extrinsic (e.g. cytokines) mechanisms (4). Hematopoietic stem cells in the bone marrow generate phenotypically distinct progenitors that are impaired in the ability to self-renew. In non-pathological settings, immature myeloid cells are largely confined to the bone marrow, have a relatively short half-life and circulate at low frequencies yetretain the capacity torapidly respond to environmental insults. Tumors hijack this homeostatic process by secreting inflammatory mediators that create a state of “emergency hematopoiesis” with preferential expansion of the myeloid, rather than the lymphoid, lineage. Such cancer-conditioned myeloid cells aid and abet chronic inflammation and exacerbate cancer progression.
The cytokine GM-CSF has long been recognized to induce the expansion of immature myeloid cells that promote allograft or transplantable tumor growth by inhibiting T lymphocytes (5–7). These cells have subsequently been termedmyeloid-derived suppressor cells (MDSC), a loosely defined and heterogeneous population of immature myeloid cells with suppressive activity. MDSC are now recognized as a paramount disease-specific tolerance mechanism during both acute and chronic inflammatory conditions. MDSC contribute to immune evasion via suppression of T cell responses (8–12) and also influence tumor remodeling, invasion, metastasis and cancer stemness independent of T cell inhibition (13–15). Thus, MDSC represent a common axis with broad therapeutic application.
MDSC subsets and immunosuppression
There are two subsets of MDSC:monocytic MDSC (Mo-MDSC) and granulocytic MDSC (Gr-MDSC) (16). These subsets can be readily discriminated by distinct phenotypic profiles and morphologies (Table 1). Reagents used in mice to identify the collective population of MDSC (e.g. αGr-1 and αCD11b) do not clearly distinguish between these two subsets so antibodies against additional markers are necessary to study these distinct lineages separately (Table 1). In humans, these subsets can also be distinguished by CD14 and CD15 expression (Tables 1 and 2). It is now clear that both Gr-MDSC and Mo-MDSC are independently suppressive (12, 16, 17). Gr-MDSC have been reported to mediate suppression largely via production of reactive oxygen species (ROS) and Arginase (16). Mo-MDSC have a more expansive suppressive arsenal that could reflect a greater cellular plasticity. This armamentarium includes expression of inducible nitric oxide synthase (iNOS), Arginase (Arg) (16, 18), TGFβ (19), indoleamine-pyrrole 2,3-dioxygenase (IDO) (20), reactive oxygen species (ROS) (21) and factors that induce STAT3 signaling (22). Mo-MDSC may also indirectly promote immunosuppression via the induction of CD4+FoxP3+ regulatory T cells (Treg) (23). The production of peroxynitrate (ONOO−), a product of NO and superoxide anion (O2−), represents another suppressive mechanism associated with MDSC and cancer (24), and may be a result of synergy between the two myeloid subsets in vivo. The suppressive activities depend in part on IFNγ signaling (16), but the relative contribution of each subset to immune suppression is context dependent and is heightened within the tumor environment. The finding that IFNγ, a cytokine expressed by effector T cells after antigen encounter, can also induce MDSC-mediated suppression underscores the need to target this population in therapies that depend on T lymphocytes for activity.
Table 1.
Immunosuppressive myeloid progenitors in cancer
| A. Mouse | ||||
|---|---|---|---|---|
| Name | Ontogeny | Phenotype | Other Markers | Progeny |
| Gr-MDSC | Granulocytic | CD45+ CD11b+ Gr1high Ly6Cint |
Ly6G+ CXCR1+ CXCR2+ |
Potentially Neutrophils |
| Mo-MDSC | Monocytic | CD45+ CD11b+ Gr1int Ly6Chigh |
CCR2+ CD115+ CD62L+ CX3CR1−/low |
High plasticity: Macrophages, Dendritic cells, resident monocytes, fibrocytes |
| Inflammatory monocytes | Monocytic | CD45+ CD11b+ Gr1int Ly6Chigh |
CCR2+ CD115+ CD62L+ CX3CR1−/low |
High plasticity: Macrophages, Dendritic cells, Resident monocytes, Fibrocytes, Mo-MDSC |
| Resident monocytes1 | Monocytic | CD45+ CD11b+ Ly6C− CX3CR1high |
CCR2− CD62L− |
M2 Macrophages |
| Fibrocytes | Monocytic | CD45+ CD34+ Collagen+ |
CXCR1+ CXCR4+ |
Fibroblasts |
| B. Human | ||||
|---|---|---|---|---|
| Name | Ontogeny | Phenotype | Other Markers | Progeny |
| Gr-MDSC | Granulocytic | CD45+ Lin− CD11b+ CD14− HLA- DRlowCD33+ |
CD15+ CD66b+ |
Potentially Neutrophils |
| Mo-MDSC | Monocytic | CD45+ Lin− CD11b+CD14+ HLA-DRlow CD33+ |
CD66b+ | High plasticity: Macrophages, Dendritic cells, resident monocytes, fibrocytes |
| Inflammatory monocytes | Monocytic | CD45+ Lin− CD14+ HLA-DR+ CD33+ |
CCR2+ CD115+ CD62L+ CD15−/low CXCR4+ |
High plasticity: Macrophages, Dendritic cells, Resident monocytes, Fibrocytes, Mo-MDSC |
| Resident monocytes1 | Monocytic | CD45+ CD14+ CD16+ CX3CR1high CD33dim |
CD115+ CD62L− CXCR4+ |
M2 Macrophage |
| Fibrocytes | Monocytic | CD45+ CD34+ Collagen+ |
α-SMA+ HLA-DR+ CXCR1+ CXCR4+ |
Fibroblasts |
Although resident monocytes (RM) have not been well studied during cancer, during other inflammatory contexts, RM have a propensity to differentiate into wound-healing (M2) macrophages after extravasation and thus may contribute to macrophage heterogeneity in solid tumors.
Table 2.
Immature myeloid progenitors in representative human cancers
| Cancer | Subset | Putative suppressive mechanism | Phenotype | Other Markers | Ref |
|---|---|---|---|---|---|
| Metastatic melanoma | Mo-MDSC | -- | CD45+ Lin− CD14+ HLA-DR−/low |
CD15dimCD66b− CD33+ Arg− CD16−/low |
(17) |
| Metastatic melanoma | Gr-MDSC | -- | CD45+ Lin− CD14− CD15+ CD33+ HLA-DR−/low |
Arg+ CD16−/low |
(17) |
| Metastatic Melanoma | Mo-MDSC | TGFβ | CD45+ CD14− HLA-DR−/low |
-- | (19) |
| Metastatic Melanoma | Mo-MDSC | PGE2 STAT3 COX2 Superoxide |
CD14+ HLA-DR−/low |
-- | (22) |
| Metastatic Melanoma | Mo-MDSC | STAT3 Arg |
CD45+ HLA-DR−/low CD14− |
CD80+CD83+ CD209+ |
(95) |
| Metastatic Melanoma | Mo-MDSC | -- | HLA-DR−/low CD86low |
-- | (96) |
| Pediatric sarcomas | Fibrocytes | IDO | CD14− CD66b+ CD33int/low CD15+ CD123− CD11c+ |
α-SMA+ Collagen+ CXCR1+ MMP9+ TSLPR+ CD127+ |
(20) |
| Non-small-cell lung cancer | Mo-MDSC | ROS | CD11b+ CD14+ HLA-DR−/low |
CD86low CD16low |
(21) |
| Non-small-cell lung cancer | Gr-MDSC | Arg1 iNOS suppression of CD3ζ |
CD11b+ CD14− CD15+ CD33+ |
SSChigh IL-4R+ IFNγR+ |
(97) |
| Glioblastoma | Mo-MDSC | TGFβ IL-10 B7H1 |
Lin− HLA-DR−/low CD33+ |
B7H1+ | (29) |
| Renal cell carcinoma | Gr-MDSC | Low arginine: ornithine ratio in plasma correlated with decreased CD3ζ | CD11b+ CD15+ CD14− CD33+ |
-- | (98) |
| Colorectal cancer | Gr-MDSC | -- | Lin−/low HLA-DR− CD11b+ CD33+ |
CD14− CD13+ CD117+ CD39+ SSChigh |
(99) |
| GI malignancies1 | Mo-MDSC | -- | CD14+ HLA-DR−/low |
-- | (26) |
| GI malignancies1 | Gr-MDSC | -- | CD15+ CD14− CD11b+ CD33+ |
-- | (26) |
| Metastatic pancreas, colon, & breast adenocarcinomas | Gr-MDSC | H202-mediated suppression of CD3ζ | CD15+ CD11b+ Low density |
-- | (36) |
| Ovarian cancer | Mo-MDSC | -- | CD11b+ CD14+ CD33+ CXCR4+ |
MDSC correlated w/PGE2 and CXCL12 in ascites | (54) |
| Numerous solid tumors | -- | -- | CD11b+ Lin−/Lo HLA-DR− CD33+ |
-- | (100) |
| Multiple myeloma | Mo-MDSC | PDE5 NOS2 Arg |
CD14+ cells | -- | (101) |
| Hepatocellular carcinoma | -- | -- | Lin−/low CD33+ HLA-DR− |
COX-2, MMP-13, and VEGF correlated with MDSC | (102) |
| Hepatocellular carcinoma | Gr-MDSC | -- | CD11b+CD14 − HLA-DR−CD33+ |
-- | (103) |
| GM-CSF induced MDSC from normal blood | Gr-MDSC | NOS TGFβ, NADPH oxidase Arg |
CD11b+ HLA-DR−/low CD33+ |
CD66b+ | (38) |
Colorectal, pancreatic, hepatocellular, and gastric cancer.
The relative contribution of each MDSC subset in mediating immunosuppression depends on the disease state and inflammatory milieu, but these capabilities are frequently assessed only in vitro. While both subsets have immunosuppressive activity in malignancy, suppressive monocytes (which have an overlapping phenotype with Mo-MDSC, as discussed below) mediate transplantation tolerance (25) and, in some models, have superior suppressive capacity (16). In contrast, some studies in humans indicate Gr-MDSC may be slightly more suppressive (26). Differences in the composition of factors may dictate the specific subset that mediates suppression. Cell number and frequency in vivo also bear consideration, as suppression is dependent upon cell ratios. Thus, if Mo-MDSC are superior suppressors in vitro, but are less frequent than Gr-MDSC in vivo, then targeting the more prevalent population may have greater therapeutic value. Indeed, studies using a genetically engineered mouse model of PDA (27) showed that both Mo-MDSC and Gr-MDSC accumulate during disease progression (12). Specific depletion of Gr-MDSC induced endogenous CD8 T cell activation and infiltration into established PDA resulting in tumor epithelial cell death and stromal remodeling (12). Thus, simply abrogating MDSC can awaken an endogenous cytotoxic T cell response indicating that immune surveillance to cancer may fail, in part, because of a defined subset of cancer-conditioned myeloid cells.
Gr-MDSC depletion in PDA was unexpectedly accompanied by a concomitant rise in Mo-MDSC suggesting homeostatic regulation between these two subsets (12). Gr-MDSC may directly regulate the monocytic population, which could indirectly inhibit the progeny of Mo-MDSC, including dendritic cells (DC) and macrophages (Figure 1). Alternatively, since both subsets respond to GM-CSF (our unpublished observations) and GM-CSF secretion by PDA is necessary for the establishment of implanted pancreatic tumor cells(10, 11), Gr-MDSC may act as a sink. Thus, the targeted depletion of Gr-MDSC could indirectly increase systemic levels of GM-CSF available to stimulate Mo-MDSC. The finding that the two MDSC subsets are homeostatically regulated during cancer progression (12) has clinical implications. Therapies designed to target one immature population may induce alternative suppressive populations justifying the simultaneous monitoring of these subsets in individual patients.
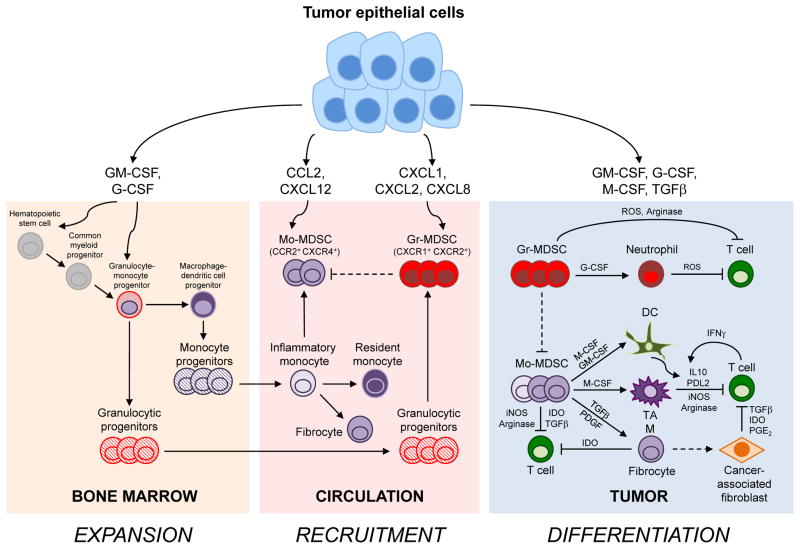
Figure 1.
Tumor epithelial cells induce the expansion, recruitment and differentiation of monocytic and granulocytic cells with distinct ontogenies, plasticities and fates. A simplified diagram of the cellular pathways instructing myeloid cell complicity in cancer is presented.
Expansion: In normal physiology, bone marrow mesenchymal cells express low and localized concentrations of cytokines, including GM-CSF, G-CSF, SCF, and Flt3L, to regulate hematopoiesis (not shown). Carcinoma cells overexpress many of these same factors resulting in elevated and sustained systemic levels and the subsequent expansion of immature myeloid cells. This chronic inflammation induces myeloid cell egress from the bone marrow and extramedullary hematopoiesis. The pleiotropic cytokine, GM-CSF, can signal to myeloid cells at various stages of differentiation with distinct cellular outcomes. GM-CSF induces the proliferation and differentiation of hematopoietic stem cells and their progenitors into MDSC that can accumulate in pathology. G-CSF, another myelopoietic cytokine, promotes granulocyte maturation and can induce the accumulation of Gr-MDSC. GM-CSF and to a lesser extent, G-CSF, can promote Gr-MDSC survival but not proliferation. S100 proteins, VEGF and IL-6 also contribute to MDSC expansion (not shown), perhaps in synergy with GM-CSF.
Recruitment: Solid tumors secrete chemokines that attract myeloid cells. The chemokines CXCL1, CXCL2 and CXCL8 recruit Gr-MDSC. Both normal and tumor epithelium express CCL2 to attract inflammatory monocytes and Mo-MDSC. Inflammatory monocytes give rise to resident monocytes. In cancer, tumor-derived factors can convert normal monocytes into Mo-MDSC or suppressive fibrocytes. Tumors also express CXCL12 to recruit CXCR4+ inflammatory monocytes, Mo-MDSC and fibrocytes. CXCR4 is also expressed on T cells and may inhibit T cell accumulation within the tumor bed (not shown).
Differentiation: After extravasation into normal tissues, monocytes differentiate into macrophages or DC that can either promote immunity or induce T cell tolerance depending on the context. The complex tumor inflammatory milieu instructs myeloid cells to become immunosuppressive Mo-MDSC, macrophages, DC and fibroblasts. Granulocytes and Gr-MDSC may differentiate into neutrophils following extravasation. M-CSF and Th2 cytokines (IL-4, IL13) promote conversion of monocytes into immunosuppressive macrophages; TAM may also be derived from tissue resident macrophages and or resident monocytes. Monocytes can be induced to form regulatory DC by Th2 cytokines or suppressive fibrocytes by TGFβ and PDGF. Fibrocytes may also seed the cancer-associated fibroblast populations with suppressive activity. IFNγ secretion, a hallmark of cytotoxic T cells, also activates a negative-feedback loop that mitigates T cell responses.
Mo-MDSC, monocytic myeloid-derived suppressor cells; Gr-MDSC, granulocytic myeloid-derived suppressor cells; DC, dendritic cell; TAM, tumor-associated macrophage; GM-CSF, granulocyte-macrophage colony-stimulating factor; G-CSF, granulocyte colony-stimulating factor; M-CSF, macrophage colony-stimulating factor; TGFβ, transforming growth factor beta; PGE2, prostaglandin E2; ROS, reactive oxygen species; IDO, indoleamine 2,3-dioxygenase; PDGF, platelet-derived growth factor; iNOS, inducible nitric oxide synthase; PDL2, programmed death ligand 2.
MDSC ontogeny
Based on the origins of bona fide monocytes, Mo-MDSC likely descend from the macrophage-dendritic cell progenitor that has lost granulocyte potential and gives rise to monocytes, macrophages, and DC (28) (Figure 1). One feature distinguishing Mo-MDSC from normal monocytes in humans is the lower expression of HLA-DR (14, 17), a critical molecule for antigen presentation to T lymphocytes. Exposure of normal circulating monocytes to tumor-conditioned media causes downregulation of HLA-DR (14, 17) and CD14 (29) supporting the contention that Mo-MDSC can be derived from normal circulating monocytes. This transition may be dependent on the activation of the STAT3 pathway (14). The downregulation of CD14 could also make it more difficult to distinguish this population from Gr-MDSC. Intriguingly, CD14+HLA-DR−/low cells can be isolated from normal blood. These cells can induce the conversion of CD4+FoxP3− T cells to CD4+FoxP3+ Tregvia expression of cell-bound TGFβ and genes involved in retinoic acid metabolism (23). Thus, Mo-MDSC may simply reflect an altered differentiation state of monocytes.
There are two subsets of monocytes at steady state: inflammatory monocytes (IM) and resident monocytes (RM) (30), which differ in expression of Ly6C and the chemokine receptors CCR2 and CX3CR1 (Table 1, Figure 1). Mo-MDSC are phenotypically similar to the IM subset (Table 1). At steady state, IM are precursors to RM (31), potentially reflecting a less mature and more plastic phenotype. RM patrol the endothelium and have an intrinsic bias to adopt an immunosuppressive macrophage phenotype (M2) reminiscent of tumor-associated macrophages (TAM) (31). In healthy animals, IM circulate and reside in the subscapular space of the spleen where they are poised to rapidly respond and migrate toward sites of inflammation following infection (32). Following extravasation, IM can differentiate into either M1 (anti-tumorigenic, pro-inflammatory) or M2 (pro-tumorigenic, anti-inflammatory) macrophages depending on the context. IM differentiate into M1 macrophages following migration into infected tissues (33). In contrast, elevated levels of T helper 2 (Th2) cytokinesIL-4 and IL-13 can induce IM differentiation toward an M2 phenotype (34), underscoring IM plasticity (Figure 1). Increased accumulation of IM correlates with advanced disease and poor prognosis in PDA patients (35), consistent with an etiological overlap between Mo-MDSC and IM.
Identification of unique markers to distinguish Gr-MDSC from normal granulocytes has proven more elusive. Gr-MDSC may be less dense than normal granulocytes. However, normal granulocytes can also change in density and become suppressive upon activation (36). Recently, a screening of differential markers on MDSC showed that the expression of extracellular S100 proteins may specifically identify both MDSC subsets in mice (37). Ongoing efforts to identify distinguishing features of human Gr-MDSC could aid in the development of novel therapies. MDSC may include not only immature monocyte and granulocyte progenitors along a continuum of differentiation, but also altered monocytes and granulocytes that have been conditioned by tumor-dependent factors. As the detailed phenotypes of distinct subsets continue to emerge, reconciling the generic term MDSC with the more standard and accepted cell subset classifications may help to unify divergent fields that study myeloid cells in distinct disease contexts and avoid descriptions and experimental conditions that indiscriminately pool various subsets.
MDSC trafficking and accumulation: roles of chemokines and cytokines
A complex composition of tumor-dependent factors contributes to the phenotypic and functional heterogeneity of MDSC in distinct malignancies, but some common pathways have emerged (Figure 1). GM-CSF is overexpressed in many malignancies (5–7, 10, 11). Signaling via the GM-CSF receptor induces the proliferation and differentiation of progenitors into MDSC (10) and also promotes the survival of differentiated Gr-MDSC (12). GM-CSF in combination with IL-6 or G-CSF induces expression of the C/EBPβ transcription factor and the subsequent expansion of MDSC (9, 38, 39). Some studies suggest that GM-CSF may preferentially induce Mo-MDSC rather than Gr-MDSC (40–42). However, GM-CSF and IL-6 were recently shown to induce the expression of microRNAs (miR-155 and miR-21) in both MDSC subsets. These microRNAs synergistically promoted the expansion of both Gr-MDSC and Mo-MDSC by modulating key phosphatases, SHIP and PTEN (43), revealing potential targets to interfere with both MDSC populations simultaneously. Of note, GM-CSF in combination with IL-4 induces monocyte differentiation into DC with potent immunostimulatory properties, justifying the use of GM-CSF in vaccines (44). Tumor-derived factors are, however, implicated in inducing tolerogenic DC (Figure 1 and reviewed in (45)). The paradoxical roles for GM-CSF can be ascribed to the in vivo context. GM-CSF expression by an irradiated tumor cell vaccine may result in the localized and transient presence of this cytokine in the context of dying tumor cells, where as sustained systemic levels of GM-CSF favors MDSC expansion and survival.
A variety of other factors have been implicated in MDSC generation including S100A8/A9, IL-6, IL-1β, G-CSF, VEGF, PGE2 and TGFβ (3). The S100A8/A9 proteins inhibit DC differentiation, enhance MDSC accumulation and promote pancreatic carcinogenesis in susceptible animals via binding to the receptor for advanced glycation end products (RAGE) (46–48). Another common pathway to induce suppressive granulocytes is mediated by G-CSF (49). Breast cancer cells express G-CSF that expand Gr-MDSC that promote tumor growth and angiogenesis (50). Thus, the observed overexpression of G-CSF by PDA epithelial cells may play a similar role in promoting the preferential accumulation of Gr-MDSC (12).
The chemokine receptor profile is distinct between Mo-MDSC and Gr-MDSC and can be employed to further distinguish these subsets. Since CCR2 is expressed on IM, Mo-MDSC and TAM but not Gr-MDSC, this pathway may be more exclusive for cells of the macrophage dendritic cell lineage. TGFβ signaling on bone marrow cells favors the induction of MDSC that utilize CCR2 for trafficking into skin tumors (51). CCR2-expressing cells are responsive to the chemokine CCL2, promoting the egress of monocytes from the bone marrow (52) and the infiltration of IM into Kras-driven lung tumors (53) and implanted PDA cell lines (35). Mo-MDSC and fibrocytes also express CXCR4 (54) that binds CXCL12, a chemokine that is often overexpressed in cancer. Recently, a CXCR4 antagonist (AMD3100) in combination with anti-PDL1, an antibody that blocks inhibitory signaling in T cells, transiently overcame immunosuppression in an autochthonous mouse model of PDA (55). The proposed mechanism of AMD3100 in this setting was inhibition of stromal fibroblasts, but it is likely that interfering with CXCR4 has many effects in vivo, including impacting myeloid progenitor egress from the bone marrow and/or recruitment into tumors.
Like granulocytes, Gr-MDSC express the chemokine receptors CXCR1, CXCR2 and CXCR8. These receptors are involved in the recruitment of Gr-MDSC into neoplasms and are essential for colitis-associated tumor formation (56). CXCR2 blockade inhibited Gr-MDSC migration into a murine rhabdomyosarcoma model and enhanced the therapeutic activity of anti-PD1 (57). Patients with metastatic sarcomas also had elevated levels of the CXCR2 ligand, CXCL8, which was associated with decreased survival (57). Not surprisingly, the ligands for these chemokine receptors (CXCL1, CXCL2, CXCL8) are often expressed by tumor epithelial cells, including PDA (10, 12). The pro-inflammatory cytokineIL-17 is commonly overexpressed in cancer and also is implicated in chronic inflammation and tumor development (58). IL-17 overexpression may mediate some of its tumor-promoting effects by recruiting Gr-MDSC (59) and monocytes (60). Thus, tumors express not only growth factors that induce the proliferation, differentiation and survival of myeloid progenitors, but also chemokines that recruit both MDSC subsets into the evolving cancer.
MDSC fate
The in vivo fates of distinct myeloid progenitors have primarily been studied at steady state and in models of acute inflammation or in transplantable tumors, leaving critical knowledge gaps of myeloid progenitor fate in autochthonous cancers that arise in the tissue of origin and progress through a preinvasive state. Genetically engineered mouse models of autochthonous cancer that recapitulate the natural history of nascent human cancer are ideally suited for the identification of the fate of complex myeloid populations in preinvasive, invasive and metastatic disease. Such studies may be particularly relevant for solid tumors in which the tissue resident macrophages that arise during embryogenesis may originally recognize the first mutated epithelial cell. IM can migrate into tumors where they subsequently differentiate into TAM (16, 53, 61, 62). TheTh2-responses that are intimately involved in solid tumors (63) can also drive the local in situ proliferation of intratumoral macrophages (64).
M-CSF (CSF1) is highly expressed by both normal and mutated epithelial cells. This cytokine promotes macrophage development (65, 66) and myeloid cell recruitment into tumors. M-CSF correlates with poor prognosis in many cancers (reviewed in (67)) and can induce monocyte differentiation into macrophages that are highly phagocytic and have low MHC class II expression, indicative of minimal antigen presentation (68). M-CSF induces polarization of human CD14+ blood monocytes to secrete the immunosuppressive cytokine IL-10 and not IL-12 (69), a secretory phenotype of M2 macrophages that may subvert tumor immunity. However, the complex chronic inflammatory milieu associated with autochthonous tumors in vivo generates much more complex populations. Tissue macrophages can also express the dendritic cell marker CD11c, hampering the ability to distinguish these distinct cell lineages in tumors (70). Despite this, M-CSF has been implicated in the differentiation of inflammatory DC from IM (71). This subset of DC, also referred to as Tip-DC (TNFα- and iNOS-producing), accumulates in the spleen during infection (72) and inflammatory diseases and promotes T cell immunity.
Although the evidence is derived from studies across various model systems, Mo-MDSC/IM may also differentiate into mature myeloid cells that promote tumor immunity, indicating that depletion of some myeloid populations during other forms of therapy may not be beneficial. This notion is supported by recent findings that CCR2+ IM rapidly migrate into transplantable tumors in response to chemotherapy and serve as functional antigen presenting cells to promote tumor immunity (73). In addition, low-dose irradiation of tumors induced the accumulation of iNOS+ macrophages that contributed to the effectiveness of adoptive T cell therapy (74). These results reveal a less well-appreciated salutary role for myeloid cells in cancer and indicate that simply removing myeloid populations, particularly if performed in combination with cytotoxic therapies that induce immunogenic cell death, may be self-defeating. Alternatively, in other studies, tumor-infiltrating myeloid cells can impart resistance to chemotherapy (75–77) and support cancer stemness (14, 15). Thus, in some settings, depletion of TAM can also have therapeutic benefit.
IM can also give rise to fibrocytes, an unusual class of hematopoietic-derived fibroinflammatory cells with properties of both myeloid cells and fibroblasts (Table 1, Figure 1 and reviewed in (78)). Fibrocytes express the activated fibroblast marker α-smooth muscle actin, extracellular matrix components including collagen, as well as the pan-hematopoietic marker CD45. In some settings, fibrocytes can serve as potent antigen presenting cells due to the expression of HLA-DR and costimulatory molecules (79, 80). On the other hand, an immunosuppressive population of fibrocytesis increased in the circulation of sarcoma patients (20). In this setting, the fibrocytes mediate T cell suppression by expression of IDO, an immunomodulatory enzyme that degrades tryptophan. The collective properties of fibrocytes raise the question of whether they overlap with at least a fraction of fibroblasts that contribute to immune suppression in solid tumor models (55, 81). CD14+ monocytes derived from healthy donors will differentiate into fibrocytes after exposure to IL-4 (20), TGFβ as well as PDGF. Immunosuppressive fibrocytesin humans can co-express the granulocytic markers CD15 and CD66b (20), suggesting a potential lineage relationship with granulocytes. The extraordinary plasticity of the Mo-MDSC makes it enticing to speculate that Mo-MDSC could differentiate into Gr-MDSC in settings of pathology. However, in the accepted steady-state developmental pathways for monocytes and granulocytes each subset arises from distinct progenitors (Figure 1).
CLINICAL-TRANSLATIONAL ADVANCES
Both subsets of MDSC are expanded systemically in a number of human malignancies (Table 2). Targeting myeloid cells in malignancy represents an active and expanding area of investigation that should lay the groundwork for defining the safety and efficacy of such strategies either alone or in combination with immunotherapy and/or chemotherapy (Table 3). Promising strategies to date have been identified almost exclusively in implantable tumor models and it is important to keep in mind that successful approaches in this setting may have limited clinical benefit in patients. This disconnect is highlighted in PDA in which the desmoplastic stroma of the autochthonous disease contributes to inordinately high interstitial fluid pressures that compress blood vessels and create a biophysical barrier to drug penetration – findings that are not recapitulated in implantable tumors (82). In this regard, the observation that targeted depletion of a single MDSC subset in autochthonous PDA activates cytotoxic T cells and tumor cell death is encouraging and warrants further study into how best to translate such myeloid-targeted therapies (12). The concern that targeting one population can have unforeseen consequences on other suppressive subsets may be especially relevant for therapies designed to target the myeloid lineage in which many of the progenitors are in a transitory state and may be susceptible to an overlapping spectrum of homeostatic and/or tumor-derived modulators.
Table 3.
Clinical trials targeting myeloid cells in cancer
| Cancer | Therapy | Target | Effect | MDSC Phenotype | Ref |
|---|---|---|---|---|---|
| Metastatic melanoma | Vemurafenib1 | B-RAF inhibitor | Decrease in Gr-MDSC and Mo-MDSC | Mo-MDSC: CD14+ HLA-DR−/low Gr-MDSC: CD14− CD66b+ Arg+ |
(17) |
| Metastatic melanoma | Ipilimumab1 | Anti – CTLA-4 | Lower Mo-MDSC frequency correlated with a more positive response to immunotherapy | Mo-MDSC: Lin− CD14+ HLA-DR−/low |
(85) |
| RCC | ATRA + IL-2 | Various | Decreased MDSC frequency in blood | Lin− HLA−DR− CD33+ |
(104) |
| RCC | Sunitinib (Tyrosine kinase inhibitor) | VEGF PDGF c-kit CSF1R |
Reduced MDSC and Treg; Impact on distinct MDSC populations is not clear: as CD33 is expressed on both subsets | Mo-MDSC: CD33+ HLA-DR− Gr-MDSC: CD14− CD15+ |
(105) |
| RCC | Sunitinib (Tyrosine kinase inhibitor) | VEGF PDGF c-kit CSF1R |
Mo-MDSC decreased in frequency following treatment | Lin− HLA-DR−/low CD14+ |
(106) |
| HNSCC | 25-hydroxyvitamin D3 | Pleiotropic effects | Decreased CD34+ cells; increased HLA-DR, increased IL-12 and IFNγ in plasma, improved T-cell blasts | CD34+ | (107) |
| SCLC | ATRA + Vaccine | Numerous targets | Decreased MDSC frequency ~2-fold in blood | Lin− HLA-DR− CD33+ or CD11b+ CD14− CD33+ |
(108) |
| PDA | Zoledronic acid | Farnesyl pyrophosphate synthase | No effect on Gr-MDSC in blood at doses studied | CD45+ Lin− CD11b+ CD33+ CD15+ |
(92) |
| PDA | CDDO-Me2 + gemcitabine | iNOS COX2 NFκB |
No change in % MDSC; increased T cell proliferation | Lin− HLA-DR−/low CD33+ or Lin− CD14− CD11b+ CD33+ |
(109) |
| Multiple Myeloma3 | PDE5 inhibitor (tadalafil) | iNOS Arg |
Decreased iNOS, Arg, ROS, & nitrotyrosine; increased TCRζ expression and IFNγ | CD14+ IL-4R+ |
(110) |
These therapies were not designed to target MDSC but did decrease MDSC frequency that correlated with disease response suggesting that MDSC number can be surrogate marker for therapeutic response.
CDDO-Me:synthetic triterpenoid C-28 methyl ester of 2-cyano-3,12-dioxooleana-1,9,-dien-28-oic acid
Case study
There are presently a number of strategies under investigation to target MDSC in malignancy (Table 3 and reviewed in (3)). Such strategies include interfering with suppressive activity (PDE-5 inhibitors, COX-2 inhibitors, and the synthetic triterpenoid CDDO-Me); inhibiting induction from progenitors (Zoledronic acid, sunitinib); inhibiting egress from the bone marrow and recruitment into tumors (blockade of CCR2 or CSF/CSF1R); and modulating differentiation (ATRA, anti-CD40). Cytotoxic drugs including gemcitabine or 5-FUhave been suggested to transiently target proliferating populations of MDSC (83), yet such non-specific approaches have limitations; these chemotherapies can also eliminate effector T cells that may be induced to proliferate and may additionally cause a rebound expansion of myeloid progenitors.
MDSC are being monitored in some recent clinical trials and may serve as a prognostic indicator of patient response to therapy. In metastatic melanoma, a specific inhibitor of mutant B-rafV600E (Vemurafenib) reduced the frequency of both Gr-MDSC and Mo-MDSC in the blood (17), with the changes in the monocytic subset correlating more closely with response to therapy. The effects of this inhibitor on MDSC may be due to simply lowering the tumor burden, thereby indirectly impacting overall inflammatory cytokine levels presumed critical for MDSC maintenance. Alternatively, this therapy may change the quality of the cytokine milieu produced by individual melanoma cells (i.e. GM-CSF, IL-1β, IL-6, TNFα, VEGF, FLT3-L, TGFβ) (17, 38, 39, 84). Anti-CTLA-4 (Ipilimumab), an immunotherapy designed to activate T cells, also reduced Mo-MDSC frequency in melanoma patients, correlating with improved response (85).
Several clinical trials targeting the CSF/CSF1R pathway are underway. Inhibiting this pathway yielded striking results in an autochthonous murine glioblastoma model (86). A selective inhibitor of CSF1 reduced TAM number and increased CD8 T cell infiltration in a transgenic breast cancer model (87). Surprisingly, radiotherapy induced prostate tumors to increase CSF1 levels, resulting in the enhanced recruitment of myeloid progenitors that undesirably contributed to tumor growth (88). Thus, in some therapeutic settings, interfering with myeloid recruitment may be beneficial. While preclinical results are encouraging, the heterogeneity of myeloid cells in tumors may present additional obstacles to TAM depletion via CSF1, as not all TAM populations are susceptible to this pathway.
Inducing MDSC differentiation toward a functional state that is stimulatory to T cells is conceptually more feasible than sustained depletion of a population that is constantly being regenerated. An agonistic antibody to the CD40 costimulatory protein has demonstrated some recent success in patients with PDA (89). The therapeutic effect of anti-CD40 was reproduced in the genetically engineered KPC mouse model where it induced systemic activation of myeloid cells, infiltration into tumors, acquisition of an M1 tumoricidal phenotype and tumor cell death resulting in stromal involution. Surprisingly, the observed tumor responses appeared to be independent of T cells (89), suggesting that modulating the myeloid lineage may be sufficient to generate at least transient anti-tumor activity. Another approach to induce myeloid differentiation is an antibody that targets phosphatidylserine exposed on the surface of apoptotic tumor cells (Bavituximab). Phosphatidylserine signals to macrophages and DC and induces inhibitory cytokine production there by thwarting immune surveillance. Inhibiting phosphatidylserine signaling via antibody blockade can modulate TAM to have therapeutic activity in preclinical studies (90).
Zoledronic acid (ZA), an aminobisphosphonate, has been shown to inhibit myelopoeisis including the generation of CD11b+Gr-1+ myeloid cells, decrease tumor growth and improve survival in a transplantable PDA model (91). This compound was recently evaluated as aneoadjuvantin PDA patients with resectable disease. However, ZA did not decrease the percentage of Gr-MDSC in the blood or marrow nor did it improve overall survival (92). The potential impact of ZA on other cell subsets in the blood including IM or Mo-MDSC that also accumulate systemically in PDA patients (35, 93) remains unanswered. Further assessment of MDSC isolated from clinical tumor specimens should advance our understanding of the relative contribution of MDSC subsets in distinct malignancies. This approach was recently described in head and neck cancer (94). Cell percentages alone can be misleading due to fluctuations in other cell populations and measuring MDSC numbers whenever possible will be informative in ways frequencies will not.
CCL2, another candidate target, is produced at high levels by transformed epithelial cells, which may reflect a dependence of tumor growth on recruitment of myeloid cells through paracrine signaling (33, 35, 83). CCL2 induces the mobilization of bone marrow progenitor cells into the blood as well as the recruitment of IM and/or Mo-MDSC where they differentiate into macrophages or DC following extravasation. Thus, targeting CCR2 could potentially: 1) inhibit the egress of progenitors from the bone marrow into circulation; 2) prevent the extravasation and accumulation of myeloid cells into neoplasms; and/or 3) disruptadialogue between tumor epithelial cells and tumor macrophages. CCL2/CCR2 blockade decreased IM and TAM and increased survival in a transplantable PDA model (92). The percentage of IM in the blood correlates directly with lymph node metastases and inversely with PDA patient survival (92), suggesting that circulating IM may be a cellular surrogate for disease burden. Additionally, CCL2 levels in patients with renal cell carcinoma correlate with MDSC levels and response to immunotherapy (83). ACCR2 inhibitor is currently in Phase 1b clinical trials in combination with FOLFIRINOX in patients with borderline resectable and locally advanced PDA. However, if the therapeutic activity of inhibiting CCL2 depends upon a stimulated endogenous T cell response, combining CCL2/CCR2 blockade with concurrent cytotoxic therapy may inadvertently mitigate efficacy. On the other hand, if the effects are T cell independent, as was found with anti-CD40, then it may have some therapeutic benefit.
CONCLUSIONS
Targeting immunosuppression may be required, though perhaps not sufficient, to stimulate anti-tumor immunity. Among the clinical conundrums that immediately arise is how best to integrate the targeting of suppressive myeloid cells with cytotoxic regimens. Combining these modalities may be self-defeating, as cytotoxic drugs will also target the effector T cells that are induced to proliferate - one of the major mechanisms immune therapies are designed to promote. Additionally, if we are to benefit from the immunogenic cell death that cytotoxic chemotherapies can induce, and which requires myeloid cells for antigen presentation and T-cell activation, then the indiscriminate and global depletion of myeloid cells will be counterproductive. The successful clinical translation of treatment regimens that incorporate myeloid-centric, cytotoxic, and T cell reagents will require rational preclinical modeling with studious attention to the sequencing, frequency and dosing of the various components. We are currently exploring strategies to target specific immunosuppressive cells and/or their inhibitory mediators as a means to enhance adoptive T cell-based technologies and provide the patient with a de novo anti-tumor T cell response. Although clinical reagents are still in development, eventual Gr-MDSC depletion, in concert with a method to modulate cells of the mononuclear-phagocyte system to become competent antigen presenting cells, may provide the most favorable context for both endogenous and adoptively transferred T cells to achieve substantive and long-term clinical benefit.
Acknowledgments
This work was supported by Fred Hutchinson Cancer Research Center/University of Washington Cancer Consortium Cancer Center Support Grant CA015704 (S.R. Hingorani and P.D. Greenberg). Support was also provided by the Giles W. and Elise G. Mead Foundation (S.R. Hingorani), a gift from Maryanne Tagney-Jones and David Jones (S.R. Hingorani), the NIH/NCI (CA18029 and CA33084; to P.D. Greenberg), a grant from the Korean Research Institute of Bioscience and Biotechnology (P.D. Greenberg), the Irvington Institute Fellowship Program of the Cancer Research Institute (I.M. Stromnes), and the Jack and Sylvia Paul Estate Fund to Support Collaborative Immunotherapy Research (I.M. Stromnes).
Footnotes
Disclosure of Potential Conflicts of Interest
P.D. Greenberg has ownership interest (including patents) in and is a consultant/advisory board member for Juno Therapeutics. No potential conflicts of interest were disclosed by the other authors.
DISCLAIMER
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.June CH. Adoptive T cell therapy for cancer in the clinic. J Clin Invest. 2007;117:1466–76. doi: 10.1172/JCI32446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stromnes IM, Schmitt TM, Chapuis AG, Hingorani SR, Greenberg PD. Re-adapting T cells for cancer therapy: from mouse models to clinical trials. Immunol Rev. 2014;257:1–20. doi: 10.1111/imr.12141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12:253–68. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Orkin SH, Zon LI. Hematopoiesis: an evolving paradigm for stem cell biology. Cell. 2008;132:631–44. doi: 10.1016/j.cell.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu L, McCaslin D, Starzl TE, Thomson AW. Bone marrow-derived dendritic cell progenitors (NLDC 145+, MHC class II+, B7-1dim, B7-2−) induce alloantigen-specific hyporesponsiveness in murine T lymphocytes. Transplantation. 1995;60:1539–45. doi: 10.1097/00007890-199560120-00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fu F, Li Y, Qian S, Lu L, Chambers F, Starzl TE, et al. Costimulatory molecule-deficient dendritic cell progenitors (MHC class II+, CD80dim, CD86−) prolong cardiac allograft survival in nonimmunosuppressed recipients. Transplantation. 1996;62:659–65. doi: 10.1097/00007890-199609150-00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bronte V, Chappell DB, Apolloni E, Cabrelle A, Wang M, Hwu P, et al. Unopposed production of granulocyte-macrophage colony-stimulating factor by tumors inhibits CD8+ T cell responses by dysregulating antigen-presenting cell maturation. J Immunol. 1999;162:5728–37. [PMC free article] [PubMed] [Google Scholar]
- 8.Gabrilovich DI, Velders MP, Sotomayor EM, Kast WM. Mechanism of immune dysfunction in cancer mediated by immature Gr-1+ myeloid cells. J Immunol. 2001;166:5398–406. doi: 10.4049/jimmunol.166.9.5398. [DOI] [PubMed] [Google Scholar]
- 9.Marigo I, Bosio E, Solito S, Mesa C, Fernandez A, Dolcetti L, et al. Tumor-induced tolerance and immune suppression depend on the C/EBPbeta transcription factor. Immunity. 2010;32:790–802. doi: 10.1016/j.immuni.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 10.Bayne LJ, Beatty GL, Jhala N, Clark CE, Rhim AD, Stanger BZ, et al. Tumor-derived granulocyte-macrophage colony-stimulating factor regulates myeloid inflammation and T cell immunity in pancreatic cancer. Cancer Cell. 2012;21:822–35. doi: 10.1016/j.ccr.2012.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pylayeva-Gupta Y, Lee KE, Hajdu CH, Miller G, Bar-Sagi D. Oncogenic Kras-induced GM-CSF production promotes the development of pancreatic neoplasia. Cancer Cell. 2012;21:836–47. doi: 10.1016/j.ccr.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stromnes IM, Brockenbrough JS, Izeradjene K, Carlson MA, Cuevas C, Simmons RM, et al. Targeted depletion of an MDSC subset unmasks pancreatic ductal adenocarcinoma to adaptive immunity. Gut. 2014 doi: 10.1136/gutjnl-2013-306271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kujawski M, Kortylewski M, Lee H, Herrmann A, Kay H, Yu H. Stat3 mediates myeloid cell-dependent tumor angiogenesis in mice. J Clin Invest. 2008;118:3367–77. doi: 10.1172/JCI35213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Panni RZ, Sanford DE, Belt BA, Mitchem JB, Worley LA, Goetz BD, et al. Tumor-induced STAT3 activation in monocytic myeloid-derived suppressor cells enhances stemness and mesenchymal properties in human pancreatic cancer. Cancer Immunol Immunother. 2014;63:513–28. doi: 10.1007/s00262-014-1527-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cui TX, Kryczek I, Zhao L, Zhao E, Kuick R, Roh MH, et al. Myeloid-derived suppressor cells enhance stemness of cancer cells by inducing microRNA101 and suppressing the corepressor CtBP2. Immunity. 2013;39:611–21. doi: 10.1016/j.immuni.2013.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Movahedi K, Guilliams M, Van den Bossche J, Van den Bergh R, Gysemans C, Beschin A, et al. Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct T cell-suppressive activity. Blood. 2008;111:4233–44. doi: 10.1182/blood-2007-07-099226. [DOI] [PubMed] [Google Scholar]
- 17.Schilling B, Sucker A, Griewank K, Zhao F, Weide B, Gorgens A, et al. Vemurafenib reverses immunosuppression by myeloid derived suppressor cells. Int J Cancer. 2013;133:1653–63. doi: 10.1002/ijc.28168. [DOI] [PubMed] [Google Scholar]
- 18.Youn JI, Collazo M, Shalova IN, Biswas SK, Gabrilovich DI. Characterization of the nature of granulocytic myeloid-derived suppressor cells in tumor-bearing mice. J Leukoc Biol. 2012;91:167–81. doi: 10.1189/jlb.0311177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Filipazzi P, Valenti R, Huber V, Pilla L, Canese P, Iero M, et al. Identification of a new subset of myeloid suppressor cells in peripheral blood of melanoma patients with modulation by a granulocyte-macrophage colony-stimulation factor-based antitumor vaccine. J Clin Oncol. 2007;25:2546–53. doi: 10.1200/JCO.2006.08.5829. [DOI] [PubMed] [Google Scholar]
- 20.Zhang H, Maric I, DiPrima MJ, Khan J, Orentas RJ, Kaplan RN, et al. Fibrocytes represent a novel MDSC subset circulating in patients with metastatic cancer. Blood. 2013;122:1105–13. doi: 10.1182/blood-2012-08-449413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang A, Zhang B, Wang B, Zhang F, Fan KX, Guo YJ. Increased CD14(+) HLA-DR (−/low) myeloid-derived suppressor cells correlate with extrathoracic metastasis and poor response to chemotherapy in non-small cell lung cancer patients. Cancer Immunol Immunother. 2013;62:1439–51. doi: 10.1007/s00262-013-1450-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mao Y, Poschke I, Wennerberg E, Pico de Coana Y, Egyhazi Brage S, Schultz I, et al. Melanoma-educated CD14+ cells acquire a myeloid-derived suppressor cell phenotype through COX-2-dependent mechanisms. Cancer Res. 2013;73:3877–87. doi: 10.1158/0008-5472.CAN-12-4115. [DOI] [PubMed] [Google Scholar]
- 23.Hoechst B, Gamrekelashvili J, Manns MP, Greten TF, Korangy F. Plasticity of human Th17 cells and iTregs is orchestrated by different subsets of myeloid cells. Blood. 2011;117:6532–41. doi: 10.1182/blood-2010-11-317321. [DOI] [PubMed] [Google Scholar]
- 24.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–74. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garcia MR, Ledgerwood L, Yang Y, Xu J, Lal G, Burrell B, et al. Monocytic suppressive cells mediate cardiovascular transplantation tolerance in mice. J Clin Invest. 2010;120:2486–96. doi: 10.1172/JCI41628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duffy A, Zhao F, Haile L, Gamrekelashvili J, Fioravanti S, Ma C, et al. Comparative analysis of monocytic and granulocytic myeloid-derived suppressor cell subsets in patients with gastrointestinal malignancies. Cancer Immunol Immunother. 2013;62:299–307. doi: 10.1007/s00262-012-1332-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hingorani SR, Wang L, Multani AS, Combs C, Deramaudt TB, Hruban RH, et al. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell. 2005;7:469–83. doi: 10.1016/j.ccr.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 28.Fogg DK, Sibon C, Miled C, Jung S, Aucouturier P, Littman DR, et al. A clonogenic bone marrow progenitor specific for macrophages and dendritic cells. Science. 2006;311:83–7. doi: 10.1126/science.1117729. [DOI] [PubMed] [Google Scholar]
- 29.Rodrigues JC, Gonzalez GC, Zhang L, Ibrahim G, Kelly JJ, Gustafson MP, et al. Normal human monocytes exposed to glioma cells acquire myeloid-derived suppressor cell-like properties. Neuro Oncol. 2010;12:351–65. doi: 10.1093/neuonc/nop023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 31.Yona S, Kim KW, Wolf Y, Mildner A, Varol D, Breker M, et al. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity. 2013;38:79–91. doi: 10.1016/j.immuni.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Swirski FK, Nahrendorf M, Etzrodt M, Wildgruber M, Cortez-Retamozo V, Panizzi P, et al. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science. 2009;325:612–6. doi: 10.1126/science.1175202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Auffray C, Sieweke MH, Geissmann F. Blood monocytes: development, heterogeneity, and relationship with dendritic cells. Annu Rev Immunol. 2009;27:669–92. doi: 10.1146/annurev.immunol.021908.132557. [DOI] [PubMed] [Google Scholar]
- 34.Egawa M, Mukai K, Yoshikawa S, Iki M, Mukaida N, Kawano Y, et al. Inflammatory monocytes recruited to allergic skin acquire an anti-inflammatory M2 phenotype via basophil-derived interleukin-4. Immunity. 2013;38:570–80. doi: 10.1016/j.immuni.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 35.Sanford DE, Belt BA, Panni RZ, Mayer A, Deshpande AD, Carpenter D, et al. Inflammatory monocyte mobilization decreases patient survival in pancreatic cancer: a role for targeting the CCL2/CCR2 axis. Clin Cancer Res. 2013;19:3404–15. doi: 10.1158/1078-0432.CCR-13-0525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schmielau J, Finn OJ. Activated granulocytes and granulocyte-derived hydrogen peroxide are the underlying mechanism of suppression of t-cell function in advanced cancer patients. Cancer Res. 2001;61:4756–60. [PubMed] [Google Scholar]
- 37.Qin H, Lerman B, Sakamaki I, Wei G, Cha SC, Rao SS, et al. Generation of a new therapeutic peptide that depletes myeloid-derived suppressor cells in tumor-bearing mice. Nat Med. 2014;20:676–81. doi: 10.1038/nm.3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lechner MG, Liebertz DJ, Epstein AL. Characterization of cytokine-induced myeloid-derived suppressor cells from normal human peripheral blood mononuclear cells. J Immunol. 2010;185:2273–84. doi: 10.4049/jimmunol.1000901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lechner MG, Megiel C, Russell SM, Bingham B, Arger N, Woo T, et al. Functional characterization of human Cd33+ and Cd11b+ myeloid-derived suppressor cell subsets induced from peripheral blood mononuclear cells co-cultured with a diverse set of human tumor cell lines. J Transl Med. 2011;9:90. doi: 10.1186/1479-5876-9-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morales JK, Kmieciak M, Knutson KL, Bear HD, Manjili MH. GM-CSF is one of the main breast tumor-derived soluble factors involved in the differentiation of CD11b−Gr1− bone marrow progenitor cells into myeloid-derived suppressor cells. Breast Cancer Res Treat. 2010;123:39–49. doi: 10.1007/s10549-009-0622-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Highfill SL, Rodriguez PC, Zhou Q, Goetz CA, Koehn BH, Veenstra R, et al. Bone marrow myeloid-derived suppressor cells (MDSCs) inhibit graft-versus-host disease (GVHD) via an arginase-1-dependent mechanism that is up-regulated by interleukin-13. Blood. 2010;116:5738–47. doi: 10.1182/blood-2010-06-287839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dolcetti L, Peranzoni E, Ugel S, Marigo I, Fernandez Gomez A, Mesa C, et al. Hierarchy of immunosuppressive strength among myeloid-derived suppressor cell subsets is determined by GM-CSF. Eur J Immunol. 2010;40:22–35. doi: 10.1002/eji.200939903. [DOI] [PubMed] [Google Scholar]
- 43.Li L, Zhang J, Diao W, Wang D, Wei Y, Zhang CY, et al. MicroRNA-155 and MicroRNA-21 promote the expansion of functional myeloid-derived suppressor cells. J Immunol. 2014;192:1034–43. doi: 10.4049/jimmunol.1301309. [DOI] [PubMed] [Google Scholar]
- 44.Dranoff G. GM-CSF-based cancer vaccines. Immunol Rev. 2002;188:147–54. doi: 10.1034/j.1600-065x.2002.18813.x. [DOI] [PubMed] [Google Scholar]
- 45.Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nat Rev Cancer. 2012;12:265–77. doi: 10.1038/nrc3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hiratsuka S, Watanabe A, Aburatani H, Maru Y. Tumour-mediated upregulation of chemoattractants and recruitment of myeloid cells predetermines lung metastasis. Nat Cell Biol. 2006;8:1369–75. doi: 10.1038/ncb1507. [DOI] [PubMed] [Google Scholar]
- 47.Cheng P, Corzo CA, Luetteke N, Yu B, Nagaraj S, Bui MM, et al. Inhibition of dendritic cell differentiation and accumulation of myeloid-derived suppressor cells in cancer is regulated by S100A9 protein. J Exp Med. 2008;205:2235–49. doi: 10.1084/jem.20080132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vernon PJ, Loux TJ, Schapiro NE, Kang R, Muthuswamy R, Kalinski P, et al. The receptor for advanced glycation end products promotes pancreatic carcinogenesis and accumulation of myeloid-derived suppressor cells. J Immunol. 2013;190:1372–9. doi: 10.4049/jimmunol.1201151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Luyckx A, Schouppe E, Rutgeerts O, Lenaerts C, Fevery S, Devos T, et al. G-CSF stem cell mobilization in human donors induces polymorphonuclear and mononuclear myeloid-derived suppressor cells. Clin Immunol. 2012;143:83–7. doi: 10.1016/j.clim.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 50.Shojaei F, Wu X, Qu X, Kowanetz M, Yu L, Tan M, et al. G-CSF-initiated myeloid cell mobilization and angiogenesis mediate tumor refractoriness to anti-VEGF therapy in mouse models. Proc Natl Acad Sci U S A. 2009;106:6742–7. doi: 10.1073/pnas.0902280106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fan Q, Gu D, Liu H, Yang L, Zhang X, Yoder MC, et al. Defective TGF-beta signaling in bone marrow-derived cells prevents hedgehog-induced skin tumors. Cancer Res. 2014;74:471–83. doi: 10.1158/0008-5472.CAN-13-2134-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tsou CL, Peters W, Si Y, Slaymaker S, Aslanian AM, Weisberg SP, et al. Critical roles for CCR2 and MCP-3 in monocyte mobilization from bone marrow and recruitment to inflammatory sites. J Clin Invest. 2007;117:902–9. doi: 10.1172/JCI29919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cortez-Retamozo V, Etzrodt M, Newton A, Rauch PJ, Chudnovskiy A, Berger C, et al. Origins of tumor-associated macrophages and neutrophils. Proc Natl Acad Sci U S A. 2012;109:2491–6. doi: 10.1073/pnas.1113744109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Obermajer N, Muthuswamy R, Odunsi K, Edwards RP, Kalinski P. PGE(2)-induced CXCL12 production and CXCR4 expression controls the accumulation of human MDSCs in ovarian cancer environment. Cancer Res. 2011;71:7463–70. doi: 10.1158/0008-5472.CAN-11-2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Feig C, Jones JO, Kraman M, Wells RJ, Deonarine A, Chan DS, et al. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proc Natl Acad Sci U S A. 2013;110:20212–7. doi: 10.1073/pnas.1320318110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Katoh H, Wang D, Daikoku T, Sun H, Dey SK, Dubois RN. CXCR2-expressing myeloid-derived suppressor cells are essential to promote colitis-associated tumorigenesis. Cancer Cell. 2013;24:631–44. doi: 10.1016/j.ccr.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Highfill SL, Cui Y, Giles AJ, Smith JP, Zhang H, Morse E, et al. Disruption of CXCR2-mediated MDSC tumor trafficking enhances anti-PD1 efficacy. Sci Transl Med. 2014;6:237ra67. doi: 10.1126/scitranslmed.3007974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McAllister F, Bailey JM, Alsina J, Nirschl CJ, Sharma R, Fan H, et al. Oncogenic Kras activates a hematopoietic-to-epithelial IL-17 signaling axis in preinvasive pancreatic neoplasia. Cancer Cell. 2014;25:621–37. doi: 10.1016/j.ccr.2014.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wu P, Wu D, Ni C, Ye J, Chen W, Hu G, et al. gammadeltaT17 cells promote the accumulation and expansion of myeloid-derived suppressor cells in human colorectal cancer. Immunity. 2014;40:785–800. doi: 10.1016/j.immuni.2014.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ge S, Hertel B, Susnik N, Rong S, Dittrich AM, Schmitt R, et al. Interleukin 17 receptor A modulates monocyte subsets and macrophage generation in vivo. PLoS One. 2014;9:e85461. doi: 10.1371/journal.pone.0085461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Umemura N, Saio M, Suwa T, Kitoh Y, Bai J, Nonaka K, et al. Tumor-infiltrating myeloid-derived suppressor cells are pleiotropic-inflamed monocytes/macrophages that bear M1- and M2-type characteristics. J Leukoc Biol. 2008;83:1136–44. doi: 10.1189/jlb.0907611. [DOI] [PubMed] [Google Scholar]
- 62.Movahedi K, Laoui D, Gysemans C, Baeten M, Stange G, Van den Bossche J, et al. Different tumor microenvironments contain functionally distinct subsets of macrophages derived from Ly6C(high) monocytes. Cancer Res. 2010;70:5728–39. doi: 10.1158/0008-5472.CAN-09-4672. [DOI] [PubMed] [Google Scholar]
- 63.De Monte L, Reni M, Tassi E, Clavenna D, Papa I, Recalde H, et al. Intratumor T helper type 2 cell infiltrate correlates with cancer-associated fibroblast thymic stromal lymphopoietin production and reduced survival in pancreatic cancer. J Exp Med. 2011;208:469–78. doi: 10.1084/jem.20101876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jenkins SJ, Ruckerl D, Cook PC, Jones LH, Finkelman FD, van Rooijen N, et al. Local macrophage proliferation, rather than recruitment from the blood, is a signature of TH2 inflammation. Science. 2011;332:1284–8. doi: 10.1126/science.1204351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, et al. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176:1693–702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pollard JW. Role of colony-stimulating factor-1 in reproduction and development. Mol ReprodDev. 1997;46:54–60. doi: 10.1002/(SICI)1098-2795(199701)46:1<54::AID-MRD9>3.0.CO;2-Q. discussion -1. [DOI] [PubMed] [Google Scholar]
- 67.Mantovani A, Sica A. Macrophages, innate immunity and cancer: balance, tolerance, and diversity. Curr Opin Immunol. 2010;22:231–7. doi: 10.1016/j.coi.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 68.Goud TJ, Schotte C, van Furth R. Identification and characterization of the monoblast in mononuclear phagocyte colonies grown in vitro. J Exp Med. 1975;142:1180–99. doi: 10.1084/jem.142.5.1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Verreck FA, de Boer T, Langenberg DM, Hoeve MA, Kramer M, Vaisberg E, et al. Human IL-23-producing type 1 macrophages promote but IL-10-producing type 2 macrophages subvert immunity to (myco)bacteria. Proc Natl Acad Sci U S A. 2004;101:4560–5. doi: 10.1073/pnas.0400983101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2011;11:723–37. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Greter M, Helft J, Chow A, Hashimoto D, Mortha A, Agudo-Cantero J, et al. GM-CSF controls nonlymphoid tissue dendritic cell homeostasis but is dispensable for the differentiation of inflammatory dendritic cells. Immunity. 2012;36:1031–46. doi: 10.1016/j.immuni.2012.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Serbina NV, Salazar-Mather TP, Biron CA, Kuziel WA, Pamer EG. TNF/iNOS-producing dendritic cells mediate innate immune defense against bacterial infection. Immunity. 2003;19:59–70. doi: 10.1016/s1074-7613(03)00171-7. [DOI] [PubMed] [Google Scholar]
- 73.Ma Y, Mattarollo SR, Adjemian S, Yang H, Aymeric L, Hannani D, et al. CCL2/CCR2-dependent recruitment of functional antigen-presenting cells into tumors upon chemotherapy. Cancer Res. 2014;74:436–45. doi: 10.1158/0008-5472.CAN-13-1265. [DOI] [PubMed] [Google Scholar]
- 74.Klug F, Prakash H, Huber PE, Seibel T, Bender N, Halama N, et al. Low-dose irradiation programs macrophage differentiation to an iNOS(+)/M1 phenotype that orchestrates effective T cell immunotherapy. Cancer Cell. 2013;24:589–602. doi: 10.1016/j.ccr.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 75.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Weizman N, Krelin Y, Shabtay-Orbach A, Amit M, Binenbaum Y, Wong RJ, et al. Macrophages mediate gemcitabine resistance of pancreatic adenocarcinoma by upregulating cytidine deaminase. Oncogene. 2013 Sep 2; doi: 10.1038/onc.2013.357. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 77.Mitchem JB, Brennan DJ, Knolhoff BL, Belt BA, Zhu Y, Sanford DE, et al. Targeting tumor-infiltrating macrophages decreases tumor-initiating cells, relieves immunosuppression, and improves chemotherapeutic responses. Cancer Res. 2013;73:1128–41. doi: 10.1158/0008-5472.CAN-12-2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Reilkoff RA, Bucala R, Herzog EL. Fibrocytes: emerging effector cells in chronic inflammation. Nat Rev Immunol. 2011;11:427–35. doi: 10.1038/nri2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chesney J, Bacher M, Bender A, Bucala R. The peripheral blood fibrocyte is a potent antigen-presenting cell capable of priming naive T cells in situ. Proc Natl Acad Sci U S A. 1997;94:6307–12. doi: 10.1073/pnas.94.12.6307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Balmelli C, Ruggli N, McCullough K, Summerfield A. Fibrocytes are potent stimulators of anti-virus cytotoxic T cells. J Leukoc Biol. 2005;77:923–33. doi: 10.1189/jlb.1204701. [DOI] [PubMed] [Google Scholar]
- 81.Kraman M, Bambrough PJ, Arnold JN, Roberts EW, Magiera L, Jones JO, et al. Suppression of antitumor immunity by stromal cells expressing fibroblast activation protein-alpha. Science. 2010;330:827–30. doi: 10.1126/science.1195300. [DOI] [PubMed] [Google Scholar]
- 82.Hingorani SR, Potter JD. Pancreas cancer meets the thunder god. Sci Transl Med. 2012;4:156ps21. doi: 10.1126/scitranslmed.3004956. [DOI] [PubMed] [Google Scholar]
- 83.Ugel S, Peranzoni E, Desantis G, Chioda M, Walter S, Weinschenk T, et al. Immune tolerance to tumor antigens occurs in a specialized environment of the spleen. Cell Rep. 2012;2:628–39. doi: 10.1016/j.celrep.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 84.Sumimoto H, Imabayashi F, Iwata T, Kawakami Y. The BRAF-MAPK signaling pathway is essential for cancer-immune evasion in human melanoma cells. J Exp Med. 2006;203:1651–6. doi: 10.1084/jem.20051848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Meyer C, Cagnon L, Costa-Nunes CM, Baumgaertner P, Montandon N, Leyvraz L, et al. Frequencies of circulating MDSC correlate with clinical outcome of melanoma patients treated with ipilimumab. Cancer Immunol Immunother. 2014;63:247–57. doi: 10.1007/s00262-013-1508-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pyonteck SM, Akkari L, Schuhmacher AJ, Bowman RL, Sevenich L, Quail DF, et al. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat Med. 2013;19:1264–72. doi: 10.1038/nm.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Strachan DC, Ruffell B, Oei Y, Bissell MJ, Coussens LM, Pryer N, et al. CSF1R inhibition delays cervical and mammary tumor growth in murine models by attenuating the turnover of tumor-associated macrophages and enhancing infiltration by CD8 T cells. Oncoimmunology. 2013;2:e26968. doi: 10.4161/onci.26968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Xu J, Escamilla J, Mok S, David J, Priceman S, West B, et al. CSF1R signaling blockade stanches tumor-infiltrating myeloid cells and improves the efficacy of radiotherapy in prostate cancer. Cancer Res. 2013;73:2782–94. doi: 10.1158/0008-5472.CAN-12-3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Beatty GL, Chiorean EG, Fishman MP, Saboury B, Teitelbaum UR, Sun W, et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science. 2011;331:1612–6. doi: 10.1126/science.1198443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yin Y, Huang X, Lynn KD, Thorpe PE. Phosphatidylserine-targeting antibody induces m1 macrophage polarization and promotes myeloid-derived suppressor cell differentiation. Cancer Immunol Res. 2013;1:256–68. doi: 10.1158/2326-6066.CIR-13-0073. [DOI] [PubMed] [Google Scholar]
- 91.Porembka MR, Mitchem JB, Belt BA, Hsieh CS, Lee HM, Herndon J, et al. Pancreatic adenocarcinoma induces bone marrow mobilization of myeloid-derived suppressor cells which promote primary tumor growth. Cancer Immunol Immunother. 2012;61:1373–85. doi: 10.1007/s00262-011-1178-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sanford DE, Porembka MR, Panni RZ, Mitchem JB, Belt BA, Plambeck-Suess SM, et al. A study of zoledronic acid as neo-adjuvant, perioperative therapy in patients with resectable pancreatic ductal adenocarcinoma. J Cancer Ther. 2013;4:797–803. doi: 10.4236/jct.2013.43096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Basso D, Fogar P, Falconi M, Fadi E, Sperti C, Frasson C, et al. Pancreatic tumors and immature immunosuppressive myeloid cells in blood and spleen: role of inhibitory co-stimulatory molecules PDL1 and CTLA4. An in vivo and in vitro study. PLoS One. 2013;8:e54824. doi: 10.1371/journal.pone.0054824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Vasquez-Dunddel D, Pan F, Zeng Q, Gorbounov M, Albesiano E, Fu J, et al. STAT3 regulates arginase-I in myeloid-derived suppressor cells from cancer patients. J Clin Invest. 2013;123:1580–9. doi: 10.1172/JCI60083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Poschke I, Mougiakakos D, Hansson J, Masucci GV, Kiessling R. Immature immunosuppressive CD14+HLA-DR−/low cells in melanoma patients are Stat3hi and overexpress CD80, CD83, and DC-sign. Cancer Res. 2010;70:4335–45. doi: 10.1158/0008-5472.CAN-09-3767. [DOI] [PubMed] [Google Scholar]
- 96.Ugurel S, Uhlig D, Pfohler C, Tilgen W, Schadendorf D, Reinhold U. Down-regulation of HLA class II and costimulatory CD86/B7-2 on circulating monocytes from melanoma patients. Cancer Immunol Immunother. 2004;53:551–9. doi: 10.1007/s00262-003-0489-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Liu CY, Wang YM, Wang CL, Feng PH, Ko HW, Liu YH, et al. Population alterations of L-arginase- and inducible nitric oxide synthase-expressed CD11b+/CD14(−)/CD15+/CD33+ myeloid-derived suppressor cells and CD8+ T lymphocytes in patients with advanced-stage non-small cell lung cancer. J Cancer ResClinOncol. 2010;136:35–45. doi: 10.1007/s00432-009-0634-0. [DOI] [PubMed] [Google Scholar]
- 98.Zea AH, Rodriguez PC, Atkins MB, Hernandez C, Signoretti S, Zabaleta J, et al. Arginase-producing myeloid suppressor cells in renal cell carcinoma patients: a mechanism of tumor evasion. Cancer Res. 2005;65:3044–8. doi: 10.1158/0008-5472.CAN-04-4505. [DOI] [PubMed] [Google Scholar]
- 99.Zhang B, Wang Z, Wu L, Zhang M, Li W, Ding J, et al. Circulating and tumor-infiltrating myeloid-derived suppressor cells in patients with colorectal carcinoma. PLoS One. 2013;8:e57114. doi: 10.1371/journal.pone.0057114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Diaz-Montero CM, Salem ML, Nishimura MI, Garrett-Mayer E, Cole DJ, Montero AJ. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy. Cancer Immunol Immunother. 2009;58:49–59. doi: 10.1007/s00262-008-0523-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Serafini P, Borrello I, Bronte V. Myeloid suppressor cells in cancer: recruitment, phenotype, properties, and mechanisms of immune suppression. Semin Cancer Biol. 2006;16:53–65. doi: 10.1016/j.semcancer.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 102.Shen P, Wang A, He M, Wang Q, Zheng S. Increased circulating Lin CD33 HLA-DR myeloid-derived suppressor cells in hepatocellular carcinoma patients. Hepatology Res. 2014;44:639–50. doi: 10.1111/hepr.12167. [DOI] [PubMed] [Google Scholar]
- 103.Kalathil S, Lugade AA, Miller A, Iyer R, Thanavala Y. Higher frequencies of GARP(+)CTLA-4(+)Foxp3(+) T regulatory cells and myeloid-derived suppressor cells in hepatocellular carcinoma patients are associated with impaired T-cell functionality. Cancer Res. 2013;73:2435–44. doi: 10.1158/0008-5472.CAN-12-3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mirza N, Fishman M, Fricke I, Dunn M, Neuger AM, Frost TJ, et al. All-trans-retinoic acid improves differentiation of myeloid cells and immune response in cancer patients. Cancer Res. 2006;66:9299–307. doi: 10.1158/0008-5472.CAN-06-1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ko JS, Zea AH, Rini BI, Ireland JL, Elson P, Cohen P, et al. Sunitinib mediates reversal of myeloid-derived suppressor cell accumulation in renal cell carcinoma patients. Clin Cancer Res. 2009;15:2148–57. doi: 10.1158/1078-0432.CCR-08-1332. [DOI] [PubMed] [Google Scholar]
- 106.van Cruijsen H, van der Veldt AA, Vroling L, Oosterhoff D, Broxterman HJ, Scheper RJ, et al. Sunitinib-induced myeloid lineage redistribution in renal cell cancer patients: CD1c+ dendritic cell frequency predicts progression-free survival. Clin Cancer Res. 2008;14:5884–92. doi: 10.1158/1078-0432.CCR-08-0656. [DOI] [PubMed] [Google Scholar]
- 107.Lathers DM, Clark JI, Achille NJ, Young MR. Phase 1B study to improve immune responses in head and neck cancer patients using escalating doses of 25-hydroxyvitamin D3. Cancer Immunol Immunother. 2004;53:422–30. doi: 10.1007/s00262-003-0459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Iclozan C, Antonia S, Chiappori A, Chen DT, Gabrilovich D. Therapeutic regulation of myeloid-derived suppressor cells and immune response to cancer vaccine in patients with extensive stage small cell lung cancer. Cancer Immunol Immunother. 2013;62:909–18. doi: 10.1007/s00262-013-1396-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nagaraj S, Youn JI, Weber H, Iclozan C, Lu L, Cotter MJ, et al. Anti-inflammatory triterpenoid blocks immune suppressive function of MDSCs and improves immune response in cancer. Clin Cancer Res. 2010;16:1812–23. doi: 10.1158/1078-0432.CCR-09-3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Noonan KA, Ghosh N, Rudraraju L, Bui M, Borrello I. Targeting immune suppression with PDE5 inhibition in end-stage multiple myeloma. Cancer Immunol Research. 2014 Mar 28; doi: 10.1158/2326-6066.CIR-13-0213. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]