Abstract
Hepatitis C virus (HCV) infection results in a chronic carrier state in 80% of individuals infected with the virus and presently affects over 170 million people worldwide. Approximately 20% of those chronically infected will ultimately progress to develop cirrhosis and death due to end-stage liver disease or hepatocellular carcinoma (HCC). Unlike many other chronic viral infections, effective treatments for HCV are available. Cure from the infection is known as a sustained virologic response (SVR). SVR is associated with reversal of the long-term outcomes of chronic liver disease, decrease in incidence of HCC, and decrease HCV attributable mortality. The current FDA approved therapies for hepatitis C virus genotype 1 (GT-1) include pegylated interferon (PEG-IFN) and ribavirin (RBV) in combination with a directly acting antiviral agent (DAA). New therapeutic advances are being made aiming to simplify management, improve the tolerability of treatment, and shorten the duration of therapy. Moreover, treatment regimens that will effectively eradicate hepatitis C without the use of interferon formulations (IFN) are being developed. In this review, we report the transition of HCV therapeutics from an interferon-α based combination therapy to an all-oral, directly acting antiviral therapy.
Keywords: boceprevir, hepatitis C, pegylated interferon, ribavirin, simeprevir, sofosbuvir, sustained virologic response, telaprevir
Introduction
Hepatitis C virus (HCV) infects an estimated 170 million individuals worldwide [Mohd Hanafiah et al. 2013] and about 4 million people in the United States [Armstrong et al. 2006]. Most individuals with acute HCV infection will progress to chronicity and over time will develop liver fibrosis. Progressive liver fibrosis can lead to cirrhosis and decompensated liver disease in due course. A total of 25% of all cirrhotic patients will also develop hepatocellular carcinoma (HCC). Today, HCV is the primary cause of liver transplantation in the United States and Western Europe [Alter et al. 1999; Davis et al. 2010; Salmon-Ceron et al. 2009].
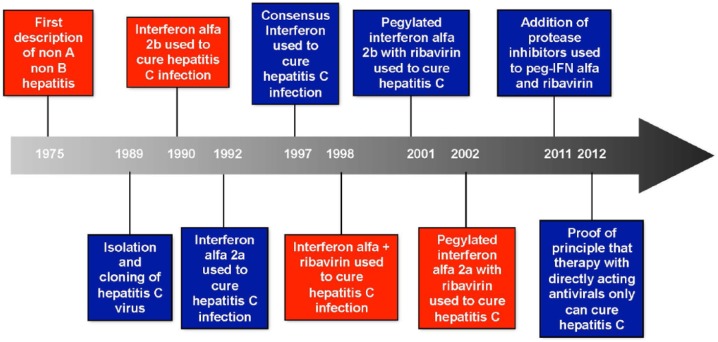
HCV exists as six closely related, yet distinct known genotypes (GTs). The most common GTs found in the United States are GT-1, -2, and -3 (GT-1–3) [Germer et al. 2011; Delwart et al. 2012; Manos et al. 2012]. Interestingly, GT-3 is associated with the development of hepatic steatosis [Rubbia-Brandt et al. 2000]. Since the discovery of HCV in 1989 [Choo et al. 1989], strategies to eradicate HCV have evolved rapidly. The need for a simple therapeutic regimen with fewer side effects allowing lower dropout rates, and improved overall efficacy cannot be over emphasized (Figure 1).
Figure 1.
Evolution of treatment for hepatitis C.
Sustained virologic response (SVR) is defined as the absence of detectable levels of plasma HCV RNA 12 weeks after the completion of therapy [Swain et al. 2010]. Patients who achieve SVR have stable virologic remission over the years following treatment and experience reversal of liver fibrosis and better liver-related outcomes [Swain et al. 2010]. SVR is therefore the equivalent to successful treatment of HCV. In monoinfected individuals, SVR has been linked to a decrease liver-related morbidity such as: decrease in liver decompensation, decrease in required liver transplantation, incidence of HCC and a decrease in all cause and liver-related mortality [Zator and Chung, 2013].
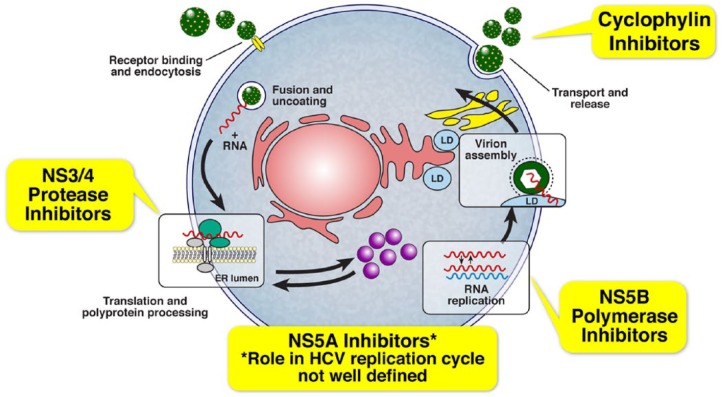
Type I interferons were initially used to treat hepatitis C successfully [Renault and Hoofnagle, 1989]. Subsequently, ribavirin (RBV), an antiviral agent, was added to improve cure rates [Mchutchison et al. 1998; Poynard et al. 1998]. Interferon α (IFN) is administered as subcutaneous injection while RBV is administered orally. Several steps of HCV lifecycle are blocked in vitro by IFN and their products, as well as by RBV (Figure 2). Recently, directly acting antivirals (DAAs) that target various stages of the HCV lifecycle have been developed. Two of these antivirals, telaprevir (TPV) and boceprevir (BOC), have been approved by the FDA for use in combination with pegylated interferon (PEG-IFN) and RBV for patients with HCV GT-1 and are associated with modest rates of cure, but significant dose-limiting adverse events (Table 1). Furthermore, SVR rates with interferon-based combination therapy have historically been poor in subgroups of HCV-infected patients such as African Americans, Hispanics, patients coinfected with HIV, patients with advanced liver disease, and those who have previously failed HCV treatment [Chung et al. 2004; Muir et al. 2004]. The basis for poor response manifested by various suboptimal outcomes to IFN-based therapy in these patients is not completely understood (Table 2). There is an urgent need for more effective treatment options for chronic HCV infection in all infected patients, regardless of baseline prognostic factors, since untreated, the disease may progress to liver fibrosis, cirrhosis, decompensated liver disease, HCC, and death.
Figure 2.
Drug targets in the lifecycle of the hepatitis C virus.
Table 1.
Agents available to treat HCV: mechanisms, activity and major adverse events.
| Name of the antiviral agent | Potential mechanism | Candidates | Spectrum | Major side-effects | Comments |
|---|---|---|---|---|---|
| Interferon | Directly and indirectly suppress HCV replication. | Interferon-α2a, Interferon-α2b, Peginterferon-α2a, Peginterferon-α2b | HCV GT-1 to GT-6 | Constitutional, hematologic (neutropenia, anemia) neuropsychiatric, nausea, rash, and cough | GT-2 and GT-3 are more sensitive than GT-1 |
| Ribavirin | Unclear although multiple mechanisms have been proposed that include inducing host immunity, reducing HCV mutagenicity. | Ribavirin | GT-1 to GT-6 | Anemia, teratogenicity | |
| HCV Protease Inhibitors | HCV NS3/NS4A serine protease inhibitor. Blocks processing of HCV polyprotein and production of new infectious virions. | Boceprevir, Telaprevir, Simeprevir, Asunaprevir+, Faldaprevir+, ABT-450+ | GT-1b > GT-1a | Anemia, dysgeusia, rash | Low barrier to resistance and not to be used as monotherapy |
| HCV NS5A Inhibitors | HCV NS5A inhibitor. Prevents formation of HCV replication complex which is vital for viral RNA replication, and virion assembly. | Daclatasvir+ | All HCV genotypes | Rash | Low barrier to resistance |
| Ledipasvir | |||||
| ABT 267+ | |||||
| HCV NS5B Polymerase Inhibitors | RNA dependent RNA polymerase inhibitor. Competitively binds to the catalytic site of HCV NS5B RNA polymerase and block HCV replication | Sofosbuvir, | All HCV genotypes | Fatigue | High barrier to resistance |
| Headache | Low barrier to develop resistance | ||||
| RNA-dependent RNA polymerase inhibitors. Nonnucleoside analogs. Block HCV replication by binding to an allosteric site of HCV RNA polymerase. | ABT-333+ | GT 1b > 1a | Rash | ||
| Deleobuvir+ | Nausea | ||||
| Vomiting |
Agents not yet approved by the FDA at the time of review.
FDA, US Food and Drug Administration; GT, genotype; HCV, hepatitis C virus HCV; RNA, ribonucleic acid.
Table 2.
Clinical responses to directly acting antiviral treatment.
| Response | Definition | Outcome |
|---|---|---|
| Viral breakthrough | Detectable HCV RNA after previously undetectable HCV RNA while receiving therapy | Failure with emergence of viral resistance |
| Relapse | Undetectable HCV RNA on therapy with detectable HCV RNA after stopping therapy | Failure with or without emergence of viral resistance |
| Sustained virologic response | Undetectable HCV RNA 12 weeks after stopping therapy | Functional cure for hepatitis C infection |
HCV, hepatitis C virus HCV; RNA, ribonucleic acid.
HCV therapeutics are rapidly emerging with approaches that are highly successful for the eradication of HCV without the use of injectable IFN formulations and RBV. This evolution is expected to revolutionize the care of HCV by offering simple regimens that are better tolerated and provide high rates of SVR, with shortened treatment duration. This transformation of DAA therapy will likely increase the number of patients who will engage in care and receive HCV treatment.
Sooner or later, it is expected that treatment for chronic HCV be so simplified that management will move from specialists to general internists. In the near future, HCV treatment may be delivered to patients by their primary care providers. The objective of this review is to describe the steady, fast-paced progress in HCV therapeutics from an IFN-based therapy to IFN-sparing or IFN-free regimens.
Pegylated interferon and ribavirin
At the start of the millennium, two major advances in the management of hepatitis C took place: one, the approval by FDA of PEG-IFN for the treatment of HCV infection, that allowed weekly subcutaneous injection instead of the previous daily or thrice weekly injection with standard IFN; and the other, the use of weight-based RBV (1–1.2 g/day). By the mid-2000s, it was established that PEG-IFN-α2a or PEG-IFN-α2b could be used in combination with weight-based RBV for GT-1-infected patients, or with flat-dosed RBV for GT-2- or GT-3-infected patients, and that this combination was better than treatment with standard IFN and RBV.
HCV lifecycle and new targets for DAA therapy
HCV preferentially infects hepatocytes and undergoes transcription to form a complementary negative sense RNA molecule, which forms the template for the production of positive stranded RNA molecules (Figure 2). HCV genome is composed of approximately 9000 nucleotides and generates structural and nonstructural (NS) proteins. The structural proteins are used to assemble new viral particles and the NS proteins support viral RNA replication. The NS3/4A is a serine protease (NS3) and cofactor (NS4A) that catalyzes the post-translational processing of NS proteins from the polyprotein, which is important for viral replication. The products released go on to form a replicative complex (NS5A) responsible for producing viral RNA using the RNA dependent/RNA polymerase (NS5B). Finally, virions are assembled, packaged and released.
HCV NS3/4A serine protease inhibitors
The NS3/4A serine protease is required for self-cleavage during viral replication. Targeting this protease may therefore restore IFN responsiveness as well as inhibiting viral replication. TPV, BOC, and simeprevir are all examples of HCV protease inhibitors.
HCV NS5B inhibitors
The HCV polymerase inhibitors are another promising DAA class. These molecules are divided into nucleoside/nucleotide competitive polymerase inhibitors and allosteric inhibitors of RNA polymerase. Nucleoside/nucleotide polymerase inhibitors have a high barrier to resistance and appear to be effective across a broad range of genotypes. Allosteric polymerase inhibitors have a lower barrier of resistance and appear to be genotype specific. Whereas the nucleoside inhibitors bind to the polymerase’s active site, the allosteric inhibitors bind to allosteric sites of the enzyme. This induces conformational changes that downregulate the polymerase’s activity. Different binding sites disposed in a right-hand motif with the thumb (thumb 1 and thumb 2), finger and palm (palm 1 and palm 2) domains are potential targets of allosteric inhibitors. As a result of different target sites, mechanism of inhibition, and potency differences, allosteric inhibitors have a low genetic barrier to resistance compared with nucleoside/nucleotide analogs (Figure 2). Sofosbuvir is an example of nucleotide analog.
HCV NS5A inhibitors
Because of its critical involvement in viral replication and assembly, NS5A has been identified as a target for viral inhibition, leading to development of therapeutic agents. Inhibition of NS5A at picomolar concentrations has been associated with significant reductions in HCV RNA levels in cell culture-based models, which makes these agents among the most potent antiviral molecules yet developed. NS5A inhibitors have pan-genotypic activity, i.e. they suppress replication of all HCV genotypes, but their antiviral effectiveness against genotypes other than 1 may vary from one molecule to another. Daclatasvir and ledipasvir are examples of NS5A inhibitors.
Combination therapy with PEG-IFN, RBV and a DAA
Combination therapy with PEG-IFN, RBV and a HCV NS3/4A serine protease inhibitor
BOC and TPV, both selective inhibitors of HCV NS3/4 serine protease, were developed and found to be effective in treating HCV-infected GT-1 patients in combination with PEG-IFN and RBV. Both agents are not indicated for use in GT-2 and GT-3 patients. Combination therapy using TPV or BOC with PEG-IFN and RBV resulted in higher rates of SVR for the treatment of HCV GT-1 in treatment naive patients (range 61–75%) compared with treatment with PEG-IFN + RBV (range 38–49%) [Hezode et al. 2009; Mchutchison et al. 2009; Kwo et al. 2010; Jacobson et al. 2011; Poordad et al. 2011; Sherman et al. 2011; Kumada et al. 2012].
Both TPV and BOC are used quite differently as part of combination therapy. For example, TPV + PEG-IFN + RBV were used as combination triple therapy for 12 weeks, followed by PEG-IFN + RBV alone for the duration of treatment. However, BOC is added to PEG-IFN + RBV for the duration of treatment after a 4-week lead-in phase with PEG-IFN + RBV alone. SVR outcomes were measured following response-guided therapy. Response-guided therapy is defined as shortened duration of treatment (24 versus 48 weeks for TPV and 28 versus 48 weeks for BOC) for patients who achieved early viral load declines. In this regard, those patients who achieve undetectable HCV RNA levels at weeks 8 and 24 and weeks 4 and 12 for TPV- and BOC-containing regimens, respectively, can be treated for 24 and 28 weeks, respectively. SVR rates were found to be similar with response-guided treatment as well as extended treatment [Bacon et al. 2011; Poordad et al. 2011; Sherman et al. 2011]. There are several host factors that determine poor response to IFN-containing therapy, such as African American race. Only one study reported [Poordad et al. 2011] a subanalysis of African American patients (n = 159 participants), which showed an improvement in SVR with BOC + PEG + RBV (SVR 53%) over PEG + RBV alone (SVR 23%). Hence, the addition of BOC could improve SVR rates among African Americans infected with hepatitis C GT-1. Both agents were studied among those patients who have experienced treatment with IFN and RBV in the past [Mchutchison et al. 2010; Bacon et al. 2011; Zeuzem et al. 2011; Flamm et al. 2013]. Among the previous IFN-experienced GT-1 patients, previous partial responders and relapsers had significant improvement in SVR rates when treated with TPV- or BOC-containing regimens for up to 48 weeks (range 69–83%) as compared with PEG-IFN + RBV alone in all studies (range 20–29%), while previous null responders had modest increases in SVR using TVP-based therapy (range 39–56% versus range 9–17%) [Mchutchison et al. 2010; Zeuzem et al. 2011]. Hence, addition of either BOC or TPV resulted in significantly higher rates of SVR among partial responders and relapsers. To date, there are no extensive data on the treatment of HCV infection in African American patients, Hispanic patients, or patients with advanced liver disease using regimens that include TPV or BOC. Treatment recommendations for these subpopulations are mainly based on expert opinion. The addition of BOC to PEG-IFN + RBV regimens results in increased incidence of anemia, dysgeusia, and neutropenia compared with PEG-IFN + RBV alone [Kwo et al. 2010; Poordad et al. 2011]. The addition of TPV to PEG-IFN + RBV regimens is associated with increased fatigue, pyrexia, nausea, diarrhea, hemorrhoids, pruritus ani, rashes, alopecia, insomnia, and anemia [Mchutchison et al. 2009; Jacobson et al. 2011]. Given the diversity in adverse events reported, no formal statistical comparison of adverse events on various treatment regimens was performed, but the rates of discontinuation of medications was higher in treatment arms containing TPV or BOC than PEG-IFN + RBV alone (range 9–26% versus 8–25% versus 2–16%, respectively). In summary, the addition of a protease inhibitor increases the adverse event profile of HCV treatment and affects compliance and ability to complete treatment duration in most patients. In this regard, the CUPIC trial demonstrated that patients with cirrhosis who are more likely to benefit immediately from HCV treatment are also more likely to experience more adverse events [Hezode, 2014].
PEG-IFN + RBV + BOC for 28 or 48 weeks for GT-1 patients.
PEG-IFN + RBV + TPV for 24 or 48 weeks for GT-1 patients.
Combination therapy with PEG-IFN, RBV and a second-generation protease inhibitor, simeprevir
Simeprevir, an HCV NS3/4 serine protease inhibitor, was recently developed and used to treat hepatitis C GT-1 patients in combination with PEG-IFN and RBV. There were four separate clinical trials that evaluated this combination and demonstrated superior rates of SVR (79–86%) when 12 weeks of simeprevir was used with PEG-IFN and RBV, followed by 12–36 weeks of PEG-IFN and RBV as compared with PEG-IFN and RBV alone in treatment-naïve patients and relapsers [Fried et al. 2013; Hayashi et al. 2014; Jannsen-Therapeutics, 2013]. In these studies, 90% of patients had an early viral load decline (HCV RNA undetectable at 4 and 12 weeks) and were eligible to stop therapy at 24 weeks using the response-guided therapy approach. This response rate is much higher than what was obtained with the use of first-generation protease inhibitors, BOC and TPV. There was no difference in SVR in patients treated with either 12 or 24 weeks of simeprevir in combination with PEG-IFN and RBV, which justifies the use of simeprevir for the initial 12 weeks followed by PEG-IFN and RBV for the rest of the duration of treatment. These results demonstrate that use of simeprevir along with PEG-IFN and RBV allows for a 24 weeks treatment regime. Combination therapy with simeprevir was also evaluated in the treatment of previous null and partial responders. The addition of simeprevir (12–48 weeks) + PEG-IFN + RBV (48 weeks) in previous null and partial responders and relapsers showed high rates of SVR (67–80%) compared with PEG-IFN + RBV [Jacobson et al. 2013a]. However, similar to what was observed with BOC- and TPV-based combination therapies, SVR rates were lower in subgroups of null responders (41–59%) and partial responders (65–86%) than previous relapsers (76–89%). Due to the advances in techniques that allowed us to evaluate HCV polymorphisms and mutations, SVR rates for simeprevir-based regimens were evaluated based on preexisting mutations in the NS3/4A region. Interestingly, SVR rates were lower in treatment-naïve and treatment experienced HCV GT-1a patients with a baseline NS3 Q80K polymorphism compared with patients without the polymorphism (26–31% difference in SVR). This polymorphism is rarely observed in GT1b and hence response rates for GT1b were higher than GT1a in these studies. Simeprevir was approved by FDA in late 2013 for the treatment of HCV chronic infection as a component of a combination antiviral treatment regimen. Simeprevir therapy is reserved for those patients infected with all GT-1b and GT-1a without Q80K polymorphism. This would require all GT-1a-infected patients to undergo sequencing analysis to rule out the presence of Q80K mutation.
The use of second-generation protease inhibitor (simeprevir) for the treatment of HCV chronic infection as a component of a combination antiviral treatment regimen allows for a shorter length of therapy, usually 24 weeks in most people. Simeprevir is also associated with less-severe and less-frequent gastrointestinal, dermatological and hematological side effects [Jannsen-Therapeutics, 2013] when compared with the first-generation counterparts (BOC and TVP).
PEG-IFN + RBV + simeprevir for 12 weeks followed by PEG-IFN + RBV for an additional 12–36 weeks for GT-1b and GT-1a patients without Q80K mutation in NS3/4A patients.
Combination therapy using PEG-IFN, RBV and a novel HCV NS5B polymerase inhibitor, sofosbuvir
Recently, a novel, first-in-class HCV RNA dependent RNA polymerase inhibitor (NS5B), sofosbuvir, was approved by FDA for the treatment of chronic hepatitis C as a component of a combination antiviral treatment. Phase III clinical trial using combination therapy with PEG-IFN, weight-based RBV and sofosbuvir for 12 weeks resulted in high SVR rates in GT-1, GT-4, GT-5 and GT-6 patients (89–90%) [Kowdley et al. 2013; Lawitz et al. 2013a, 2013b]. Even though there was no comparator arm, the rates of SVR were significantly higher than what would have been historical control arm for the patients. There was no added benefit to extending duration of treatment to 24 weeks or use the response-guided therapy approach (SVR 89–91%). The addition of sofosbuvir to PEG-IFN and RBV reduces the total duration of treatment and adds little to the overall adverse event profile.
PEG-IFN + RBV + sofosbuvir for 12 weeks for GT-1, GT-4, GT-5 and GT-6 patients.
IFN-free DAA therapy
The field of HCV therapeutics has exploded with newer potent antiviral agents allowing for a variety of combination therapy with or without the use of concomitant IFNs or RBV. Furthermore, novel DAA therapy offers the additional advantage of short duration, low pill burden and favorable side-effect profile. Hence, DAA-only therapeutics may provide us with a simple and highly effective treatment for HCV. These combinations have allowed to shorten the duration of therapy even further and reduce the pill burden with the promise of making HCV therapy simpler and highly effective for those in the general population infected with hepatitis C. Recently, treatments of shortened durations with new classes of potent DAAs (Figure 2), have been shown to result in SVR with or without PEG + RBV. A recent clinical trial showed high SVR rates (up to 83%) when daclatasvir, an NS5A replication complex inhibitor of HCV, was used with PEG + RBV for 48 weeks in GT-1 treatment-naïve patients [Pol et al. 2012]. In patients infected with HCV GT-1 four small studies evaluated various IFN-free treatment regimens in HCV GT-1-infected, treatment-naïve patients resulting in high SVR rates (range 84–100%) [Chayama et al. 2012; Lok et al. 2012; Gane et al. 2013, Poordad et al. 2013]. A combination of daclatasvir and asuneprevir (NS3/4A inhibitor) was effective in treating GT1b patients [Lok et al. 2012]. However, this regimen was not successful in treating GT-1a subjects. A combination of ABT450/r (NS3/4A inhibitor), ABT 267 (an NS5A inhibitor) and ABT-333 (an NS5B inhibitor) with RBV resulted in high rates of SVR in treatment-naïve and treatment-experienced GT-1 patients [Kowdley et al. 2014].
Single DAA therapy (sofosbuvir and RBV)
The first attempt to use an interferon free regimen for hepatitis C was attempted recently using a combination of sofosbuvir and RBV. Sofosbuvir has several unique characteristics that allowed novel combination therapy regimens without the use of interferon. Sofosbuvir is a one-pill-a-day regimen that similar to interferon is active against all genotypes [Lawitz et al. 2013b]. It has an excellent safety profile and is not associated with emergence of resistance resulting in viral breakthroughs. Hence, sofosbuvir was used in a regimen that allowed treatment of hepatitis C without use of Peg-IFN. All oral combination therapy with sofosbuvir and RBV for 12-24 weeks in HCV GT-1 was evaluated in recent studies [Gane et al. 2013; Osinusi et al. 2013]. SVR rates using weight-based dosing of RBV with sofosbuvir were higher in GT-1 treatment-naïve patients (SVR 68–84%) [Lalezari et al. 2013; Osinusi et al. 2013]. One unique study compared sofosbuvir and RBV was studied in patients with traditional bad prognostic factors and resulted in high rates of SVR [Osinusi et al. 2013]. Most experience for this combination was conducted for GT-2 and GT-3 patients. In patients with HCV GT-2 or GT-3, a study showed higher rates of SVR after sofosbuvir + RBV for 12 weeks as compared with PEG + RBV for 24 weeks. For HCV GT-2-infected patients, SVR rates were (SVR 97% versus 78%) for each treatment group respectively. However, for HCV GT-3-infected patients, the improvement in SVR rates in the sofosbuvir group was not observed (SVR 56% versus 63%, respectively) [Lawitz et al. 2013b]. Extending the duration of sofosbuvir + RBV in subjects with HCV GT-2 infection minimally change the outcome (SVR 86% versus 94% at 12 and 16 weeks, respectively) [Jacobson et al. 2013b]. Extending the duration of treatment from 12 to 16 weeks in the group of patients with HCV GT-3 infection resulted in a significant increase in the SVR rate (SVR 30% versus 62%, respectively) [Jacobson et al. 2013b]. Another study confirmed the high SVR rate for sofosbuvir + RBV in treatment-naïve and treatment-experienced GT-2 patients (SVR 93%) and showed an improved SVR rate when this combination is used for 24 weeks in patient with HCV GT-3 (SVR 80% ) [Gilead-Sciences, 2013].
Sofosbuvir + RBV for 12 weeks for GT-2 patients.
Sofosbuvir + RBV for 24 weeks for GT-3 patients.
Sofosbuvir + RBV for 24 weeks for GT-1 patients.
Dual DAA combination therapy
More recently, combinations of these DAAs have been effectively used without the use of IFN and RBV to achieve high rates of SVR. In an open-label study, 88 HCV-infected treatment-naïve patients (44 HCV GT-1 and 44 GT-2/3) were assigned to daclatasvir + sofosbuvir with or without RBV for 24 weeks. The study expanded to include 123 additional patients that were randomly assigned to daclatasvir + sofusbuvir, with or without RBV for 12 weeks (82 patients were treatment naïve) or 24 weeks (41 patients with prior virologic failure with TPV or BOC + PEG-IFN + RBV). For HCV GT-1, high SVR rates (SVR 98%) were seen in both treatment-naïve and treatment-experienced patients, at 12 weeks after the end of therapy [Sulkowski et al. 2014]. High SVR rates were also seen at 12 weeks after the end of therapy for HCV GT-2- and GT-3-infected patients with SVR of 92% and 89%, respectively. High rates of SVR at week 12 were observed in HCV GT-1a and GT-1b (SVR 98% and 100%, respectively). SVR rates in patients with CC and non-CC IL28B genotypes were also high (SVR 94% and 98%, respectively) [Sulkowski et al. 2014]. In this open-label study, once-daily oral sofosbuvir and daclatasvir was associated with high SVR in patients infected with HCV GT-1, GT-2 and GT-3, including patients with no response to prior therapy with TPV or BOC [Sulkowski et al. 2014].
Triple DAA combination therapy
In another phase IIB, randomized, open-label trial of faldeprevir (a NS3/4A protease inhibitor) and deleobuvir (a nonnucleoside NS5B polymerase inhibitor) [Zeuzem et al. 2013], 362 treatment-naïve patients infected with HCV GT-1 were assigned to one of five groups: faldeprevir 120 mg once daily + deleovubir 600 mg three times daily + RBV for 16, 28, or 40 weeks (TID16W, TID28W, or TID40W, respectively); faldeprevir 120 mg once daily + deleobuvir at 600 mg twice daily + RBV for 28 weeks (BID28W); or faldeprevir 120 mg once daily + deleobuvir 600 mg three times daily, without RBV for 28 weeks (TID28W-NR). The primary endpoint SVR at 12 weeks after completion of therapy was met in 59% in the TID16W group, 59% of patients in the TID28W group, 52% of patients in the TID40W group, 69% of patients in the BID28W group, and 39% of patients in the TID28W-NR group. In this study, SVR rates were 56–85% among patients with HCV GT-1b infection, as compared with 11–47% among patients with HCV GT-1a infection. SVR rates in patients with CC and non-CC IL28B genotypes were 58–84% and 33–64%, respectively. The SVR at 12 weeks after the completion of therapy was 52–59% among patients who received IFN-free treatment with faldaprevir + deleobuvir +RBV.
In regimens using daclatasvir or sofosbuvir with PEG-IFN + RBV, the range of patients who discontinued therapy increased with treatment duration from 12 weeks (range 0–6%) to 24 weeks (range 2–14%) to 48 weeks (range 8–33%). More data from ongoing clinical trials are needed before a safety profile for these combinations can be established.
Concluding remarks
HCV therapy is steadily moving from an immune-based, long-term therapy with significant adverse events and modest efficacy to an all oral, well-tolerated, DAA, short-term, and more efficacious regimen. Today, an IFN-sparing regimen is only recommended for those infected with GT-2 and GT-3 and for GT-1 if they are intolerant or ineligible to be treated with IFN α. However, it is highly likely that an IFN-free regimen will be available for treatment of all genotypes of HCV in the near future. To date, several DAA have been developed and are currently being evaluated in various combinations in clinical trials. The recommendations for HCV therapy could soon become complex and confusing. The authors refer the reader to follow the Joint IAS-USA-IDSA-AASLD expert panel guidelines available online as a dynamic document which will be regularly updated with new information on current management strategies for chronic hepatitis C (see http://www.aasld.org). Changes to guidelines for treatment of HCV can be expected as new regimens are developed and new agents are approved by FDA. In addition, given the recently published CDC guidelines [CDC, 2012] recommending birth-cohort screening for HCV infection, many new HCV diagnoses can be expected in the United States. The increase awareness of HCV infection and the increased detection by testing will identify large numbers of chronically infected individuals. The recent advances in HCV therapeutics are in the verge of a paradigm shift in the treatment of chronic hepatitis C into a routinely curable disease. The availability of shorter, simpler, well-tolerated treatment regimens can have a major impact in reducing the morbidity and mortality associated with HCV infection. This has major implications in public health and in personalized medicine tailored for the specific needs of a particular HCV-infected patient.
A major factor to consider is the cost of curing HCV, which at the moment is substantial. It is unrealistic to expect that 170 million people with chronic hepatitis C infection worldwide can be cured with existing regimens. Moving forward, there will have to be a global strategy to test and diagnose HCV infected individuals, link them to care, and provide them with effective treatments that are simple to dispense, to take, and to adhere to. At this time, deferring HCV treatment in patients with early liver disease, while impending simpler and more effective and affordable regimens are available, seems reasonable.
Footnotes
Conflict of interest statement: The authors do not have any conflicts of interest to report.
Funding: This research was supported [in part] by the Intramural Research Program of the NIH (National Institute of Allergy and Infectious Diseases, and the Clinical Research Center).
Disclaimer: The content of this publication does not necessarily reflect the views of policies of the Department of Health and Human Services or the Department of Veterans Affairs, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Contributor Information
Karoll J. Cortez, Veteran Affairs Medical Center, Baltimore, MD, USA
Shyam Kottilil, Immunopathogenesis Section, Laboratory of Immunoregulation, NIAID, NIH, Building 10, Room 11N204, Bethesda, MD 20892, USA.
References
- Alter M., Kruszon-Moran D., Nainan O., Mcquillan G., Gao F., Moyer L., et al. (1999) The Prevalence of Hepatitis C Virus Infection in the United States, 1988 through 1994. N Engl J Med 341: 556–562. [DOI] [PubMed] [Google Scholar]
- Armstrong G., Wasley A., Simard E., Mcquillan G., Kuhnert W., Alter M. (2006) The Prevalence of Hepatitis C Virus Infection in the United States, 1999 through 2002. Ann Intern Med 144: 705–714. [DOI] [PubMed] [Google Scholar]
- Bacon B., Gordon S., Lawitz E., Marcellin P., Vierling J., Zeuzem S., et al. (2011) Boceprevir for previously treated chronic HCV genotype 1 infection. N Engl J Med 364: 1207–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC (2012) Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945–1965. MMWR 61: 1–18. [PubMed] [Google Scholar]
- Chayama K., Takahashi S., Toyota J., Karino Y., Ikeda K., Ishikawa H., et al. (2012) Dual therapy with the nonstructural protein 5a inhibitor, daclatasvir, and the nonstructural protein 3 protease inhibitor, asunaprevir, in hepatitis C virus genotype 1B-infected null responders. Hepatology 55: 742–748. [DOI] [PubMed] [Google Scholar]
- Choo Q., Kuo G., Weiner A., Overby L., Bradley D., Houghton M. (1989) Isolation of a CDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science 244: 359–362. [DOI] [PubMed] [Google Scholar]
- Chung R., Andersen J., Volberding P., Robbins G., Liu T., Sherman K., et al. (2004) Peginterferon alfa-2a plus ribavirin versus interferon alfa-2a plus ribavirin for chronic hepatitis C in HIV-coinfected persons. N Engl J Med 351: 451–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis G., Alter M., El-Serag H., Poynard T., Jennings L. (2010) Aging of hepatitis C virus (HCV)-infected persons in the United States: a multiple cohort model of HCV prevalence and disease progression. Gastroenterology 138: 513–521, 521, e511–516. [DOI] [PubMed] [Google Scholar]
- Delwart E., Slikas E., Stramer S., Kamel H., Kessler D., Krysztof D., et al. (2012) Genetic diversity of recently acquired and prevalent HIV, hepatitis B virus, and hepatitis C virus infections in US blood donors. J Infect Dis 205: 875–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flamm S., Lawitz E., Jacobson I., Bourliere M., Hezode C., Vierling J., et al. (2013) Boceprevir with peginterferon alfa-2a-ribavirin is effective for previously treated chronic hepatitis C genotype 1 infection. Clin Gastroenterol Hepatol 11: 81–U216. [DOI] [PubMed] [Google Scholar]
- Fried M., Buti M., Dore G., Flisiak R., Ferenci P., Jacobson I., et al. (2013) Once-daily simeprevir (TMC435) with pegylated interferon and ribavirin in treatment-naive genotype 1 hepatitis C: The Randomized Pillar Study. Hepatology 58: 1918–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gane E., Stedman C., Hyland R., Ding X., Svarovskaia E., Symonds W., et al. (2013) Nucleotide polymerase inhibitor sofosbuvir plus ribavirin for hepatitis C. N Engl J Med 368: 34–44. [DOI] [PubMed] [Google Scholar]
- Germer J., Mandrekar J., Bendel J., Mitchell P., Yao J. (2011) Hepatitis C virus genotypes in clinical specimens tested at a national reference testing laboratory in the United States. J Clin Microbiol 49: 3040–3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilead-Sciences (2013) Sovaldi Full Prescribing Information, Valence Data.
- Hayashi N., Seto C., Kato M., Komada Y., Goto S. (2014) Once-daily simeprevir (TMC435) with peginterferon/ribavirin for treatment-naive hepatitis C genotype 1-infected patients in Japan: The DRAGON Study. J Gastroenterol 49(1): 138–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hezode C., Forestier N., Dusheiko G., Ferenci P., Pol S., Goeser T., et al. (2009) Telaprevir and peginterferon with or without ribavirin for chronic HCV infection. N Engl J Med 360: 1839–1850. [DOI] [PubMed] [Google Scholar]
- Hezode C., Ontaine H., Dorival C., Zoulim F., Larrey D., Canva V., et al. for the CUPIC Study Group. (2014) Effectiveness of telaprevir or boceprevir in treatment-experienced patients with HCV genotype 1 infection and cirrhosis. Gastroenterology. DOI: 10.1053/j.gastro.2014.03.051. [DOI] [PubMed] [Google Scholar]
- Jacobson I., Dore G., Foster G., Fried M., Radu M., Rafalskiy V., et al. (2013a) Abstract 1425 simeorevir (TM435) with peginterferon/ribavirin for chronic HCV genotype-1 infection in treatment-naïve patients: results from QUEST-1, a phase III trial. J Hepatol 58(Suppl. 1): S574. [Google Scholar]
- Jacobson I., Gordon S., Kowdley K., Yoshida E., Rodriguez-Torres M., Sulkowski M., et al. (2013b) Sofosbuvir for hepatitis C genotype 2 or 3 in patients without treatment options. N Engl J Med 368: 1867–1877. [DOI] [PubMed] [Google Scholar]
- Jacobson I., Mchutchison J., Dusheiko G., Di Bisceglie A., Reddy K., Bzowej N., et al. (2011) Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med 364: 2405–2416. [DOI] [PubMed] [Google Scholar]
- Jannsen-Therapeutics (2013) Olysio Full Prescribing Information, Promise Data, Quest 1 Data, Quest 2 Data. http://www.olysio.com/shared/product/olysio/prescribing-information.pdf
- Kowdley K., Lawitz E., Crespo I., Hassanein T., Davis M., Demicco M., et al. (2013) Sofosbuvir with pegylated interferon alfa-2a and ribavirin for treatment-naive patients with hepatitis C genotype-1 infection (ATOMIC): an open-label, randomised, multicentre phase 2 trial. Lancet 381: 2100–2107. [DOI] [PubMed] [Google Scholar]
- Kowdley K., Lawitz E., Poordad F., Cohen D., Nelson D., Zeuzem S., et al. (2014). Phase 2B trial of interferon-free therapy for hepatitis C virus genotype 1. N Engl J Med 370: 222–232. [DOI] [PubMed] [Google Scholar]
- Kumada H., Toyota J., Okanoue T., Chayama K., Tsubouchi H., Hayashi N. (2012) Telaprevir with peginterferon and ribavirin for treatment-naive patients chronically infected with HCV of genotype 1 in Japan. J Hepatol 56: 78–84. [DOI] [PubMed] [Google Scholar]
- Kwo P., Lawitz E., Mccone J., Schiff E., Vierling J., Pound D., et al. (2010) Efficacy of boceprevir, an NS3 protease inhibitor, in combination with peginterferon alfa-2b and ribavirin in treatment-naive patients with genotype 1 hepatitis C infection (SPRINT-1): an open-label, randomised, multicentre phase 2 trial. Lancet 376: 705–716. [DOI] [PubMed] [Google Scholar]
- Lalezari J., Nelson D., Hyland R., Lin M., Rossi S., Symonds W., et al. (2013) 845 once daily sofosbuvir plus ribavirin for 12 and 24 weeks in treatment-naïve patients with HCV infection: the Quantum study. J Hepatol 58: S346. [Google Scholar]
- Lawitz E., Lalezari J., Hassanein T., Kowdley K., Poordad F., Sheikh A., et al. (2013a) Sofosbuvir in combination with peginterferon alfa-2a and ribavirin for non-cirrhotic, treatment-naive patients with genotypes 1, 2, and 3 hepatitis c infection: a randomised, double-blind, phase 2 trial. Lancet Infect Dis 13:401–408. [DOI] [PubMed] [Google Scholar]
- Lawitz E., Mangia A., Wyles D., Rodriguez-Torres M., Hassanein T., Gordon S., et al. (2013b) Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med 368: 1878–1887. [DOI] [PubMed] [Google Scholar]
- Lok A., Gardiner D., Lawitz E., Martorell C., Everson G., Ghalib R., et al. (2012) Preliminary study of two antiviral agents for hepatitis C genotype 1. N Engl J Med 366: 216–224. [DOI] [PubMed] [Google Scholar]
- Manos M., Shvachko V., Murphy R., Arduino J., Shire N. (2012) Distribution of hepatitis C virus genotypes in a diverse US integrated health care population. J Med Virol 84: 1744–1750. [DOI] [PubMed] [Google Scholar]
- Mchutchison J., Everson G., Gordon S., Jacobson I., Sulkowski M., Kauffman R., et al. (2009) Telaprevir with peginterferon and ribavirin for chronic HCV genotype 1 infection. N Engl J Med 360: 1827–1838. [DOI] [PubMed] [Google Scholar]
- Mchutchison J., Gordon S., Schiff E., Shiffman M., Lee W., Rustgi V., et al. (1998) Interferon alfa-2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. Hepatitis Interventional Therapy Group. N Engl J Med 339: 1485–1492. [DOI] [PubMed] [Google Scholar]
- Mchutchison J., Manns M., Muir A., Terrault N., Jacobson I., Afdhal N., et al. (2010) Telaprevir for previously treated chronic HCV infection. N Engl J Med 362: 1292–1303. [DOI] [PubMed] [Google Scholar]
- Mohd Hanafiah K., Groeger J., Flaxman A., Wiersma S. (2013) Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology 57: 1333–1342. [DOI] [PubMed] [Google Scholar]
- Muir A., Bornstein J., Killenberg P. (2004) Peginterferon alfa-2b and ribavirin for the treatment of chronic hepatitis C in blacks and non-Hispanic whites. N Engl J Med 350: 2265–2271. [DOI] [PubMed] [Google Scholar]
- Osinusi A., Meissner E., Lee Y., Bon D., Heytens L., Nelson A., et al. (2013) Sofosbuvir and ribavirin for hepatitis C genotype 1 in patients with unfavorable treatment characteristics: a randomized clinical trial. JAMA 310: 804–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pol S., Ghalib R., Rustgi V., Martorell C., Everson G., Tatum H., et al. (2012) Daclatasvir for previously untreated chronic hepatitis C genotype-1 infection: a randomised, parallel-group, double-blind, placebo-controlled, dose-finding, phase 2A trial. Lancet Infect Dis 12: 671–677. [DOI] [PubMed] [Google Scholar]
- Poordad F., Lawitz E., Kowdley K., Cohen D., Podsadecki T., Siggelkow S., et al. (2013) Exploratory study of oral combination antiviral therapy for hepatitis C. N Engl J Med 368: 45–53. [DOI] [PubMed] [Google Scholar]
- Poordad F., Mccone J., Jr, Bacon B., Bruno S., Manns M., Sulkowski M., et al. (2011) Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med 364: 1195–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poynard T., Marcellin P., Lee S., Niederau C., Minuk G., Ideo G., et al. (1998) Randomised trial of interferon alpha2b plus ribavirin for 48 weeks or for 24 weeks versus interferon alpha2b plus placebo for 48 weeks for treatment of chronic infection with hepatitis C virus. International Hepatitis Interventional Therapy Group (IHIT). Lancet 352: 1426–1432. [DOI] [PubMed] [Google Scholar]
- Renault P., Hoofnagle J.H. (1989) Side effects of alpha interferon. Semin Liver Dis 9: 273–277. [DOI] [PubMed] [Google Scholar]
- Rubbia-Brandt L., Quadri R., Abid K., Giostra E., Male P., Mentha G., et al. (2000) Hepatocyte steatosis is a cytopathic effect of hepatitis C virus genotype 3. J Hepatol 33: 106–115. [DOI] [PubMed] [Google Scholar]
- Salmon-Ceron D., Rosenthal E., Lewden C., Bouteloup V., May T., Burty C., et al. (2009) Emerging role of hepatocellular carcinoma among liver-related causes of deaths in HIV-infected patients: the French National Mortalite 2005 Study. J Hepatol 50: 736–745. [DOI] [PubMed] [Google Scholar]
- Sherman K., Flamm S., Afdhal N., Nelson D., Sulkowski M., Everson G., et al. (2011) Response-guided telaprevir combination treatment for hepatitis C virus infection. N Engl J Med 365: 1014–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulkowski M., Gardiner D., Rodriguez-Torres M., Reddy K., Hassanein T., Jacobson I., et al. (2014) Daclatasvir plus sofosbuvir for previously treated or untreated chronic HCV infection. N Engl J Med 370: 211–221. [DOI] [PubMed] [Google Scholar]
- Swain M., Lai M., Shiffman M., Cooksley W., Zeuzem S., Dieterich D., et al. (2010) A sustained virologic response is durable in patients with chronic hepatitis C treated with peginterferon alfa-2a and ribavirin. Gastroenterology 139: 1593–1601. [DOI] [PubMed] [Google Scholar]
- Zator Z., Chung R. (2013) After the cure: management of HCV after achievement of SVR. Curr HIV/AIDS Rep 10: 428–435. [DOI] [PubMed] [Google Scholar]
- Zeuzem S., Andreone P., Pol S., Lawitz E., Diago M., Roberts S., et al. (2011) Telaprevir for retreatment of HCV infection. N Engl J Med 364: 2417–2428. [DOI] [PubMed] [Google Scholar]
- Zeuzem S., Soriano V., Asselah T., Bronowicki J., Lohse A., Mullhaupt B., et al. (2013) Faldaprevir and deleobuvir for HCV genotype 1 infection. N Engl J Med 369: 630–639. [DOI] [PubMed] [Google Scholar]